Abstract
Arginase can cause vascular dysfunction by competing with nitric oxide synthase for l-arginine and by increasing cell proliferation and collagen formation, which promote vascular fibrosis/stiffening. We have shown that increased arginase expression/activity contribute to vascular endothelial cell (EC) dysfunction. Here, we examined the roles of the two arginase isoforms, arginase I and II (AI and AII, respectively), in this process. Experiments were performed using streptozotocin-induced diabetic mice: wild-type (WT) mice and knockout mice lacking the AII isoform alone (AI+/+AII−/−) or in combination with partial deletion of AI (AI+/−AII −/−). EC-dependent vasorelaxation of aortic rings and arterial fibrosis and stiffness were assessed in relation to arginase activity and expression. Diabetes reduced mean EC-dependent vasorelaxation markedly in diabetic WT and AI+/+AII−/− aortas (53% and 44% vs. controls, respectively) compared with a 27% decrease in AI+/−AII −/− vessels. Coronary fibrosis was also increased in diabetic WT and AI+/+AII−/− mice (1.9- and 1.7-fold vs. controls, respectively) but was not altered in AI+/−AII −/− diabetic mice. Carotid stiffness was increased by 142% in WT diabetic mice compared with 51% in AI+/+AII−/− mice and 19% in AI+/−AII −/− mice. In diabetic WT and AI+/+AII−/− mice, aortic arginase activity and AI expression were significantly increased compared with control mice, but neither parameter was altered in AI+/−AII −/− mice. In summary, AI+/−AII −/− mice exhibit better EC-dependent vasodilation and less vascular stiffness and coronary fibrosis compared with diabetic WT and AI+/+AII−/− mice. These data indicate a major involvement of AI in diabetes-induced vascular dysfunction.
Keywords: fibrosis, oxidative stress, vascular stiffness
in vascular endothelial cells (ECs), nitric oxide (NO) synthase (NOS) uses l-arginine to produce NO, which maintains blood flow and reduces inflammation (21, 22). Reduced availability of l-arginine to NOS and the resultant reduction of NO production have been implicated in the vascular dysfunction associated with diabetes and other cardiovascular disease states. Acute l-arginine supplementation can prevent or reverse EC dysfunction and restore EC-dependent vasodilation in diabetes (1, 31). Reduced availability of l-arginine for NOS can occur via increased activity or expression of arginase, an enzyme that competes with NOS for l-arginine, producing ornithine and urea.
Two isoforms of arginase exist in mammals, arginase I and II (AI and AII, respectively) (8, 23). AI, which is located in the cytoplasm, is expressed most abundantly in liver, whereas AII is a mitochondrial enzyme expressed primarily in the kidney. Both AI and AII have been found in EC populations (11, 34). During diabetes, impaired vascular function is closely associated with oxidative stress and inflammation (12, 28), both of which have been associated with elevated arginase activity and expression (4, 18).
Enhanced arginase activity appears to be involved in conditions characterized by vascular endothelial dysfunction (VED), such as diabetes, pulmonary hypertension, ischemia-reperfusion, and aging (5, 19, 29, 33, 42). Additionally, high chronic l-arginine intake can induce arginase expression/activity, thereby inducing vascular dysfunction (20, 21, 30, 35). Competition between arginase and NOS for their common substrate, arginine, suggests a cause-and-effect relationship in which increased arginase activity/expression decreases arginine bioavailabilty for NOS. We (33) previously tested this concept in diabetic rats and high-glucose (HG)-treated bovine coronary ECs (BCECs). We showed that diabetes-induced impairment of coronary vasorelaxation to ACh was correlated with increases in ROS, arginase activity, and AI expression in the aorta and liver. Treatment of diabetic coronary arteries with arginase inhibitors reversed the impaired vasodilation to ACh. Treatment of BCECs with HG (25 mM) also increased arginase activity and superoxide levels and diminished NO production in ECs. Additionally, transfection of BCECs with AI small interfering RNA prevented the elevation of arginase activity in HG-treated cells and normalized NO production, suggesting an involvement of AI in the reduced NO production. Our results indicate that increased arginase activity in diabetes contributes to VED by decreasing l-arginine availability to NOS.
In addition to the arginase-induced decreases in NO formation, elevated ornithine production, resulting from increased arginase function, gives rise to an enhanced synthesis of polyamines and proline (16, 47). Increases in polyamines can enhance cell proliferation, whereas proline supports collagen production. These processes can contribute to vascular fibrosis and stiffness, which are characteristics of diabetes-induced vascular pathology (24, 36).
Based on this evidence, we hypothesized that an upregulation of arginase expression and activity is involved in diabetes-induced vascular dysfunction, increased superoxide production, coronary fibrosis, and vascular stiffness. This was tested in streptozotocin (STZ)-diabetic wild-type (WT) mice and arginase knockout mice with either partial deletion of AI and complete deletion of AII (AI+/−AII−/−) or complete deletion of AII alone (AI+/+AII−/−).
METHODS
Animals and Induction of Diabetes
Experiments were performed using C57BL/6J WT mice, AI+/+AII−/− mice, and AI+/−AII−/− mice. AI homozygous knockout mice do not survive beyond 2 wk due to hyperammonemia. The C57BL/6J AI+/− and AII−/− mice developed by Cederbaum et al. (15) and O'Brien et al. (27) were provided by Dr. Stephen Cederbaum and have been backcrossed for at least 10 generations. At 10 wk old, mice were rendered diabetic with STZ (65 mg/kg ip, every other day for up to 4 injections). Mice were considered diabetic once blood glucose values of >350 mg/dl were achieved. Our study protocol was approved by the Institutional Animal Care and Use Committee of the Georgia Health Sciences University.
Tissue Harvest
After 8 wk of diabetes, mice were anesthetized with ketamine (100 mg/kg ip) and xylazine (10 mg/kg ip), and blood samples were obtained via cardiac puncture using heparinized syringes. Plasma was isolated by centrifugation. Tissues were harvested for immediate use (aorta), fixed in formalin (heart), or snap frozen in liquid nitrogen (heart and aorta) and stored at −80°C for later assay. Carotid arteries were initially placed in Krebs buffer at 0°C and then preserved/vitrified via stepwise cold exposure to increasing concentrations of propanediol [10–40% (wt/wt) for 30 min each with constant 15% (wt/wt) trehalose] in low ionic strength HEPES buffer at 0°C and stored at −80°C until use (32). At the time of use, stored carotid arteries were allowed to warm to 0°C and exposed to the reverse order of propanediol concentrations used for preservation (30 min each) ending in Krebs buffer.
Arginase Activity
Frozen mouse aortas were pulverized and homogenized on ice in lysis buffer [1:4 (wt/vol), 50 mM Tris·HCl, 0.1 mM EDTA, and 0.1 mM EGTA, pH 7.5] containing protease inhibitors. Homogenates were centrifuged for 10 min at 14,000 g, and the resulting supernatants were used for assay. Arginase activity was measured using a colorimetric determination of urea production from l-arginine, as previously described (13). Briefly, 25 μl of 10 mM MnCl2 were added to 25 μl of tissue homogenate, and samples were heated at 56°C for 10 min to activate arginase; 50 μl of 0.5 M l-arginine were then added to each tube, and samples incubated at 37°C for 1 h to hydrolyze l-arginine. Then, 400 μl of an acid solution mixture (10% H2SO4, 30% H3PO4, and 70% H2O) was added to stop the reaction. Afterward, 25 μl of 9% α-isonitrosopropiophenone were then added to each sample, and samples were heated at 100°C for 45 min. Samples were then placed in the dark at room temperature for 10 min, after which 200 μl of samples were loaded into 96-well plates and absorbance was read at 540 nm in a microplate reader (BioTek Instruments). Results were standardized according to sample protein concentration, as determined by a protein assay (Bio-Rad). Values were corrected for basal urea levels obtained in the absence of l-arginine and MnCl2.
Western Blot Analysis
Western blot analysis of arginase and nitrotyrosine levels was performed using frozen aortas. These aortas were pulverized and homogenized on ice in RIPA buffer (Upstate) containing protease inhibitors, NaF, and sodium orthovanadate. Homogenates were centrifuged for 10 min at 14,000 g, and supernatants were used for blots. For arginase expression, samples were subjected to electrophoresis, transferred to nitrocellulose membranes, and reacted with AI or AII primary antibodies (1:250, Santa Cruz Biotechnology) overnight followed by 1 h of incubation with donkey anti-rabbit horseradish peroxidase-labeled secondary antibody (1:4,000, GE Healthcare). All membranes were probed with α-actin antibody to assess the level of protein loading. Protein expression was quantified using densitometric analysis with ImageJ [National Institutes of Health (NIH)].
Vascular Function
Mouse aortas were immediately placed in ice-cold Krebs-Henseleit buffer, cleaned, and cut into 3-mm segments. Aortic rings were mounted in oxygenated tissue chambers (Danish-Myo Technology) containing Krebs-Henseleit buffer and allowed to equilibrate for 1 h at a resting tension of 5 mN, with the buffer changed every 15 min. After 1 h of equilibration, vessels were contracted with phenylephrine (PE; 10−6 M) to test vessel viability. Contractile responses were measured and compared among experimental groups. In the precontracted aorta, endothelium-dependent vasorelaxation was tested by adding increasing concentrations of ACh at 3-min intervals. To test endothelium-independent vasorelaxation, increasing concentrations of sodium nitroprusside (SNP) were used. Vessels were washed and equilibrated for 1 h between successive curves. Vasorelaxation responses are expressed as percentages of PE-induced contraction.
Passive Mechanical Properties of Arteries
The properties of carotid arteries were examined. After stepwise removal from vitrification solutions, carotid artery segments were cannulated at both ends, and mechanical characteristics were determined in Ca2+-free Krebs solution composed of (in mM) 118.3 NaCl, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, and 25 NaHCO3 using a pressure myograph. Vessel wall thickness (WTh; in μm) was calculated as follows: WTh = (OD − ID)/2, where OD and ID are outer and inner carotid artery diameters (in μm), respectively, as measured by video microscopic imaging. Transmural luminal pressure was converted from mmHg to N/m2, where 1 mmHg = 1.334 × 102 N/m2, for the calculation of circumferential stress (σ). σ, or the amount of hydrostatic force applied over a cross-sectional area of the luminal surface, was calculated as follows: σ = PIL × ID/2WTh, where PIL is transmural luminal pressure. Circumferential strain (ε), or the ratio of the observed change in the luminal diameter compared with the initial luminal diameter, was calculated as follows: ε = ID − ID20/ID20, where ID20 is the internal carotid artery diameter at the lowest luminal pressure (20 mmHg). Each measurement was taken as a hydrostatic sample at increments of 10 mmHg, ranging from 40 to 120 mmHg. Each stress-strain relationship was defined by the following equation: σ = α × eβχ, where α is the intercept, β is the “slope” of the exponential fit, and χ is wall strain. The β-coefficient was used as a relative measure of vascular stiffness, or vascular compliance. Each β-coefficient is the average plot of individual stress-strain data obtained from one carotid artery/animal.
Coronary Fibrosis
Hearts were embedded in paraffin blocks after fixation in 10% formalin. Paraffin-embedded sections (5 μm thick) were deparaffinized with xylene and rehydrated by immersion in a graded series of ethanol washes. Sections were stained by Masson's trichrome (Accustain Kit, Sigma-Aldrich) according to the manufacturer's protocol. Collagen deposition around the coronary vessels was detected by blue staining. The area of collagen staining relative to the vessel surface area was quantified using ImageJ (NIH). Perivascular fibrosis data are expressed as the collagen-to-vessel surface area ratio.
Hydroxyproline Levels
Hydroxyproline levels in cardiac tissue homogenates were assayed with a commercial ELISA kit for mouse hydroxyproline according to the manufacturer's instructions (Cederlane Labs). Sample levels were measured in a microplate reader at 450 nm, and the concentration of hydroxyproline in cardiac mouse samples was determined by comparing the optical density of the samples to standard curves. Values were standardized according to sample protein concentrations.
Lipid Peroxidation Levels
Lipid peroxidation was assessed in mouse plasma samples using a Lipid Hydroperoxide Assay Kit (Cayman Labs) according to the manufacturer's instructions. In brief, after chloroform extraction of lipid hydroperoxides from plasma, hydroperoxide levels were measured using redox reactions with ferrous ions and detected at 500 nm in a microplate reader.
Statistical Analysis
Data are given as means ± SE. Statistical analysis was performed by one-way ANOVA with a Tukey post test. In some experiments, statistical differences were determined by a Student's t-test. Results were considered significant when P values were <0.05.
RESULTS
Arginase Activity and Expression
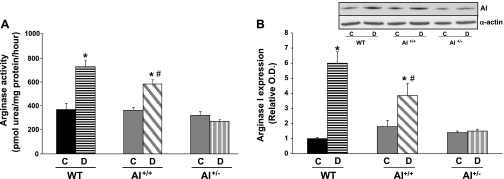
Arginase activity in the aorta of diabetic WT mice was increased twofold compared with levels in nondiabetic control mice (Fig. 1A). In diabetic AI+/+AII−/− mice, aortic arginase activity was elevated by 1.61-fold versus nondiabetic controls. In contrast, arginase activity was not altered in AI+/−AII−/− mice compared with their nondiabetic controls. There were no significant differences among the three control groups.
Fig. 1.
Effect on diabetes on arginase activity (A) and arginase I (AI) protein expression (B) in the aorta from wild-type (WT), AI+/+ , and AI+/− mice. Solid bars indicate responses in control nondiabetic mice (C). Hatched bars indicate responses in diabetic mice (D). Densitometric analysis was calculated as the fold increase of the WT control. A representative Western blot showing AI protein expression is included (B, top). n = 4–6 mice/group. *P < 0.05 vs. control for that genotype; #P < 0.05 vs. AI+/− diabetic mice.
AI and AII expressions were also assessed (Fig. 1B). AI expression was elevated in diabetic WT mice (6.1-fold) compared with nondiabetic WT mice. AI expression was also elevated in diabetic AI+/+AII−/− mice (2.2-fold) versus nondiabetic group levels. There were no differences between the diabetic and nondiabetic AI+/−AII−/− groups. Expression of AI in nondiabetic AI+/+AII−/− and AI+/−AII−/− mice was modestly greater than that in nondiabetic WT mice. The basal elevation of AI was likely a compensation for the complete lack of AII in these mice. As expected, AII expression was not evident in aortas from either arginase knockout group (data not shown) and not different between control and diabetic WT groups.
Vascular Function
Vasorelaxation.
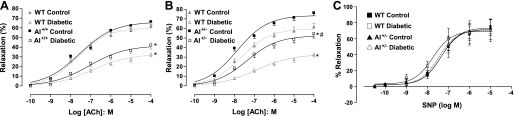
Endothelium-dependent and -independent vasodilator function was examined in the aorta of mice. In WT mice (Fig. 2A), the endothelium-dependent vasodilator ACh produced a maximum relaxation (Emax) of 61.8 ± 3.7% in the control group and 32.1 ± 2.9% in the diabetic group. In AI+/+AII−/− mice (Fig. 2A), the Emax to ACh was 66.2 ± 3.3% in the control group, which was not different than that in WT controls, and the Emax in the diabetic group was 42.2 ± 4.1%, which was similar to that of WT diabetics. In AI+/−AII−/− mice (Fig. 2B), the Emax to ACh in controls was 75.9 ± 3.1%, a value significantly greater than WT controls. The Emax for diabetic mice of this genotype was 54.3 ± 4.3%, a value much greater than that of the diabetic WT group.
Fig. 2.
Effect of diabetes on endothelium-dependent relaxation to increasing concentrations of ACh in the aorta from WT mice (A and B), AI+/+AII−/− (AI+/+) mice (A), and AI+/−AII−/− (AI+/−) mice (B). The dashed line indicates responses in diabetic mice; the solid line indicates responses in control nondiabetic mice. The shaded lines in A and B show responses in WT mice for comparison. C: effect of diabetes on endothelium-independent relaxation to sodium nitroprusside (SNP) in the aorta from WT and AI+/− mice. The dashed line and open symbols indicate responses in diabetic mice; the solid line and filled symbols indicate responses in nondiabetic control mice. n = 5–6 mice/group. *P < 0.05 vs. control of that genotype; #P < 0.05 between the two diabetic groups.
We also examined mean vasorelaxation responses of the aorta to ACh over the range of 10−9–10−4 M in diabetic mice of each genotype compared with their respective control/nondiabetic responses (Fig. 2, A and B). When calculated as percentage of their respective control responses over the range of ACh concentrations, the WT group value was 46.8 ± 3.6% and the AI+/+AII−/− value was 56.3 ± 4.1%. However, the AI+/−AII−/− value was significantly better at 72.5 ± 4.8% of control responses (P < 0.05 vs. other genotypes). Thus, the vasorelaxant responses of AI+/−AII−/− mice to ACh were better protected from the effects of diabetes.
Vasorelaxation responses to the endothelium-independent NO donor SNP were not affected by diabetes in any of the genotypes (Fig. 2C).
Vasoconstriction.
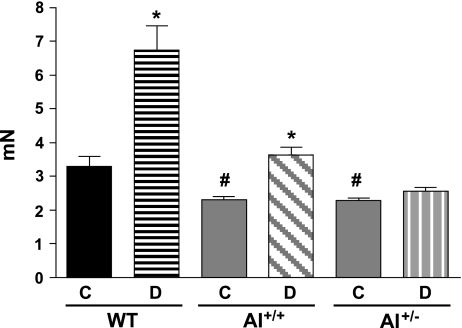
Contractile responses to PE (10−6 M) were approximately twofold greater in aortas of diabetic versus nondiabetic WT mice (Fig. 3). Among nondiabetic mice, aortic contractile responses to PE were 1.35- to 1.4-fold higher in WT mice versus both of the knockout strains, which did not differ in responses. Contractile responses of aortas from diabetic AI+/+AII−/− mice were 1.52-fold greater than those from their nondiabetic controls. However, contractile responses of AI+/−AII−/− aortas were not different between the diabetic and nondiabetic groups.
Fig. 3.
Effect of diabetes on the maximum contractile response to phenylephrine (10−6 M) in the aorta from WT, AI+/+, and AI+/− mice. The contractile response is given as developed tension (in nN). The solid bars indicate responses in control nondiabetic mice; the hatched bars indicate responses in diabetic mice. n = 4–6 mice/group. *P < 0.05 vs. control of that genotype; #P < 0.05 vs. control WT mice.
Carotid Artery Stiffness
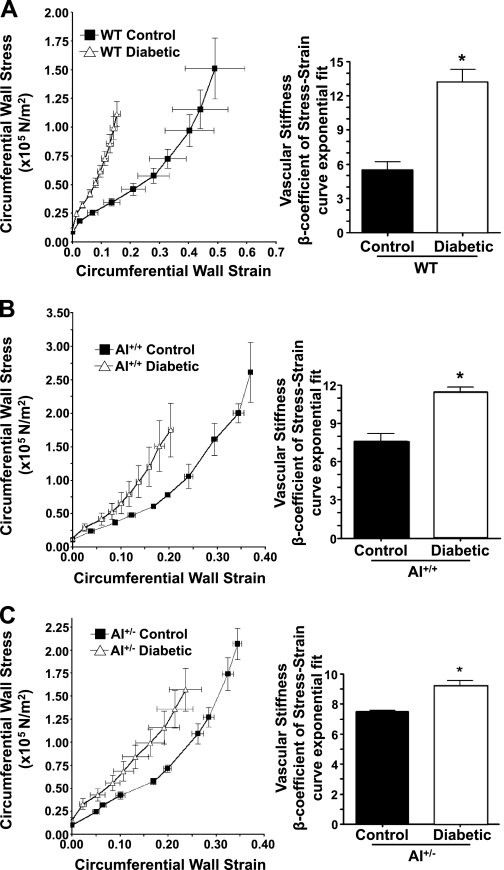
Passive mechanical properties of carotid arteries from diabetic and age-matched nondiabetic mice of each genotype were determined after 8 wk of diabetes. Stiffness was determined as the change in circumferential wall strain per unit change in circumferential wall stress as hydrostatic pressure was incrementally increased. The greater the slope of this relationship (β-coefficient), the greater the stiffness and the lesser the compliance. In WT mice, arteries from diabetic mice exhibited a 142% greater β-coefficient value than nondiabetic mice (Fig. 4A). For the AI+/+AII−/− groups, diabetic mice exhibited a 51% greater β-coefficient value than nondiabetic controls, which had a greater β-coefficient value than WT controls (7.4 vs. 5.4; Fig. 4B). In AI+/−AII−/− mice, diabetic mice had only a 19% greater β-coefficient that their control counterparts, which exhibited a β-coefficient similar to AI+/+AII−/− controls (Fig. 4C). Thus, diabetes enhances the stiffness of carotid arteries of WT mice to a greater extent than AI+/+AII−/− or AI+/−AII −/− mice. The latter genotype exhibited the smallest increase in carotid artery stiffness in response to diabetes among the three genotypes.
Fig. 4.
Determination of carotid artery stiffness in control and diabetic mice of WT, AI+/+, and AI+/− genotypes. Stiffness was assessed as a change in circumferential wall strain per unit change in circumferential wall stress as hydrostatic pressure was incrementally increased in vessels. Curves of this relationship are shown for WT (A), AI+/+ (B), and AI+/− (C) mice that were either control nondiabetic (filled symbols) or diabetic (open symbols). The slopes of these relationship (β-coefficient) are shown in the adjacent histogram. n = 5 mice/group. *P < 0.05 vs. the control group of a given genotype.
Coronary Perivascular Fibrosis
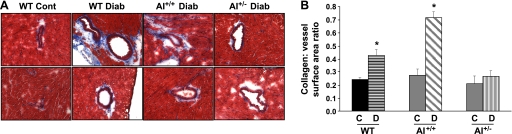
Fibrosis, as defined by enhanced levels of tissue collagen, is important in vascular remodeling and negatively affects vascular function. As shown in Fig. 5A, diabetic WT mice exhibited enhanced coronary perivascular fibrosis, as evident by the increased blue staining of collagen around vessels compared with control WT mice. Collagen staining was also elevated in AI+/+AII−/− diabetic mice to a level similar to that observed in diabetic WT mice. However, collagen staining was not altered in diabetic AI+/−AII−/− mice versus their respective controls. Additionally, the ratio of coronary perivascular fibrosis to total vessel surface area increased markedly for diabetic WT mice (1.9-fold) and AI+/+AII−/− mice (1.7-fold), whereas only marginal changes were observed in AI+/−AII−/− knockout mice (Fig. 5B). These data indicate that arginase activation is associated with increased collagen production and that AI is the predominant isoform contributing to the proline pathway for collagen synthesis.
Fig. 5.
Assessment of coronary perivascular fibrosis in control (Cont) and diabetic (Diab) mice of WT, AI+/+, and AI+/− genotypes was performed using Masson's trichrome staining (blue color for collagen). A: representative photographs of left ventricular paraffin-embedded sections. B: quantitative data providing the ratio of the perivascular collagen area to the vessel wall surface area. n = 4–5 mice/group. *P < 0.05 vs. all control groups.
Hydroxyproline Levels
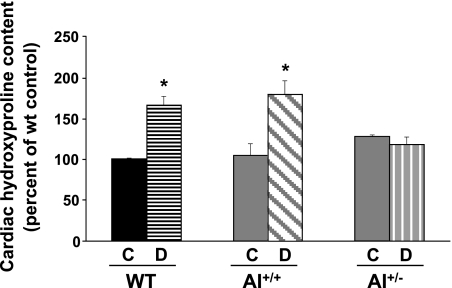
Levels of hydroxyproline are indicative of tissue collagen content. In diabetic WT and AI+/+AII−/− mice, myocardial levels of hydroxyproline were elevated similarly (by 1.66- and 1.71-fold) compared with their respective controls (Fig. 6). There were no differences in cardiac hydroxyproline levels between diabetic and nondiabetic AI+/−AII−/− mice. This profile of hydroxyproline levels among the groups was the same as that observed for coronary fibrosis.
Fig. 6.
Determination of left ventricular levels of hydroxyproline in control and diabetic mice of WT, AI+/+, and AI+/− genotypes. Results represent the percentage of control WT values. n = 5–6 mice/group. *P < 0.05 vs. all control groups.
Plasma Oxidant Levels
Levels of lipid hydroperoxide in plasma provide a measure of the systemic production of oxidative species. Diabetes elevated plasma lipid hydroperoxide above nondiabetic levels to 42.3 ± 2.5 μM in WT mice versus 26.4 ± 1.6 μM in controls (P ≤ 0.05), but it did not alter levels in AI+/−AII−/− mice (33.7 ± 2.8 vs. 27.9 ± 1.8 μM in nondiabetic controls). Plasma hydroperoxide levels were not different between nondiabetic control WT and AI+/−AII−/− mice.
DISCUSSION
Arginase Activity/Expression
Diabetes elevated vascular (aortic) arginase activity and expression in our WT mice, similar to our observations in a previous study of diabetic rats (33). Arginase activity and AI expression were also elevated in diabetic AI+/+AII−/− mice but not in AI+/−AII−/− mice, indicating that enhanced AI expression accounts for elevated enzyme activity. However, it is important to note that AII may play a role in the diabetes-induced increase in AI in our model. Diabetic AI+/+AII−/− mice exhibited less elevation of arginase activity and AI expression than WT mice. This suggests that AII contributes to the diabetes-induced enhancement of AI expression and activity in diabetic WT mice. The interplay between the two arginase isoforms leading to AI transcriptional regulation in diabetes requires further investigation. We did observe that nondiabetic AII knockout mice exhibited higher AI expression than nondiabetic WT mice but that arginase activity in the two strains was not different. The increased AI expression under control conditions may be a compensatory response to the lack of AII to maintain basal activity.
Vascular Function
Our present findings of elevated arginase activity and expression associated with vascular dysfunction in diabetic WT mice are in agreement with our previous study (33) showing that STZ-induced diabetes in rats causes impaired coronary EC-dependent vasodilation and elevated vascular arginase activity and expression. Our present data further demonstrate the prominent role of AI in VED resulting from diabetes. Diabetic mice that lacked one copy of the AI gene and both copies of AII (AI+/−AII−/−) showed substantially greater endothelium-dependent vasorelaxation than was seen in either WT mice or mice deficient in AII alone (AI+/+AII−/−). Endothelium-independent vasodilation to the NO donor SNP was not affected by diabetes or by the absence of either the AI or AII genes. In contrast, and as expected, the contractile responses of aortas to PE were enhanced by diabetes (9). Impaired endothelial function is also responsible for this enhancement, as reduced NO production in response to PE allows greater contraction. This profile of PE-induced contraction in diabetic mice of the three genotypes mirrored the profile of impaired vasorelaxant responses to ACh observed in diabetes. WT aortas exhibited the greatest enhancement of PE-induced contraction and the most impairment of ACh-induced vasorelaxation during diabetes.
These data strongly indicate that AI is the isoform primarily responsible for VED in these diabetic mice. Others (34) have reported that mice lacking AII genes are largely protected from atherosclerosis-induced VED, vascular stiffness, and enhanced endothelial ROS production. Additionally, Goto-Kakizaki diabetic rats have been shown to exhibit impaired coronary function, which was restored by an arginase inhibitor, and elevated aortic AII, but not AI, expression (16). However, a study (35) of myocardial ischemia-reperfusion and coronary endothelial dysfunction in mice showed only the involvement of AI. We (25) have also shown that STZ-induced diabetes-induced impairment of coronary vasorelaxation to ACh in rats was correlated with increases in ROS, arginase activity, and AI expression in the aorta. Additionally, a recent study (2) of diabetic human coronary arterioles demonstrated the involvement of upregulated AI in reduced NO production and vasodilation. Together, these results suggest that the involvement of arginase isoforms in vascular dysfunction varies depending on the model and disease condition.
Vascular Fibrosis and Stiffness
Coronary arterial perivascular fibrosis is a serious complication of diabetes (24) that contributes to vascular dysfunction (19). Vascular remodeling with pathological fibrosis may be caused by increased collagen production and/or impaired collagen turnover due to deficient matrix metalloproteinase activation (31, 36). Elevated vascular stiffness is a known cardiovascular risk factor (3). We found that 8-wk diabetic WT mice have markedly increased perivascular collagen deposition around coronary vessels compared with control WT mice. We also found no differences in coronary fibrosis between diabetic WT and diabetic AI+/+AII−/− mice. However, less perivascular collagen deposition was observed in AI+/−AII−/− mice, indicating that AI is the predominant isoform contributing to collagen synthesis. This fibrotic process appears to be caused by an elevation of arginase activity, which increases the production of ornithine and the subsequent production of pyrroline-5-carboxylate and proline, a precursor of collagen. Preventing the elevation of arginase activity or expression prevents the vascular fibrotic response and preserves vascular compliance (1, 7).
Increased vascular stiffness contributes to the elevation of systolic and mean arterial blood pressure as well as the incidence of vascular rupture and thrombosis (36). Our finding of greater stiffness (reduced compliance) of isolated carotid arteries from diabetic WT mice versus their nondiabetic WT controls is in line with our findings of greater coronary vascular fibrosis in this genotype. Compared with WT mice, we did observe a smaller elevation of diabetes-induced stiffness in AI+/+AII−/− mice versus their controls, which suggests that AII activity contributes to stiffness in diabetes. Stiffness of carotid arteries from diabetic AI+/−AII−/− mice was only modestly elevated compared with their controls, which correlates with a low degree of coronary fibrosis in these mice. There was a progressive reduction in vascular stiffness values (β-coefficients; Fig. 4, A–C) when diabetic WT, AI+/+AII−/−, and AI+/−AII−/− tissues were compared versus their controls, indicating that both isoforms are involved in diabetes-induced vascular stiffness. However, this reduced stiffening to diabetes in all AII−/− vessels partially resulted from a moderate elevation of basal stiffness in the arteries of control, nondiabetic AII−/− versus control WT mice. We do not know the basis of the enhanced basal vascular stiffness in mice lacking AII. However, it may relate to the elevated systemic blood pressure and sympathetic nervous function observed in AII knockout mice (17).
The present results indicate that arginase plays a critical role in collagen synthesis and fibrosis by providing the substrate for the collagen precursor proline. This conclusion is supported by our finding of elevated hydroxyproline levels in diabetic hearts of WT and AI+/+AII−/− mice compared with diabetic AI+/−AII−/− or control WT mice. These findings correlate with the Masson's trichrome staining results for collagen. However, we cannot exclude the possibility of impaired collagen degradation and turnover in diabetes. Another mechanism may account for the effect of elevated arginase activity on coronary fibrosis, since elevated arginase can diminish NO biosynthesis and NO-dependent processes. Several studies (5, 20, 33) have reported that long-term inhibition of NOS induces perivascular fibrosis and arterial stiffness in experimental animal models and humans. This effect is associated with elevated plasminogen activator inhibitor 1 production and the subsequent inhibition of matrix metalloproteinases, which leads to decreased matrix degradation and increased collagen deposition (23). It is probable that the overall improvement in vascular function of diabetic AI+/−AII−/− mice, due to enhanced levels of NO production, is a central event in preventing coronary perivascular fibrosis (collagen deposition) and the impairment of vascular compliance.
It is important to note that ANG II, a well-recognized mediator of diabetic vascular complications including coronary fibrosis, is a known activator of arginase (26, 30). We have found that ANG II can exert its actions on arginase through the activation of a p38 MAPK pathway. This pathway has been reported to be involved in the fibrotic actions of ANG II in heart tissue (10, 29). Elevation of arginase activity and protein expression in response to ANG II and HG also have been shown to involve the activation of the small GTPase RhoA and its effector Rho kinase (25, 26).
ROS
Diabetes and elevated levels of arginase activity and expression are associated with increased levels of ROS (15). Our study showed that whole body levels of ROS are elevated in diabetic WT mice, as indicated by measurements of plasma lipid hydroperoxide levels. In our study, we showed that preventing the elevation of AI activity, by partial gene deletion of AI in AI+/−AII−/− mice, prevents diabetes-induced increases of plasma lipid hydroperoxide levels. These data indicate an important role for AI in the enhanced oxidative milieu during diabetes.
ROS generation from mitochondria as well as from cytosolic NADPH oxidase have been shown to contribute to oxidative organ damage in diabetes (6, 14). However, we believe that endothelial NOS uncoupling due to enhanced arginase activity and reduced l-arginine is also an important source of superoxide formation in the vasculature. Determining the role of arginase on signaling pathways associated with NADPH oxidase-derived and mitochondrial ROS requires further study.
Summary
Our data indicate that elevated arginase function has an important role in the vascular complications of diabetes. Diabetic AI+/−AII−/− mice exhibited less arginase activity/expression, less impairment of endothelium-dependent vasodilation, less tissue oxidation, and less coronary fibrosis and vascular stiffness than diabetic WT and AI+/+AII−/− mice. Thus, AI appears to be the primary arginase isoform involved in diabetes-induced vascular dysfunction.
GRANTS
This work was supported by National Institutes of Health Grants RO1-HL-70215 and RO1-EY-11766 (to R. W. Caldwell and R. B. Caldwell).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.J.R., S.D.C., D.W.S., R.B.C., and R.W.C. conception and design of research; M.J.R., J.A.I., D.H.P., and M.I.A. performed experiments; M.J.R., J.A.I., D.H.P., M.I.A., R.B.C., and R.W.C. analyzed data; M.J.R., R.B.C., and R.W.C. interpreted results of experiments; M.J.R., J.A.I., D.H.P., and M.I.A. prepared figures; M.J.R., J.A.I., R.B.C., and R.W.C. drafted manuscript; M.J.R., J.A.I., D.H.P., S.D.C., D.W.S., R.B.C., and R.W.C. edited and revised manuscript; M.J.R., J.A.I., and R.W.C. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors appreciate the excellent assistance of Dr. Lin Yao and Anil Bhatta (Georgia Health Sciences University).
REFERENCES
- 1. Bagnost T, Ma L, da Silva RF, Rezakhaniha R, Houdayer C, Stergiopulos N, Andre C, Guillaume Y, Berthelot A, Demougeot C. Cardiovascular effects of arginase inhibition in spontaneously hypertensive rats with fully developed hypertension. Cardiovasc Res 87: 569–577, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Beleznai T, Feher A, Spielvogel D, Lansman SL, Bagi Z. Arginase 1 contributes to diminished coronary arteriolar dilation in patients with diabetes. Am J Physiol Heart Circ Physiol 300: H777–H783, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benetos A, Waeber B, Izzo J, Mitchell G, Resnick L, Asmar R, Safar M. Influence of age, risk factors, and cardiovascular and renal disease on arterial stiffness: clinical applications. Am J Hypertens 15: 1101–1108, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Bivalacqua TJ, Hellstrom WJ, Kadowitz PJ, Champion HC. Increased expression of arginase II in human diabetic corpus cavernosum: in diabetic-associated erectile dysfunction. Biochem Biophys Res Commun 283: 923–927, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Boffa JJ, Tharaux PL, Placier S, Ardaillou R, Dussaule JC, Chatziantoniou C. Angiotensin II activates collagen type I gene in the renal vasculature of transgenic mice during inhibition of nitric oxide synthesis: evidence for an endothelin-mediated mechanism. Circulation 100: 1901–1908, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54: 1615–1625, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Caldwell RB, Zhang W, Romero MJ, Caldwell RW. Vascular dysfunction in retinopathy-an emerging role for arginase. Brain Res Bull 81: 303–309, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cederbaum SD, Yu H, Grody WW, Kern RM, Yoo P, Iyer RK. Arginases I and II: do their functions overlap? Mol Genet Metab 81, Suppl 1: S38–S44, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Chang KS, Stevens WC. Endothelium-dependent increase in vascular sensitivity to phenylephrine in long-term streptozotocin diabetic rat aorta. Br J Pharmacol 107: 983–990, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen J, Mehta JL. Angiotensin II-mediated oxidative stress and procollagen-1 expression in cardiac fibroblasts: blockade by pravastatin and pioglitazone. Am J Physiol Heart Circ Physiol 291: H1738–H1745, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Chicoine LG, Paffett ML, Young TL, Nelin LD. Arginase inhibition increases nitric oxide production in bovine pulmonary arterial endothelial cells. Am J Physiol Lung Cell Mol Physiol 287: L60–L68, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Cohen RA, Tong X. Vascular oxidative stress: the common link in hypertensive and diabetic vascular disease. J Cardiovasc Pharmacol 55: 308–316, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Corraliza IM, Campo ML, Soler G, Modolell M. Determination of arginase activity in macrophages: a micromethod. J Immunol Methods 174: 231–235, 1994 [DOI] [PubMed] [Google Scholar]
- 14. Gao L, Mann GE. Vascular NAD(P)H oxidase activation in diabetes: a double-edged sword in redox signalling. Cardiovasc Res 82: 9–20, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Goodarzi MT, Navidi AA, Rezaei M, Babahmadi-Rezaei H. Oxidative damage to DNA and lipids: correlation with protein glycation in patients with type 1 diabetes. J Clin Lab Anal 24: 72–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gronros J, Jung C, Lundberg JO, Cerrato R, Ostenson CG, Pernow J. Arginase inhibition restores in vivo coronary microvascular function in type 2 diabetic rats. Am J Physiol Heart Circ Physiol 300: H1174–H1181, 2011 [DOI] [PubMed] [Google Scholar]
- 17. Huynh NN, Andrews KL, Head GA, Khong SM, Mayorov DN, Murphy AJ, Lambert G, Kiriazis H, Xu Q, Du XJ, Chin-Dusting JP. Arginase II knockout mouse displays a hypertensive phenotype despite a decreased vasoconstrictory profile. Hypertension 54: 294–301, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Jiang M, Jia L, Jiang W, Hu X, Zhou H, Gao X, Lu Z, Zhang Z. Protein disregulation in red blood cell membranes of type 2 diabetic patients. Biochem Biophys Res Commun 309: 196–200, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Kania G, Blyszczuk P, Eriksson U. Mechanisms of cardiac fibrosis in inflammatory heart disease. Trends Cardiovasc Med 19: 247–252, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Kinlay S, Creager MA, Fukumoto M, Hikita H, Fang JC, Selwyn AP, Ganz P. Endothelium-derived nitric oxide regulates arterial elasticity in human arteries in vivo. Hypertension 38: 1049–1053, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Kuhlencordt PJ, Gyurko R, Han F, Scherrer-Crosbie M, Aretz TH, Hajjar R, Picard MH, Huang PL. Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation 104: 448–454, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Lefer DJ, Jones SP, Girod WG, Baines A, Grisham MB, Cockrell AS, Huang PL, Scalia R. Leukocyte-endothelial cell interactions in nitric oxide synthase-deficient mice. Am J Physiol Heart Circ Physiol 276: H1943–H1950, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Li H, Meininger CJ, Hawker JR, Jr, Haynes TE, Kepka-Lenhart D, Mistry SK, Morris SM, Jr, Wu G. Regulatory role of arginase I and II in nitric oxide, polyamine, and proline syntheses in endothelial cells. Am J Physiol Endocrinol Metab 280: E75–E82, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Regan TJ, Lyons MM, Ahmed SS, Levinson GE, Oldewurtel HA, Ahmad MR, Haider B. Evidence for cardiomyopathy in familial diabetes mellitus. J Clin Invest 60: 884–899, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Romero MJ, Platt DH, Tawfik HE, Labazi M, El-Remessy AB, Bartoli M, Caldwell RB, Caldwell RW. Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circ Res 102: 95–102, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shatanawi A, Romero M, Iddings J, Chandra S, Umapathy N, Verin A, Caldwell R, Caldwell R. Angiotensin II increases arginase activity and expression through RhoA/Rho kinase and p38 mitogen-activated protein kinase (MAPK) pathways. Am J Physiol Cell Physiol 300: C1181–C1192, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shi O, Morris SM, Jr, Zoghbi H, Porter CW, O'Brien WE. Generation of a mouse model for arginase II deficiency by targeted disruption of the arginase II gene. Mol Cell Biol 21: 811–813, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tabit CE, Chung WB, Hamburg NM, Vita JA. Endothelial dysfunction in diabetes mellitus: molecular mechanisms and clinical implications. Rev Endocr Metab Disord 11: 61–74, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thandavarayan RA, Watanabe K, Ma M, Gurusamy N, Veeraveedu PT, Konishi T, Zhang S, Muslin AJ, Kodama M, Aizawa Y. Dominant-negative p38α mitogen-activated protein kinase prevents cardiac apoptosis and remodeling after streptozotocin-induced diabetes mellitus. Am J Physiol Heart Circ Physiol 297: H911–H919, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Toque HA, Romero MJ, Tostes RC, Shatanawi A, Chandra S, Carneiro Z, Inscho E, Webb RC, Caldwell RB, Caldwell RW. p38 Mitogen-activated protein kinase (MAPK) increases arginase activity and contributes to endothelial dysfunction in corpora cavernosa from angiotensin-II treated mice. J Sex Med 7: 3857–3867, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Van Linthout S, Seeland U, Riad A, Eckhardt O, Hohl M, Dhayat N, Richter U, Fischer JW, Bohm M, Pauschinger M, Schultheiss HP, Tschope C. Reduced MMP-2 activity contributes to cardiac fibrosis in experimental diabetic cardiomyopathy. Basic Res Cardiol 103: 319–327, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Wusteman MC, Simmonds J, Vaughan D, Pegg DE. Vitrification of rabbit tissues with propylene glycol and trehalose. Cryobiology 56: 62–71, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Zatz R, Baylis C. Chronic nitric oxide inhibition model six years on. Hypertension 32: 958–964, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang C, Hein TW, Wang W, Chang CI, Kuo L. Constitutive expression of arginase in microvascular endothelial cells counteracts nitric oxide-mediated vasodilatory function. FASEB J 15: 1264–1266, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Zhang C, Wu J, Xu X, Potter BJ, Gao X. Direct relationship between levels of TNF-α expression and endothelial dysfunction in reperfusion injury. Basic Res Cardiol 105: 453–464, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol 25: 932–943, 2005 [DOI] [PubMed] [Google Scholar]