Abstract
Loss of fluid shear stress (ischemia) to the lung endothelium causes endothelial plasma membrane depolarization via ATP-sensitive K+ (KATP) channel closure, initiating a signaling cascade that leads to NADPH oxidase (NOX2) activation and ROS production. Since wortmannin treatment significantly reduces ROS production with ischemia, we investigated the role of phosphoinositide 3-kinase (PI3K) in shear-associated signaling. Pulmonary microvascular endothelial cells in perfused lungs subjected to abrupt stop of flow showed membrane depolarization and ROS generation. Stop of flow in flow-adapted mouse pulmonary microvascular endothelial cells in vitro resulted in the activation of PI3K and Akt as well as ROS generation. ROS generation in the lungs in situ was almost abolished by the PI3K inhibitor wortmannin and the PKC inhibitor H7. The combination of the two (wortmannin and H7) did not have a greater effect. Activation of NOX2 was greatly diminished by wortmannin, knockout of Akt1, or dominant negative PI3K, whereas membrane depolarization was unaffected. Ischemia-induced Akt activation (phosphorylation) was not observed with KATP channel-null cells, which showed minimal changes in membrane potential with ischemia. Activation of Akt was similar to wild-type cells in NOX2-null cells, which do not generate ROS with ischemia. Cromakalim, a KATP channel agonist, prevented both membrane depolarization and Akt phosphorylation with ischemia. Thus, Akt1 phosphorylation follows cell membrane depolarization and precedes the activation of NOX2. These results indicate that PI3K/Akt and PKC serve as mediators between endothelial cell membrane depolarization and NOX2 assembly.
Keywords: mechanotransduction, endothelium, NADPH oxidase, membrane potential, phosphorylation, phosphoinositide 3-kinase, reactive oxygen species, protein kinase C
the plasma membrane of endothelial cells (ECs) in situ is continuously subjected to a variable shear stress that is associated with luminal blood flow. Our studies (2, 4, 5, 27, 32, 35, 39, 45) have focused on the signaling response to the abrupt decrease of shear that occurs with the stop of flow (i.e., ischemia). Studies of intact perfused rat and mouse lungs and flow-adapted ECs in primary culture have shown that the cessation of flow results in an immediate response that is characterized by the generation of ROS associated with the activation of EC NADPH oxidase (NOX) (4, 35, 45). These experiments on ischemia were done under conditions that maintained normoxia so that ROS generation in this model was not the result of a changed metabolic state but rather reflected the effect of altered mechanotransduction.
The NOX enzyme family has been categorized into seven members (NOX1–NOX5 and DUOX-1 and DUOX-2) depending on the membrane-associated flavoprotein components (7, 20, 38). The prototype (NOX2) is composed of two integral membrane proteins: p22phox and glycoprotein (gp)91phox, which together constitute cytochrome b558, and four cytosolic proteins (Rac1 or Rac2, p40phox, p47phox, and p67phox) (20, 23). Upon activation, NOX2-related cytosolic proteins translocate to the plasma membrane and associate with integral membrane subunits to form the functional enzyme (23, 26). Our studies (4, 31, 44) using gp91phox-null mice have indicated that NOX2 is the NOX that generates ROS in the endothelium during ischemia.
Based on our studies of ischemia (5, 6, 13, 35, 45), the signaling cascade that results in ROS production is initiated by EC membrane depolarization associated with the inactivation of ATP-sensitive K+ (KATP) channels. We have postulated that this KATP channel response reflects the loss of fluid shear stress as sensed by caveolae and that the membrane depolarization, in turn, triggers the assembly and activation of NOX2 (13, 30, 45). However, the intermediate steps that link depolarization and NOX2 activation are not clear. Previous studies (1, 8, 22, 36, 41) have implicated phosphoinositide 3-kinase (PI3K) in the activation of NOX2 in various cell systems. We (44) have previously shown in the isolated mouse lung that wortmannin, a PI3K inhibitor, prevents the translocation of NOX2 subunits and reduces ROS production with ischemia. The present study further evaluated the role of PI3K and its downstream effector, the serine threonine kinase Akt (also called PKB), as well as PKC, in the signaling events that lead to ischemia-mediated activation of ROS generation by mouse pulmonary microvascular ECs (PMVECs).
MATERIALS AND METHODS
Materials.
Bis-(1,3-dibutylbarbituric acid)trimethine oxonol (bis-oxonol), dichlorodihydrofluoroscein diacetate (H2DCF-DA), diphenyleneiodonium chloride (DPI) and Alexa fluor 594-conjugated wheat germ agglutinin were purchased from Invitrogen (Eugene, OR). Cromakalim (a KATP channel agonist) and wortmannin were from Sigma (St. Louis, MO). H7 dihydrochloride was from Calbiochem (San Diego, CA). The PI3K activity assay kit was from Echelon Biosciences (Salt Lake City, UT). The antibodies used were anti-Rac1, anti-von Willebrand factor (vWF), and anti-p85 from Upstate Biotechnology (Lake Placid, NY), anti-phosphorylated (p)Ser273 Akt from Cell Signaling (Beverly, MA), Akt from Santa Cruz Biotechnology (Santa Cruz, CA) and Millipore (Billerica, MA), anti-platelet-EC adhesion molecule (PECAM-1) sheep polyclonal from Centacor (Malvern, PA), anti-PECAM monoclonal from BD Biosciences (San Jose, CA), FITC-labeled anti-CD31 (PECAM-1) from Millipore (Billerica, MA), anti-vascular endothelial (VE)-cadherin from Transduction Labs (San Diego, CA), anti-Flk-1 and anti-Flk-2 from Chemicon (Temecula, CA), and fluorophore-labeled goat anti-mouse or anti-rabbit IgG from Invitrogen.
Animals.
Animal use was reviewed and approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. Male C57BL/6 mice weighing ∼20 g were obtained from Jackson Labs (Bar Harbor, ME). Breeding pairs of mice deficient in KATP channels generated by targeted disruption of the gene for Kir6.2 were obtained from Chiba University (29) and bred in the University of Pennsylvania animal facilities as previously described (45). Kir6.2 is the pore-forming subunit of the KATP channel in lung ECs (10, 13). Kir6.2−/− mice have been backcrossed for five generations to the C57BL/6 background. Except for differences in coat color related to the chromosomal location of the Kir6.2 locus, there were no obvious phenotypic differences between the wild-type and gene-targeted mice. NOX2-null mice (made by gene targeting for gp91phox) were obtained from Jackson Labs. These mice have been used as a model of chronic granulomatous disease (17). Akt1- and Akt2-null mice were generated in our facilities and have been previously described (15); they have been backcrossed for 10 generations to the C57BL/6 background.
Isolation of mouse PMVECs.
Mouse PMVECs were isolated by collagenase treatment of minced mouse lungs and cell adhesion to Dynabeads (Dynal, Oslo, Norway) coated with polyclonal anti-rat PECAM as previously described (13, 30). Isolated cells were maintained for several passages under static culture conditions in DMEM supplemented with 10% FBS, EC growth supplement, nonessential amino acids, and penicillin-streptomycin. The endothelial phenotype of the preparation was confirmed by cellular uptake of the EC-specific marker DiI-acetylated LDL (DiIAcLDL) and by immunostaining for EC marker proteins: PECAM-1, vWF, VE-cadherin, Flt-1 (VEGF receptor-1), and Flk-1 (VEGF receptor-2). Confluent mouse PMVECs were incubated with DiIAcLDL at a final concentration of 10 μg/ml for 1 h at 37°C, washed three times with fresh medium, and evaluated by epifluorescence microscopy at excitation/emission wavelengths of 545/585 nm. For immunostaining, cultured cells were split and incubated with either a primary antibody or with nonimmune IgG for 3 h at 37°C, washed, incubated with a secondary antibody (goat anti-mouse or anti-rabbit IgG conjugated with Alexa fluor 488 or FITC at 1:100 dilution), and then examined under a Nikon Optiphot microscope.
Isolated lung perfusion and intravital microscopy.
The isolated perfused lung technique used in this study for mouse lungs has been previously described (13, 44, 45). Briefly, mice were anesthetized with 50 mg/kg ip pentobarbital sodium and continuously ventilated through a tracheal cannula with 5% CO2 in air (BOC Group, Murray Hill, NJ). The chest was opened, and the pulmonary circulation was cleared of blood by gravity flow of perfusion through a cannula inserted in the main pulmonary artery, exiting from the transected left ventricle. The perfusate was Krebs-Ringer bicarbonate solution [composed of (in mM) 118.45 NaCl, 4.74 KCl, 1.17 MgSO4·7H2O, 1.18 KH2PO4, and 24.87 NaHCO3] supplemented 10 mM glucose and 5% dextran to maintain osmolarity. The lungs were dissected free and placed in a chamber with ports connected to a ventilator and to a peristaltic pump that maintained the lung perfusion rate at 2 ml/min (44).
Intravital imaging was carried out as previously described (4, 13, 35, 37, 44, 45). The lung preparation was placed onto a chamber on the stage of an epifluorescence microscope equipped with an optical filter changer (lambda 10-2, Sutter Instruments, Novato, CA), a Hamamatsu ORCA-100 digital camera (Hamamatsu, Bridgewater, NJ), and MetaMorph imaging software (Universal Imaging, Downingtown, PA). Excitation of the lung surface was accomplished with a mercury lamp fiber optic light source. The change in bis-oxonol fluorescence (480 ± 20-nm excitation/535 ± 25-nm emission) was used to determine changes in EC membrane potential, and 2′,7′-dichlorofluorescin (DCF) fluorescence (485 ± 5-nm excitation/510 ± 10-nm emission) was used for ROS production. Isolated lungs were preperfused with bis-oxonol (0.2 μM) or H2DCF-DA (5 μM) for 30 min to allow for uptake of the fluorophore. Bis-oxonol intercalates into the cell membrane, while intracellular cleavage of the diacetate from H2DCF-DA results in cell trapping of H2DCF. The remaining intravascular dye was removed by 10 min of perfusion of the lungs with dye-free buffer. In some experiments, wortmannin (100 nM) or H7 dihydrochloride (100 μM) or a combination of the two was added to the dye loading solution in the preperfusion period. Wortmannin was used to inhibit PI3K, and H7 dihydrochloride was used as an inhibitor of PKC. The combination of the two was used to ascertain if there was any contribution of PI3K- and PKC-independent pathways in the generation of ROS with pulmonary ischemia. For each lung perfusion experiment, images from six randomly selected fields were averaged to obtain a mean value for fluorescence.
Flow adaptation of PMVECs.
ECs were plated on a Pronectin-treated optically clear plastic slide, which was placed in a temperature-controlled (37°C) confocal imaging chamber (Warner Instruments, Hamden, CT). Flow adaption of cells was accomplished in the chamber by the flow of medium at 10 dyn/cm2 for 24 h through in-flow and out-flow perfusion ports. For live cell imaging of flow-adapted ECs, the chamber was mounted on a microscope stage, allowing the monitoring of cell fluorescence with changes of flow. The Po2 level in the confocal chamber remained satisfactory for as long as 1 h after the stop of flow, as monitored using a phosphorescence probe (PdP1) that showed increasing phosphorescence with decreased perfusate Po2 (11, 28).
ECs also were flow adapted in an artificial capillary system (Cellmax Quad, Cellco, Germantown, MD) to obtain a relatively large number of cells for performing biochemical assays. This method allows ischemia without cellular anoxia or nutrient deprivation and has been previously described (31, 32, 39).
PI3K activity assay.
Cellular PI3K activity was measured using a kit supplied by Echelon Biosciences, as previously described (44). The assay uses a competitive ELISA to measure the amount of phosphatidylinositol (3,4,5)-triphosphate [PI(3,4,5)P3] produced from phosphatidylinositol (4,5)-bisphosphate (PIP2). ECs were lysed, and anti-PI3K antibody was added followed by a 50% slurry of protein A agarose beads to immunoprecipitate PI3K, which was used for the assay. PI(3,4,5)P3 standards and controls supplied in the kit were used to generate a standard curve.
Infection of PMVECs with Adp85 dominant negative PI3K.
Cells were infected with an adenoviral vector containing cDNA encoding dominant negative PI3K (mutant p85 subunit), which was obtained from Harold Franch (Emory School of Medicine, Atlanta, GA) (21). The amplified virus was titered and used for expression experiments. For adenoviral infections, 2 × 105 PMVECs were plated in a 60-mm culture dish, and cells were rinsed with serum-free medium and then infected (100 particles/cell) with adenovirus encoding dominant negative PI3K or empty vector.
Rac1 localization after ischemia.
Localization of Rac1 GTPase in mouse PMVECs after infection with adenovirus encoding dominant negative PI3K was evaluated under conditions of cell membrane depolarization induced either by ischemia or by high K+ and compared with control cells (noninfected or infected with vector only). Cells were flow adapted (10 dyn/cm2) for 24 h and subjected to stop of flow or continuously perfused with 24 mM K+ for 30 min. Cells were then fixed with ice-cold methanol-acetone (1:1) and immunostained using a monoclonal Rac1 antibody (1:200). The EC membrane was visualized using Alexa fluor 594-conjugated wheat germ agglutinin (4 μg/ml). A yellow colabel in the merged image indicates the colocalization of Rac with the EC membrane. The fluorescence intensity of Rac1 on the EC membrane was quantitated by Metamorph software, which marked the colabeled region, and was normalized to the length of the cell perimeter.
RESULTS
EC membrane depolarization with ischemia in wild-type and Akt-null lungs and the effects of inhibitors.
The membrane polarity-sensitive dye bis-oxonol was used for the imaging of subpleural microvessels in mouse lungs to detect changes in membrane polarization with ischemia. No change in fluorescence was found during continuous perfusion. However, an increase in fluorescence was observed within 30–60 s after the cessation of flow, and the fluorescence signal remained relatively constant during the subsequent 4 min of observation (Fig. 1). This result indicates a stable level of membrane depolarization subsequent to flow cessation. The KATP channel agonist cromakalim blocked depolarization with ischemia in wild-type lungs (Fig. 1), as previously shown (45). This agonist prevents the KATP channel inactivation that normally accompanies ischemia.
Fig. 1.
Depolarization of the endothelial cell (EC) membrane with ischemia in isolated lungs from wild-type (WT) and Akt-null mice. Lungs were preperfused for 30 min with the membrane potential-sensitive dye bis-oxonol (20 nM), and, where indicated, wortmannin (100 μM) or cromakalim (30 μM) were added. Images were acquired before (time 0) and at 0.5, 1, 2, 3, 4, and 5 min after the global cessation of flow (ischemia). Increased bis-oxonol fluorescence indicates cell membrane depolarization. Continuously perfused lungs (without ischemia) were used as a control. A: images were obtained at time 0 and at 0.5, 1, and 2 min of ischemia, as indicated at the top. Images are shown in pseudocolor with intensity as indicated by the sidebar. The imposed lines outline the margins of the microvessels. All images were acquired with the same exposure settings. Scale bar = 10 μm. B: fluorescence intensity quantified by MetaMorph software was plotted as a function of postischemic time from 0 to 5 min. The intensity for each time point was calculated as the mean value for six randomly selected fields. The dashed line indicates control perfusion with continuous flow (no ischemia). The plotted results represent means ± SE from 3 mice.
We next investigated whether PI3K/Akt activity has a role in EC membrane depolarization with ischemia. Pretreatment of isolated lungs with the PI3K inhibitor wortmannin had no effect on the observed changes in bis-oxonol fluorescence (Fig. 1). Likewise, the change in fluorescence with ischemia in Akt1-null lungs was similar to wild-type lungs (Fig. 1). The depolarization response in Akt1-null lungs was abrogated by cromakalim but was unaffected by the presence of wortmannin, as in wild-type lungs (Fig. 1). These results indicate that depolarization of the EC membrane occurs with ischemia and is independent of both PI3K and Akt activities.
Ischemia results in PI3K/Akt activation in vitro.
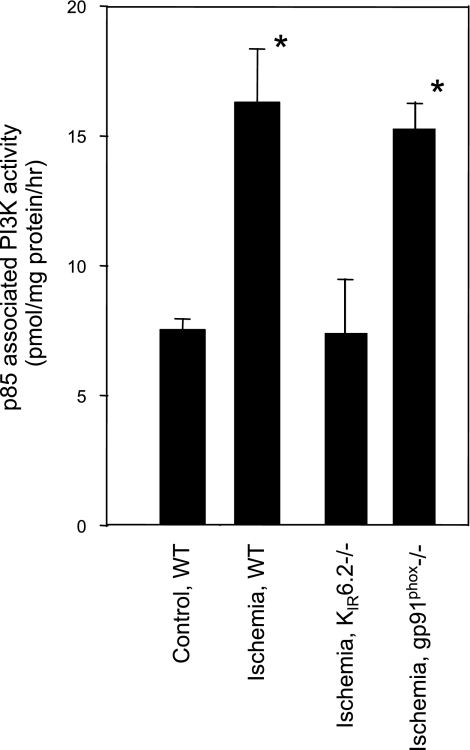
The relationship of PI3K activation to EC membrane depolarization was further evaluated in vitro using flow-adapted PMVECs subjected to ischemia; this model has been shown previously to result in cell membrane depolarization that depends on KATP channels (13, 30). Cell lysates were treated with an anti-p85 antibody to immunoprecipitate PI3K, which was then assayed for activity. Ischemia in wild-type cells resulted in PI3K activation, but this response was not observed in cells that lacked the KATP channel (Fig. 2). KATP channel-null ECs do not show significant depolarization with ischemia (13, 30, 45). Hence, depolarization is required for ischemia-induced activation of PI3K in ECs.
Fig. 2.
Phosphoinositide 3-kinase (PI3K) activity in mouse pulmonary microvascular ECs (PMVECs) is increased with ischemia. Flow-adapted WT, ATP-sensitive K+ (KATP) channel (Kir6.2)-null, or NADPH oxidase [NOX2; glycoprotein (gp)91phox]-null cells were subjected to stop of flow. Cells were immediately removed from the cartridges, lysed, incubated with anti-p85 antibody to immunoprecipitate PI3K, and assayed for PI3K activity with phosphatidylinositol (4,5)-biphosphate (PIP2) as the substrate. WT cells cultured under static conditions were the control. *P < 0.01 vs. control cells.
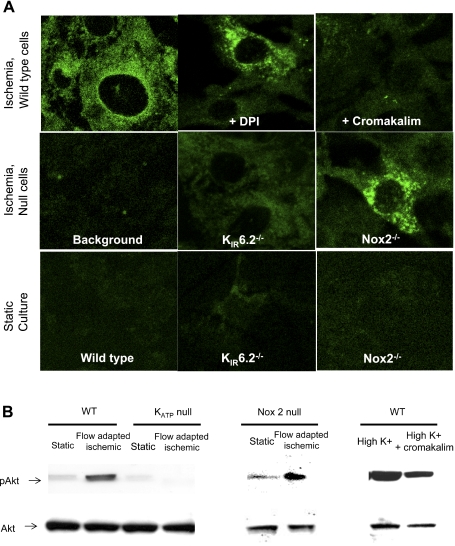
We next evaluated the possible ischemia-mediated activation (phosphorylation) of Akt, the downstream partner of PI3K. To detect activation, cells were fixed, immunostained with anti-pSer473 Akt followed by secondary anti-mouse IgG-Alexa fluor 488 and imaged by confocal microscopy. Flow-adapted wild-type cells showed phosphorylation of Akt with ischemia, whereas no Akt phosphorylation was observed in KATP channel-null cells or wild-type cells pretreated with cromakalim (Fig. 3A). The presence of cromakalim or deletion of the KATP channel largely prevented cell membrane depolarization with ischemia (13, 30). However, cells that depolarized with ischemia, but did not produce ROS, such as DPI-treated cells (DPI pretreatment prevents NADPH oxidase activation), showed Akt phosphorylation (Fig. 3A), indicating that phosphorlation occurred in the absence of ROS but not in the absence of depolarization. Cells cultured under static conditions were used as a negative control. Similar results were obtained by immunoblotting static and ischemic cells from wild-type, KATP channel-null, and NOX2 (gp91phox)-null animals for pAkt (Fig. 3B). Quantitation of the lanes revealed that the phosphorylation of Akt in ischemic cells of wild-type and NOX2-null animals (i.e., cells that depolarized with ischemia) was 2.8- and 4-fold higher than their static controls, respectively. This was not observed in cells that do not depolarize with ischemia (i.e., KATP channel-null cells). In addition, when wild-type cells were depolarized (by high K+), phosphorylation of Akt was observed, which was reduced (by 51%) in cells pretreated with cromakalim. In all these experiments, the total Akt content in these cells remained unchanged. Thus, activation of Akt with ischemia, as for PI3K, represents a downstream response to membrane depolarization.
Fig. 3.
Activation of Akt1 with ischemia in WT, Kir6.2 (KATP channel)-null, and NOX2-null PMVECs. WT cells were also pretreated for 30 min with 10 μM diphenyleneiodonium chloride (DPI) or 30 μM cromakalim, as indicated. ECs cultured under flow (24 h, 10 dyn/cm2) were subjected to abrupt flow cessation (ischemia). A: activation of Akt1 was detected by green immunofluorescence using an antibody specific to phosphorylated (p)Ser473 Akt. Cells cultured under static conditions were the control. Background is treatment with nonspecific IgG. B: representative blots of static cells, ischemic cells, and depolarized cells (cells treated with high K+ in the presence and absence of the KATP agonist cromakalim). Immunoblots were probed using anti-pSer473 Akt and Akt antibodies. The gap indicates either separate blots or that several nonrelevant lanes were removed.
ROS generation with ischemia in isolated lungs.
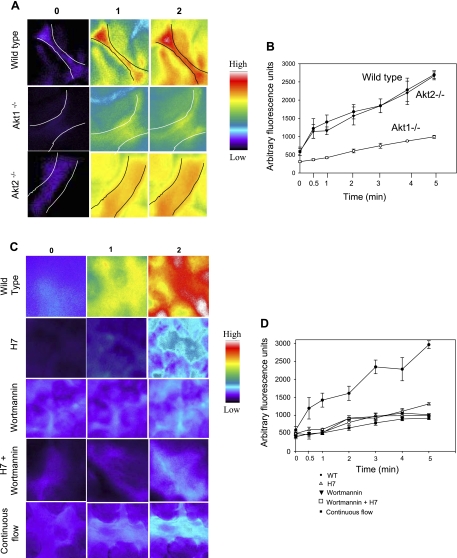
We next investigated the relationship between activation of PI3K/Akt and ROS production with ischemia. ROS generation in isolated lungs was monitored by the oxidation of the ROS-sensitive dye H2DCF to DCF. Wild-type lungs showed a rapid increase in DCF fluorescence with ischemia, indicating ROS production (Fig. 4), as previously shown (44, 45). ROS production with ischemia was significantly reduced in lungs with knockout of Akt1, whereas the response of Akt2-null lungs to ischemia was similar to wild-type lungs (Fig. 4, A and B). Thus, Akt1 is required for the activation of endothelial ROS production with ischemia. Based on the Akt2-null results, the marked decrease of ROS generation in Akt1-null lungs results specifically from loss of the protein and is not a general effect of an Akt-null phenotype.
Fig. 4.
ROS production with ischemia in the endothelium of subpleural capillaries of the intact lung. A: lungs from WT, Akt1-null, and Akt2-null mice were preperfused for 30 min with dichlorodihydrofluoroscein diacetate (H2DCF-DA; 5 mM), and 2′,7′-dichlorofluorescin (DCF) fluorescence was monitored continuously. Increased fluorescence indicates H2DCF oxidation to DCF, representing ROS production. Images were obtained before and at 1-min intervals for 5 min after the global cessation of flow (ischemia). The images shown were acquired before (time 0) and at 1 and 2 min after the onset of ischemia, as indicated at the top. All images were acquired with the same exposure time. The images are in pseudocolor, as shown by the intensity bar on the sidebar. B: DCF fluorescence intensity as a function of postischemic time. The fluorescence intensity was quantified by MetaMorph software. Each data point represents the mean of six random fields. Results are means ± SE from 4 mice. C: lungs were pretreated with the PKC inhibitor H7 (100 μM), the PI3K inhibitor wortmannin (100 nM), or a combination of the two and then imaged with DCF under similar conditions. D: DCF fluorescence intensity as a function of postischemic time.
Since PKC has also been reported to mediate ROS generation in vascular ECs, we investigated if the PI3K/Akt pathway intersects with PKC. To do this, we pretreated lungs with the PKC inhibitor H7 dihydrochloride and monitored ROS production with ischemia. ROS production with pulmonary ischemia was significantly reduced with PKC inhibition (Fig. 4, C and D). This inhibition was comparable with that achieved with the inhibitor of PI3K. The combination of the two inhibitors had no greater effect than either alone, indicating that PI3K and PKC are in the same rather than parallel pathways for ROS production with ischemia.
Rac1 translocation and membrane depolarization.
Rac1 is a cytoplasmic protein that translocates to the cell membrane during activation of the NOX2 enzyme complex. Rac1 is the responsible isoform in PMVECs, and its translocation is required for ROS production with ischemia (44). We (44) have previously reported that membrane depolarization in PMVECs initiates Rac1 translocation from the cytosol to the membrane, and this translocation can be blocked by wortmannin. To extend these results, we monitored Rac1 translocation in dominant negative PI3K-infected cells subjected to ischemia (Fig. 5A). Rac1 translocation also was evaluated after treatment of cells with 24 mM KCl (high K+) as a membrane depolarizing agent (Fig. 5B). Based on the yellow colocalization signal, wild-type cells and AdlacZ (adenovirus control)-infected cells showed Rac1 translocation with both ischemia (Fig. 5A) and high K+ (Fig. 5B). On the other hand, Rac1 translocation with these agonists was significantly suppressed by dominant negative PI3K infection of PMVECs. Thus, PI3K activity is required for Rac1 translocation and subsequent NOX2 activation in response to EC membrane depolarization.
Fig. 5.
Rac1 colocalization with a plasma membrane marker wheat germ agglutinin after ischemia or high K+ in mouse PMVECs. Cells were noninfected (WT) or infected with adenovirus without (AdLacZ) or with dominant negative PI3K (AdDNp85). Data are mean values for 3–4 fields. Results are means ± SE for 3 independent experiments. A: Rac1 localization with ischemia. Cells were flow adapted (10 dyn/cm2) for 24 h, subjected to stop of flow for 30 min, fixed, and immunostained using a monoclonal anti-Rac1 antibody (1:200). Left, Rac1 was detected by FITC-labeled anti-mouse IgG secondary antibody (green). The EC membrane was visualized using Alexa fluor 594-conjugated wheat germ agglutinin (WGA; red). The yellow colabel in the merged image indicates the colocalization of Rac1 with the EC membrane. Right, Metamorph software was used to mark the colabeled region, and the fluorescence intensity of Rac1 on the EC membrane was determined. Fluorescence intensity was normalized to the measured cell perimeter. B: Rac localization on the EC membrane upon high-K+ (24 mM) treatment. Images and quantitation were obtained as described in A.
ROS are not required for the activation of PI3K/Akt and ROS with ischemia.
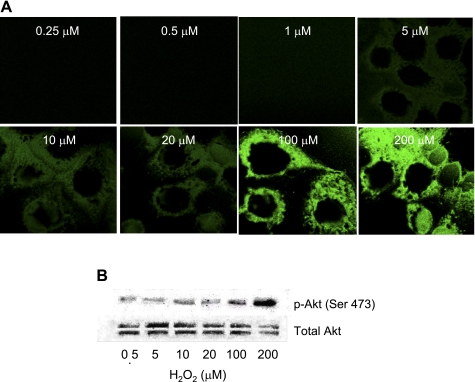
The results of the perfused lung experiments (Fig. 4) indicated that PI3K/Akt and PKC are necessary for ROS production with ischemia but do not exclude the possibility that ROS, in turn, may contribute to PI3K/Akt activation, essentially a positive feedback mechanism. This possibility was investigated in isolated cells that were devoid of NOX2 (gp91phox null) and do not produce ROS with ischemia. Flow-adapted NOX2-null PMVECs showed the activation of PI3K (Fig. 2) and Akt1 (Fig. 3) with ischemia that was similar to wild-type PMVECs. Thus, the failure to generate ROS had no effect on the activation of PI3K/Akt. Because of previous reports obtained with other systems (14, 19, 25, 42), we determined if exogenous H2O2 can lead to Akt phosphorylation in mouse PMVECs. Wild-type PMVECs were exposed for 2–3 min to H2O2 at concentrations from 0.25 to 100 μM. Akt activation (phosphorylation) was observed, but only when H2O2 was present at relatively high concentrations (>10 μM; Fig. 6). These results are compatible with previous reports using exogenous H2O2 (9, 34, 42). Although exogenous H2O2 may have an effect that differs from the local generation of H2O2, these results suggest that H2O2 does not activate Akt at concentrations that might be encountered physiologically. Thus, PI3K, Akt, and PKC appear to be downstream of cell membrane depolarization and upstream of ROS generation.
Fig. 6.
Effect of H2O2 on the phosphorylation of Akt upon exposure of PMVECs cultured under static conditions. The response to increasing concentrations of H2O2 is shown by immunofluorescence (A) and immunoblot (B) with anti-pSer473 Akt antibody (1:1,000). The secondary antibody was goat anti-mouse IgG used at a dilution of 1:5,000.
DISCUSSION
Our laboratory has been engaged in studies to delineate the response of PMVECs to altered shear. In this connection, we have established isolated perfused lung and in vitro flow-adapted cellular models that are useful to study the response to alterations of the mechanical component of flow without the attendant tissue hypoxia or anoxia that accompanies the stop of blood flow in vascular beds of other organs (2, 4–6, 10, 13, 31, 35, 37, 44, 45). Removal of fluid shear stress from flow-adapted cells represents a relevant in vitro model in the sense that the normal endothelium in situ is constantly exposed to flow (shear) and the cessation of blood flow occurs with various pathologies, such as pulmonary embolism or lung transplantation. In contrast, the response to initiation of shear with cells grown under static conditions is a less physiological model even though the resulting response may follow pathways similar to those accompanying flow cessation (16). The signaling events that accompany the changes in fluid shear stress to ECs are considered to be an example of mechanotransduction.
Our studies have relied heavily on the use of fluorophores, especially DCF in the present study, to detect ROS generation in the intact lung. DCF oxidation is often considered an inadequate readout for ROS generation; however, our earlier imaging studies showed a correspondence of the DCF signal with ROS generation as evaluated by several methods in our models of ischemia in lungs and flow-adapted ECs. First, the DCF signal did not increase in lungs or cells that do not generate ROS with stopped flow (NOX2-null cells, caveolin-null cells, and DPI treatment) (30, 44, 45). Second, increased fluorescence compatible with ROS was demonstrated with several other fluorophores (amplex red and dihydroethidine) (3, 35, 44, 45). Third, an increase in ROS with stopped flow in flow-adapted ECs was demonstrated by SOD-dependent reduction of cytochrome c (27). DCF, unlike hydroethidine, does not detect superoxide but does detect H2O2, the presumed dismutation product of superoxide generated by NOX2 activation with pulmonary ischemia. Hence, DCF oxidation in our model appears to be dependent on the presence of ROS (H2O2).
We (4, 35, 44, 45) have previously reported using isolated lungs or flow-adapted cells in vitro that abrupt the cessation of flow causes a rapid (within 1 min) increase in ROS production by the pulmonary endothelium. Fluorescence imaging of membrane polarity in the intact lung showed that EC membrane depolarization preceded ROS generation and occurred within seconds of stopped flow (2, 5, 35, 44, 45). Depolarization required the presence of intact caveolae (30). By patch clamp of flow-adapted cells in vitro, stop of flow resulted in an immediate decrease of Kir current, indicating essentially immediate depolarization with an abrupt loss of shear. Cells with “knockout” of Kir6.2 had almost no change in membrane potential and markedly reduced ROS production with ischemia (13, 45). Thus, membrane depolarization is followed by ROS production, associated with a loss of fluid shear stress, and this relationship has been observed both for the pulmonary endothelium in situ as well as flow-adapted PMVECs in vitro. The absence of ROS generation with ischemia in lungs and flow-adapted pulmonary ECs from NOX2 gene-targeted mice indicate that this NOX isoform is the source of ROS (5, 31). The relationship between EC membrane potential and NOX2 activation was confirmed by exposing cells to high extracellular K+ to depolarize the EC membrane (30, 44). Although previous studies established that cell membrane depolarization signals for NOX activation, the pathway connecting these events was not clear.
We (44) have previously shown that EC membrane depolarization induced by high K+ results in PI3K activation. The present study investigated PI3K, Akt, and PKC activation as links between EC membrane depolarization and NOX activation using inhibitors of PI3K (wortmannin) and PKC (H7), infection with dominant negative PI3K, and Akt gene targeting. We (44) have previously reported that treatment of mouse lungs or isolated mouse flow-adapted PMVECs with the PI3K/Akt inhibitor wortmannin reduces ROS production with ischemia. In the present study, the results using a dominant negative construct showed that the translocation of Rac1 with depolarization (the initial step in NOX2 activation) requires PI3K. We also showed that inhibition of PKC (by H7) markedly diminished ROS generation with ischemia, with a magnitude comparable with that of PI3K inhibition by wortmannin. The combination of wortmannin and H7 had no greater effect than either alone. These results indicate that PI3K/Akt and PKC are in the same pathway and are required for ROS generation with ischemia. PKC has been reported to be involved in NOX2 activation in vascular ECs via the phosphorylation of p47phox (43). Another possible target of PKC is the phosphorylation of peroxiredoxin 6 (40), a recently described component of the NOX2 activation cascade (12).
Although wortmannin prevented NOX2 activation, it had no effect on cell membrane depolarization. PI3K activity was significantly enhanced in cells that showed depolarization with ischemia (wild-type and NOX2-null cells) but was unaffected with ischemia in the absence of depolarization (KATP channel-null cells). Thus, membrane depolarization results in PI3K activation. But how are membrane potential and PI3K activity linked? A previous study (46) has indicated that an imposed electrical field can modulate PI3K activity in HL60 cells. This latter study also showed that exposure to an electrical field modified the potential difference across membranes and led to PI3K, Src, and Akt activation. However, in cells where the p110 subunit of PI3K was deleted, the activation of Akt and Src under a similar electrical stimulus was significantly impaired. PI3K may have one or more domains that are sensitive to alterations in membrane polarity. Another possibility for the membrane potential effect relates to substrate availability. Membrane depolarization has been shown to activate phosphatase activity in both Xenopus oocytes and Chinese hamster ovary cells transfected with a voltage sensor containing phosphatase from Ciona intestinalis, resulting in perturbation of the PIP2 pool (18, 24, 33). Thus, it is possible that membrane depolarization associated with ischemia activates PI3K directly through a membrane potential-sensitive domain or indirectly through the activation of phosphoinositide phosphatases that modulate PIP2 concentrations.
Depolarization of the lung endothelium with ischemia was also unaffected by deletion of Akt1. Furthermore, pretreatment of Akt1-null lungs with the KATP channel agonist cromakalim prevented the depolarization response with ischemia, similar to the wild-type response. Thus, EC membrane depolarization with ischemia is independent of Akt. On the other hand, ROS generation after ischemia in Akt1-null lungs was significantly reduced compared with either wild-type lungs or Akt2-null lungs. Thus, Akt1 activation with ischemia, like PI3K activation, is downstream of cell membrane depolarization but upstream of NOX2 activation.
To summarize, our data indicate that flow cessation results in cell membrane depolarization, which is followed by PI3K/Akt and PKC activation, which, in turn, causes NOX assembly and ROS generation. We speculate that the activity of PI3K is regulated either by a voltage-sensitive domain in the protein or by phosphatase-mediated changes in substrate concentration and that this change in activity depends on changes in the EC membrane potential.
GRANTS
This work was supported by National Institutes of Health Grants HL-75587 and DK-56886.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.C., E.B., M.J.B., and A.B.F. conception and design of research; S.C. analyzed data; S.C. interpreted results of experiments; S.C. prepared figures; S.C. drafted manuscript; S.C. edited and revised manuscript; S.C. and A.B.F. approved final version of manuscript; N.H., K.M.D., E.M.S., and W.L. performed experiments.
ACKNOWLEDGMENTS
The authors thank Dr. Harold Franch for supplying dominant negative PI3K, Dr. John Noel and Dr. Hui Wang for technical assistance, and Victoria Brown for administrative support.
Results from this study have been previously presented in part at the 2008, 2009, and 2010 Experimental Biology meetings in San Diego, CA, New Orleans, LA, and Anaheim, CA, respectively.
REFERENCES
- 1. Ago T, Kuribayashi F, Hiroaki H, Takeya R, Ito T, Kohda D, Sumimoto H. Phosphorylation of p47phox directs phox homology domain from SH3 domain toward phosphoinositides, leading to phagocyte NADPH oxidase activation. Proc Natl Acad Sci USA 100: 4474–4479, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. al-Mehdi AB, Ischiropoulos H, Fisher AB. Endothelial cell oxidant generation during K+-induced membrane depolarization. J Cell Physiol 166: 274–280, 1996 [DOI] [PubMed] [Google Scholar]
- 3. Al-Mehdi AB, Shuman H, Fisher AB. Intracellular generation of reactive oxygen species during nonhypoxic lung ischemia. Am J Physiol Lung Cell Mol Physiol 272: L294–L300, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Al-Mehdi AB, Zhao G, Dodia C, Tozawa K, Costa K, Muzykantov V, Ross C, Blecha F, Dinauer M, Fisher AB. Endothelial NADPH oxidase as the source of oxidants in lungs exposed to ischemia or high K+. Circ Res 83: 730–737, 1998 [DOI] [PubMed] [Google Scholar]
- 5. Al-Mehdi AB, Zhao G, Fisher AB. ATP-independent membrane depolarization with ischemia in the oxygen-ventilated isolated rat lung. Am J Respir Cell Mol Biol 18: 653–661, 1998 [DOI] [PubMed] [Google Scholar]
- 6. Al-Mehdi AB, Zhao G, Tozawa K, Fisher AB. Depolarization-associated iron release with abrupt reduction in pulmonary endothelial shear stress in situ. Antioxid Redox Signal 2: 335–345, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Bokoch GM, Knaus UG. NADPH oxidases: not just for leukocytes anymore! Trends Biochem Sci 28: 502–508, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Brown GE, Stewart MQ, Liu H, Ha VL, Yaffe MB. A novel assay system implicates PtdIns(3,4)P2, PtdIns(3)P, and PKCδ in intracellular production of reactive oxygen species by the NADPH oxidase. Mol Cell 11: 35–47, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Cai H, Li Z, Davis ME, Kanner W, Harrison DG, Dudley SC., Jr Akt-dependent phosphorylation of serine 1179 and mitogen-activated protein kinase kinase/extracellular signal-regulated kinase 1/2 cooperatively mediate activation of the endothelial nitric-oxide synthase by hydrogen peroxide. Mol Pharmacol 63: 325–331, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Chatterjee S, Al-Mehdi AB, Levitan I, Stevens T, Fisher AB. Shear stress increases expression of a KATP channel in rat and bovine pulmonary vascular endothelial cells. Am J Physiol Cell Physiol 285: C959–C967, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Chatterjee S, Chapman KE, Fisher AB. Lung ischemia: a model for endothelial mechanotransduction. Cell Biochem Biophys 52: 125–138, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chatterjee S, Feinstein SI, Dodia C, Sorokina E, Lien YC, Nguyen S, Debolt K, Speicher D, Fisher AB. Peroxiredoxin 6 phosphorylation and subsequent phospholipase A2 activity are required for agonist-mediated activation of NADPH oxidase in mouse pulmonary microvascular endothelium and alveolar macrophages. J Biol Chem 286: 11696–11706, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chatterjee S, Levitan I, Wei Z, Fisher AB. KATP channels are an important component of the shear-sensing mechanism in the pulmonary microvasculature. Microcirculation 13: 633–644, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Chen JX, Zeng H, Lawrence ML, Blackwell TS, Meyrick B. Angiopoietin-1-induced angiogenesis is modulated by endothelial NADPH oxidase. Am J Physiol Heart Circ Physiol 291: H1563–H1572, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBα is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem 276: 38349–38352, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Davies PF, Barbee KA, Volin MV, Robotewskyj A, Chen J, Joseph L, Griem ML, Wernick MN, Jacobs E, Polacek DC, dePaola N, Barakat AI. Spatial relationships in early signaling events of flow-mediated endothelial mechanotransduction. Annu Rev Physiol 59: 527–549, 1997 [DOI] [PubMed] [Google Scholar]
- 17. Dinauer MC, Li LL, Bjorgvinsdottir H, Ding C, Pech N. Long-term correction of phagocyte NADPH oxidase activity by retroviral-mediated gene transfer in murine X-linked chronic granulomatous disease. Blood 94: 914–922, 1999 [PubMed] [Google Scholar]
- 18. Falkenburger BH, Jensen JB, Hille B. Kinetics of PIP2 metabolism and KCNQ2/3 channel regulation studied with a voltage-sensitive phosphatase in living cells. J Gen Physiol 135: 99–114, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Feng J, Damrauer SM, Lee M, Sellke FW, Ferran C, Abid MR. Endothelium-dependent coronary vasodilatation requires NADPH oxidase-derived reactive oxygen species. Arterioscler Thromb Vasc Biol 30: 1703–1710, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fisher AB, Zhang Q. NADPH and NADPH oxidase. In: Encyclopedia of Respiratory Medicine, edited by Geoffrey JL, Steven DS. Oxford: Academic, 2006, p. 77–84 [Google Scholar]
- 21. Franch HA, Raissi S, Wang X, Zheng B, Bailey JL, Price SR. Acidosis impairs insulin receptor substrate-1-associated phosphoinositide 3-kinase signaling in muscle cells: consequences on proteolysis. Am J Physiol Renal Physiol 287: F700–F706, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Frey RS, Gao X, Javaid K, Siddiqui SS, Rahman A, Malik AB. Phosphatidylinositol 3-kinase gamma signaling through protein kinase Cζ induces NADPH oxidase-mediated oxidant generation and NF-κB activation in endothelial cells. J Biol Chem 281: 16128–16138, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 86: 494–501, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Halaszovich CR, Schreiber DN, Oliver D. Ci-VSP is a depolarization-activated phosphatidylinositol-4,5-bisphosphate and phosphatidylinositol-3,4,5-trisphosphate 5′-phosphatase. J Biol Chem 284: 2106–2113, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Kou B, Ni J, Vatish M, Singer DR. Xanthine oxidase interaction with vascular endothelial growth factor in human endothelial cell angiogenesis. Microcirculation 15: 251–267, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Lassegue B, Griendling KK. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol 30: 653–661, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Manevich Y, Al-Mehdi A, Muzykantov V, Fisher AB. Oxidative burst and NO generation as initial response to ischemia in flow-adapted endothelial cells. Am J Physiol Heart Circ Physiol 280: H2126–H2135, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Matsuzaki I, Chatterjee S, Debolt K, Manevich Y, Zhang Q, Fisher AB. Membrane depolarization and NADPH oxidase activation in aortic endothelium during ischemia reflect altered mechanotransduction. Am J Physiol Heart Circ Physiol 288: H336–H343, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Miki T, Nagashima K, Tashiro F, Kotake K, Yoshitomi H, Tamamoto A, Gonoi T, Iwanaga T, Miyazaki J, Seino S. Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proc Natl Acad Sci USA 95: 10402–10406, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Milovanova T, Chatterjee S, Hawkins BJ, Hong N, Sorokina EM, Debolt K, Moore JS, Madesh M, Fisher AB. Caveolae are an essential component of the pathway for endothelial cell signaling associated with abrupt reduction of shear stress. Biochim Biophys Acta 1783: 1866–1875, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Milovanova T, Chatterjee S, Manevich Y, Kotelnikova I, Debolt K, Madesh M, Moore JS, Fisher AB. Lung endothelial cell proliferation with decreased shear stress is mediated by reactive oxygen species. Am J Physiol Cell Physiol 290: C66–C76, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Milovanova T, Manevich Y, Haddad A, Chatterjee S, Moore JS, Fisher AB. Endothelial cell proliferation associated with abrupt reduction in shear stress is dependent on reactive oxygen species. Antioxid Redox Signal 6: 245–258, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Murata Y, Okamura Y. Depolarization activates the phosphoinositide phosphatase Ci-VSP, as detected in Xenopus oocytes coexpressing sensors of PIP2. J Physiol 583: 875–889, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sadidi M, Lentz SI, Feldman EL. Hydrogen peroxide-induced Akt phosphorylation regulates Bax activation. Biochimie 91: 577–585, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Song C, Al-Mehdi AB, Fisher AB. An immediate endothelial cell signaling response to lung ischemia. Am J Physiol Lung Cell Mol Physiol 281: L993–L1000, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Suh CI, Stull ND, Li XJ, Tian W, Price MO, Grinstein S, Yaffe MB, Atkinson S, Dinauer MC. The phosphoinositide-binding protein p40phox activates the NADPH oxidase during FcγIIA receptor-induced phagocytosis. J Exp Med 203: 1915–1925, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tozawa K, al-Mehdi AB, Muzykantov V, Fisher AB. In situ imaging of intracellular calcium with ischemia in lung subpleural microvascular endothelial cells. Antioxid Redox Signal 1: 145–154, 1999 [DOI] [PubMed] [Google Scholar]
- 38. Vignais PV. The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell Mol Life Sci 59: 1428–1459, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wei Z, Costa K, Al-Mehdi AB, Dodia C, Muzykantov V, Fisher AB. Simulated ischemia in flow-adapted endothelial cells leads to generation of reactive oxygen species and cell signaling. Circ Res 85: 682–689, 1999 [DOI] [PubMed] [Google Scholar]
- 40. Wu Y, Feinstein SI, Manevich Y, Chowdhury I, Pak JH, Kazi A, Dodia C, Speicher DW, Fisher AB. Mitogen-activated protein kinase-mediated phosphorylation of peroxiredoxin 6 regulates its phospholipase A2 activity. Biochem J 419: 669–679, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yamamori T, Inanami O, Nagahata H, Kuwabara M. Phosphoinositide 3-kinase regulates the phosphorylation of NADPH oxidase component p47phox by controlling cPKC/PKCδ but not Akt. Biochem Biophys Res Commun 316: 720–730, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Yang B, Oo TN, Rizzo V. Lipid rafts mediate H2O2 prosurvival effects in cultured endothelial cells. FASEB J 20: 1501–1503, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Yu G, Peng T, Feng Q, Tyml K. Abrupt reoxygenation of microvascular endothelial cells after hypoxia activates ERK1/2 and JNK1, leading to NADPH oxidase-dependent oxidant production. Microcirculation 14: 125–136, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Zhang Q, Chatterjee S, Wei Z, Liu WD, Fisher AB. Rac and PI3 kinase mediate endothelial cell-reactive oxygen species generation during normoxic lung ischemia. Antioxid Redox Signal 10: 679–689, 2008 [DOI] [PubMed] [Google Scholar]
- 45. Zhang Q, Matsuzaki I, Chatterjee S, Fisher AB. Activation of endothelial NADPH oxidase during normoxic lung ischemia is KATP channel dependent. Am J Physiol Lung Cell Mol Physiol 289: L954–L961, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Zhao M, Song B, Pu J, Wada T, Reid B, Tai G, Wang F, Guo A, Walczysko P, Gu Y, Sasaki T, Suzuki A, Forrester JV, Bourne HR, Devreotes PN, McCaig CD, Penninger JM. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-γ and PTEN. Nature 442: 457–460, 2006 [DOI] [PubMed] [Google Scholar]