Abstract
Purpose
To evaluate the development of visual acuity, dysphotopsia phenomena, and subjective sensations in patients implanted with the Tecnis® ZMB00 (Advanced Medical Optics Inc, Santa Ana, CA) multifocal intraocular lens.
Methods
In a sample of 70 eyes, distance and near visual acuity, with and without correction, contrast sensitivity, and patient satisfaction, were analyzed at 15, 30, and 60 days post-surgery.
Results
Mean uncorrected distance visual acuity (logarithm of minimum angle of resolution) at 15, 30, and 60 days was 0.194 ± 0.054, 0.119 ± 0.026, and 0.076 ± 0.014 (P < 0.0001), respectively, and with correction, 0.051 ± 0.007 vs 0.041 ± 0.004 vs 0.022 ± 0.002 (P < 0.0001), respectively. At 60 days, 94.3% of eyes could read 1.00 close-up without correction. Patient satisfaction in terms of dysphotopsia effects and visual acuity was excellent. The mean contrast sensitivity was 1.64 ± 0.10 (logarithmic units) measured with the Pelli–Robson test.
Conclusion
This type of multifocal intraocular lens was very effective at the distances analyzed, producing excellent objective and subjective results.
Keywords: higher-order aberrations, diffractive multifocal intraocular lens, visual acuity, dysphotopsia
Introduction
In the last 20 years, our knowledge of the fundamental pathophysiological causes of the deterioration of visual function with age has advanced considerably. In particular, much is now known about contrast sensitivity as a parameter for evaluating the quality of a patient’s vision.1 Guirao et al2 and Glasser and Campbell3 showed that the pathogenesis of vision deterioration relies on an increase in the spherical aberration of the eye, with a loss of balance between corneal and lenticular aberration. In the young eye, the negative spherical aberration of the lens compensates for the positive spherical aberration of the cornea to produce a single retinal focal point. As the years pass, the lenticular spherical aberration becomes progressively more positive, while the corneal spherical aberration remains relatively stable.4 We have studied the one-piece Tecnis® ZMB00 intraocular lens (Advanced Medical Optics Inc, Santa Ana, CA). This lens offers a modified prolate anterior surface that adds −0.27 μm of spherical aberration to the eye, thus emulating the optics of the young eye.
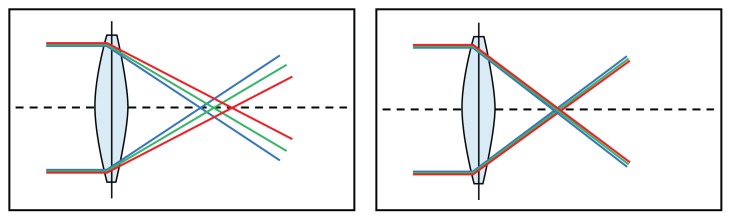
The lens uses a hydrophobic acrylic material with a high Abbe number of 55.5 Chromatic dispersion is lower in this material and can result in an improvement of up to 12% in contrast sensitivity compared with other materials,6 thus minimizing the impact of chromatic aberration (Figure 1).
Figure 1.
Lenses with and without chromatic aberration.
Notes: This wavelength-dependent refractive error depends on optic material. Materials with a higher Abbe number have lower chromatic dispersion.
The posterior surface has a full diffractive optic. This facilitates a constantly balanced light distribution between the distance and near foci and visual performance independent of variations in pupil size.
The lens blocks ultraviolet radiation but allows the passage of blue light, which is fundamental to good scotopic sensitivity.7 Blue light is also important for regulating circadian rhythm.8 In 2002, a new type of photoreceptor pigment, called melanopsin, was detected in the cell bodies and dendrites of retinal ganglion cells.9 This pigment is involved in the regulation of circadian rhythm10 and has its absorption maximum in blue light.
The hydrophobic acrylic material used in the manufacture of ZMB00 does not show any glistening or calcifications that would affect visual acuity, produce stray light, or diminish contrast sensitivity.12–15
Patients and methods
A prospective study was performed in 70 eyes (70 patients) implanted with the Tecnis ZMB00 multifocal lens.
The development of postoperative distance and near visual acuity with and without correction was studied at 15, 30, and 60 days. Postoperative contrast sensitivity was studied with the Pelli–Robson test (Haag-Streit, Koeniz, Switzerland) at 60 days. Possible adverse effects (dysphotopsia, halos, glare) were assessed with patient satisfaction questionnaires at every postoperative visit. The distance and near visual acuity results achieved were determined by means of subjective evaluation by the patient and surgeon at each visit.
The inclusion criterion for this study was patients with N01–N04 cataracts according to the Lens Opacities Classification System III. The exclusion criterion used in this study protocol was any patient who experienced impairment of visual acuity in some way due to previous systemic or ocular pathology.
In accordance with the Declaration of Helsinki, all patients received an explanation of what was going to be done and signed the necessary informed consent form.
All patients underwent ultrasound phacoemulsification performed by the same surgeon at the Quirón University Hospital of Madrid.
Using topical anesthesia, a clear corneal incision was made with a 2.75 mm calibrated diamond knife along the steep meridian. Biometry, using the IOLMaster® (Carl Zeiss, CZM-Jena, Germany), was performed. The refractive power of the emmetropic intraocular lens was calculated using the SRK-T, Hoffer Q, or Haigis formulae in relation to axial length.
Measurements
Visual acuity was measured using the logarithm of the minimum angle of resolution (logMAR) at 4 m and the standard ETDRS chart (Precision Vision, La Salle, IL) back-lit at 85 cd/m2, with the level of luminance as recommended by the Food and Drug Administration and the National Eye Institute for eye examinations.
The near visual acuity was measured using a Rosenbaum chart.
Patient satisfaction and the incidence of photic phenomena at each postoperative follow-up visit was recorded and evaluated. Specifically, patients were asked the following questions: (1) How would you rate your near vision? (2) How would you rate your distance vision? (3) How would you rate your overall vision? (Answers for questions 1–3 were: A, very good; B, good; C, sufficient; D, insufficient.) (4) Do you have visual symptoms such as glare or halos? (Answers were: A, none; B, mild; C, moderate; D, severe).
At 60 days, contrast sensitivity at 3 m with the best distance correction was evaluated using the Pelli–Robson test in photopic conditions (85 cd/m2). The test chart consists of eight lines of six letters each, but each line contains two three-letter sets at different contrast sensitivity levels. Reading from left to right and top to bottom, log contrast sensitivity increases in 0.15 log steps from 0.05 to 2.30. All letters in the test are the same size, subtending 5° of visual angle at a distance of 3 m, with a line width of 1°.
Statistical analysis of data
Statistical analysis of the data was carried out with SPSS software (v 17.0; IBM Corporation, Somers, NY). A t-test was used to compare related samples. This technique is especially indicated for the analysis of differences between the means of two variables within the same study group. P values of less than 0.05 were taken as the reference for statistical significance.
Results
The average age of the patients in the group was 67 ± 7 years.
Mean uncorrected distance visual acuity (logMAR) at 15, 30, and 60 days was 0.194 ± 0.054 vs 0.119 ± 0.026 vs 0.076 ± 0.014 (P < 0.0001), respectively (Table 1). Of the eyes operated, 74.3% had a distance vision of 0.1 logMAR or better at 60 days of surgery. The spherical equivalent obtained at 60 days post-surgery was −0.08 ± 0.56 D. The development of uncorrected distance visual acuity from day 15 to day 60 of the postoperative follow-up was very satisfactory and improved by 0.118 logMAR (P < 0.0001). The mean visual acuity (logMAR) with postoperative correction of distance vision at 15, 30, and 60 days was 0.051 ± 0.007 vs 0.041 ± 0.004 vs 0.022 ± 0.002 (P < 0.0001), respectively.
Table 1.
Development of uncorrected and best-corrected distance visual acuity (logMAR)
| Follow-up | Uncorrected visual acuity | Best-corrected visual acuity |
|---|---|---|
| 15 days | 0.194 ± 0.054 | 0.051 ± 0.007 |
| 30 days | 0.119 ± 0.026 | 0.041 ± 0.004 |
| 60 days | 0.076 ± 0.014 | 0.022 ± 0.002 |
Abbreviation: logMAR, logarithm of the minimum angle of resolution.
The proportions of patients with best-corrected and uncorrected near visual acuity Jaeger values at 15, 30, and 60 days are shown in Table 2.
Table 2.
Proportion of patients (%) with best-corrected and uncorrected near visual acuity Jaeger values at 15, 30, and 60 days
| Follow-up | J1 | J2 | J3 | J4 | |
|---|---|---|---|---|---|
| Uncorrected visual acuity | 15 days | 17.14 | 47.14 | 18.57 | 17.14 |
| 30 days | 48.57 | 34.29 | 15.71 | 1.43 | |
| 60 days | 78.57 | 18.57 | 2.86 | 0.00 | |
| Best-corrected visual acuity | 15 days | 57.14 | 40.00 | 2.86 | 0.00 |
| 30 days | 85.71 | 12.86 | 1.43 | 0.00 | |
| 60 days | 94.29 | 4.29 | 1.43 | 0.00 |
Most of the eyes (94.3%) could read Jaeger J1 without correction at 60 days after surgery. None of the eyes read Jaeger J4 at the final follow-up and only two eyes read Jaeger J3. It is interesting to note the positive development experienced by the eyes that were eventually able to read Jaeger J1 in the course of the three follow-up visits; vision improved by an average of 30.7% (P < 0.0001) in each examination.
The results of the visual satisfaction questionnaires indicated that 90.0% of patients rated their distance vision as good to very good and 97.1% rated their near vision as good to very good. Therefore, their overall vision was very satisfactory. None of the patients in the sample rated their distance or near vision as insufficient, as shown in Tables 3 and 4.
Table 3.
Subjective evaluation of distance visual acuity by patients (%)
| Distance vision subjective evaluation test (patient evaluation) | ||||
|---|---|---|---|---|
|
| ||||
| Period | A | B | C | D |
| 15 days | 47.14 | 14.29 | 37.14 | 1.43 |
| 30 days | 54.29 | 30.00 | 15.71 | 0.00 |
| 60 days | 60.00 | 30.00 | 10.00 | 0.00 |
Notes: A, very good; B, good; C, sufficient; D, insufficient.
Table 4.
Subjective evaluation of near visual acuity by patients (%)
| Near vision subjective evaluation test (patient evaluation) | ||||
|---|---|---|---|---|
|
| ||||
| Period | A | B | C | D |
| 15 days | 47.14 | 25.71 | 25.71 | 1.43 |
| 30 days | 50.00 | 31.43 | 18.57 | 0.00 |
| 60 days | 74.29 | 22.86 | 2.86 | 0.00 |
Notes: A, very good; B, good; C, sufficient; D, insufficient.
According to the surgeon satisfaction survey, visual performance achieved by patients was rated as very good for near vision in 98.6% and very good for distance vision in 100%.
Photic adverse effects disappeared in most cases at 60 days; 88.6% had none, 10.0% had mild effects, and only 1.4% had moderate effects.
Contrast sensitivity16 was almost unaffected by the implant, reaching values of 1.64 ± 0.10 (P < 0.0001) in 75.7% of cases 60 days after surgery (Table 5). The average contrast sensitivity for the entire sample was 1.63 ± 0.10. In a previous study that was carried out with the Tecnis ZM900 intraocular lens,17 contrast sensitivity was 1.44 ± 0.26 as evaluated with the Pelli–Robson test under the same conditions, but at 210 days. This means that better contrast sensitivity values were obtained with the Tecnis ZMB00 multifocal intraocular lens in a much shorter follow-up time.
Table 5.
Contrast sensitivity values at 60 days
| Eyes | Contrast sensitivity |
|---|---|
| 2 | 1.58 ± 0.11 |
| 15 | 1.60 ± 0.11 |
| 53 | 1.64 ± 0.10 |
| 70 | 1.63 ± 0.10 |
Posterior capsular opacity did not occur in any patients studied during the entire follow-up period, so it did not influence data analysis.
Discussion
In this study, a sample of 70 eyes implanted with Tecnis ZMB00 multifocal intraocular lenses was analyzed during a follow-up of 15, 30, and 60 days, respectively. Of the eyes operated, 74.3% achieved distance vision of 0.097 logMAR or more at 60 days after surgery, and 94.3% could read Jaeger J1 without requiring vision correction at 60 days. Of the patients, 90% rated their distance vision without correction as good to very good at 60 days of postoperative follow-up, and 97.1% had the same opinion of their near vision at 60 days of postoperative follow-up. The surgeon rated overall vision as good for distance vision in 100% and for near vision in 98.6%. Of the total, 88.6% stated in the evaluation questionnaire that they had not experienced adverse effects, and only 1.4% experienced moderate effects. Of the operated eyes, 75.71% had a contrast sensitivity of 1.64 ± 0.10 (logarithmic units) measured with the Pelli–Robson test, the average of all eyes being 1.63 ± 0.10.
In an earlier study of three-piece multifocal diffractive lenses,17 longer neuroadaptation rates (time period that the implant wearer requires to adapt to the new visual situation) than with the new intraocular lens were found. Patients with the Tecnis ZMB00 multifocal intraocular lens achieved similar neuroadaptation values at 60 days of follow-up to patients with the older ZM900 lens at 210 days, probably due to design improvements, optical characteristics, and the difference in the incision size. This neuroadaptive process may take several months in some cases, however, and therefore a longer follow-up may be necessary to assess the full potential of the new intraocular lens. It is interesting to note that visual recovery after lens implantation may be significantly accelerated by specific vision training programs, as described by Kaymak et al.18 These authors showed that after 2 weeks of training, the trained eyes (nondominant eye) functioned significantly better than the fellow eye in terms of visual acuity and contrast sensitivity.
These findings coincide with those of Tan and Fong,19 who found improved contrast sensitivity and visual acuity in low-myopia eyes after neural vision therapy. These results reinforce the idea that neural plasticity has a primary role in the adaptation of the visual system to changing conditions or in the acquisition of new capabilities. However, as in any learning process, results can vary with time and personal effort and are constrained by the inherent limits of each individual.
Simultaneous compensation of both spherical and chromatic aberrations was achieved in a single procedure with better visual results than the sum of the separate corrections. 20–23 This was achieved due to the prolate design of the anterior surface and the low chromatic dispersion of the material used to manufacture the lens compared with other materials. Near and distance visual acuities were good, and the contrast sensitivity was almost unaffected, so the patient’s overall visual performance was excellent.
The eyes studied did not require any refractive correction to further improve vision.
All measurements for this study were monocular. This fact further reinforces the value of the results because binocular visual acuity is better than monocular in multifocal intraocular lens patients.
To conclude, this study shows a high grade of patient satisfaction, as evaluated using distance and near visual acuity tests, and subjective visual perceptions, indicating that implantation of this lens was a good experience for the patient. From the surgeon’s point of view, and supported by subjective satisfaction questionnaires, the ZMB00 multifocal intraocular lens was an effective device for the surgical correction of patients’ accommodation disorders, and one that allowed them to be independent of corrective lenses.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Owsley C, Sekuler R, Siemsen D. Contrast sensitivity throughout adulthood. Vision Res. 1983;23:689–699. doi: 10.1016/0042-6989(83)90210-9. [DOI] [PubMed] [Google Scholar]
- 2.Guirao A, Redondo M, Artal P. Optical aberrations of the human cornea as a function of age. J Opt Soc Am A Opt Image Sci Vis. 2000;17:1697–1702. doi: 10.1364/josaa.17.001697. [DOI] [PubMed] [Google Scholar]
- 3.Glasser A, Campbell MC. Presbyopia and the optical changes in the human crystalline lens with age. Vision Res. 1998;38:209–229. doi: 10.1016/s0042-6989(97)00102-8. [DOI] [PubMed] [Google Scholar]
- 4.Alió JL, Schimchak P, Negri HP, et al. Crystalline lens optical dysfunction through aging. Ophthalmology. 2005;112:2022–2029. doi: 10.1016/j.ophtha.2005.04.034. [DOI] [PubMed] [Google Scholar]
- 5.Zhao H, Mainster M. The effect of chromatic dispersion on pseudophakic optical performance. Br J Ophthalmol. 2007;91:1225–1229. doi: 10.1136/bjo.2007.118745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao H, Piers PA, Mainster MA. The additive effects of different optical design elements contributing to contrast loss in pseudophakic eyes implanted with different aspheric IOLs. Presented at XXVII Congress of the ESCRS; September 12–16, 2009; Barcelona, Spain. [Google Scholar]
- 7.Terwee T, Weeber H, van der Mooren M, et al. Visualization of the retinal image in an eye model with spherical and aspheric, diffractive, and refractive multifocal intraocular lenses. J Refract Surg. 2008;24:223–232. doi: 10.3928/1081597X-20080301-03. [DOI] [PubMed] [Google Scholar]
- 8.Mainster MA. Violet and blue light blocking intraocular lenses: photoprotection vs photoreception. Br J Ophthalmol. 2006;90:784–792. doi: 10.1136/bjo.2005.086553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 10.Provencio I, Rodriguez IR, Jiang G, et al. A novel human opsin in the inner retina. J Neurosci. 2000;20:600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berson DM. Strange vision: ganglion cells as circadian photoreceptors. Trends Neurosci. 2003;26:314–320. doi: 10.1016/S0166-2236(03)00130-9. [DOI] [PubMed] [Google Scholar]
- 12.Turner PL, Mainster MA. Circadian photoreception: ageing and the eye’s important role in systemic health. Br J Ophthalmol. 2008;92:1439–1444. doi: 10.1136/bjo.2008.141747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyata A, Yaguchi S. Equilibrium water content and glistenings in acrylic intraocular lenses. J Cataract Refract Surg. 2004;30:1768–1772. doi: 10.1016/j.jcrs.2003.12.038. [DOI] [PubMed] [Google Scholar]
- 14.Gunenc U, Oner FH, Tongal S, Ferliel M. Effects on visual function of glistenings and folding marks in AcrySof intraocular lenses. J Cataract Refract Surg. 2001;27:1611–1614. doi: 10.1016/s0886-3350(01)00995-6. [DOI] [PubMed] [Google Scholar]
- 15.Christiansen G, Durcan FJ, Olson RJ, et al. Glistenings in the AcrySof intraocular lens: pilot study. J Cataract Refract Surg. 2001;27:728–733. doi: 10.1016/s0886-3350(00)00700-8. [DOI] [PubMed] [Google Scholar]
- 16.Mäntyjärvi M, Laitinen T. Normal values for the Pelli–Robson contrast sensitivity test. J Cataract Refract Surg. 2001;27:261–266. doi: 10.1016/s0886-3350(00)00562-9. [DOI] [PubMed] [Google Scholar]
- 17.Palomino Bautista C, Carmona González D, Castillo Gómez A, Bescos JA. Evolution of visual performance in 250 eyes implanted with the Tecnis ZM900 multifocal IOL. Eur J Ophthalmol. 2009;19(5):762–768. doi: 10.1177/112067210901900513. [DOI] [PubMed] [Google Scholar]
- 18.Kaymak H, Fahle M, Ott G, et al. Intraindividual comparison of the effect of training on visual performance with ReSTOR and Tecnis diffractive multifocal IOLs. J Refract Surg. 2008;24:287–293. doi: 10.3928/1081597X-20080301-11. [DOI] [PubMed] [Google Scholar]
- 19.Tan DT, Fong A. Efficacy of neural vision therapy to enhance contrast sensitivity function and visual acuity in low myopia. J Cataract Refract Surg. 2008;34:570–577. doi: 10.1016/j.jcrs.2007.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Artal P, Manzanera S, Piers P, et al. Visual effect of the combined correction of spherical and longitudinal chromatic aberrations. Opt Express. 2010;18:1637–1648. doi: 10.1364/OE.18.001637. [DOI] [PubMed] [Google Scholar]
- 21.López-Gil N, Montés-Micó R. New intraocular lens for achromatizing the human eye. J Cataract Refract Surg. 2007;33:1296–1302. doi: 10.1016/j.jcrs.2007.03.041. [DOI] [PubMed] [Google Scholar]
- 22.Yoon GY, Williams DR. Visual performance after correcting the monochromatic and chromatic aberrations of the eye. J Opt Soc Am A Opt Image Sci Vis. 2002;19:266–275. doi: 10.1364/josaa.19.000266. [DOI] [PubMed] [Google Scholar]
- 23.Manzanera S, Piers P, Wheeler H, Artal P. Visual benefit of the combined correction of spherical and chromatic aberrations [abstract] Ophthalmol Vis Sci. 2007;48:E-Abstract 1513. [Google Scholar]