Abstract
Background
Glaucoma can be associated with an increase in the occurrence of ocular surface disease (OSD) symptoms. The objective of this study was to examine the prevalence of ocular surface complaints in patients with glaucoma who used topical intraocular pressure (IOP)-lowering therapies.
Methods
In this multicenter, international, noninterventional study, adults with glaucoma or ocular hypertension who were using 1 or more topical IOP-lowering medications completed the Ocular Surface Disease Index (OSDI) questionnaire during a regularly scheduled clinic visit. OSDI scores (ranging from 0 to 100) were calculated for each patient. An OSDI score ≥13 indicated a clinically relevant presence of OSD.
Results
Of the 448 patients who were evaluated, 53.3% were women, 61.6% had a diagnosis of primary open-angle glaucoma, and the mean age was 63 years. The overall OSD prevalence rate in the evaluable population was 59.2%, with 25.7%, 13.2%, and 20.3% of the patients reporting mild, moderate, or severe OSD symptoms, respectively. Patients with glaucoma diagnoses of less than 6 years had a significantly lower mean OSDI score relative to patients with glaucoma diagnoses of 6 years or more (18 [mild OSD] versus 23 [moderate OSD], respectively; P = 0.03). As the number of IOP-lowering treatments increased from one or two medications to three or four medications, the mean OSDI score increased from mild to moderate, though the difference in scores was not statistically significant (P = 0.15).
Conclusions
OSD was highly prevalent in this population of glaucoma patients who were using IOP-lowering medications. Longer duration since diagnosis was significantly correlated with worsening of OSD symptoms. Increases in the number of medications applied also showed a clinically relevant increase in OSD symptom severity.
Keywords: OSDI, correlation, time since diagnosis, number of medications
Introduction
Ocular surface disease (OSD) is a multifactorial ocular condition that results from inadequate tear film production and/or increased tear evaporation, and may involve tear film degradation as well as damage to the ocular surface.1 Ocular surface damage may be triggered by noninfectious irritation of the conjunctival and corneal surface, which, in conjunction with a compromised tear film, may aggravate the signs and symptoms of OSD and may leave the ocular surface vulnerable to further injury.2–4
Individuals with OSD may experience a number of ocular symptoms at varying levels of severity, including dryness, burning/stinging, itching, irritation, tearing, photophobia, foreign body sensation, grittiness, redness, and blurred vision.5 Additionally, patients with OSD may or may not have clinically meaningful signs, such as rapid tear film breakup, high tear osmolarity, and increased ocular surface staining.1 Studies suggest that OSD negatively impacts visual function; the ability to carry out daily tasks (eg, driving and participating in sports and other leisure activities, such as reading and cooking); and overall quality of life.6,7
Approximately 15% of the general elderly population experiences some level of OSD.8 Patients with glaucoma and ocular hypertension, however, have been shown to suffer OSD at a higher prevalence rate than patients without these ocular conditions.2 The etiology of OSD in glaucoma patients is thought to be multifactorial: while both conditions are common in the elderly, the presence of additional anterior segment ocular disorders (eg, allergy, blepharitis, dry eye, or eyelid anatomical abnormalities) may further contribute to the onset of OSD. In addition, substantial attention has been focused on the chronic use of topical ocular medications in this population.2,4,5 Specifically, medications intended to lower intraocular pressure (IOP) are generally administered several times a day, and patients with chronic conditions like glaucoma and ocular hypertension require long-term use and/or multiple medications to achieve and maintain the desired IOP-lowering effects.9 Topical ocular IOP-lowering drugs, however, can trigger or exacerbate OSD by inducing ocular surface damage, especially if they contain preservatives, particularly benzalkonium chloride (BAK).5,9,10 The objective of the current study was therefore to examine the overall prevalence of OSD in an international population of glaucoma patients who were taking one or more IOP-lowering medications.
Methods
Study design
This was a multicenter, international, noninterventional, single-visit study designed to evaluate the prevalence of OSD in patients with glaucoma. Upon entry, all participating patients provided their written informed consent. An Institutional Review Board/Independent Ethics Committee in each associated country approved the protocol and the participating investigator. The study was conducted in accordance with Good Clinical Practices and the ethical principles described within the Declaration of Helsinki.
During a regularly scheduled clinic visit, patients who met all of the inclusion and none of the exclusion criteria completed the Ocular Surface Disease Index (OSDI) questionnaire. Demographic information, medical histories, and concomitant medication usage, including artificial tear usage, were recorded for each patient.
Patients
Overall, up to 600 patients were planned for enrollment at up to 12 investigational centers located in Europe, Asia, Australia, and Latin America. Eligible patients included men and women of any race or ethnicity who were 18 years of age and older, were diagnosed with glaucoma (closed-angle, open-angle, pseudoexfoliation, or pigment dispersion) or ocular hypertension, and, at the time of enrollment, were using topical ocular medications to lower IOP. Additionally, all patients must have had a best-corrected visual acuity of at least 20/60 in each eye.
Assessments
Patients who met the entry criteria completed the OSDI. Specifically, this instrument is a 12-item, disease-specific quality of life questionnaire that is used to quantify the impact of dry eye on vision-related quality of life. The questionnaire includes three subscales: ocular discomfort (OSDI-symptoms); functioning (OSDI-function); and environmental triggers (OSDI-triggers). The individual items within the subscales refer to a 1-week recall period; possible responses to each item refer to the frequency of the associated disturbance. Each response was recorded using a scale that ranged from 0 (none of the time) to 4 (all of the time). The average score was transformed to a scale ranging from 0 to 100, with higher scores representing greater disabilities. The impact of dry eye was then assessed categorically as normal (scores of 0–12), mild (scores of 13–22), moderate (scores of 23–32), or severe (scores of 33–100), as previously described.11–13 The OSDI questionnaire has been reported to have excellent test-retest reliability and to effectively classify clinically normal, mild to moderate, and severe OSD.14,15
Statistics
All analyses were conducted using data from patients who satisfied the entry criteria (ie, the evaluable patients). The numbers and percentages of glaucoma patients who had OSDI scores indicating a normal ocular surface or indicating the presence of mild, moderate, or severe OSD were tabulated. Correlations between the mean OSDI score and the time since glaucoma diagnosis, as well as the number of IOP-lowering medications used, were also tabulated. Specifically, the mean OSDI scores were summarized by the categorical time since glaucoma diagnosis (< mean number of years since diagnosis and ≥ mean number of years since diagnosis) and, separately, by the categorical number of IOP-lowering medications used (1 or 2 medications and 3 or 4 medications); comparisons within subgroups were performed using either an analysis of variance or a t-test with inferences drawn at an alpha level of 0.05.
Results
Patient characteristics and disposition
A total of 458 patients were enrolled across eight investigational centers located in Argentina, Australia, China, Colombia, Germany, India, Mexico, and Spain. Of the enrolled patients, 448 met all of the entry criteria and were included in the analyses. The evaluable patients were 19 to 90 years of age, inclusive, with a mean (standard deviation [SD]) age of 63 (14) years. Overall, 56.0% of the patients were Caucasian and 53.3% of the patients were women. Most of the patients (78.6%) had a diagnosis of either primary open-angle glaucoma (61.6%) or ocular hypertension (17.0%) as shown in Table 1.
Table 1.
Demographic and baseline characteristics (evaluable patients)
| Total (n = 448) | |
|---|---|
| Age, years | |
| Mean (standard deviation) | 63 (14) |
| Range (minimum, maximum) | (19, 90) |
| Sex, n (%) | |
| Men | 209 (46.7) |
| Women | 239 (53.3) |
| Race, n (%) | |
| Caucasian | 251 (56.0) |
| Black | 0 (0.0) |
| Asian | 117 (26.1) |
| Other | 80 (17.9) |
| Glaucoma diagnosis, n (%) | |
| Ocular hypertension | 76 (17.0) |
| Primary open-angle glaucoma | 276 (61.6) |
| Open-angle glaucoma with pseudoexfoliation | 53 (11.8) |
| Open-angle glaucoma with pigment-dispersion | 6 (1.3) |
| Closed-angle glaucoma | 37 (8.3) |
| Time since glaucoma diagnosis, years (n = 387) | |
| Mean (standard deviation) | 6.0 (5.4) |
| < 6 years, n (%) | 250 (64.6) |
| ≥ 6 years, n (%) | 137 (35.4) |
| Number of IOP-lowering medications | |
| Mean (standard deviation) | 1.9 (1.3) |
| 1 or 2, n (%) | 348 (77.7) |
| 3 or 4, n (%) | 83 (18.5) |
| ≥ 5, n (%) | 17 (3.8) |
Abbreviation: IOP, intraocular pressure.
Prevalence of ocular surface disease
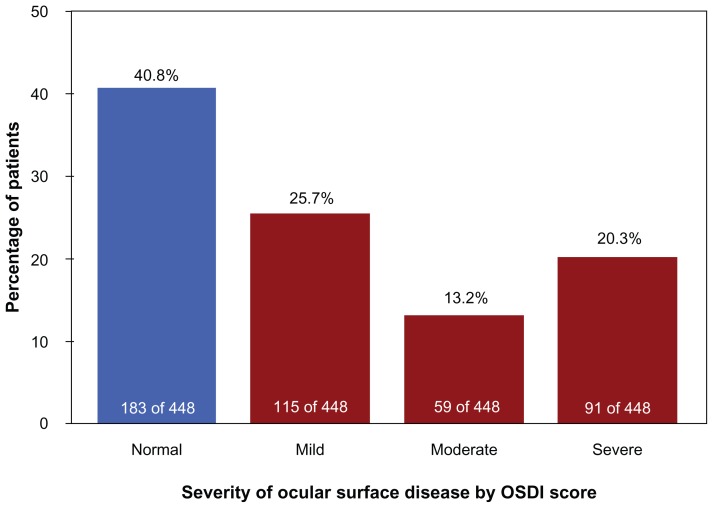
More than half of the total study population had abnormal OSDI scores (265 of 448 patients), with an overall OSD prevalence rate of 59.2% (95% confidence interval: 54.6%–63.7%). The mean age of patients with normal OSDI scores was 62 (14) years, which was not significantly different (P = 0.18) from the mean age of patients with abnormal OSDI scores, at 64 (14) years. Based on their OSDI scores, of the 448 evaluated patients, 115 (25.7%) had mild OSD, 59 (13.2%) had moderate OSD, and 91 (20.3%) had severe OSD (Figure 1).
Figure 1.
Number and percentage of glaucoma patients with Ocular Surface Disease Index scores indicating normal ocular surface or the presence of mild, moderate, or severe dry eye/ocular surface disease complaints (evaluable patients).
Abbreviation: OSDI, Ocular Surface Disease Index.
Correlation of ocular surface disease with time since glaucoma diagnosis
The mean (SD) time since glaucoma diagnosis in this population was 6.0 (5.4) years. Using this as the basis for dividing patients into categories of durations since their glaucoma diagnosis (ie, ≥6 years and <6 years), a direct correlation was shown between an increase in time since glaucoma diagnosis and higher (worse) OSDI scores (Table 2). On average, patients who had a glaucoma diagnosis of less than 6 years had a mean (SD) OSDI score of 18 (16) units, which is indicative of mild OSD, while patients who had a glaucoma diagnosis of 6 years or longer had a mean (SD) OSDI score of 23 (21) units, which is indicative of moderate OSD. The OSDI scores were significantly different between groups (P = 0.03), but the ages were not significantly different between groups (P = 0.11) (Table 2).
Table 2.
Correlation between Ocular Surface Disease Index score and the time since glaucoma diagnosis (evaluable patients)
| Time since glaucoma diagnosis (years) | Na | OSDI | Age, years | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Mean | SD | P-valueb | Mean | SD | P-valuec | ||
| <6 years | 250 | 18 | 16 | 0.03 | 61 | 14 | 0.11 |
| ≥6 years | 137 | 23 | 21 | 64 | 14 | ||
Notes:
The time since glaucoma diagnosis for 61 patients was unknown;
P-value was calculated from an analysis of variance;
P-value was calculated by t-test.
Abbreviations: OSDI, Ocular Surface Disease Index; SD, standard deviation.
Correlation of ocular surface disease with number of IOP-lowering medications
On average, patients used 1.9 (1.3) IOP-lowering medications. A direct correlation was shown between an increase in the number of IOP-lowering medications used by patients and higher (worse) OSDI scores. As the number of IOP-lowering medications increased from one or two to three or four, the mean (SD) OSDI score increased from 20 [17] units (mild severity) to 23 [21] units (moderate severity). Although the difference in scores may have been clinically relevant in terms of the designations on the OSDI scale, the difference was not statistically significant (P = 0.15) (Table 3). Note that 17 patients who reported using 5 or more IOP-lowering medications were not evaluated, since this claim seemed unlikely to have reflected current use and more likely to have reflected a history of prescription uses.
Table 3.
Correlation between the Ocular Surface Disease Index score and the number of IOP-lowering medications used (evaluable patients)
Notes:
Data for 17 patients who reported use of 5 or more medications were not included in this tabulation;
P-value was calculated from an analysis of variance.
Abbreviations: OSDI, Ocular Surface Disease Index; SD, standard deviation; IOP, intraocular pressure.
Correlation of ocular surface disease with race/geography
Patients who were Latino or of mixed race/ethnicity, all of whom were enrolled at clinics in Latin America, had a mean (SD) OSDI score of 29 (21) units, indicating moderate OSD. This score was significantly higher than the score for Asian patients (mean [SD] = 17 [17] units; P = 0.0001) and Caucasian patients (mean [SD] = 20 [17] units; P = 0.0009): both of these groups had OSDI scores indicating mild OSD. There were no significant differences in OSDI scores between Asian and Caucasian patients (data not shown in tables or figures).
Discussion
OSD is commonly observed in individuals who suffer from glaucoma and/or ocular hypertension. The prevalence rate of OSD reported in this international study (wherein 59.2% of patients with glaucoma had OSD) is consistent with the prevalence rate of OSD (59%) reported in a previous cross-sectional study of patients in the United States who had open-angle glaucoma or ocular hypertension and who completed the OSDI questionnaire.11 Additionally, in a study of more than 20,000 adults in Germany, approximately 53% of the patients with glaucoma were also diagnosed with dry eye based on clinical tests including Schirmer’s test, corneal fluorescein staining, and measurements of tear meniscus and tear film break up time.16 While the approach was different, the result is consistent with that obtained in the current study. Further, the prevalence of severe OSD in the study reported here (20.3% of patients with glaucoma) is consistent with the prevalence of severe OSD in a previously conducted study (27%) that similarly used the OSDI questionnaire.11
Preservatives are added to ophthalmic products packaged in multi-dose containers in order to increase their shelf life and decrease their risk of contamination.10 Although preserved ophthalmic products have been approved as safe for use on the basis of results from short-term to medium-term clinical studies, frequent and long-term instillation of preserved ophthalmic products (over the course of many years) may result in ocular surface damage caused by the preservative.17 BAK is the most widely used preservative in ophthalmic preparations due to its broad spectrum of antimicrobial efficacy and its effect on the tight junctions between epithelial cells in the cornea.18 However, BAK has been shown to induce toxic effects on the cornea and ocular tissues.19–21 These toxic effects tend to occur particularly after chronic topical ocular use of BAK-containing products for the treatment of chronic diseases, such as dry eye and glaucoma.
Further, the use of multiple topical ocular therapies may increase ocular surface damage due to possible additive effects, as well as increase the BAK load (due to multiple BAK-preserved ocular preparations).9 This is particularly relevant for patients suffering from glaucoma and ocular hypertension, since patients with these conditions routinely use multiple topical ocular IOP-lowering agents, which can be administered several times a day for long periods of time. As such, the potential for these patients to develop dry eye secondary to the use of topical ocular preserved therapies is clinically meaningful. Newer ophthalmic preservatives have been developed (eg, Purite®, [Allergan, Irvine, CA]; SofZia® buffer system, [Alcon, Fort Worth, TX]; and Polyquad® preservative, [Alcon]) and have been used in reformulating BAK-preserved IOP-lowering medications. This advancement can offer glaucoma patients alternate therapies that can reduce their exposure to BAK and is especially important to help preserve the ocular surface health of patients who use products or multiple products over many years.19,20
In the present study, patients who had glaucoma histories of less than 6 years had a mean OSDI score indicative of mild OSD, while patients who had longer glaucoma histories had a mean OSDI score that was significantly worse (P = 0.03), indicating moderate OSD. The change from mild to moderate OSD appeared to be associated only with duration of treatment (P = 0.03), not with patient age (P = 0.11). This correlation with duration of diagnosis is consistent with previous studies reporting that the occurrence of dry eye increased with the durations of glaucoma disease and glaucoma treatment.12,16
Although there was a clinically relevant difference observed in the OSDI score for patients who used one or two IOP-lowering medications (mild OSD severity) when compared with patients who used three or four IOP-lowering medications (moderate OSD severity), this difference was not statistically significant (P = 0.15). This result is less conclusive than previous studies showing a strong correlation between the number of IOP-lowering medications used and the presence of dry eye.12,13,16 However, given the issue of poor medication adherence,22 patients who reported taking their medications may not have been truly compliant to the dosing regimen, and may therefore not have experienced the adverse effects of topical ocular drug exposure to the fullest extent possible.
Finally, in the present study, Asian and Caucasian patients had mean OSDI scores indicative of mild OSD, while Latino or mixed-race patients (all of whom were enrolled at Latin American clinics) had a mean OSDI score that was significantly worse (P ≤ 0.0009), indicating moderate OSD. A majority of the patients enrolled in the study were Caucasian (56.0%), with the remaining patients distributed unequally between Asian (26.1%) and other (17.9%) races.
Some limitations of this study were related to the multitude of variables. This was evident in the wide variation (ie, large standard deviations) in the mean OSDI scores for the population, which rendered data interpretation more complicated. The pathogenesis of OSD in glaucoma patients is thought to be multifactorial; while the role of preserved topical ocular drops in OSD is the most well studied, other anterior segment ocular conditions (eg, blepharitis, allergies, infections, or anatomic abnormalities of the eyelid[s]) are known to exacerbate OSD.2 Collecting data about comorbid conditions and determining the impact of all these factors on the OSD prevalence was beyond the scope of the objectives outlined for this study. For the data that were collected, the study could have benefited from a multivariate analysis to determine the contribution of the various factors that lead to OSD, including age, sex, number of medications, and time since diagnosis.
Other limitations of the study were related to the subjective nature of the data. Relying on self-reported values or patient histories for the numbers of IOP-lowering medications yielded data that were less reliable than values recorded by electronic dosage-tracking technologies would have been. Moreover, the OSDI is a subjective tool; adding objective assessments of the ocular surface could have strengthened the study, although the correlation between such signs and symptoms has been reported to be poor.23 Despite its various limitations, this study clearly supports previous studies that have observed a high prevalence of OSD among glaucoma patients and have recognized the increase in OSD with both increasing time since glaucoma diagnosis, and the use of multiple topical ocular preparations.
Conclusions
The data from this international study indicated that more than half of the patients with glaucoma experienced some level of OSD. There was a statistically significant correlation between increased time since glaucoma diagnosis and worsening of OSD symptoms. Further, increases in the number of medications applied also showed a clinically relevant increase in OSD symptom severity.
Acknowledgments
Medical writing support, which was funded by Alcon Research, was provided by Cullen T Vogelson and Usha Sivaprasad of Illuminated Research, LLC (Fort Worth, TX).
Footnotes
Disclosure
Alcon Research, Ltd, funded this study and provided the services of a medical writer.
References
- 1.The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop. Ocul Surf. 2007;2007;5(2):75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 2.Stewart WC, Stewart JA, Nelson LA. Ocular surface disease in patients with ocular hypertension and glaucoma. Curr Eye Res. 2011;36(5):391–398. doi: 10.3109/02713683.2011.562340. [DOI] [PubMed] [Google Scholar]
- 3.Lemp MA. Advances in understanding and managing dry eye disease. Am J Ophthalmol. 2008;146(3):350–356. doi: 10.1016/j.ajo.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 4.Gayton JL. Etiology, prevalence, and treatment of dry eye disease. Clin Ophthalmol. 2009;3:405–412. doi: 10.2147/opth.s5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skalicky SE, Goldberg I, McCluskey P. Ocular surface disease and quality of life in patients with glaucoma. Am J Ophthalmol. 2012;135(1):1–9. doi: 10.1016/j.ajo.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 6.Friedman NJ. Impact of dry eye disease and treatment on quality of life. Curr Opin Ophthalmol. 2010;21(4):310–316. doi: 10.1097/ICU.0b013e32833a8c15. [DOI] [PubMed] [Google Scholar]
- 7.Pflugfelder SC. Prevalence, burden, and pharmacoeconomics of dry eye disease. Am J Manag Care. 2008;14(3 Suppl):S102–S106. [PubMed] [Google Scholar]
- 8.Schein OD, Hochberg MC, Muñoz B, et al. Dry eye and dry mouth in the elderly: a population-based assessment. Arch Intern Med. 1999;159(12):1359–1363. doi: 10.1001/archinte.159.12.1359. [DOI] [PubMed] [Google Scholar]
- 9.Baudouin C, Labbe A, Liang H, Pauly A, Brignole-Baudouin F. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010;29(4):312–334. doi: 10.1016/j.preteyeres.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Lemp MA. Management of dry eye disease. Am J Manag Care. 2008;14(3 Suppl):S88–S101. [PubMed] [Google Scholar]
- 11.Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008;17(5):350–355. doi: 10.1097/IJG.0b013e31815c5f4f. [DOI] [PubMed] [Google Scholar]
- 12.Fechtner RD, Godfrey DG, Budenz D, Stewart JA, Stewart WC, Jasek MC. Prevalence of ocular surface complaints in patients with glaucoma using topical intraocular pressure-lowering medications. Cornea. 2010;29(6):618–621. doi: 10.1097/ICO.0b013e3181c325b2. [DOI] [PubMed] [Google Scholar]
- 13.Rossi GC, Tinelli C, Pasinetti GM, Milano G, Bianchi PE. Dry eye syndrome-related quality of life in glaucoma patients. Eur J Ophthalmol. 2009;19(4):572–579. doi: 10.1177/112067210901900409. [DOI] [PubMed] [Google Scholar]
- 14.Rossi GCM. How to diagnose the ocular surface disease in treated glaucoma patients. Eur Ophthalmic Rev. 2011;5(1):38–42. [Google Scholar]
- 15.Schiffman RM, Christianson MD, Jacobsen G. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000;118(5):615–621. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 16.Erb C, Gast U, Schremmer D. German register for glaucoma patients with dry eye. I. Basic outcome with respect to dry eye. Graefes Arch Clin Exp Ophthalmol. 2008;246(11):1593–1601. doi: 10.1007/s00417-008-0881-9. [DOI] [PubMed] [Google Scholar]
- 17.Furrer P, Mayer JM, Gurny R. Ocular tolerance of preservatives and alternatives. Eur J Pharm Biopharm. 2002;53(3):263–280. doi: 10.1016/s0939-6411(01)00246-6. [DOI] [PubMed] [Google Scholar]
- 18.Kaur IP, Lal S, Rana C, Kakkar S, Singh H. Ocular preservatives: associated risks and newer options. Cutan Ocul Toxicol. 2009;28(3):93–103. doi: 10.1080/15569520902995834. [DOI] [PubMed] [Google Scholar]
- 19.Noecker R. Effects of common ophthalmic preservatives on ocular health. Adv Ther. 2001;18(5):205–215. doi: 10.1007/BF02853166. [DOI] [PubMed] [Google Scholar]
- 20.Yee RW. The effect of drop vehicle on the efficacy and side effects of topical glaucoma therapy: a review. Curr Opin Ophthalmol. 2007;18(2):134–139. doi: 10.1097/ICU.0b013e328089f1c8. [DOI] [PubMed] [Google Scholar]
- 21.Servat JJ, Bernardino CR. Effects of common topical antiglaucoma medications on the ocular surface, eyelids and periorbital tissue. Drugs Aging. 2011;28(4):267–282. doi: 10.2165/11588830-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 22.Robin AL, Covert D. Does adjunctive glaucoma therapy affect adherence to the initial primary therapy? Ophthalmology. 2005;112(5):863–868. doi: 10.1016/j.ophtha.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 23.Nichols KK, Nichols JJ, Mitchell GL. The lack of association between signs and symptoms in patients with dry eye disease. Cornea. 2004;23(8):762–770. doi: 10.1097/01.ico.0000133997.07144.9e. [DOI] [PubMed] [Google Scholar]