Abstract
Molecular studies examining the impact of mitochondrial morphology on the mammalian heart have previously focused on dynamin related protein-1 (Drp-1) and mitofusin-2 (Mfn-2), while the role of the other mitofusin isoform, Mfn-1, has remained largely unexplored. In the present study, we report the generation and initial characterization of cardiomyocyte-specific Mfn-1 knockout (Mfn-1 KO) mice. Using electron microscopic analysis, we detect a greater prevalence of small, spherical mitochondria in Mfn-1 KO hearts, indicating that the absence of Mfn-1 causes a profound shift in the mitochondrial fusion/fission balance. Nevertheless, Mfn-1 KO mice exhibit normal left-ventricular function, and isolated Mfn-1 KO heart mitochondria display a normal respiratory repertoire. Mfn-1 KO myocytes are protected from mitochondrial depolarization and exhibit improved viability when challenged with reactive oxygen species (ROS) in the form of hydrogen peroxide (H2O2). Furthermore, in vitro studies detect a blunted response of KO mitochondria to undergo peroxide-induced mitochondrial permeability transition pore opening. These data suggest that Mfn-1 deletion confers protection against ROS-induced mitochondrial dysfunction. Collectively, we suggest that mitochondrial fragmentation in myocytes is not sufficient to induce heart dysfunction or trigger cardiomyocyte death. Additionally, our data suggest that endogenous levels of Mfn-1 can attenuate myocyte viability in the face of an imminent ROS overload, an effect that could be associated with the ability of Mfn-1 to remodel the outer mitochondrial membrane.
Keywords: mitochondrial dynamics, GTPase, dynamin, membrane permeability, apoptosis, cardiomyocytes, reactive oxygen species
merging and partitioning the mitochondrial membranes are ongoing processes that govern the structure and morphology of the organelle in a wide range of organisms and cell types (8, 48). Extensive mitochondrial fusion and matrix continuity are currently viewed as adaptive mechanisms that allow mitochondria to cope with increased energetic demands and cellular stressors (60, 67). Furthermore, coupling selective fusion with fission is believed to constitute a means for mitochondrial quality control (68). In mammals, fusion of the outer mitochondrial membrane (OMM) between adjacent mitochondria is controlled by mitofusins (Mfn-1 and Mfn-2; Refs. 10, 36, 56). Disrupting mitochondrial fusion via Mfn-1 or Mfn-2 ablation alters the distribution and morphology of mitochondria and precipitates a stochastic process of mitochondrial dysfunction (9, 10). Recently, the requirement of mitochondrial fusion for normal neuronal function and for mitochondrial DNA (mtDNA) maintenance in skeletal muscle has been documented in mice (11, 12).
Despite the widespread utilization of fusion by mitochondria, the mechanism of membrane merging (i.e., tethering, formation of a fusion pore and lipid rearrangement) and how mitofusins participate in these processes remain incompletely understood. In this regard, it has been shown that the C-terminal coiled-coil region of mitofusins is necessary in the initial tethering between opposing mitochondria and that guanosine triphosphate (GTP) hydrolysis during mitofusin engagement is essential for subsequent OMM fusion steps (32, 43). More recently, it has been shown that the two isoforms are able to complement each other in the fusion process such that the formation of Mfn-1:Mfn-2 heterologous complexes can be highly effective in promoting fusion in vitro (24). The effect of mitofusins in membrane fusion is counterbalanced by dynamin-related protein-1 (Drp-1), a mediator of mitochondrial division (61, 70). Although mitofusins and Drp-1 catalyze opposite reactions, they are structurally similar in that they encompass a GTPase domain, and the mutation of key residues in these domains can significantly alter the dynamics of mitochondrial morphology in cells (46, 56, 57, 62).
Excessive mitochondrial fragmentation, typified by the presence of numerous small and spherical mitochondria, is an early event occurring during regulated cell death (apoptosis) (14, 39) and has been described in experimental models of heart failure (55). Reversing mitochondrial diminution via mitofusin activation or Drp-1 inhibition can prevent the progression of cell death in some contexts (19, 28, 35, 49, 64) but not in others (6, 52, 58). On the other hand, fragmentation alone is probably not sufficient to trigger cell death, because many cells harboring extensively fragmented mitochondrial networks maintain apparent viability (10, 31, 36, 37). Thus the question of whether mitochondrial fragmentation is sufficient to affect apoptosis remains controversial (53, 63). Furthermore, it has been suggested that mitochondria-shaping proteins can influence cell death progression through activities that do not primarily involve mitochondrial fragmentation (34). For example, the mitochondrial fission factor Fis-1 has been shown to relay apoptosis-inducing signals before fragmentation occurs (27).
In heart, mitochondria are abundantly present where they form a crystalline pattern and display limited motility (69). However, cardiac mitochondria appear capable for interorganellar communication through the coordination of membrane potential (3, 5). To better understand the role of mitochondrial dynamics in cardiac myocytes, we have undertaken a mouse genetic approach to ablate mitofusins in cardiac myocytes. Previously, we constructed and characterized mice lacking Mfn-2 in cardiac myocytes (50). This modification led to hearts that displayed modest cardiac hypertrophy and mild functional deterioration. Mfn-2-deficient mitochondria were pleiomorphic and characteristically enlarged, and myocytes from these hearts were protected from mitochondrial permeability transition pore (MPTP) opening and cell death. Here, we constructed mice lacking Mfn-1 in cardiac myocytes. These hearts are structurally and functionally normal. In marked contrast to Mfn-2-deficient hearts, however, mitochondria in Mfn-1-deficient hearts are smaller, yet myocytes are protected from reactive oxygen species (ROS)-induced death. Furthermore, the assessment of isolated mitochondria showed greater resistance to tert-butyl hydroxyperoxide (tBH)-induced MPTP opening. These findings highlight a striking functional difference between Mfn-1 and Mfn-2 in controlling mitochondria size in myocytes but also reveal considerable parallels regarding the involvement of mitofusins in regulating the mitochondrial response against ROS-induced dysfunction. Finally, our data challenge the notion that smaller mitochondria predispose to stress-induced cell death.
MATERIALS AND METHODS
Animals.
Mice carrying the Mfn-1loxP allele (stock Mfn1tm2Dcc; identification no. 029901-UCD) were obtained from the Mutant Mouse Regional Resource Center at University of California, Davis, and their generation has been previously described (11). These mice were mated with Myh6-cre transgenic mice designed to express cre recombinase selectively in cardiac myocytes and to drive site-specific recombination of loxP sequences as early as embryonic day 9.5 (1, 21). Mice with the genotype Mfn-1flox/flox;Myh6-cre+/− are termed Mfn-1 KO. Mice with the genotype Mfn-1flox/flox;Myh6-cre−/− (littermates) or Mfn-1+/+;Myh6-cre+/− are collectively termed Mfn-1 wild-type (WT) and were used interchangeably in the present study. The genetic background of the mice is 129S/C56BL6/BlackSwiss. The mice were housed in a 12-h light-dark cycle and temperature-controlled room and all handling procedures conformed to the regulations of and were approved by the Institutional Animal Care and Use Committee of Boston University School of Medicine.
Echocardiography.
The procedure to obtain echocardiograms and measurements from lightly anesthetized mice using the Vevo770 system (http://www.visualsonics.com/vevo770) has been described previously (50). Cardiac mass (CM) was calculated from long axis views of the myocardium (epicardium and endocardium) in diastole (d) and systole (s) according to the equation:
where T is the average wall thickness and 1.05 is the specific gravity constant of the myocardium. The volumes in end-diastole and end-systole (EDV and ESV, respectively) were calculated according to Simpson's equation that utilizes the area of the chamber at four levels perpendicular to the long axis. These volumes were used to calculate the ejection fraction according to the equation
The fractional shortening (FS) was calculated from M-mode views of the myocardium at the papillary muscle level (short axis view) according to the equation:
where LVID is the LV internal diameter.
Electron microscopy.
Rod-shaped pieces (∼1 mm in diameter and 2 mm in length) were obtained from the LV free wall of adult male mice, fixed in Karnovsky's solution (2% paraformaldehyde, 2.5% glutaraldehyde, and 0.1 M phosphate buffer; Electron Microscopy Sciences) and processed until embedding into epon plastic (Embed-812; Electron Microscopy Sciences), as previously described (50). Ultra-thin sections containing predominantly longitudinal myofibers were stained with 1% (wt/vol) uranyl acetate for 20 min followed by Reynold's lead citrate for 5 min. Micrographs were collected using a Philips CM12 transmission electron microscope at 120 kV and ×6,300 magnification. Scanned micrographs were analyzed using ImageJ software to manually generate masks of mitochondrial contours that were used for the calculation of mitochondrial area, maximum mitochondrial diameter, mitochondrial perimeter, and total mitochondrial number.
Isolation of adult cardiac myocytes.
Adult cardiac myocytes (ACMs) were isolated from hearts of male mice weighting 30 g or more, as previously described (50, 51). Briefly, the hearts were excised and perfused through the aorta with a buffer containing the following (in mM): 115 NaCl, 4.7 KCl, 0.6 KH2PO4, 0.6 Na2HPO4, 1.2 MgSO4-7H2O, 0.032 phenol red, 10 KHCO3, 10 HEPES, 12 NaHCO3, 20 glucose, 30 taurine, and 10 butanedione monoxime, pH 7.46, at 37°C. For digestion of the extracellular matrix, the perfusion buffer was supplemented with 19.5 units of Liberase TH (Roche), 0.5 pM EDTA-free trypsin (Invitrogen), 25 μM CaCl2, and 25 μM blebbistatin (Sigma). Ventricles were gently dissociated into rod-shaped myocytes that were suspended into perfusion buffer containing FBS (Hyclone) and were gradually reintroduced to CaCl2. For their plating, myocytes were suspended in culture medium containing MEM (Invitrogen; contains 1.8 mM CaCl2) supplemented with 1% insulin-transferrin-selenium, 2 mM l-glutamine, 4 mM NaHCO3, 10 mM HEPES, 2% penicillin/streptomycin, 5% FBS, 0.2% bovine calf serum, and 25 μM blebbistatin and seeded onto dishes previously coated with mouse laminin (BD Biosciences). After an initial plating step for 1 h at 37°C and 2% CO2, the nonadherent cells were aspirated and fresh culture medium was added for an additional 2-h period before the beginning of treatments.
Confocal microscopy and image processing.
Single myocytes were seeded on glass bottom dishes (MatTek) that were previously coated with laminin and incubated in culture medium supplemented with 100 nM tetramethylrhodamine methylester (TMRM; Molecular Probes) for 15 min at 37°C. That medium was replaced with TMRM-free medium, and myocytes were imaged with a Zeiss LSM 710 confocal microscope using the Plan apochromat ×63, 1.40 Oil DIC objective. A myocyte area of 67.48 × 67.48 μm was blindly selected for each dish for continuous imaging. Illumination of TMRM was performed with the 543 nm laser (the pinhole set at 50 μm), and fluorescence was detected between 554 and 652 nm. The laser power varied between 0.2 to 12% for Mfn-1 WT (means: 2.7 ± 0.8) and between 0.2 to 14% for Mfn-1 KO (means: 2.8 ± 1.0). Following initial imaging, H2O2 was injected into the dish at a final concentration of 200 μM and time-lapse imaging commenced immediately at intervals of 2.4 s for 30 min to monitor the change in fluorescence intensity. Time-lapse images were analyzed offline using ImageJ software to generate mitochondrial depolarization curves within a region of 25 × 25 μm for each myocyte assessed. The area under the curve was chosen as a quantitative measurement of mitochondrial depolarization.
Myocyte viability assay.
Freshly isolated ACMs were prepared as described above reintroduced to calcium and resuspended in MEM-based medium that contained 1.2 mM CaCl2, 12 mM NaHCO3, 1% penicillin/streptomycin, 2.5% FBS, and 25 μM blebbistatin. ACMs were seeded on laminin-coated dishes (12 μg laminin/35-mm dish) at a density of 375,000 rod-shaped myocytes per dish. After an initial plating step at 37°C and 2% CO2 for 1 h, nonadherent cells were removed and fresh medium containing 20 μM H2O2 or PBS were added to the remaining cells. Two hours later, cells were switched to medium containing 0.04% trypan-blue solution (Sigma) for 10 min and multiple fields per dish were photographed using a camera-equipped light microscope. Counting of blue vs. nonblue myocytes was performed with ImageJ to calculate a death score (percentage of cells that stained blue) per field. For comparisons, distribution curves were generated using multiple fields per genotype per treatment, or alternatively the average death score per animal per genotype per treatment was used.
Mitochondrial isolation and assessment of MPTP.
Subsarcolemmal mitochondria (SSM) and interfibrillar mitochondria (IFM) were isolated from adult mouse hearts as previously described in detail (50). Size and membrane potential of SSM and IFM was determined by flow cytometry as previously reported (13, 50). Calcium uptake and tBH-induced calcium release were assessed in isolated SSM and IFM. In short, mitochondria were resuspended in 2.0 ml calcium-free buffer containing 100 mM KCl, 50 mM MOPS, 5 mM KH2PO4, 5 μM EGTA, 1 mM MgCl2, 5 mM glutamate, and 5 mM malate and assayed for Ca2+ uptake in a fluorescence spectrophotometer at 37°C. Tert-butyl hydrogen peroxide (400 mM) was infused at a rate of 0.2 μl/min, and the concentration of free Ca2+ in the medium was calculated by monitoring the fluorescence of the Ca2+ indicator calcium green-5N (CaGN-5N; Molecular Probes) with an excitation and emission of 488 and 530.
Measurement of mitochondrial enzyme activities.
Activities of citrate synthase, isocitrate dehydrogenase, and medium-chain acyl coenzyme A (acyl-CoA) dehydrogenase were measured from heart homogenates and isolated mitochondria as previously described (47).
Determination of total glutathione levels in heart.
To determine glutathione levels in heart, a commercially available kit was used (Abcam) and the procedure was performed according to manufacturer's specifications. Briefly, 40 mg of heart tissue were rapidly collected and homogenized in 200 μl in ice-cold assay buffer. Following deproteination, the samples were neutralized and the supernatant was assessed for total glutathione (reduced and oxidized). Quantification of glutathione in the samples was performed spectrophotometrically, and the standard curve that was generated ranged from 4 ng to 6 μg of glutathione.
Western blotting and quantitative real-time PCR.
The procedures for the isolation, separation, transfer to membranes, and detection of proteins (20 μg) from whole cardiac extracts were previously described (50). The antibodies used in the present study (diluted at 1,000:1 in 3% blocking solution) include the anti-Mfn-1 (University of California, Davis/NIH; NeuroMabs; clone no. N111/24), anti-Mfn-2 (Sigma, N-terminal-specific), anti-Drp-1 (BD Transduction Laboratories), anti-cre (Novagen), anti-GAPDH (Cell Signaling), anti-porin/VDAC1 (Abcam), anti-uncoupling protein-3 (Thermo Scientific), and anti-FOF1-ATPase subunit-α (Invitrogen). For quantitative real-time PCR, total RNA was extracted from mouse hearts with fibrous-tissue mini kit (Qiagen) and 840 ng RNA were reversed transcribed into cDNA using Quantitect reverse transcription kit (Qiagen). Quantification of cDNAs was performed with the SYBR green reagent (Applied Biosystems) and the StepOne real time system. Primers were designed using Primer3 and evaluated for gene specificity using BLAST. GAPDH was used as the housekeeping gene.
Statistical analysis.
Handling and representation of the data were performed using SPSS software. All values shown are means ± SE. For comparisons between two groups (i.e., WT vs. KO), the independent-samples two-tailed t-test was used (nonpaired). For comparisons between four groups (i.e., two genotypes and two treatments), one-way ANOVA was used followed by the least significant differences post hoc test. Differences were considered statistically significant for P < 0.05 or below. To compare the distribution curves between genotypes, the Kolmogorov-Smirnov test was applied.
RESULTS
Mfn-1 ablation and transcription pattern analysis.
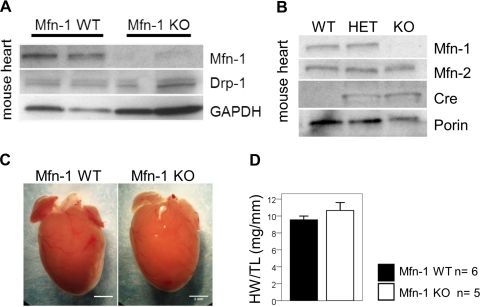
Mice with targeted deletion of Mfn-1 in cardiac myocytes are viable and survive through adulthood, in agreement with earlier observations with globally deleted Mfn-1 mutant mice (11). Western blot analyses showed a complete absence of Mfn-1 from cardiac extracts of Mfn-1 KO mice, suggesting an effective abrogation of the Mfn-1 gene (Fig. 1, A and B). This event did not lead to appreciable changes in the level of Drp-1 protein (Fig. 1A) or Mfn-2 (Fig. 1B). Despite the deletion of Mfn-1, the hearts did not display significant gross abnormalities (Fig. 1C). Furthermore, there was no reproducibly detectable hypertrophy when normalizing the total heart weight to tibia length (Fig. 1D; P = 0.291).
Fig. 1.
Characteristics of the mitofusin-1 (Mfn-1) knockout (KO) heart. A: Western blot analysis performed to detect endogenous levels of Mfn-1 and dynamin-related protein-1 (Drp-1) in mouse heart extracts. GAPDH is used as a loading control. B: Western blot analysis performed to detect cre-specific reduction of Mfn-1 in the heart. Porin/VDAC1 is used as a mitochondria loading control (WT, wild-type; HET, heterozygous for Mfn-1flox; KO, homozygous for Mfn-1flox and positive for cre). C: gross appearance of hearts from 5-mo-old Mfn-1 WT and KO mice; scale bar = 2 mm. D: size of hearts is reported as the ratio of heart weight (HW) to tibia length (TL; n = 6 WT; 4 mice were homozygous Mfn-1flox without cre and 2 were homozygous Mfn-1+/+ with cre and n = 5 mice homozygous Mfn-1flox with cre).
To identify cellular pathways likely to be affected by the deletion of Mfn-1 in hearts, quantitative real-time PCR analysis was performed and the results are reported in Table 1. As shown, Mfn-1-deficient hearts displayed a 95% reduction in Mfn-1 mRNA, while the mRNAs of other classical (Mfn-2, Drp-1, and Opa-1) and recently described (Mtp-18 and Slp-2) mitochondrial fusion/fission mediators decreased slightly or remained unchanged. Transcripts encoding mitochondria biogenesis regulators (i.e., Tfam and Pgc-1α) did not change significantly, while genes encoding for electron transport chain (ETC) components such as Ndufb-5, Nd-5, and Cox4-1 were significantly attenuated. However, this was not a general feature because the transcription of other ETC and ATPase subunits was not significantly affected (i.e., Cox4–2, Cox-5b, and Atp-5o; Table 1). Finally, the mRNAs of Anp and Bnp were markedly elevated, but those of β-Mhc and α-skeletal muscle actin were unchanged.
Table 1.
Transcriptional regulation of relevant genes
| Transcript | Fold Induction/Reduction (Mfn-1 KO vs. WT) | P | Functional Class |
|---|---|---|---|
| Mfn-1 | 0.074 | 0.001 | Mitochondrial fusion/fission |
| Mfn-2 | 0.838 | 0.017 | |
| Opa-1 | 0.796 | 0.040 | |
| Drp-1 | 0.805 | 0.041 | |
| Mtp-18 | 1.047 | 0.587 | |
| Slp-2 | 1.028 | 0.721 | |
| Tfam | 0.516 | 0.098 | Mitochondrial biogenesis |
| Pgc-1α | 0.409 | 0.078 | |
| Err-α | 1.250 | 0.307 | |
| Endo-G | 0.768 | 0.018 | MTDNA maintenance |
| Pol-γ | 1.002 | 0.986 | |
| Ndufb-5 | 0.744 | 0.040 | ETC/ATP synthase |
| Nd-5 | 0.647 | 0.033 | |
| Cox4-1 | 0.609 | 0.026 | |
| Cox4-2 | 0.718 | 0.090 | |
| Cox-5b | 0.937 | 0.261 | |
| Atp-5o | 0.640 | 0.067 | |
| Anp | 5.699 | 0.001 | Stress indicators |
| Bnp | 5.427 | 0.001 | |
| β-Mhc | 0.793 | 0.058 | |
| α-Sk.actin | 1.100 | 0.738 | |
| Sod-2 | 0.676 | 0.007 | ROS detoxification |
| Bcl-2 | 1.345 | 0.001 | Apoptosis/autophagy |
| Beclin-1 | 0.865 | 0.353 | |
| LC-3b | 0.843 | 0.126 |
Values represent fold reduction/induction in the mitofusin-1 (Mfn-1) knockout (KO) group (n = 5) relative to wild type (WT; n = 4). Comparisons for statistical significance were performed with two-tailed Student's t-test.
Mfn-2, mitofusin 2; Opa-1, optic atrophy 1 long variant; Drp-1, dynamin-related protein 1; Mtp-18, mitochondrial protein 18; Slp-2, stomatin like protein 2; Tfam, transcriptional factor A mitochondrial; Pgc-1α, PPAR-γ coactivator 1α; Err-α, estrogen receptor related factor-α; Endo-G, endonuclease-γ; MTDNA, mitochondrial DNA; Pol-γ, polymerase-γ; Ndufb-5, NADH-dehydrogenase (ubiquinone) subcomplex 1β, polypeptide 5; Nd-5, NADH-dehydrogenase subunit 5, mitochondrially encoded; Cox4-1, cytochrome c oxidase, subunit 4, isoform 1; Cox4-2, cytochrome c oxidase, subunit 4, isoform 2; Cox-5b, cytochrome c oxidase subunit 5b; Atp5o, F1FO-ATPase complex, oligomycin sensitivity-conferring subunit, polypeptide 5; Anp, atrial natriuretic peptide; Bnp, brain natriuretic peptide; β-MHC, β-myosin heavy chain; α-Sk actin; α-skeletal actin; Sod2, superoxide dismutase, mitochondrial; LC-3b; microtubule associated protein 1 light chain 3b; ETC, electron transport chain; ROS, reactive oxygen species.
Mfn-1-deficient adult myocytes contain small and spherical mitochondria.
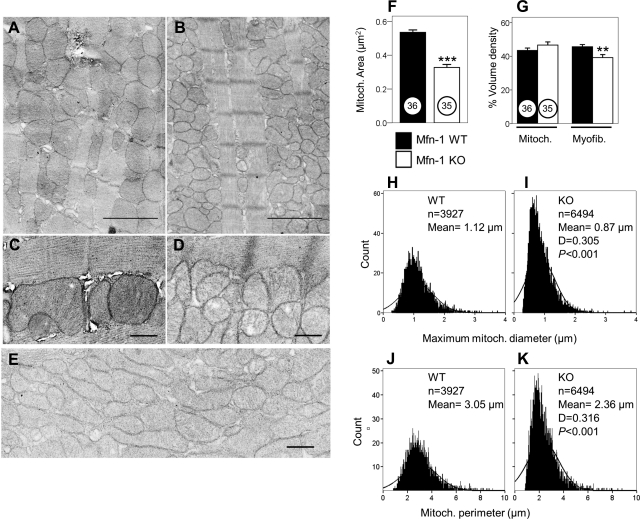
Analysis of the ultramicroscopic structure of hearts from adult Mfn-1 WT and Mfn-1 KO mice detected the formation of small ovoid or spherical mitochondria when Mfn-1 was genetically removed (compare Fig. 2A with Fig. 2B or Fig. 2C with Fig. 2D). Although the presence of fragmented mitochondria was quite prominent in most fields, in some areas of Mfn-1 KO myocytes the mitochondria assumed different morphologies to form clusters of lamellar often interdigitating mitochondria with poorly defined boundaries (Fig. 2E). This pattern was not observed in any Mfn-1 WT sections. Detailed analysis of the mitochondrial dimensions and averaging over a large population of mitochondria demonstrated a significant reduction in the cross sectional area of individual mitochondria per field examined in Mfn-1 KO samples (Fig. 2F; P < 0.001). Despite these unusual structural features, the overall mitochondrial mass estimated by mitochondrial volume density analysis was not different between Mfn-1 WT and KO samples (Fig. 2G, mitochondria; P = 0.176). With the use of the same analysis, a small but significant decrease in the myofibril volume density was detected (Fig. 2G, myofibrils; P < 0.01).
Fig. 2.
Electron microscopy images showing mitochondria and myofibrils from adult hearts. A: 5-mo-old Mfn-1 WT. B: littermate Mfn-1 KO, the scale bars in A and B = 2 μm. C: interfibrillar mitochondria in Mfn-1 WT heart. D: interfibrillar mitochondria in Mfn-1 KO heart, scale bars in C and D = 0.5 μm. E: distinct area of Mfn-1 KO heart (myofibrils present but not shown) containing unusually elongated/lamellar mitochondria; scale bar = 0.5 μm. F: area per individual mitochondrion measured from electron micrographs taken from 2 animals per genotype. Numbers in circles show the number of fields used per genotype (***P < 0.001). G: mitochondrial and myofibril volume density estimated by the grid method expressed as a percentage (grid contains 9 × 10 dots; **P < 0.01). H: histogram showing the distribution of maximum mitochondrial diameter of all the mitochondria identified (n = 3,927) in WT samples. I: histogram showing the distribution of maximum mitochondrial diameter of all the mitochondria identified (n = 6,494) in KO samples. D is value for most extreme differences determined by Kolmogorov-Smirnov analysis (P < 0.001) that the 2 histograms belong to the same distribution. J–K: same as in H and I for Mfn-1 WT and Mfn-1 KO, respectively, only that the histograms were created using mitochondrial perimeter.
Frequency distribution curves, constructed for maximum mitochondrial diameter, were shifted to the left when Mfn-1 was ablated, consistent with the recurrent presence of smaller mitochondria in Mfn-1 KO hearts (compare Fig. 2H with Fig. 2I for Mfn-1 WT and KO tissue, respectively; P < 0.001). Similar observations could also be made regarding the mitochondrial perimeter (compare Fig. 2J with Fig. 2K, WT and KO tissue, respectively; P < 0.001). Another feature that emerged from this analysis was the marked increase in the number of identifiable mitochondria when Mfn-1 was deleted (estimated number: 58 vs. 98 mitochondria/100 μm2 in WT and KO hearts, respectively). The detection of smaller mitochondria that occur in higher numbers is in agreement with the measurements showing that the overall mitochondrial volume density does not differ between WT and Mfn-1 KO samples.
Function of Mfn-1 KO hearts closely resembles that of Mfn-1 WT.
The functional significance of Mfn-1 ablation in cardiac myocytes was assessed in adult mice using noninvasive echocardiography. The experimental groups included 11 Mfn-1 WT and 13 Mfn-1 KO male mice that were ∼18 wk old at the time of echocardiography (Table 2). The heart rate during echocardiography was not significantly different between Mfn-1 WT and KO mice (results not shown). The cardiac mass calculated from B-mode long axis views of the LV was similar in hearts with or without Mfn-1, and this was also the case after correcting for body weight. Diastolic and systolic LV volumes were not affected by Mfn-1 deletion, and as a result, the ejection fraction and the cardiac output were indistinguishable between Mfn-1 WT and KO mice (Table 2). The fractional shortening, calculated from M-mode tracings, was also similar between groups. The peak aortic and pulmonic valve flows did not differ significantly in the Mfn-1 KO hearts. Finally, examination of diastolic function in Mfn-1 WT and KO hearts did not reveal any differences (e.g., early mitral inflow, E; Table 2), suggesting that the overall systolic and diastolic heart function is unlikely to be affected by the ablation of Mfn-1 from myocytes.
Table 2.
Echocardiographic analysis
| Mfn-1 WT (n = 11) | Mfn-1 KO (n = 13) | |
|---|---|---|
| Age, day | 125 ± 11 | 129 ± 11 |
| BW, g | 36.3 ± 5.3 | 34.7 ± 3.8 |
| Cardiac mass, mg | 165.7 ± 14.9 | 167.58 ± 16.0 |
| CM/BW, mg/g | 4.63 ± 0.62 | 4.86 ± 0.55 |
| EDV, μl | 76.11 ± 15.6 | 84.23 ± 18.15 |
| ESV, μl | 32.43 ± 9.25 | 38.32 ± 15.96 |
| EF, % | 57.47 ± 6.90 | 56.28 ± 9.39 |
| CO, ml/min | 22.53 ± 4.70 | 22.79 ± 3.59 |
| FS, % | 56.65 ± 9.19 | 55.23 ± 11.80 |
| PAF, mm/s | 1252 ± 213 | 1403 ± 315 |
| PPF, mm/s | 842 ± 136 | 820 ± 123 |
| E, mm/s | 583 ± 126 | 513 ± 101 |
| A, mm/s | 447 ± 95 | 420 ± 86 |
| ET, ms | 49.50 ± 5.33 | 45.14 ± 4.35 |
| E', mm/s | 11.89 ± 2.41 | 11.36 ± 2.29 |
| A', mm/s | 11.56 ± 1.02 | 11.57 ± 1.10 |
Results are means ± SD. For derivation of the different parameters please refer to materials and methods. Comparisons for statistical significance were performed with two-tailed Student's t-test.
BW, body weight; CM, cardiac mass; EDV, end-diastolic volume; ESV, end-systolic volume; EF, ejection fraction; CO, cardiac output; FS, fractional shortening; PAF, peak aortic flow; PPF, peak pulmonic flow; E, early mitral inflow; A, late mitral inflow; ET, ejection time; E', early relaxation velocity at the mitral annulus; A', late relaxation velocity at the mitral annulus.
Rate of mitochondrial depolarization downstream of ROS challenge is decreased in Mfn-1 KO myocytes.
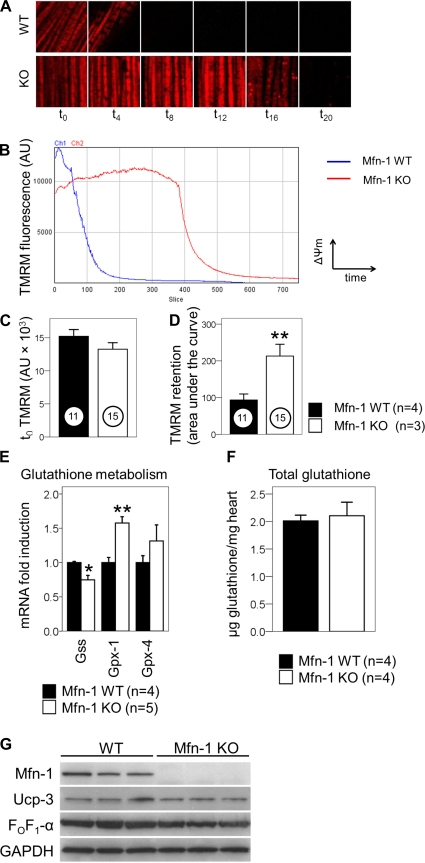
We have previously demonstrated that the ablation of Mfn-2 in cardiac myocytes is associated with a marked delay in the loss of mitochondrial membrane potential (ΔΨm) (50). To examine this parameter in myocytes lacking Mfn-1, ACMs were isolated from Mfn-1 WT and KO mice and challenged with H2O2-induced stress (200 μM for 30 min). H2O2 has been extensively used to induce mitochondrial ROS overload, membrane depolarization, and ultimately cell death in myocytes (2, 38, 66). The ability of mitochondria to retain the mitochondrial dye TMRM was quantified by repetitive confocal imaging over a 30-min period after H2O2 injection. The treatment with H2O2 and a concomitant photochemical ROS production due to TMRM illumination (72) effectively lead to mitochondrial depolarization within intact myocytes, as shown in Fig. 3A. Compared with WT, Mfn-1 KO myocytes contained mitochondria that were refractory to depolarization (Fig. 3A, compare the fluorescence intensity in the different time points). When plotted against time, the depolarization curve of Mfn-1 KO myocytes significantly shifted to the right, indicating a delay in this process (Fig. 3B). This was not due to alterations in basal membrane potential (ΔΨm) because the TMRM fluorescence was similar between the two groups at t0 (Fig. 3C). The ability of mitochondria to retain TMRM was quantified as the area under the curve. This analysis showed that the TMRM retention values are significantly higher in Mfn-1 KO myocytes (Fig. 3D; 11 WT or 15 KO myocytes from 7 different experiments; P < 0.01). To examine whether the ablation of Mfn-1 alters the scavenging capacity for H2O2, we examined the expression of genes related to glutathione, a central intracellular H2O2 detoxifier. As shown in Fig. 3E, the ablation of Mfn-1 did not result to consistent increases in genes regulating glutathione availability. Although glutathione peroxidase-1 was significantly increased, glutathione synthase was decreased. Additionally, the expression of the mitochondria-targeted glutathione peroxidase-4 was unchanged (Fig. 3E). Furthermore, direct quantification of glutathione levels in heart extracts did not reveal any differences between groups (Fig. 3F). Mild uncoupling of the ETC is thought to confer a protective effect in mitochondria undergoing ROS challenge (42, 66). To test whether alterations in uncoupling proteins occur during Mfn-1 deletion, we examined the protein levels of uncoupling protein-3 (UCP-3). As shown in Fig. 3G, the levels of UCP-3 were unaffected by the deletion of Mfn-1. Collectively, it can be concluded from this analysis that Mfn-1 facilitates mitochondrial depolarization downstream of H2O2, but this does not involve alterations in glutathione availability or ETC uncoupling.
Fig. 3.
Mitochondrial depolarization downstream of H2O2 is delayed in single myocytes, but this does not involve altered H2O2 scavenging or electron transport chain uncoupling. A: representative images of myocyte areas (×25 μm) containing mitochondria undergoing depolarization with different kinetics (Mfn-1 WT above and Mfn-1 KO below). H2O2 was injected at t0 and imaging began immediately. Images shown were taken at the indicated time points (minutes) following H2O2 injection. B: graph showing dissipation of tetramethylrhodamine methylester (TMRM) fluorescence of a selected region of mitochondria after H2O2 exposure. On the y-axis, TMRM fluorescence [arbitrary units (AU)] is a quantitative indicator of inner membrane potential (ΔΨm) for the selected group of mitochondria. On the x-axis, time after H2O2 injection is shown as consecutive slices (time interval: 2.4 s). Channel 1 (blue) shows the course of depolarization in Mfn-1 WT myocytes, and channel 2 (red) shows the depolarization of Mfn-1 KO myocytes at the same conditions. C: TMRM fluorescence at t0 is quantified in myocytes from each group as a measure of basal membrane potential (ΔΨm; P = 0.189). D: capacity of mitochondria to lose TMRM is quantified as the area under the curve from traces such as those shown in B. Confocal microscopy experiment was done using 4 WT and 3 KO mice that were 2.5–5 mo old. Number of myocytes assessed are shown in circles for each group (6 myocytes were Mfn-1flox without cre, 5 were Mfn-1+ with cre, and 15 were homozygous Mfn-1flox with cre). E: relative levels of mRNAs encoding for proteins with the capacity to alter glutathione levels are quantified in whole-heart extracts. Gss, glutathione synthase; Gpx-1, glutathione peroxidase-1; Gpx-4, glutathione peroxidase-4, mitochondrial variant. F: levels of total glutathione (reduced and oxidized) in whole heart extracts are shown in μg/mg for the 2 experimental groups (2 mice were Mfn-1flox without cre, 2 mice were Mfn-1+ with cre, and 4 mice were homozygous Mfn-1flox with cre, aged 3.5–4 mo old). G: protein abundance of uncoupling protein-3 (UCP-3) and α-subunit of the FOF1-ATPase (FOF1-α) in Mfn-1 WT and KO hearts. *P < 0.05, **P < 0.01.
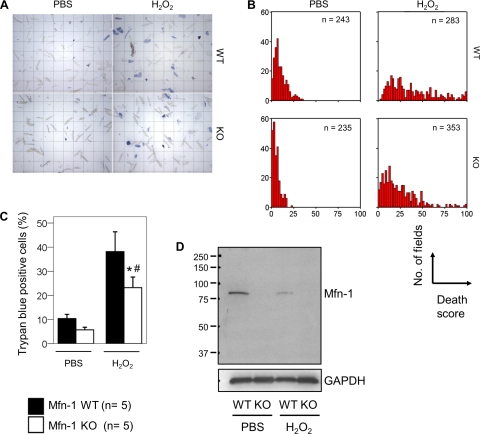
Mfn-1 KO myocytes are protected from ROS-induced cell death.
Irreversible membrane depolarization is closely associated with mitochondrial dysfunction and cell death. Therefore, the delayed mitochondrial depolarization observed in Mfn-1 KO myocytes could have an impact on myocyte survival. To examine myocyte viability, ACMs from Mfn-1 WT and KO mice were exposed to PBS or to H2O2-induced stress (20 μM for 2 h) to induce mitochondrial dysfunction and cell death (23). Myocyte death following these treatments was assessed microscopically on the basis of trypan-blue staining (Fig. 4A). Multiple fields per genotype per treatment were scored according to the percentage of trypan-blue positive cells (0–100% dead myocytes). In PBS-control conditions, the majority of fields quantified in Mfn-1 WT and KO myocytes tended to cluster to the left part of the x-axis (Fig. 4B). However, in H2O2-treated conditions, this distribution collapses and spreads rightward because many fields have a higher percentage of dead cells. Although this occurred with the Mfn-1 WT myocytes, Mfn-1 KO myocytes had improved tolerance to H2O2 seen as a significant decrease in the number of dead cells (Fig. 4B, compare the distribution of the red bars in the panels in the right; WT and KO). In agreement with these observations, the mean percentage of trypan-blue myocytes in H2O2-treated myocytes was significantly different when comparing averages between animals (Fig. 4C; P < 0.05 WT vs. KO and P < 0.05 PBS vs. H2O2). Recent evidence suggests that Mfn-1 is targeted for degradation by the proteasome in response to intracellular stress (22, 65). In agreement with these observations, we find that the levels of Mfn-1 protein were attenuated in Mfn-1 WT myocytes exposed to H2O2 (Fig. 4D). Thus the removal of Mfn-1 may be advantageous for the cell during stress, providing an explanation why myocytes rendered deficient in Mfn-1 (Mfn-1 KO) are more tolerant to ROS-related insults.
Fig. 4.
Viability assay of single adult cardiac myocytes. Experiment was done with 5 WT and 5 KO mice 2.5–5 mo old. A: microscopic fields containing single myocytes from WT and KO hearts previously treated in parallel with PBS or H2O2. Viable (unstained) vs. dead (blue) myocytes were counted, and each field was scored for the percentage of blue cells (death score). B: distribution analysis of all the fields scored (shown by n) according to their death score (0–100%). Clustering at the left of the x-axis indicates a low death index. C: average death score per treatment and genotype (n = 5 Mfn-1 WT or KO mice; *P < 0.05 WT vs. KO and #P < 0.05 PBS vs. H2O2). D: Western blot analysis performed to detect Mfn-1 protein levels in Mfn-1 WT and KO single myocytes following treatment with PBS and H2O2. GAPDH is used as a protein loading control. Numbers at left indicate respective molecular masses (37–250 kDa).
Characteristics of isolated mitochondria.
The protein yields of subsarcolemmal mitochondria (SSM) and interfibrillar mitochondria (IFM) did not decrease significantly upon Mfn-1 ablation (Table 3), although the Mfn-1 KO IFM appeared to have slightly higher protein content than WT (11.6 ± 0.8 vs. 14.1 ± 0.78 mg mitochondrial protein/g wet mass respectively; P = 0.051; Table 3). Further, the activities in whole tissue homogenates were similar for the mitochondrial oxidative enzymes citrate synthase (3.84 ± 0.25 vs. 4.88 ± 0.48 μmol·mg protein−1·min−1 for WT and KO, respectively), isocitrate dehydrogenase (0.60 ± 0.15 and 0.85 ± 0.14 μmol·mg protein−1·min−1), and medium chain acyl-CoA dehydrogenase (0.28 ± 0.02 and 0.31 ± 0.03 μmol·mg protein−1·min−1), consistent with the notion that the overall mitochondrial mass did not change in Mfn-1 KO hearts (for example, Fig. 2G). Mitochondrial size was found to be smaller by flow cytometry for both SSM and IFM in the Mfn-1 KO hearts than in WT (Table 3), consistent with the electron microscopy analysis (Fig. 2F). The membrane potential of SSM and IFM was not affected by Mfn-1 deletion (Table 3). Mitochondrial respiration was unaffected by deletion of Mfn-1, with the exception of a greater state III respiration in the IFM from Mfn-1 KO with succinate + rotenone and a higher respiratory control ratio with glutamate + malate (Table 3). Thus deletion of Mfn-1 resulted in smaller mitochondria but did not have a deleterious effect on mitochondrial content or oxidative capacity.
Table 3.
Function of isolated cardiac mitochondria
| Subsarcolemmal Mitochondria |
Interfibrillar Mitochondria |
|||
|---|---|---|---|---|
| Mfn-1 WT (n = 6) | Mfn-1 KO (n = 6) | Mfn-1 WT (n = 6) | Mfn-1 KO (n = 6) | |
| Yield, mg mitochondrial protein/g wet mass, | 18.2 ± 1.0 | 16.5 ± 5.8 | 11.6 ± 0.8 | 14.1 ± 0.78 |
| Mitochondria diameter, arbitrary units | 534 ± 13 | 490 ± 6* | 487 ± 8 | 464 ± 4* |
| ΔΨm (JC-1 aggregate/monomer) | 3.36 ± 0.43 | 2.86 ± 0.30 | 3.26 ± 0.53 | 3.18 ± 0.40 |
| Enzyme activities, nmol·mg mitochondrial protein−1 ·min−1 | ||||
| Citrate synthase | 1.79 ± 0.28 | 2.17 ± 0.28 | 2.64 ± 0.98 | 2.34 ± 0.57 |
| Medium chain Acyl-CoA dehydrogenase | 0.22 ± 0.02 | 0.22 ± 0.01 | 0.23 ± 0.02 | 0.23 ± 0.05 |
| Respiration, nmol·mg mitochondrial protein−1·min−1 | ||||
| Glutamate + malate: state III | 97.2 ± 6.6 | 95.4 ± 4.4 | 99.5 ± 3.8 | 117 ± 7.3 |
| Glutamate + malate: state IV | 39 ± 3.1 | 33.4 ± 2.3 | 46.7 ± 2.7 | 39.3 ± 2.3 |
| Glutamate + malate: RCR | 2.55 ± 0.25 | 2.94 ± 0.27 | 2.15 ± 0.11 | 3.00 ± 0.20* |
| Palmitylcarnitine + malate: state III | 59.1 ± 5.8 | 83.7 ± 10 | 77.3 ± 20 | 69.3 ± 7.7 |
| Palmitylcarnitine + malate: state IV | 24.9 ± 0.93 | 31.5 ± 2.7 | 32 ± 5 | 28 ± 2.8 |
| Palmitylcarnitine + malate: RCR | 2.41 ± 0.3 | 2.42 ± 0.4 | 2.29 ± 0.34 | 2.57 ± 0.34 |
| Succinate + rotenone: state III | 417.4 ± 29.4 | 454.4 ± 17.7 | 454.2 ± 29.3 | 530.7 ± 17* |
| Succinate + rotenone: state IV | 167.8 ± 10.5 | 178.9 ± 12.2 | 198.8 ± 22.6 | 215 ± 11.4 |
| Succinate + rtenone: RCR | 2.37 ± 0.07 | 2.66 ± 0.1 | 2.20 ± 0.05 | 2.56 ± 0.15 |
Values are means ± SE. Each data point (total of 6 per genotype) represents mitochondria pooled from 2 hearts. ΔΨm, Basal membrane potential; JC-1, 5,5′,6,6′-tetrachloro-1,1′,3,3′ -tetraethylbenzimidazolylcarbocyanine iodide; RCR, respiratory control ratio
P < 0.05, between genotypes (Mfn-1 WT and KO) by unpaired two-tailed t-test within the same mitochondrial group (subsarcolemmal mitochondria and interfibrillar mitochondria).
ROS-induced MPTP opening is decreased in isolated interfibrillar mitochondria.
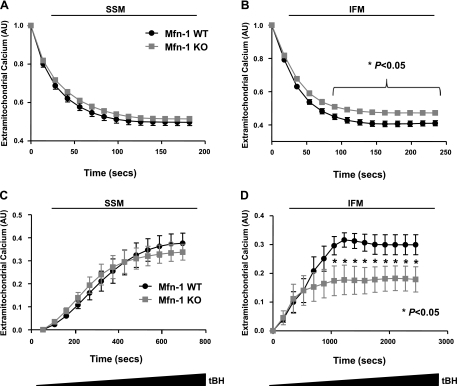
The ability to uptake Ca2+ is used to assess the physiological state of mitochondria (7). As shown in Fig. 5A, Mfn-1 KO SSM exhibit a similar Ca2+ uptake as WT SSM. On the other hand, the same assay detected a modest reduction in the ability of Mfn-1 KO IFM to uptake extra-mitochondrial Ca2+ (Fig. 5B). To assess the ability of mitochondria to undergo ROS-induced MPTP, we utilized the continuous infusion of tBH. Addition of tBH triggered the rapid release of Ca2+ from mitochondria, which was not different between Mfn-1 WT and KO SSM for the duration of the assay (Fig. 5C) but was found to be significantly blunted in Mfn-1 KO IFM (Fig. 5D), consistent with the resistance to ROS-induced mitochondrial dysfunction and death observed in isolated myocytes (Figs. 3 and 4).
Fig. 5.
Calcium-uptake capacity and reactive oxygen species (ROS) sensitivity of mitochondria isolated from Mfn-1 WT and KO hearts. A: rate of Ca2+ uptake by subsarcolemmal mitochondria (SSM) is monitored as a decrease in fluorescence of the extra-mitochondrial Ca2+ indicator (CaGN-5N; 200 nM). B: decrease in CaGN-5N fluorescence over time due to the uptake of Ca2+ by interfibrillar mitochondria (IFM). C: SSM mitochondria were primed for mitochondrial permeability transition pore (MPTP) opening with a low concentration of Ca2+ (22.5 μM) and were subsequently exposed to increasingly higher doses of tBH. Mitochondrial Ca2+ release as a result of tBH-induced MPTP was monitored as an increase in CaGN-5N fluorescence. D: increase in CaGN-5N fluorescence due to tBH-induced MPTP opening in IFM. AU, arbitrary fluorescence units; tBH, tert-butyl-hydroxyperoxide. *P < 0.05, between the 2 genotypes for the indicated time points.
DISCUSSION
Here we report the generation and functional characterization of mice that lack the mitochondrial fusion protein Mfn-1 in cardiac myocytes. Mice lacking Mfn-1 exhibited normal heart function, but the mitochondria within Mfn-1-deficient myocytes were small and spherical. These findings are in marked contrast to our previous analysis of Mfn-2-deficient myocytes that displayed larger mitochondria (50). Despite mitochondrial fragmentation, Mfn-1-deficient myocytes were protected from ROS-induced mitochondrial depolarization and cell death.
Why are mitochondria smaller in Mfn-1-deficient myocytes yet enlarged in myocytes that are deficient for Mfn-2? One possibility is that the degree of mitochondrial size is dependent on the relative levels of mitofusin GTPase activity within the cell. Earlier studies (26) comparing mitofusins have shown that the two homologs exhibit considerable differences in terms of binding and hydrolyzing GTP, where Mfn-1 has eightfold higher GTPase activity than Mfn-2. Furthermore, it has been shown that endogenous Mfn-1 can interact with mutant Mfn-2 to form fusion-competent complexes in cells, whereas endogenous Mfn-2 is inefficient in this process (15). Collectively, these data suggest that Mfn-1 is more effective in driving mitochondrial fusion than Mfn-2. Because it has been demonstrated that heterotypic interactions between Mfn-1 and Mfn-2 are favored for mitochondrial fusion compared with homotypic interactions (24), the balance of Mfn-1 relative to Mfn-2 may be an important feature that determines mitochondrial size. In this regard, it is possible that under certain conditions, the diminished GTPase activity of Mfn-2 may serve to retard the fusion activity of Mfn-1 protein within the heterotypic complex. For example, if an Mfn-2 threshold is surpassed in a cell that expresses both isoforms, excessive Mfn-2 expression could reverse the mitochondrial fusion activity of Mfn-1 by functioning in a manner that is analogous to a dominant-negative isoform.
In the human and mouse heart, both Mfn-1 and Mfn-2 are highly expressed (Ref. 56 and our unpublished observations), and it is conceivable that by deleting either of the mitofusins will displace the ratio from its set point and increase/decrease the efficiency of fusion. Consistent with this hypothesis, we (50) have previously generated mice with cardiomyocyte-specific deletion of Mfn-2. Lack of Mfn-2 resulted in formation of large globular mitochondria indicating that an excess of Mfn-1 activity (via removal of Mfn-2) leads to mitochondrial enlargement, possibly by enhancing fusion between mitochondria during biogenesis. Conversely, in the present study where Mfn-1 is ablated under identical conditions, we find that mitochondria become smaller and spherical (Fig. 2), indicating that a relative excess of Mfn-2 activity can lead to mitochondrial fragmentation. It is important to note that when one mitofusin is ablated, the levels of the other isoform do not change significantly (Fig. 1B; Ref. 50). Our observation that deletion of mitofusins leads to distinct mitochondrial morphologies is in concordance with earlier findings with mitofusin-deficient mouse embryonic fibroblasts (MEF; Ref. 10), where it was noted that Mfn-1-deficient MEFs contain small spherical mitochondria and Mfn-2-deficient MEFs contain large globular mitochondria. Similarly, the diminished GTPase activity of Mfn-2 may explain why Mfn-2 overexpression, in the presence of endogenous Mfn-1, induces the breakdown of mitochondrial tubules into small spherical structures relative to the WT condition (18, 25, 54, 57). In light of the above considerations, we suggest that in myocytes (and probably in other cells) excessive Mfn-1 activity leads to mitochondrial enlargement, whereas excessive Mfn-2 activity leads to mitochondrial diminution.
Notably, these shifts in Mfn-1/Mfn-2 balance and the accompanying morphological transitions do not lead to significant impairment in the biochemical properties of mitochondria, and heart function is not severely impaired either in Mfn-1 KO or Mfn-2 KO mice. In this regard, we report here that Mfn-1 KO hearts are functionally normal by echocardiography (Table 2) and cardiac hypertrophy is not detectable. However, in the case of Mfn-2 KO mice the hearts were modestly hypertrophied (50). Given the contrast in mitochondrial size between Mfn-2 and Mfn-1 KO hearts, and the absence of hypertrophy in the latter, it could be suggested that the modest hypertrophy detected in Mfn-2 KO hearts is related to the mitochondrial enlargement. More specifically, it might be that hypertrophy of Mfn-2 KO myocytes occurs as the consequence of an intracellular burden imposed to myocytes due to the inclusion of aberrantly enlarged mitochondria. It has been previously reported (29) that experimental enlargement of mitochondria alters the mechanical properties of cardiac myocytes. Furthermore, the notion that mitochondria can cause cellular dysfunction due to their increased size has been suggested for cerebellar neurons (30). Interestingly, Mfn-2 conditionally deficient neurons are found to undergo degeneration in the cerebellum, and mitochondria in these cells form conglomerates within the soma that appear unable to enter the dendrites due to their size (11). On the other hand, in that same study no abnormalities were detectable in Mfn-1-deficient mice, indicating that Mfn-1-deficient neurons are functionally intact. In this regard, it would be interesting to examine whether mitochondria in Mfn-1 KO neurons have normal size or whether they are reduced in size as we detect in the Mfn-1 KO heart.
Mitochondrial dynamics appear to be intimately related to cell death pathways, but the details of this interaction remain largely obscure. A common theme that emerges from the study of cells undergoing apoptosis is that they exhibit mitochondrial fragmentation that occurs almost simultaneously with the release of cytochrome c (41). Notably, it has been reported that repressing mitochondrial fragmentation by inhibiting Drp-1 interferes but does not block the apoptotic process (17, 19, 28, 52). Although it remains to be determined how smaller mitochondria facilitate the apoptotic process, it is now clear that mitochondrial fragmentation alone does not necessarily lead to cell death or any other apparent cellular dysfunction in several contexts (4, 59). Our current model provides an experimental system to test the relationship between mitochondrial fragmentation and cell death induction in cardiac myocytes, since the Mfn-1 KO myocytes acquire increased numbers of fragmented mitochondria (Fig. 2). Accordingly, by subjecting these myocytes to ROS-induced stress we do not detect any evidence of sensitivity towards cell death. In contrast, Mfn-1 KO myocytes are found to be protected against ROS-induced membrane depolarization (Fig. 3), and cell death is significantly attenuated in Mfn-1 KO myocytes exposed to H2O2 (Fig. 4). Taken together, our findings are in agreement with the notion that preexisting mitochondrial fragmentation is not sufficient to predispose myocytes to death. More importantly, the ablation of Mfn-1 confers resistance against ROS-induced mitochondrial depolarization and correlates well with a decreased rate of cardiomyocyte death.
The acquired ability of Mfn-1 KO mitochondria to tolerate deleterious amounts of H2O2 does not appear to be due to a preexisting secondary activation of redox defense pathways. In support of the above, we find that the levels of glutathione and UCP-3 in heart do not differ significantly between the two groups (Fig. 3, F and G). In addition, using isolated mitochondria, we were able to detect resistance against tBH-induced MPTP opening (Fig. 5), consistent with a delayed mitochondrial depolarization observed with single myocytes (Fig. 3D). The experiments with isolated mitochondria also revealed that the increased tolerance against ROS is likely to be more dominant or exclusively localized to IFM rather than the SSM (Fig. 5, C and D), and additional work will be required to delineate why this property is selectively detected in this Mfn-1 KO mitochondrial population. Nevertheless, the work with isolated mitochondria indicates that the ability of Mfn-1 KO myocytes to tolerate a ROS challenge is at least in part attributable to a blunted response of Mfn-1 KO mitochondria to undergo MPTP opening downstream of ROS overload.
Fusion between biological membranes, including those enveloping mitochondria, has been previously thought of as a leakage-free process where the integrity of the rearranging membranes is not breached (71). Nevertheless, there is experimental evidence to suggest that fusion may involve the formation of holes on membranes (16). For example, it has been observed that during the early steps of hemagglutinin-induced fusion the membranes exhibit a transient increase in permeability that is resolved later as fusion progresses (20). This observation is in agreement with theoretical models that predict the opening of a hole on the area near the hemifusion stalk, (i.e., an intermediate structure that connects the apposed membranes during early fusion; Ref. 45). Breaching membrane integrity is also predicted from calculations of the tension forces that develop during the formation of the hemifusion stalk (33). Therefore, although membrane fusion is beneficial for a number of cell processes that require content mixing between distinct compartments, it also entails a transient loss of membrane integrity to some extent. Along these lines, we have previously reported that Mfn-2 can significantly contribute to MPTP formation in cardiac myocytes treated with ROS and in the present study we extend these observations to Mfn-1. Taken together, our observations suggest a previously unsuspected connection between mitochondrial fusion and stress-induced MPTP formation.
Apart from MPTP formation, which requires simultaneous opening of a channel-like pore in the IMM and a pore (or hole) on the OMM, permeabilization of the OMM alone (via formation of lipidic pores) is also sufficient to trigger cell death. Along these lines, it has been recently reported that another mediator of mitochondrial dynamics, Drp-1, can promote formation of holes on liposome membranes due to its activity in creating hemifusion intermediates (44). Given this evidence, we postulate that mitofusins, through similar mechanisms that involve creation of hemifusion intermediates, can induce the nucleation of holes on the OMM. In the presence of the appropriate stimulus, these holes can be employed to accelerate mitochondrial permeability transition or even participate in mitochondrial outer membrane permeabilization to allow the release of apoptosis-inducing factors as it has been suggested for Drp-1 (40).
In conclusion, the present investigation shows that cardiomyocyte-specific deletion of Mfn-1 results in smaller mitochondria that are more resistant to oxidative stress-induced MPT and cell death. The absence of Mfn-1 did not impair left ventricular function or trigger cardiac hypertrophy. Our findings suggest that the endogenous levels of Mfn-1 have the capacity to attenuate myocyte viability downstream of oxidative stress, an effect that could be associated with the ability of Mfn-1 to destabilize the membrane during mitochondrial fusion.
GRANTS
This study was funded by National Institutes of Health Grants HL-074237 (to W. C. Stanley) and HL-102874, AG-34972, AG-15052, and HL-68758 (to K. Walsh).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.N.P., G.A.N., E.R.D., K.A.O., and R.F.R. performed experiments; K.N.P. and G.A.N. analyzed data; K.N.P. interpreted results of experiments; K.N.P. and E.R.D. prepared figures; K.N.P. and K.W. drafted manuscript; K.N.P., E.R.D., W.C.S., and K.W. edited and revised manuscript; W.C.S. and K.W. conception and design of research; K.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Donald L. Gantz and Michael T. Kirber for assistance with electron and confocal microscopy respectively. We are also very thankful to Taina Rokotuiveikau and Matthew Phillippo for assistance in mouse genotyping.
REFERENCES
- 1. Agah R, Frenkel PA, French BA, Michael LH, Overbeek PA, Schneider MD. Gene recombination in postmitotic cells. Targeted expression of Cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J Clin Invest 100: 169–179, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Akao M, O'Rourke B, Teshima Y, Seharaseyon J, Marban E. Mechanistically distinct steps in the mitochondrial death pathway triggered by oxidative stress in cardiac myocytes. Circ Res 92: 186–194, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Aon MA, Cortassa S, Marban E, O'Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem 278: 44735–44744, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Arnoult D. Mitochondrial fragmentation in apoptosis. Trends Cell Biol 17: 6–12, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Brady NR, Elmore SP, van Beek JJ, Krab K, Courtoy PJ, Hue L, Westerhoff HV. Coordinated behavior of mitochondria in both space and time: a reactive oxygen species-activated wave of mitochondrial depolarization. Biophys J 87: 2022–2034, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Breckenridge DG, Kang BH, Kokel D, Mitani S, Staehelin LA, Xue D. Caenorhabditis elegans drp-1 and fis-2 regulate distinct cell-death execution pathways downstream of ced-3 and independent of ced-9. Mol Cell 31: 586–597, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chalmers S, Nicholls DG. The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. J Biol Chem 278: 19062–19070, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Chan DC. Mitochondrial fusion and fission in mammals. Ann Rev Cell Dev Biol 22: 79–99, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem 280: 26185–26192, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol 160: 189–200, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen H, McCaffery JM, Chan DC. Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell 130: 548–562, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, Chan DC. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell 141: 280–289, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dabkowski ER, Williamson CL, Hollander JM. Mitochondria-specific transgenic overexpression of phospholipid hydroperoxide glutathione peroxidase (GPx4) attenuates ischemia/reperfusion-associated cardiac dysfunction. Free Radic Biol Med 45: 855–865, 2008 [DOI] [PubMed] [Google Scholar]
- 14. De Vos K, Goossens V, Boone E, Vercammen D, Vancompernolle K, Vandenabeele P, Haegeman G, Fiers W, Grooten J. The 55-kDa tumor necrosis factor receptor induces clustering of mitochondria through its membrane-proximal region. J Biol Chem 273: 9673–9680, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Detmer SA, Chan DC. Complementation between mouse Mfn1 and Mfn2 protects mitochondrial fusion defects caused by CMT2A disease mutations. J Cell Biol 176: 405–414, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Engel A, Walter P. Membrane lysis during biological membrane fusion: collateral damage by misregulated fusion machines. J Cell Biol 183: 181–186, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Estaquier J, Arnoult D. Inhibiting Drp1-mediated mitochondrial fission selectively prevents the release of cytochrome c during apoptosis. Cell Death Differ 14: 1086–1094, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Eura Y, Ishihara N, Yokota S, Mihara K. Two mitofusin proteins, mammalian homologues of FZO, with distinct functions are both required for mitochondrial fusion. J Biochem 134: 333–344, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell 1: 515–525, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Frolov VA, Dunina-Barkovskaya AY, Samsonov AV, Zimmerberg J. Membrane permeability changes at early stages of influenza hemagglutinin-mediated fusion. Biophys J 85: 1725–1733, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gaussin V, Van de Putte T, Mishina Y, Hanks MC, Zwijsen A, Huylebroeck D, Behringer RR, Schneider MD. Endocardial cushion and myocardial defects after cardiac myocyte-specific conditional deletion of the bone morphogenetic protein receptor ALK3. Proc Natl Acad Sci USA 99: 2878–2883, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gegg ME, Cooper JM, Chau KY, Rojo M, Schapira AH, Taanman JW. Mitofusin 1 and mitofusin 2 are ubiquitinated in a PINK1/parkin-dependent manner upon induction of mitophagy. Hum Mol Genet 19: 4861–4870, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goto K, Takemura G, Maruyama R, Nakagawa M, Tsujimoto A, Kanamori H, Li L, Kawamura I, Kawaguchi T, Takeyama T, Fujiwara H, Minatoguchi S. Unique mode of cell death in freshly isolated adult rat ventricular cardiomyocytes exposed to hydrogen peroxide. Med Mol Morphol 42: 92–101, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Hoppins S, Edlich F, Cleland MM, Banerjee S, McCaffery JM, Youle RJ, Nunnari J. The soluble form of Bax regulates mitochondrial fusion via MFN2 homotypic complexes. Mol Cell 41: 150–160, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang P, Yu T, Yoon Y. Mitochondrial clustering induced by overexpression of the mitochondrial fusion protein Mfn2 causes mitochondrial dysfunction and cell death. Eur J Cell Biol 86: 289–302, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Ishihara N, Eura Y, Mihara K. Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J Cell Sci 117: 6535–6546, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Iwasawa R, Mahul-Mellier AL, Datler C, Pazarentzos E, Grimm S. Fis1 and Bap31 bridge the mitochondria-ER interface to establish a platform for apoptosis induction. EMBO J 30: 556–568, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jagasia R, Grote P, Westermann B, Conradt B. DRP-1-mediated mitochondrial fragmentation during EGL-1-induced cell death in C. elegans. Nature 433: 754–760, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Kaasik A, Kuum M, Joubert F, Wilding J, Ventura-Clapier R, Veksler V. Mitochondria as a source of mechanical signals in cardiomyocytes. Cardiovasc Res 87: 83–91, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Kaasik A, Safiulina D, Choubey V, Kuum M, Zharkovsky A, Veksler V. Mitochondrial swelling impairs the transport of organelles in cerebellar granule neurons. J Biol Chem 282: 32821–32826, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Karbowski M, Norris KL, Cleland MM, Jeong SY, Youle RJ. Role of Bax and Bak in mitochondrial morphogenesis. Nature 443: 658–662, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Koshiba T, Detmer SA, Kaiser JT, Chen H, McCaffery JM, Chan DC. Structural basis of mitochondrial tethering by mitofusin complexes. Science 305: 858–862, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Kozlovsky Y, Chernomordik LV, Kozlov MM. Lipid intermediates in membrane fusion: formation, structure, and decay of hemifusion diaphragm. Biophys J 83: 2634–2651, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Landes T, Martinou JC. Mitochondrial outer membrane permeabilization during apoptosis: the role of mitochondrial fission. Biochim Biophys Acta 1813: 540–545, 2011 [DOI] [PubMed] [Google Scholar]
- 35. Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell 15: 5001–5011, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Legros F, Lombes A, Frachon P, Rojo M. Mitochondrial fusion in human cells is efficient, requires the inner membrane potential, and is mediated by mitofusins. Mol Biol Cell 13: 4343–4354, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lim ML, Minamikawa T, Nagley P. The protonophore CCCP induces mitochondrial permeability transition without cytochrome c release in human osteosarcoma cells. FEBS Lett 503: 69–74, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Long X, Goldenthal MJ, Wu GM, Marin-Garcia J. Mitochondrial Ca2+ flux and respiratory enzyme activity decline are early events in cardiomyocyte response to H2O2. J Mol Cell Cardiol 37: 63–70, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Mancini M, Anderson BO, Caldwell E, Sedghinasab M, Paty PB, Hockenbery DM. Mitochondrial proliferation and paradoxical membrane depolarization during terminal differentiation and apoptosis in a human colon carcinoma cell line. J Cell Biol 138: 449–469, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Martinou JC, Youle RJ. Mitochondria in apoptosis: bcl-2 family members and mitochondrial dynamics. Dev Cell 21: 92–101, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Martinou JC, Youle RJ. Which came first, the cytochrome c release or the mitochondrial fission? Cell Death Differ 13: 1291–1295, 2006 [DOI] [PubMed] [Google Scholar]
- 42. McLeod CJ, Aziz A, Hoyt RF, Jr, McCoy JP, Jr, Sack MN. Uncoupling proteins 2 and 3 function in concert to augment tolerance to cardiac ischemia. J Biol Chem 280: 33470–33476, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Meeusen S, McCaffery JM, Nunnari J. Mitochondrial fusion intermediates revealed in vitro. Science 305: 1747–1752, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Montessuit S, Somasekharan SP, Terrones O, Lucken-Ardjomande S, Herzig S, Schwarzenbacher R, Manstein DJ, Bossy-Wetzel E, Basanez G, Meda P, Martinou JC. Membrane remodeling induced by the dynamin-related protein Drp1 stimulates Bax oligomerization. Cell 142: 889–901, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Muller M, Katsov K, Schick M. A new mechanism of model membrane fusion determined from Monte Carlo simulation. Biophys J 85: 1611–1623, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Neuspiel M, Zunino R, Gangaraju S, Rippstein P, McBride H. Activated mitofusin 2 signals mitochondrial fusion, interferes with Bax activation, and reduces susceptibility to radical induced depolarization. J Biol Chem 280: 25060–25070, 2005 [DOI] [PubMed] [Google Scholar]
- 47. O'Shea KM, Khairallah RJ, Sparagna GC, Xu W, Hecker PA, Robillard-Frayne I, Des Rosiers C, Kristian T, Murphy RC, Fiskum G, Stanley WC. Dietary omega-3 fatty acids alter cardiac mitochondrial phospholipid composition and delay Ca2+-induced permeability transition. J Mol Cell Cardiol 47: 819–827, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Okamoto K, Shaw JM. Mitochondrial morphology and dynamics in yeast and multicellular eukaryotes. Annu Rev Genet 39: 503–536, 2005 [DOI] [PubMed] [Google Scholar]
- 49. Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation 121: 2012–2022, 2010 [DOI] [PubMed] [Google Scholar]
- 50. Papanicolaou KN, Khairallah RJ, Ngoh GA, Chikando A, Luptak I, O'Shea KM, Riley DD, Lugus JJ, Colucci WS, Lederer WJ, Stanley WC, Walsh K. Mitofusin-2 maintains mitochondrial structure and contributes to stress-induced permeability transition in cardiac myocytes. Mol Cell Biol 31: 1309–1328, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Papanicolaou KN, Streicher JM, Ishikawa TO, Herschman H, Wang Y, Walsh K. Preserved heart function and maintained response to cardiac stresses in a genetic model of cardiomyocyte-targeted deficiency of cyclooxygenase-2. J Mol Cell Cardiol 49: 196–209, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Parone PA, James DI, Da Cruz S, Mattenberger Y, Donze O, Barja F, Martinou JC. Inhibiting the mitochondrial fission machinery does not prevent Bax/Bak-dependent apoptosis. Mol Cell Biol 26: 7397–7408, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Parone PA, Martinou JC. Mitochondrial fission and apoptosis: an ongoing trial. Biochim Biophys Acta 1763: 522–530, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Rojo M, Legros F, Chateau D, Lombes A. Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of the transmembrane GTPase Fzo. J Cell Sci 115: 1663–1674, 2002 [DOI] [PubMed] [Google Scholar]
- 55. Sabbah HN, Sharov V, Riddle JM, Kono T, Lesch M, Goldstein S. Mitochondrial abnormalities in myocardium of dogs with chronic heart failure. J Mol Cell Cardiol 24: 1333–1347, 1992 [DOI] [PubMed] [Google Scholar]
- 56. Santel A, Frank S, Gaume B, Herrler M, Youle RJ, Fuller MT. Mitofusin-1 protein is a generally expressed mediator of mitochondrial fusion in mammalian cells. J Cell Sci 116: 2763–2774, 2003 [DOI] [PubMed] [Google Scholar]
- 57. Santel A, Fuller MT. Control of mitochondrial morphology by a human mitofusin. J Cell Sci 114: 867–874, 2001 [DOI] [PubMed] [Google Scholar]
- 58. Sheridan C, Delivani P, Cullen SP, Martin SJ. Bax- or Bak-induced mitochondrial fission can be uncoupled from cytochrome C release. Mol Cell 31: 570–585, 2008 [DOI] [PubMed] [Google Scholar]
- 59. Sheridan C, Martin SJ. Mitochondrial fission/fusion dynamics and apoptosis. Mitochondrion 10: 640–648, 2010 [DOI] [PubMed] [Google Scholar]
- 60. Skulachev VP. Mitochondrial filaments and clusters as intracellular power-transmitting cables. Trends Biochem Sci 26: 23–29, 2001 [DOI] [PubMed] [Google Scholar]
- 61. Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell 12: 2245–2256, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Smirnova E, Shurland DL, Ryazantsev SN, van der Bliek AM. A human dynamin-related protein controls the distribution of mitochondria. J Cell Biol 143: 351–358, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Suen DF, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev 22: 1577–1590, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sugioka R, Shimizu S, Tsujimoto Y. Fzo1, a protein involved in mitochondrial fusion, inhibits apoptosis. J Biol Chem 279: 52726–52734, 2004 [DOI] [PubMed] [Google Scholar]
- 65. Tanaka A, Cleland MM, Xu S, Narendra DP, Suen DF, Karbowski M, Youle RJ. Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J Cell Biol 191: 1367–1380, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Teshima Y, Akao M, Jones SP, Marban E. Uncoupling protein-2 overexpression inhibits mitochondrial death pathway in cardiomyocytes. Circ Res 93: 192–200, 2003 [DOI] [PubMed] [Google Scholar]
- 67. Tondera D, Grandemange S, Jourdain A, Karbowski M, Mattenberger Y, Herzig S, Da Cruz S, Clerc P, Raschke I, Merkwirth C, Ehses S, Krause F, Chan DC, Alexander C, Bauer C, Youle R, Langer T, Martinou JC. SLP-2 is required for stress-induced mitochondrial hyperfusion. EMBO J 28: 1589–600, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 27: 433–446, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Vendelin M, Beraud N, Guerrero K, Andrienko T, Kuznetsov AV, Olivares J, Kay L, Saks VA. Mitochondrial regular arrangement in muscle cells: a “crystal-like” pattern. Am J Physiol Cell Physiol 288: C757–C767, 2005 [DOI] [PubMed] [Google Scholar]
- 70. Wakabayashi J, Zhang Z, Wakabayashi N, Tamura Y, Fukaya M, Kensler TW, Iijima M, Sesaki H. The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice. J Cell Biol 186: 805–816, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wickner W, Schekman R. Membrane fusion. Nat Struct Mol Biol 15: 658–664, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med 192: 1001–1014, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]