Abstract
p21-activated kinase 1 (Pak1) is a serine/threonine kinase that activates protein phosphatase 2a, resulting in the dephosphorylation of cardiac proteins and increased myofilament Ca2+ sensitivity. Emerging evidence indirectly indicates a role for Pak1 in ischemia-reperfusion (I/R), but direct evidence is lacking. We hypothesize that activation of the Pak1 signaling pathway is a cardioprotective mechanism that prevents or reverses the detrimental effects of ischemic injury by inducing posttranslational modifications in myofilament proteins that ultimately improve cardiac contractility following ischemic insult. In the present study, we subjected ex vivo hearts from wild-type (WT) and Pak1-knockout (KO) mice to 20 min of global cardiac ischemia followed by 30 min of reperfusion. In the absence of Pak1, there was an exacerbation of the increased end-diastolic pressure and reduced left ventricular developed pressure occurring after I/R injury. ProQ analysis revealed an increase in troponin-T phosphorylation at baseline in Pak1-KO hearts compared with WT. Significantly decreased myosin light chain 2 (MLC2) phosphorylation in Pak1-KO hearts compared with WT after I/R injury was confirmed by Western immunoblotting. These data indicate that Pak1-KO hearts have reduced recovery of myocardial performance after global I/R injury concomitant with changes in troponin-T and MLC2 phosphorylation. Finally, a protein-protein association between Pak1 and MLC2, and Pak1 and troponin-T, was determined by coimmunoprecipitation. Thus, results of our study provide a basis for targeting a novel pathway, including Pak1, in the therapies for patients with ischemic events.
Keywords: p21-activated kinase 1, ischemia, reperfusion, cardiac, mice, global knockout
when patients experience myocardial infarction, reperfusion injury occurs when coronary flow is reestablished, resulting in reduced myocardial mechanical function. However, despite a large number of studies, the signaling mechanisms identified so far have contributed little to the improvement of the clinical outcome of these patients. Studies in our laboratory have discovered a potentially significant signaling cascade involving p21-activated kinase 1 (Pak1) (24). Pak1 is a serine/threonine kinase that has been previously demonstrated by our laboratory (24) and others (13, 18) to be activated by Cdc42/Rac1. Pak1 activates protein phosphatase 2a (PP2A) (11, 30) and leads to the dephosphorylation of cardiac proteins, including cardiac troponin-I (cTnI) and myosin-binding protein C (MyBP-C) (11). The dephosphorylation of cTnI and MyBP-C increases myofilament Ca2+ sensitivity in adult rat cardiac myocytes (10, 11) and in cardiac intact trabeculae (29). Activation of Pak1 is not associated with a change in phospholamban phosphorylation (25).
Emerging evidence indicates a role for Pak1 in ischemia-reperfusion (I/R), but it is not clear whether Pak1 activation during ischemia reduces or increases injury. On the one hand, Pak1 and PP2A activation have been suggested to be downstream signaling molecules for sphingosine 1-phosphate and bradykinin signaling in cardiac tissue, and be cardioprotective during I/R injury (5). It has been demonstrated that the phosphoinositide 3-kinase (PI3K)-dependent pathway can activate Pak1 (15) and that activation of the PI3K/protein kinase B signaling pathway protects against I/R injury (31). Additionally, Pak1 may reduce the rise in Ca2+ or enhance myofilament response to Ca2+ with I/R, which may improve the response to I/R (1). On the other hand, Pak1 has been implicated to be an upstream mediator of c-Jun NH2-terminal kinase (JNK) (19, 20), which is activated during myocardial I/R and leads to cardiomyocyte death (3). However, a recent study found no differences in JNK 1/2/3 phosphorylation in cardiac tissue due to Pak1 during baseline conditions (27). One particular prior study found Pak1 to prevent I/R-associated arrhythmias (4). However, the authors in this latter study perfused the heart with a hypoxic solution, rather than performing true ischemia. Thus, the role of Pak1 had yet to be studied under true ischemic conditions in cardiac tissue. In the present study, we determined the effect of Pak1 on cardiac function during true ischemia using a stop-flow method. Additionally, the generation of mouse models without Pak1 expression provides a powerful tool to investigate this question.
We hypothesize that activation of the Pak1 signaling pathway induces posttranslational modifications in myofilament proteins, which promote cardioprotective mechanisms that prevent or reverse the detrimental effects of ischemic injury in mouse cardiac tissue. In the present study, the contractility of hearts after I/R injury from Pak1-knockout (KO) mice was significantly further reduced compared with wild type (WT). ProQ analysis revealed a significant decrease in myosin light-chain kinase 2 (MLC2) phosphorylation in Pak1-KO hearts compared with WT after I/R injury. Understanding the role of Pak1 activation and its downstream effects is critical for the development of pharmaceutical interventions for use in patients with ischemic events. The current study has advanced the field by providing direct evidence that Pak1 signaling acts upon myofilament proteins, and is beneficial during I/R injury, in that, in the absence of Pak1, end diastolic pressure is exacerbated and left ventricular developed pressure is further decreased after I/R injury. Thus, the current study provides a novel target for future studies to explore as a therapeutic strategy for patients with ischemic events.
MATERIALS AND METHODS
All protocols were approved by the Animal Care and Use Committee of the University of Illinois and comply with the laws of The United States of America.
PAK-1-KO mouse model.
Mice with Pak-1 gene disruption at both alleles were created in SV129 background and have been described previously (14, 26). Pak1-KO mice were previously rederived in FVB background and genotyped to confirm the deletion of the Pak1 gene, and the levels of protein expression of Pak1, Pak2, and Pak3 (two less abundant protein isoforms of Pak1 in the heart) were measured in the left ventricle cardiac tissue lysate by Western immunoblotting (27). Additionally, hemodynamic parameters were previously assessed by echocardiography, which demonstrated no morphometric and hemodynamic differences between Pak1-KO mice and WT controls (27). In the current study, young adult (16.2 ± 0.3 wk) female mice were used for acquisition of functional and Western immunoblotting analysis. In the current study, age- and gender-matched FVB nontransgenic mice purchased from Charles River Laboratories were used as WT controls.
Ex vivo left ventricular pressure measurements during global cardiac I/R.
Female WT (n = 9) and Pak1-KO (n = 16) mice were intraperitoneally injected with 50 IU/g heparin 20 min before being anesthetized with 0.5 mg/g pentobarbital sodium by intraperitoneal injection. The hearts were rapidly excised, the aorta was cannulated, and the heart was perfused using ice-cold modified Krebs-Henseleit (KH) solution containing (in mM) 118 NaCl, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 0.5 Na EDTA, 25 NaHCO3, 10 glucose, and 2.5 CaCl2. Once perfused, the heart was rapidly transferred to the Langendorff setup and perfused with KH solution, which was gassed with 95% O2-5% CO2 and at 37°C.
The left atrium was removed, and a balloon connected to a pressure transducer was inserted into the left ventricle for the measurement of left ventricular pressure as described previously (1). Electrodes were attached to the right atrium to field-stimulate and pace the myocardium. The heart was paced for 20 min at the lowest rate necessary to capture the rhythm (usually 7–9 Hz) until stabilization of pressure measurements was observed. Pacing was stopped 1 min before a stop-flow method was used to induce global ischemia. After 20 min of ischemia, flow was re-established, and field stimulation for pacing was resumed 5 min after re-establishment of flow. Reperfusion continued for a total of 30 min, with continuous recording of left ventricular pressure and heart rate. The left ventricular pressure and heart rate were multiplied so as to report the rate-pressure constant, to account for varying heart rates in the different hearts. After 30 min of reperfusion, hearts were flash-frozen in liquid nitrogen and saved for later analysis of protein phosphorylation and redox states. The tail clip was saved for PCR genotype confirmation.
Analysis of phosphorylation and redox state of myofilament proteins.
ProQ Diamond (Invitrogen) stain was used to detect changes in phosphorylation states of myofilament proteins. Myofibrils were prepared from WT and Pak1-KO mouse hearts, and pellets were solubilized in a nonreducing 2× Laemmli buffer (4% SDS, 20% glycerol, 0.004% bromphenol blue, and 0.125 M Tris·HCl, pH 6.8) (12). Twenty five millimolar N-ethylmaleimide (NEM) was added to the standard rigor buffer with Triton X-100, the standard rigor wash buffer, and the 2× Laemmli buffer. An RC-DC assay (Bio-Rad) was used to determine protein concentrations. Samples were diluted at a 1:1 ratio in reducing sample buffer [8 M urea, 2 M thiourea, 0.05 M Tris, pH 6.8, 75 mM dithiothreitol (DTT), 3% SDS, and 0.05% bromphenol blue] (32), and ∼15 μg of protein were loaded on a 12% resolving one-dimensional (1D) SDS-PAGE gel (7, 28). The gels were stained with ProQ Diamond and destained according to the manufacturer's recommendations before imaging with a Typhoon 9410 scanner (GE Healthcare). Coomassie R-250 staining was used to normalize protein load to actin. Optical density of the proteins was determined using ImageQuant TL (GE Healthcare) software, and results were exported to Excel for further analysis with statistical software JMP.
Western blot analysis was used to detect glutathionylated proteins. Myofibrils were prepared from WT and Pak1-KO mouse hearts, and pellets were solubilized in a nonreducing 2× Laemmli buffer (12). Twenty five millimolar NEM was added to the standard rigor buffer with Triton X-100, the standard rigor wash buffer, and the 2× Laemmli buffer. With the use of the protein concentration determined from an RC-DC (Bio-Rad) assay, ∼40 μg of total protein were applied to 1D 12% resolving SDS-PAGE gel and transferred onto a 0.2 μM polyvinylidene difluoride (PVDF) membrane. The blot was blocked in 5% nonfat dry milk with 2.5 mM NEM for 1 h. Anti-glutathione mouse monoclonal primary antibody (Virogen) was used at 1:1,000 dilution along with anti-mouse horseradish peroxidase-conjugated secondary antibody (Sigma) at 1:100,000 dilution to detect for S-glutathionylation (9).
Western blot analysis was used to detect the phosphorylation level of MLC2. Myofibrils were prepared from WT and Pak1-KO mouse hearts, and pellets were solubilized in a nonreducing 2× Laemmli buffer (4% SDS, 20% glycerol, 0.004% bromphenol blue, and 0.125 M Tris·HCl, pH 6.8) (12). Twenty five millimolar NEM was added to the standard rigor buffer with Triton X-100, the standard rigor wash buffer, and the 2× Laemmli buffer. An RC-DC assay (Bio-Rad) was used to determine protein concentrations. Samples were diluted at a 1:1 ratio in reducing sample buffer (8 M urea, 2 M thiourea, 0.05 M Tris, pH 6.8, 75 mM DTT, 3% SDS, and 0.05% bromphenol blue) (32), and ∼20 μg of protein were loaded on a 12% resolving 1D SDS-PAGE gel (7, 28) and transferred to a 0.2 μM PVDF membrane. The blot was blocked in 5% nonfat dry milk for 1 h. Anti-phospho-MLC S20 (Abcam) was used at 1:5,000 dilution to detect phosphorylated levels of MLC2. Cardiac-specific anti-MLC2 (Abcam) was used at 1:10,000 to detect total levels of MLC2 protein. Ponceau Image was used to normalize the MLC2 level to actin.
Coimmunoprecipitation was performed to establish whether Pak1 associates with either TnT, MLC2, or both. Total proteins (200 μg) obtained from WT left ventricular cardiac tissue were incubated overnight with rabbit monoclonal total Pak-1 antibody (Cell Signaling) followed by incubation with protein A-Sepharose beads (70 μl of 50% bead slurry) with gentle rocking for 4 h at 4°C. Complexes were then precipitated in sample buffer (8 M urea, 2 M thiourea, 0.05 M Tris, pH 6.8, 75 mM DTT, 3% SDS, and 0.05% bromphenol blue) and analyzed by SDS-PAGE and Western blot. Cardiac-specific anti-MLC2 (Abcam) was used at 1:10,000 to detect coimmunoprecipitation of cardiac MLC2 with Pak1. The membrane was stripped using Thermo Scientific Restore Western Blot stripping buffer. Anti-troponin-T (TnT; Santa Cruz Biotechnology) was used at 1:200 to detect coimmunoprecipitation of TnT with Pak1.
Statistics.
Data were statistically analyzed by two-way ANOVA followed by Student's t-test using JMP statistical software where all groups are considered (Figs. 1, B–C, and 3, A–F). Data were statistically analyzed by t-test using JMP statistical software where only two groups were considered (Fig. 1D). Data were statistically analyzed by an independent sample t-test (ANOVA with two groups) using JMP statistical software to determine if there is a difference for the two groups WT and Pak1-KO over the entire reperfusion phase (Fig. 1E). A value of P < 0.05 was considered significantly different (P value required for significance appropriately adjusted by JMP statistical software for ANOVA when followed by Student's t-test). Data are represented as means ± SE.
Fig. 1.
A: representative tracing of pressure during ischemia, wild-type (WT) female mouse, 8 Hz, 37°C, experiment no. 110311e. B: left ventricular developed pressure (LVDP) in WT and p21-activated kinase 1 (Pak1)-knockout (KO) ex vivo hearts is significantly reduced after 20 min ischemia and 30 min reperfusion relative to baseline values in each group. Statistics were performed by 2-way ANOVA followed by Student's t-test using JMP statistical software. C: the rate-pressure product, calculated as heart rate multiplied by left ventricular developed pressure, in WT and Pak1-KO ex vivo hearts is significantly reduced after 20 min ischemia and 30 min reperfusion relative to baseline values in each group. Statistics were performed by 2-way ANOVA followed by Student's t-test using JMP statistical software. bpm, Beats/min. D: %recovery of left ventricular developed pressure after ischemia-reperfusion (I/R) injury, as calculated by left ventricular developed pressure after I/R divided by left ventricular developed pressure at baseline, is significantly less in Pak1-KO hearts compared with WT controls. Statistics were performed by t-test using JMP statistical software. E: change in left ventricular end diastolic pressure during reperfusion is increased in Pak1-KO hearts compared with WT. Data were statistically analyzed by an independent-samples t-test (ANOVA with 2 groups) using JMP statistical software to determine if there is a difference for the two groups WT and Pak1-KO over the entire reperfusion phase (but not at any particular time point). Data collected at 2.5 mM extracellular Ca2+ concentration, 37°C, n = 9 for WT, n = 16 for Pak1-KO. Data are reported as means ± SE. *P < 0.05 considered significant.
RESULTS
Pak1 is involved in regulation of developed pressure during I/R in mouse heart.
A representative tracing of left ventricular pressure in Langendorff-perfused hearts during global ischemia is shown in Fig. 1A. Left ventricular developed pressure (systolic pressure minus diastolic pressure) in WT and Pak1-KO ex vivo hearts was significantly reduced after 20 min ischemia and 30 min reperfusion relative to baseline values before ischemia in each group (Fig. 1B). The rate-pressure product in WT and Pak1-KO ex vivo hearts was also significantly reduced after 20 min ischemia and 30 min reperfusion relative to baseline values in each group (Fig. 1C), in agreement with the values for left ventricular developed pressure. Left ventricular developed pressure measurements after I/R were divided by the left ventricular developed pressure at baseline before ischemia to determine the percent recovery of myocardial function. The percent recovery of left ventricular developed pressure after I/R injury was significantly reduced in Pak1-KO hearts compared with WT controls (Fig. 1D). End systolic pressure in WT and Pak1-KO ex vivo hearts was significantly reduced after I/R relative to baseline values in each group. (WT decreased from 85.6 ± 1.7 mmHg at baseline to 69.8 ± 1.8 mmHg after I/R injury. Pak1-KO decreased from 90.8 ± 3.7 mmHg at baseline to 68.8 ± 3.2 mmHg after I/R injury.) One-way ANOVA results indicate that the change in end diastolic pressure across all points over the course of reperfusion was significantly higher in Pak1-KO hearts compared with WT hearts (Fig. 1E). However, this increase was not significantly different between WT and Pak1-KO hearts at any of the particular points measured during reperfusion as shown in Fig. 1E (P = 0.07 at 5 min reperfusion, P = 0.11 at 10 min reperfusion, P = 0.08 at 15 min reperfusion, P = 0.09 at 20 min reperfusion, P = 0.11 at 25 min reperfusion, and P = 0.18 at 30 min reperfusion). Positive dP/dt significantly decreased (in WT from 2,290.4 ± 94.8 mmHg/s at baseline to 1,127.3 ± 37.2 mmHg/s after I/R and in Pak1-KO from 2,585.8 ± 236.9 mmHg/s at baseline to 841.0 ± 103.4 mmHg/s after I/R) while negative dP/dt significantly increased after 20 min ischemia and 30 min reperfusion (in WT from −1,733.8 ± 72.0 mmHg/s at baseline to −946.2 ± 47.0 mmHg/s after I/R injury, and in Pak1-KO from −2,174.1 ± 164.7 mmHg/s at baseline to −867.2 ± 105.1 mmHg/s after I/R injury) relative to baseline values before ischemia in each group.
Change of phosphorylation of myofilament proteins in Pak1-KO heart.
All hearts used in the analysis of the phosphorylation and redox states of the myofilament proteins were frozen at 8 Hz. A representative gel with ProQ and Coomassie staining can be seen in Fig. 2. Compared with WT hearts not subjected to ischemia, WT hearts after I/R demonstrated a significant increase in phosphorylation of TnT (Fig. 3, B and C) and a significant decrease in phosphorylation of tropomyosin (Tm), TnI, and MCL2 (Fig. 3, D, E, and F, respectively). Pak1-KO hearts after I/R injury exhibited a significant decrease in phosphorylation of TnI and MLC2 compared with Pak1-KO hearts not subjected to ischemia (Fig. 3, E and F, respectively). However, TnT and Tm phosphorylation was not significantly different in Pak1-KO hearts at baseline and after I/R injury (Fig. 3, B–D). TnT phosphorylation was increased at baseline in Pak1-KO hearts compared with WT (Fig. 3, B and C). MLC2 phosphorylation was significantly decreased in Pak1-KO hearts compared with WT after I/R injury (Fig. 3F). MyBP-C phosphorylation was not significantly different between any of the groups studied (Fig. 3A). No significant changes in S-glutathionylation of the myofilament proteins were detected in any of the groups studied (results not shown).
Fig. 2.
ProQ and Coomassie gels demonstrating changes in phosphorylation of various myofilament proteins in WT and Pak1-KO hearts after 20 min ischemia and 30 min reperfusion compared with hearts not subjected to ischemia. Gel no. 56. MyBP-C, myosin-binding protein C; TnT, troponin-T; TnI, troponin-I; MLC2, myosin light chain 2.
Fig. 3.
Phosphorylation of myofilament proteins as detected by ProQ. A: MyBP-C phosphorylation was not significantly different after I/R in either WT or Pak1-KO hearts. B and C: TnT3 and TnT4 phosphorylation was increased at baseline in Pak1-KO hearts compared with WT hearts, and phosphorylation of WT hearts was increased after I/R compared with WT hearts at baseline. D: tropomyosin (Tm) phosphorylation decreased in WT hearts after I/R but was not significantly different in Pak1-KO hearts after I/R. E: troponin-I (TnI) phosphorylation decreased after I/R in both WT and Pak1-KO hearts but was not significantly different between WT and Pak1-KO. F: MLC2 phosphorylation decreased after I/R in both WT and Pak1-KO hearts and was significantly decreased in Pak1-KO compared with WT after I/R. Data are normalized to actin. Arbunits, arbitrary units. Statistics were performed by 2-way ANOVA followed by Student's t-test using JMP statistical software. Data are reported as means ± SE. *P < 0.05.
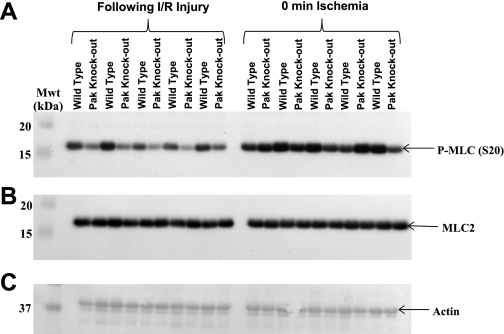
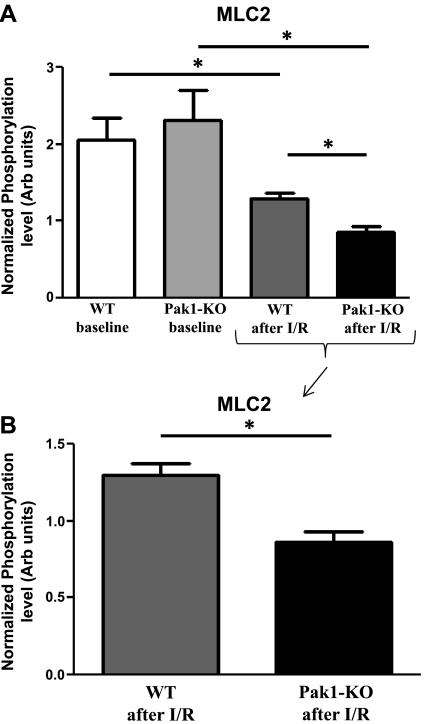
Western blot analysis of phosphorylation levels of MLC2 at serine-20 was performed to confirm the results obtained from the ProQ analysis (Fig. 4). Consistent with our ProQ findings, phosphorylation levels of MLC2 decreased after I/R injury in hearts from both WT and Pak1-KO mice compared with baseline levels (Fig. 5). MLC2 phosphorylation levels were even further decreased in hearts from Pak1-KO mice after I/R injury compared with WT after I/R injury. Again consistent with our ProQ results, MLC2 phosphorylation was not significantly different between hearts from WT and Pak1-KO mice at baseline.
Fig. 4.
Representative Western Blot demonstrating changes in MLC2 phosphorylation. A: Western blot probed for phospho (P)-MLC2 at serine-20. B: Western blot probed for total MLC2. C: Ponceau image used for normalization of MLC2 to actin.
Fig. 5.
Phosphorylation of MLC2 as detected by Western blot. A: MLC2 phosphorylation decreased after I/R injury in hearts from both WT and Pak1-KO mice. MLC2 phosphorylation was not significantly different between hearts from WT and Pak1-KO mice at baseline. B: MLC2 phosphorylation was further reduced in hearts from Pak1-KO mice after I/R injury compared with hearts from WT mice after I/R injury. Statistics were performed by 2-way ANOVA followed by Student's t-test using JMP statistical software. Data are reported as means ± SE. *P < 0.05.
Coimmunoprecipitation.
To test whether a direct association exists between Pak1 and cardiac MLC2, we performed a coimmunoprecipitation and demonstrate that these two proteins establish a direct protein-protein interaction in left ventricular WT cardiac tissue (Fig. 6, A and B). Pak1 and TnT also establish a direct protein-protein interaction in left ventricular WT cardiac tissue (Fig. 6, A and C).
Fig. 6.
Coimmunoprecipitation demonstrating an association between Pak1 and MLC2, and Pak1 and TnT. A: Western blot probed with total Pak1 antibody (Cell Signaling). B: Western blot probed with total cardiac-specific MLC2 antibody (Abcam). C: Western blot probed with total TnT antibody (Santa Cruz Biotechnology).
DISCUSSION
Our current study has advanced the field by demonstrating the beneficial effects of the Pak1 signaling pathway, which improves myocardial performance concomitant with a modulation of TnT and MLC2 during global myocardial ischemia and reperfusion injury. In the absence of Pak1, there was an exacerbation of the increased end diastolic pressure and reduced left ventricular developed pressure occurring after I/R injury. Thus, results of our study provide a basis for targeting a novel pathway including Pak1 in the therapies for patients with ischemic events.
The mechanism by which the absence of Pak1 induces a depressed recovery of function after I/R may involve a depressed level of MLC2 phosphorylation. This was the only difference we found in sarcomeric protein phosphorylation between WT and Pak-KO hearts following I/R (Fig. 3F). Our laboratory has shown that ablation of MLC2 phosphorylation decreases ventricular power, lengthens the duration of ventricular ejection, and may also modify other sarcomeric proteins (e.g., cardiac troponins) as substrates for kinases and phosphatases (21–23). The other differences that may be of significance are that Tm phosphorylation fell significantly with I/R in WT but not in Pak-KO hearts, and TnT phosphorylation increased with I/R in WT but not in Pak-KO. In previous studies, we have reported that a loss in MLC2 phosphorylation in fibers from a transgenic mouse model expressing a cardiac specific nonphosphorylatable regulatory light chain results in a decrease in maximum Ca2+-activated tension and tension cost (ATP hydrolysis/unit tension) with no change in Ca2+ sensitivity (21). Moreover, there is evidence that phosphorylation of cTnT may depress cardiac function.
An interesting finding of our study was the demonstration that, under baseline conditions, the major difference between WT and Pak-KO hearts was the phosphorylation levels of TnT3 and TnT4, both of which were higher in the Pak-1 KO myofilaments compared with WT (Fig. 3, B and C). TnT3 and TnT4 phosphorylation levels both increased in WT following I/R injury, but this increase in phosphorylation levels was not seen in Pak-KO hearts after I/R injury, suggesting a role for Pak1 in the regulation of TnT3 and TnT4 phosphorylation. Additionally, we also demonstrate that Pak1 and TnT associate in a protein-protein interaction, as assessed by coimmunoprecipitation. The results of this study suggest Pak1 plays a regulatory role in the phosphorylation level of TnT.
We demonstrate that the ablation of Pak1 causes a reduction in the percent recovery of left ventricular developed pressure after I/R injury, probably due largely to an exacerbated increase in end diastolic pressure. There is a significant baseline increase in TnT phosphorylation in the Pak1-KO hearts compared with WT, and this difference is not observed after I/R injury. Overall protein phosphorylation was detected by ProQ, and it is important to note that phosphorylation at one site of any of the proteins studied may have increased while phosphorylation at another site in the same protein may have decreased.
Phosphorylation of Tm, TnI, and MLC2 is significantly reduced in WT hearts after I/R injury compared with phosphorylation levels in WT before ischemic insult. I/R injury has been previously reported to be associated with a decrease in myofilament Ca2+ sensitivity (8), and the concurrent dephosphorylation of Tm, TnI, and MLC2 has previously been reported to be associated with a decrease in myofilament Ca2+ sensitivity (16). This could provide a possible mechanism for the decrease in left ventricular systolic and developed pressures observed in WT hearts after I/R injury. Specific increases in myofilament Ca2+ sensitivity in transgenic mouse hearts that express slow skeletal TnI treated with EMD-57033 have shown to diminish the effects of I/R on cardiac function (1). Additionally, phosphorylation of MCL2 is further decreased in hearts from Pak1-KO mice compared with WT controls after I/R injury, and a decrease in phosphorylation of MLC2 has been previously associated with a decrease in myofilament Ca2+ sensitivity (17, 23). A previous study demonstrated the phosphorylation of intact nonmuscle myosin II regulatory light chain by γ-Pak (2). This decrease in MLC2 phosphorylation and the coimmunoprecipitation demonstrating an association between MLC2 and Pak1 suggests that Pak1 plays a role in the phosphorylation of MLC2 in cardiac tissue during I/R injury and could provide a possible mechanism for the reduced recovery of pressure observed in hearts from Pak-KO mice.
The main emphasis of the current study is to demonstrate that Pak1 plays a role in I/R injury. Future studies may investigate the other mechanisms by which protein phosphorylation can be altered during I/R. Pak1 is involved in a number of cell types and signaling pathways and has been shown to form a complex with an important cytoskeletal protein (6). Pak1 is upstream of PP2A activation and extracellular signal-regulated kinase 1/2 inactivation (27). The impaired response to ischemia observed in Pak1-KO mice could also depend on changes in other signaling pathways and targets, including downstream modulation of Ca2+ cycling, in addition to the mechanisms explored in the current study. Future studies will distinguish the individual effects of these various signaling proteins and the cross talk that occurs between pathways.
In conclusion, the present study demonstrates that Pak1 signaling acts upon myofilament proteins and is beneficial during I/R injury, in that Pak1 reduces end diastolic pressure and increases left ventricular developed pressure after I/R injury. Thus, the current study provides a novel pathway including the potential target Pak1 for development of pharmaceutical interventions for use in the clinic for patients with ischemic events.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute (NHLBI) Grants P01 HL-062426 (R. J. Solaro) and RO1 HL-064035 (R. J. Solaro and B. M. Wolska). NHLBI Grant T32 HL-07692-16-20 supported M. M. Monasky. A grant from the Center for Clinical and Translational Science supported D. M. Taglieri.
DISCLOSURES
None.
ACKNOWLEDGMENTS
We thank Chad M. Warren for valuable technical support.
REFERENCES
- 1. Arteaga GM, Warren CM, Milutinovic S, Martin AF, Solaro RJ. Specific enhancement of sarcomeric response to Ca2+ protects murine myocardium against ischemia-reperfusion dysfunction. Am J Physiol Heart Circ Physiol 289: H2183–H2192, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Chew TL, Masaracchia RA, Goeckeler ZM, Wysolmerski RB. Phosphorylation of non-muscle myosin II regulatory light chain by p21-activated kinase (gamma-PAK). J Muscle Res Cell Motil 19: 839–854, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Duplain H. Salvage of ischemic myocardium: a focus on JNK. Adv Exp Med Biol 588: 157–164, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Egom EE, Ke Y, Musa H, Mohamed TM, Wang T, Cartwright E, Solaro RJ, Lei M. FTY720 prevents ischemia/reperfusion injury-associated arrhythmias in an ex vivo rat heart model via activation of Pak1/Akt signaling. J Mol Cell Cardiol 48: 406–414, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Egom EE, Ke Y, Solaro RJ, Lei M. Cardioprotection in ischemia/reperfusion injury: spotlight on sphingosine-1-phosphate and bradykinin signalling. Prog Biophys Mol Biol 103: 142–147, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Even-Faitelson L, Ravid S. PAK1 and aPKCzeta regulate myosin II-B phosphorylation: a novel signaling pathway regulating filament assembly. Mol Biol Cell 17: 2869–2881, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fritz JD, Swartz DR, Greaser ML. Factors affecting polyacrylamide gel electrophoresis and electroblotting of high-molecular-weight myofibrillar proteins. Anal Biochem 180: 205–210, 1989 [DOI] [PubMed] [Google Scholar]
- 8. Gao WD, Atar D, Backx PH, Marban E. Relationship between intracellular calcium and contractile force in stunned myocardium. Direct evidence for decreased myofilament Ca2+ responsiveness and altered diastolic function in intact ventricular muscle. Circ Res 76: 1036–1048, 1995 [DOI] [PubMed] [Google Scholar]
- 9. Hill BG, Ramana KV, Cai J, Bhatnagar A, Srivastava SK. Measurement and identification of S-glutathiolated proteins. Methods Enzymol 473: 179–197, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ke Y, Lei M, Collins TP, Rakovic S, Mattick PA, Yamasaki M, Brodie MS, Terrar DA, Solaro RJ. Regulation of L-type calcium channel and delayed rectifier potassium channel activity by p21-activated kinase-1 in guinea pig sinoatrial node pacemaker cells. Circ Res 100: 1317–1327, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Ke Y, Wang L, Pyle WG, de Tombe PP, Solaro RJ. Intracellular localization and functional effects of P21-activated kinase-1 (Pak1) in cardiac myocytes. Circ Res 94: 194–200, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Layland J, Cave AC, Warren C, Grieve DJ, Sparks E, Kentish JC, Solaro RJ, Shah AM. Protection against endotoxemia-induced contractile dysfunction in mice with cardiac-specific expression of slow skeletal troponin I. FASEB J 19: 1137–1139, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Manser E, Leung T, Salihuddin H, Zhao ZS, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature 367: 40–46, 1994 [DOI] [PubMed] [Google Scholar]
- 14. McDaniel AS, Allen JD, Park SJ, Jaffer ZM, Michels EG, Burgin SJ, Chen S, Bessler WK, Hofmann C, Ingram DA, Chernoff J, Clapp DW. Pak1 regulates multiple c-Kit mediated Ras-MAPK gain-in-function phenotypes in Nf1+/− mast cells. Blood 112: 4646–4654, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Menard RE, Mattingly RR. Cell surface receptors activate p21-activated kinase 1 via multiple Ras and PI3-kinase-dependent pathways. Cell Signal 15: 1099–1109, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Monasky MM, Biesiadecki BJ, Janssen PM. Increased phosphorylation of tropomyosin, troponin I, and myosin light chain-2 after stretch in rabbit ventricular myocardium under physiological conditions. J Mol Cell Cardiol 48: 1023–1028, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morano I, Hofmann F, Zimmer M, Ruegg JC. The influence of P-light chain phosphorylation by myosin light chain kinase on the calcium sensitivity of chemically skinned heart fibres. FEBS Lett 189: 221–224, 1985 [DOI] [PubMed] [Google Scholar]
- 18. Parrini MC, Lei M, Harrison SC, Mayer BJ. Pak1 kinase homodimers are autoinhibited in trans and dissociated upon activation by Cdc42 and Rac1. Mol Cell 9: 73–83, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Rudel T, Zenke FT, Chuang TH, Bokoch GM. p21-activated kinase (PAK) is required for Fas-induced JNK activation in Jurkat cells. J Immunol 160: 7–11, 1998 [PubMed] [Google Scholar]
- 20. Schmitz U, Thommes K, Beier I, Wagner W, Sachinidis A, Dusing R, Vetter H. Angiotensin II-induced stimulation of p21-activated kinase and c-Jun NH2-terminal kinase is mediated by Rac1 and Nck. J Biol Chem 276: 22003–22010, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Scruggs SB, Hinken AC, Thawornkaiwong A, Robbins J, Walker LA, de Tombe PP, Geenen DL, Buttrick PM, Solaro RJ. Ablation of ventricular myosin regulatory light chain phosphorylation in mice causes cardiac dysfunction in situ and affects neighboring myofilament protein phosphorylation. J Biol Chem 284: 5097–5106, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scruggs SB, Reisdorph R, Armstrong ML, Warren CM, Reisdorph N, Solaro RJ, Buttrick PM. A novel, in-solution separation of endogenous cardiac sarcomeric proteins and identification of distinct charged variants of regulatory light chain. Mol Cell Proteomics 9: 1804–1818, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scruggs SB, Solaro RJ. The significance of regulatory light chain phosphorylation in cardiac physiology. Arch Biochem Biophys 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sheehan KA, Ke Y, Solaro RJ. p21-Activated kinase-1 and its role in integrated regulation of cardiac contractility. Am J Physiol Regul Integr Comp Physiol 293: R963–R973, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Sheehan KA, Ke Y, Wolska BM, Solaro RJ. Expression of active p21-activated kinase-1 induces Ca2+ flux modification with altered regulatory protein phosphorylation in cardiac myocytes. Am J Physiol Cell Physiol 296: C47–C58, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smith SD, Jaffer ZM, Chernoff J, Ridley AJ. PAK1-mediated activation of ERK1/2 regulates lamellipodial dynamics. J Cell Sci 121: 3729–3736, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Taglieri DM, Monasky MM, Knezevic II, Sheehan KA, Lei M, Wang X, Chernoff J, Wolska BM, Ke Y, Solaro RJ. Ablation of p21-activated kinase-1 in mice promotes isoproterenol-induced cardiac hypertrophy in association with activation of Erk1/2 and inhibition of protein phosphatase 2A. J Mol Cell Cardiol 51: 988–996, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vahebi S, Kobayashi T, Warren CM, de Tombe PP, Solaro RJ. Functional effects of rho-kinase-dependent phosphorylation of specific sites on cardiac troponin. Circ Res 96: 740–747, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Varian KD, Janssen PM. Frequency-dependent acceleration of relaxation involves decreased myofilament calcium sensitivity. Am J Physiol Heart Circ Physiol 292: H2212–H2219, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Westphal RS, Coffee RL, Jr, Marotta A, Pelech SL, Wadzinski BE. Identification of kinase-phosphatase signaling modules composed of p70 S6 kinase-protein phosphatase 2A (PP2A) and p21-activated kinase-PP2A. J Biol Chem 274: 687–692, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Williams DL, Ozment-Skelton T, Li C. Modulation of the phosphoinositide 3-kinase signaling pathway alters host response to sepsis, inflammation, and ischemia/reperfusion injury. Shock 25: 432–439, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Yates LD, Greaser ML. Quantitative determination of myosin and actin in rabbit skeletal muscle. J Mol Biol 168: 123–141, 1983 [DOI] [PubMed] [Google Scholar]