Abstract
Androgens are reported to have both beneficial and detrimental effects on human cardiovascular health. The aim of this study was to characterize nongenomic signaling mechanisms in coronary artery smooth muscle (CASM) and define the ionic basis of testosterone (TES) action. TES-induced relaxation of endothelium-denuded porcine coronary arteries was nearly abolished by 20 nM iberiotoxin, a highly specific inhibitor of large-conductance, calcium-activated potassium (BKCa) channels. Molecular patch-clamp studies confirmed that nanomolar concentrations of TES stimulated BKCa channel activity by ∼100-fold and that inhibition of nitric oxide synthase (NOS) activity by NG-monomethyl-l-arginine nearly abolished this effect. Inhibition of nitric oxide (NO) synthesis or guanylyl cyclase activity also attenuated TES-induced coronary artery relaxation but did not alter relaxation due to 8-bromo-cGMP. Furthermore, we detected TES-stimulated NO production in porcine coronary arteries and in human CASM cells via stimulation of the type 1 neuronal NOS isoform. Inhibition of the cGMP-dependent protein kinase (PKG) attenuated TES-stimulated BKCa channel activity, and direct assay determined that TES increased activity of PKG in a concentration-dependent fashion. Last, the stimulatory effect of TES on BKCa channel activity was mimicked by addition of purified PKG to the cytoplasmic surface of a cell-free membrane patch from CASM myocytes (∼100-fold increase). These findings indicate that TES-induced relaxation of endothelium-denuded coronary arteries is mediated, at least in part, by enhanced NO production, leading to cGMP synthesis and PKG activation, which, in turn, opens BKCa channels. These findings provide a molecular mechanism that could help explain why androgens have been reported to relax coronary arteries and relieve angina pectoris.
Keywords: testosterone; coronary; large-conductance, calcium-activated potassium channel; guanosine 3′,5′-cyclic monophosphate
androgens are increasingly used as therapeutic agents to treat hypogonadalism, restore sexual desire/function, and enhance bone density and muscle mass (1). Despite their increased use among both men and women, our understanding of how androgens affect the cardiovascular system is lacking, and is, at times, even contradictory. It is often believed that estrogens protect against cardiovascular disease (CVD), whereas androgens are detrimental; however, endogenous testosterone (TES) concentrations are inversely related to CVD mortality, and declining TES levels are a strong independent predictive marker for men at high risk for CVD (23, 26). Recent in-depth reviews and analyses of the role of androgens in CVD reveal that there is little unequivocal evidence from either animal or human studies that androgens shorten the male lifespan (24, 43).
Nearly 70 years ago, it was reported that acute, intracoronary administration of TES relieved angina pectoris (12). More recently, it was demonstrated that acute administration of TES in patients with coronary artery disease (CAD) improves exercise-induced myocardial ischemia and that men with established CAD have lower serum TES levels compared with normal controls (32). Conversely, men undergoing short-term androgen deprivation therapy for prostate cancer exhibit increased risk for CAD (21). In contrast to these reports of beneficial effects of androgens on cardiovascular function, other clinical studies suggest that androgens raise blood pressure and promote coronary artery dysfunction in men (18). Furthermore, acute administration of TES may increase coronary vascular resistance in isolated hearts in vitro (4). In light of these inconsistent findings, it is obvious that our understanding of how androgens influence cardiovascular function, particularly function of the coronary arteries, is far from complete.
A number of studies have observed an acute relaxation effect of TES in a variety of arteries (5, 8–10, 37, 45), and, interestingly, this nongenomic relaxation response is often endothelium-independent (30). Therefore, vascular smooth muscle (VSM) is clearly a target of androgen action. Although androgens can modulate the activity of ion channels expressed in VSM cells, the signaling mechanisms underlying this modulation remain controversial. The present study was designed to identify and better characterize TES signaling mechanisms in coronary artery smooth muscle (CASM).
MATERIALS AND METHODS
Coronary arteries and single myocytes.
Fresh porcine hearts were obtained from local abattoirs. Hearts were from male and female pigs. The left anterior descending (LAD) coronary artery was excised and placed into ice-cold Krebs-Henseleit buffer solution of the following composition (in mM): 122 NaCl, 4.7 KCl, 15.5 NaHCO3, 1.2 KH2PO4, 1.2 MgCl2, 1.8 CaCl2, and 11.5 glucose, pH 7.2. Myocytes were isolated by a modification of a procedure described previously (38). Briefly, the arterial media was dissected and placed in a low-calcium dissociation medium of the following composition (in mM): 110 NaCl, 5 KCl, 0.16 CaCl2, 2 MgCl2, 10 HEPES, 10 NaHCO3, 0.5 KH2PO4, 0.5 NaH2PO4, 10 glucose, 0.49 EDTA, and 10 taurine, pH 6.9. Media strips were incubated at 37°C in 5 ml of the above solution with 6.33 mg papain, 4 mM dithiothreitol, and 0.2% BSA. Myocytes were isolated by 30 min of gentle shaking followed by trituration. Human CASM cells (Cambrex; cells not sorted by sex; passage 3–7) were grown in smooth muscle growth medium (SmGM-2) supplemented with 5% FBS.
Arterial tension studies.
Two 4- to 5-mm coronary artery rings were obtained from each LAD and prepared for isometric contractile force recordings as described previously (38). The endothelium was removed by rubbing the intimal surface and tested by observing the absence of acetylcholine-induced relaxation. The tissue bathing solution was the modified Krebs-Henseleit buffer described above (95% O2-5% CO2; 37°C). Rings were equilibrated for 90 min under an optimal resting tension of 2.0 grams. Preparations were exposed to maximally effective concentrations of a contractile agonist, e.g., prostaglandin F2α (PGF2α), to ensure stabilization of VSM. Inhibitors were allowed to equilibrate for at least 30 min before TES exposure. Vehicle was usually 50–75% ethanol, further diluted to no more than 0.1%.
Patch-clamp studies.
For cell-attached patches, cells were placed in a solution of the following composition (in mM): 140 KCl, 10 MgCl2, 0.1 CaCl2, 10 HEPES, and 30 glucose (pH 7.4; 22–25°C). Single potassium channels were measured by filling the patch pipette (2–5 MΩ) with Ringer solution of the following composition (in mM): 110 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, and 10 HEPES, and making a gigaohm seal on a single myocyte. Voltage across the patch was controlled by clamping the cell at 0 mV with the high-concentration extracellular potassium solution. In experiments of inside-out patches, the solution exposed to the cytoplasmic surface of the membrane was (in mM): 60 K2SO4, 30 KCl, 2 MgCl2, 0.16 CaCl2, 1 BAPTA (pCa 7), 10 HEPES, 5 ATP, and 10 glucose (pH 7.4; 22–25°C). The pipette solution was the same Ringer solution. Currents were filtered at 2 kHz and digitized at 10 kHz. Channel activity [open probability (NPo)] in patches with multiple large-conductance, calcium-activated potassium (BKCa) channels was determined as described previously (38), and mean NPo was determined by averaging channel activity from multiple membrane patches. The effects of TES, cyclic nucleotides, and purified cGMP-dependent protein kinase (PKG) on channel activity were observed at a variety of membrane potentials. Measurement of channel activity at +40 mV was employed to ensure a more accurate statistical analysis, as is routinely done (14, 38).
Protein kinase assay.
PKG was assayed as described previously (41). Briefly, denuded arteries were incubated in Krebs-Henseleit solution. Isobutyl methylxanthine (0.1 mM) was added in the incubation to inhibit phosphodiesterase activity. Arteries were then treated with 1 or 10 μM TES for 30 min in the absence or presence of 20 μM NG-monomethyl-l-arginine (l-NMMA), after which the smooth muscle was chopped and suspended in a 4-volume ice-cold homogenization buffer (in mM): 20 Tris·HCl, pH 7.4, 1 dithiothreitol, 1 EGTA, 1 EDTA, 1 phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, 2 μg/ml aprotonin, and 0.1% Triton X-100. The homogenate was centrifuged at 13,000 g (4°C) for 15 min, and the supernatant was used as a tissue extract for kinase activity. Protein concentrations were determined by the standard method of Lowry et al. (25). Kinase activity was determined by measuring 32Pi incorporation from [γ-32P]ATP into the serine residue of the synthetic peptide “kemptide” (22). Reactions (30°C; 10 min) occurred in a total volume of 50 μl that contained 50 mM Tris·HCl (pH 7.5), 20 μl of tissue extract, 20 mM MgCl2, 10 mM MnCl2, 100 μM kemptide, 100 μM of ATP, and 0.5 μCi [γ-32P]ATP (4 mCi/μM), 0.1 mg/ml BSA, and the 50 mM phosphatase inhibitors β-glycerophosphate, 1 mM sodium pyrophosphate, and 0.1 mM sodium vanadate. Reactions were terminated by adding 20 μl of 20% trichloroacetic acid and cooling. kemptide-directed phosphorylation was assessed by spotting 20 μl of each supernatant onto p-81 phosphocellulose paper discs, and 32P incorporation into kemptide was determined by liquid scintillation.
Fluorescence studies.
The cell-permeable form of the nitric oxide (NO) fluorescent indicator 4-amino-5-methylamino- 2′,7′-difluorescein (DAF-2 FM; Molecular Probes) was employed to examine production of NO within CASM (15, 40). Briefly, human CASM cells were incubated with 2 μM DAF-FM (diluted from a 5 mM stock solution in dimethyl sulfoxide) for 45 min (37°C; dark). Cells were washed several times with Ringer solution, and fluorescence was observed with a LSM 510 META laser scanning microscope (excitation 488 nm, emission 595 nm; Carl Zeiss) operating in the confocal mode with a ×40 0.85 numerical aperture objective (28). Images were analyzed by densitometry, and moving average analysis constructed a time course of fluorescence intensity. A time course for TES-stimulated fluorescence was measured in the absence or presence of 100 μM Nω-propyl-l-arginine [l-NPA, nitric oxide synthase (NOS) inhibitor; 30-min pretreatment] to control for nonspecific fluorescence.
Western blotting.
Expression of neuronal NOS (nNOS) or endothelial NOS (eNOS) (BD Transduction Laboratories) in CASM was detected using specific antibodies for the respective isoform (1:1,000; BD Transduction Laboratories) as described previously (39). Protein concentrations were determined by Bio-Rad DC protein assay. ADP-Sepharose beads were employed to affinity extract NOS proteins from lysates. Proteins were separated on SDS-polyacrylamide gels using a Mini Protean II (Bio-Rad) gel kit according to the manufacturer's instructions. Proteins were then transferred to a Hybond ECL membrane (Amersham Pharmacia Biotech) using a Mini-Trans-Blot Electrophoretic Transfer Cell (Bio-Rad) at 100 volts for 1 h. Blots were blocked with 5% nonfat milk overnight at 4°C. The membrane was then rinsed with Tris-buffered saline Tween (TBST) three times for 15 min and two times for 5 min. Blots were then probed with primary antibodies (nNOS, eNOS, 1:1,000; BD Transduction Laboratories) in TBST containing 1% nonfat milk protein for 1 h. After being washed, the membrane was then incubated with anti-rabbit IgG conjugated to horseradish peroxidase and visualized with an enhanced chemiluminescence system (Amersham).
Nitrite assay.
Nitrite production was measured as described previously (7) using a Greiss Reagent kit (Cayman Chemical). CASM explants were blotted on filter paper and weighed and then incubated for 90 min in Krebs buffer, and drugs were added for 30 min at the concentrations indicated. CASM was homogenized in 6% trichloroacetic acid, which was removed by washing with water saturated with diethyl ether. Total accumulation of nitrate and nitrite was calculated from a standard curve of nitrate concentrations and expressed as nitrite accumulation (μM) per milligram net CASM weight.
Statistical analysis.
All data were expressed as means ± SE. Statistical significance between two groups was evaluated by Student's t-test for paired data. Comparison between multiple groups was made by the one-way ANOVA test, with a post hoc Tukey's test to determine significant differences among the data groups. A probability of <0.05 was considered to indicate a significant difference.
RESULTS
TES-induced relaxation of porcine coronary arteries is dependent upon activation of BKCa channels.
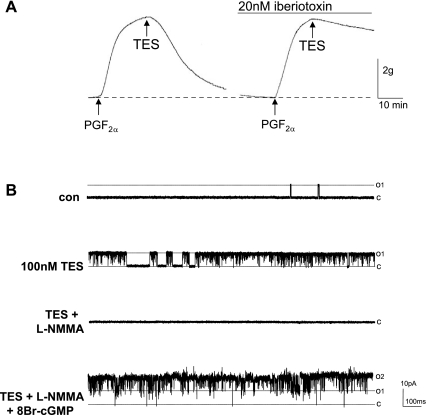
As we (8) and others (36, 45) have reported previously, micromolar concentrations of TES were required to produce maximal relaxation of precontracted coronary arteries in vitro. As shown in Fig. 1A, 25 μM TES almost completely relaxed endothelium-denuded porcine coronary arteries that had been contracted with 10 μM PGF2α. On average, the relaxation response to TES was 77.2 ± 4.1% (n = 8). Treating vessels with vehicle (<0.1% ethanol) had no effect on vessel tension. In contrast, relaxation was nearly abolished (only 12.6 ± 3.3%; n = 6) by 20 nM iberiotoxin, and single-channel patch-clamp (cell-attached patch) recordings from isolated porcine CASM myocytes demonstrated a stimulatory effect of TES on BKCa channel activity. In contrast to studies on denuded arteries, more physiological (i.e., nM) concentrations of TES stimulated channel activity dramatically in primary, isolated arterial myocytes (Fig. 1B). On average, 100 nM TES increased BKCa channel NPo by >40-fold (from 0.003 ± 0.001 to 0.131 ± 0.048; n = 10 cells; P < 0.0001). This channel has a high microscopic conductance (>200 pS), is stimulated by elevating calcium levels at the cytoplasmic surface of inside-out patches (data not shown), and is inhibited by either iberiotoxin or 1 mM tetraethylammonium (8, 14, 41).
Fig. 1.
Testosterone (TES)-induced coronary artery relaxation requires nitric oxide (NO)-stimulated large-conductance, calcium-activated potassium (BKCa) channel activity. A: representative traces of TES (25 μM)-induced maximal relaxation of endothelium-denuded coronary arteries precontracted with 10 μM prostaglandin F2α (PGF2α), in the presence or absence of iberiotoxin (20 nM; 30 min). The period of drug exposure is indicated by the line above the tracing or by arrow. B: typical BKCa channel recordings from the same cell-attached patch on a porcine coronary artery smooth muscle (CASM) cell (+40 mV) before (con) and 20 min after exposure to 100 nM TES and then after cumulative addition of 20 μM NG-monomethyl-l-arginine (l-NMMA) and 500 μM 8-bromo (Br)-cGMP. Channel openings are upward deflections from the baseline (closed) state, indicated by dashed line (c). The opening level amplitude of a single BKCa channel is indicated by the dashed line at open level 1 (O1), whereas simultaneous opening of 2 channels is indicated by the dashed line at open level 2 (O2).
NO mediates TES-induced coronary artery relaxation.
The stimulatory effect of 100 nM TES on BKCa channel activity was reversed by blocking NO production with 20 μM NG-monomethyl-l-arginine (l-NMMA). Despite the continued presence of TES, l-NMMA reversed the stimulatory effect of androgen (nearly 100% in the patch illustrated in Fig. 1B). On average, l-NMMA reversed TES-stimulated channel activity by >60% (0.052 ± 0.02; n = 3; P = 0.004). BKCa channels could then be reactivated by treating myocytes with 8-bromo (Br)-cGMP, a cell-permeable derivative of cGMP. In the continued presence of l-NMMA, 8-Br-cGMP (500 μM) restored and enhanced BKCa channel activity to an average NPo of 0.807 ± 0.008 (n = 3; Fig. 1B). Subsequent biochemical and fluorescence experiments further substantiated that TES stimulated NO production in CASM.
As shown in Fig. 2A, we found that TES increased nitrite production in porcine coronary arteries. TES (10 μM) increased nitrite accumulation by 15.5-fold (n = 4; P < 0.05). Furthermore, the stimulatory effect of TES was attenuated 84% by 20 μM l-NMMA (only a 2.5-fold increase in nitrite production over control levels; n = 4; P < 0.05). We were able to partially reverse the inhibitory influence of l-NMMA by adding 2 mM l-arginine. In the presence of both l-NMMA and l-arginine, TES increased nitrite accumulation by 10-fold over control levels (n = 4). Similarly, we detected TES-stimulated DAF-2 fluorescence (i.e., NO production) in single human CASM cells grown in culture (Fig. 2B). TES (100 nM) produced a time-dependent 50% increase in DAF-2 fluorescence (intensity increasing from 16.6 ± 1.8 to 25.1 ± 1.3, n = 9; P < 0.01). As shown in Fig. 2C, however, 100 nM TES failed to stimulate NO synthesis (intensity measurement of 12.6 ± 0.8, n = 13) in the presence of 10 μM l-NPA, an inhibitor with selectivity for type 1/nNOS. Immunoblotting (Fig. 2C, inset) detected expression of nNOS but not eNOS in these cells (n = 4).
Fig. 2.
TES increases NO production in porcine and human CASM. A: average nitrite accumulation in porcine CASM before (con) and 30 min after 10 μM TES and in the presence of 20 μM l-NMMA or l-NMMA and 2 mM l-arginine (l-arg). Bars represent mean values ± SE (n = 4). P < 0.05 compared with control (*) and compared with TES alone (#). B: 4-amino-5-methylamino- 2′,7′-difluorescein (DAF-2) fluorescence recorded from human CASM myocytes before (con) and 20 min after 100 nM TES. The time course plot is a moving average of fluorescence intensity after addition of TES. C: DAF-2 fluorescence recorded from human CASM myocytes in the presence of 10 μM Nω-propyl-l-arginine (l-NPA) and then 20 min after subsequent addition of 100 nM TES. The time course plot is a moving average of fluorescence intensity in the presence of l-NPA and TES normalized to control levels (i.e., 1.0). Inset: immunoblot detection of nitric oxide synthase isoforms expressed in human CASM (representative of n = 4 preparations). eNOS, endothelial nitric oxide synthase; nNOS, neuronal nitric oxide synthase.
Nongenomic signaling mechanisms in coronary arteries.
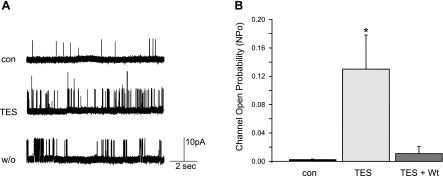
We were unable to reverse the stimulatory effect of 100 nM TES on BKCa channel activity by “wash out,” since channel openings recorded from cell-attached patches persisted 20–60 min after TES removal (n = 4; Fig. 3A). In contrast, TES-stimulated BKCa channel activity was attenuated by wortmannin, an inhibitor of phosphatidylinositol 3-kinase (PI3 kinase) (Fig. 3B). On average, BKCa channel activity stimulated by 100 nM TES was reduced ∼90% by 50 nM wortmannin (NPo 0.011 ± 0.010; n = 6; P < 0.05).
Fig. 3.
A: the stimulatory effect of TES on BKCa channel activity is not easily reversed. Representative recordings (+40 mV) before (con) and 10 min after exposure to 100 nM TES and then 20 min after washout (w/o) of TES. Channel activity was only minimally reversed even 20–60 min after TES removal (n = 3 cell-attached patches). B: TES-stimulated BKCa channel activity is inhibited by wortmannin. Summary analysis of channel open probability (NPo) before (con) and 10 min after exposure to 100 nM TES. In the presence of 50 nM wortmannin (Wt), TES had no significant effect on channel activity (n = 6; *P < 0.05 compared with control). Each bar represents the mean channel NPo ± SE.
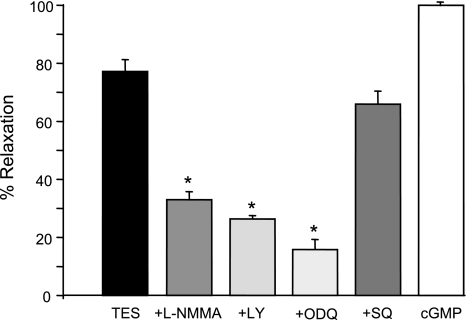
Tension studies on endothelium-denuded coronary arteries revealed that inhibition of NO signaling attenuates TES-induced relaxation. As summarized in Fig. 4, relaxation induced by 25 μM TES (77.2 ± 4.1%; n = 8) was reduced to 33 ± 2.8% (n = 6; P < 0.05) by 20 μM l-NMMA. Similarly, TES-induced relaxation was depressed by 400 μM aminoguanidine (14.4% relaxation; n = 4; P < 0.05; data not shown), another inhibitor of NOS activity. Furthermore, TES-induced relaxation was attenuated by agents that inhibit the activity of soluble guanylyl cyclase (Fig. 4). TES-induced relaxation was only 26.4 ± 1.2% (n = 6; P < 0.05) when cGMP synthesis was inhibited by 20 μM LY-83583 and was reduced even more (15.8 ± 3.5%; n = 6; P < 0.05) by 10 μM 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one, another inhibitor of guanylyl cyclase. In contrast, inhibition of adenylyl cyclase by 100 μM SQ-22536 had no significant effect on TES-induced relaxation (66 ± 4.5%; n = 4). To exclude potential nonspecific side effects of the various inhibitors, we observed that subsequent addition of 500 μM 8-Br-cGMP relaxed coronary arteries by 100% in the presence of each inhibitor (n = 4–6 for each inhibitor).
Fig. 4.
Summary of inhibitor action on TES-induced coronary artery relaxation. Relaxation response to 25 μM TES was measured in the presence of 20 μM l-NMMA (n = 6), 20 μM LY-83583 (LY, n = 6), 10 μM 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ, n = 6), or 100 μM SQ-22536 (SQ, n = 4). Relaxation to 500 μM 8-Br-cGMP was used as a positive control. Each bar represents the mean relaxation response ± SE (n = 4–6). *P < 0.05 compared with TES alone.
TES stimulates cGMP-dependent phosphorylation in CASM.
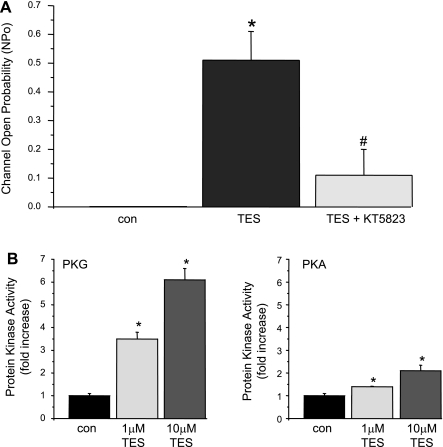
Pharmacological inhibition of PKG (with 300 nM KT-5823) attenuated TES-induced BKCa channel stimulation in cell-attached patches on primary porcine CASM myocytes (Fig. 5A). As we had observed previously, 100 nM TES increased channel NPo substantially (average of ∼0 to 0.51 ± 0.10; n = 3 cells; P < 0.006); however, this TES-stimulated BKCa channel activity was attenuated (NPo 0.11 ± 0.09; n = 3; P < 0.006; Fig. 5A) by 300 nM KT-5823, which exhibits selectivity for PKG (inhibitory constant 234 nM) at this concentration (20). TES-stimulated PKG activity was then confirmed by direct biochemical assay. We found that TES produced a concentration-dependent stimulation of PKG activity in porcine CASM (Fig. 5B, left). On average, TES (1 and 10 μM) increased PKG activity by 3.5- and 6.1-fold, respectively (n = 6 determinations; P < 0.05). Interestingly, the maximal effect of TES (i.e., 10 μM) on PKG activity was attenuated (∼ 50%) by 20 μM l-NMMA (3.2-fold; n = 6; P < 0.05; data not shown). TES also produced a concentration-dependent increase in the activity of the cAMP-dependent protein kinase (PKA) in porcine CASM, albeit to a much lesser extent (Fig. 5B, right). On average, 1 μM TES increased PKA activity by 0.4-fold (n = 6, P < 0.05), whereas 10 μM TES produced a 2.1-fold increase in kinase activity (n = 6, P < 0.05).
Fig. 5.
The cGMP-dependent protein kinase (PKG) is involved in TES-stimulated BKCa channel activity. A: summary of average BKCa channel activity (NPo) from cell-attached patch recordings (porcine CASM myocyte, +40 mV) before (con) and 20 min after exposure to 100 nM TES and then after cumulative addition of 300 nM KT-5823. Each bar represents the mean channel NPo ± SE (n = 3). P < 0.05 compared with control (*) and compared with TES alone (#). B: average activity of either PKG or cAMP-dependent protein kinase (PKA) measured in porcine CASM before (con) and 30 min after treatment with 1 or 10 μM TES (n = 6). Bars represent mean values ± SE. *P < 0.05 compared with control.
Reconstituting the effect of TES in a cell-free system using purified PKG.
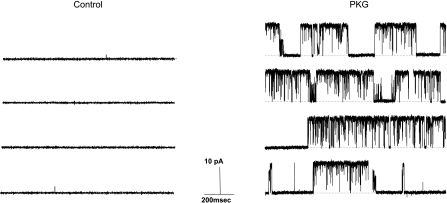
The effect of purified PKG was measured on BKCa channels isolated in an inside-out patch obtained from primary porcine coronary artery myocytes. Under these cell-free conditions, addition of 50 μM cGMP did not affect BKCa channel activity (n = 5, data not shown), thus ruling out direct effects of the nucleotide itself. As shown in Fig. 6, application of 400 U/ml of purified PKG (activated by 50 μM cGMP) stimulated the activity BKCa channels dramatically in this isolated membrane. On average, purified PKG increased channel NPo >100-fold (from 0.002 ± 0.002 to 0.265 ± 0.063; n = 5; P = 0.002), an effect similar to that observed for TES on intact myocytes (i.e., cell-attached patches). Nonactivated PKG or activated PKA had no significant effect on channel activity (data not shown).
Fig. 6.
Purified PKG opens BKCa channels. Typical recordings from the same cell-free (inside-out) patch (+40 mV) before (control) and 5 min after exposure to purified PKG (400 U/ml; activated with 50 μM cGMP). Channel openings are upward deflections from baseline (closed) state, indicated by dashed line.
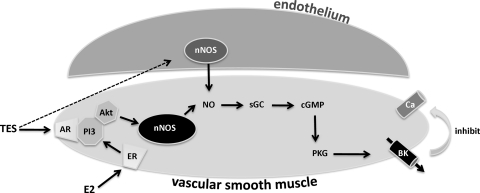
A summary diagram of the putative signal transduction mechanism stimulated by TES in CASM is provided in Fig. 7 and illustrates that gonadal steroid (i.e., TES and estrogen) signaling in CASM appears to involve activity of nNOS, leading to activation of a common effector molecule, the BKCa channel.
Fig. 7.
Summary model of nongenomic steroid [TES and 17β-estradiol (E2)] signal transduction in CASM. ER, estrogen receptor; AR, putative androgen receptor; PI3, phosphoinositide 3-kinase; Akt, protein kinase B; sGC, soluble guanylyl cyclase; BK, BKCa channel. Broken line indicates that TES may also stimulate production of NO from endothelial nitric oxide synthase (44).
DISCUSSION
Our findings from both tissue and cellular studies demonstrate that TES stimulates a rapid, nongenomic signaling mechanism in CASM that involves NO production derived from nNOS, with subsequent activation of cGMP-dependent phosphorylation via PKG. Furthermore, the fact that iberiotoxin (a highly specific antagonist of BKCa channels) is a powerful inhibitor of TES-induced relaxation suggests that BKCa channels are the primary target of TES action in CASM. We propose that potassium efflux subsequent to activation of BKCa channels closes calcium channels indirectly by inducing CASM repolarization. Previous studies employing single-channel patch-clamp techniques have also identified the BKCa channel as a target of TES action in myocytes from coronary arteries (8) or human corpus cavernosum (13), but the present study is the first (to our knowledge) to clearly demonstrate a PKG-mediated effect of TES on single potassium channels. These findings are consistent with other studies indicating that TES-induced arterial relaxation involves potassium channel stimulation (9, 13, 34, 42), but now implicate the PI3 kinase-protein kinase B (Akt)/nNOS signaling system as mediating TES-induced NO production in VSM.
Other studies indicate that TES-induced arterial relaxation can involve direct inhibition of L-type calcium channels (6, 31). More specifically, whole cell recordings of calcium currents indicate that TES acutely reduces calcium currents in primary aortic myocytes (29) and in a smooth muscle-derived cell line (33). Clearly, there is vascular heterogeneity regarding ionic mechanisms stimulated by acute TES treatment. Interestingly, though, more long-term exposure to TES increases L-type calcium channel current and expression in vascular (2) and cardiac (11) myocytes. Furthermore, there is evidence that high TES concentrations can stimulate calcium channel activity in aortic myocytes (29). These findings raise some potential questions regarding how well acute (i.e., minutes) TES inhibition of calcium channels in vitro mirrors more long-term, physiological exposure to TES; i.e., does more chronic exposure to TES actually increase calcium flux in VSM cells in vivo? If so, global increases in VSM calcium levels would raise vascular resistance and could help explain why some males receiving anabolic steroids have an elevated incidence of hypertension (35). On the other hand, it is also possible that TES-stimulated subsarcolemmal calcium accumulation (i.e., calcium sparks) in VSM cells could prolong and intensify BKCa channel activity within the arterial wall to promote vasodilation in some arteries. Such a putative role for BKCa channels in mediating calcium-dependent vascular relaxation is consistent with our demonstration that TES is a powerful stimulator of this channel in CASM. We have not measured the effects of TES on BKCa channel expression, but there is evidence that TES promotes expression of voltage-dependent potassium channels in vivo: castration lowered potassium channel expression in VSM, but restoration of physiological TES levels enhanced potassium channel expression/function in these vessels (47). Thus, it appears that long-term exposure to TES may increase the expression and activity of both potassium and calcium channels in VSM, suggesting an important role for these proteins in mediating TES-induced vasodilation under physiological conditions. Further studies are required to better delineate acute, nongenomic effects of TES from the more long-term, genomic effects and how these apparently diverse mechanisms influence vascular function.
As is commonly observed, we found that supraphysiological (i.e., μM) concentrations of TES were required to induce robust relaxation of coronary arteries in vitro. In contrast, we observed powerful effects of nanomolar concentrations of TES on single CASM cells, and others have reported similar findings in isolated arterioles and veins (30). The reason for this apparent discrepancy in effective TES concentrations is not clear but may be related to the nature of the preparation (e.g., diffusional barriers in intact arteries vs. open access to isolated cells), vascular heterogeneity, or possibly competing influences deriving from the vascular endothelium (which were absent in our studies of isolated myocytes). Nonetheless, we found that, in single cells, TES stimulates BKCa channel activity at concentrations closer to a “physiological” range and observed that our findings in myocytes correlated well with our observation that iberiotoxin was highly effective at inhibiting relaxation induced by even supraphysiological levels of TES. Thus, these consistent findings strongly suggest that BKCa channels mediate the relaxant effect of TES on CASM regardless of the concentration employed.
Nongenomic TES signaling in VSM is also somewhat controversial. For example, NOS inhibitors have limited effect on TES-induced relaxation of human radial (34), rat pulmonary (19), rabbit coronary (45), or rabbit carotid (27) arteries. In contrast, TES-induced relaxation is at least partially dependent upon NO production in canine coronary arteries (5), rat aorta (9), and rat mesenteric arteries (37). In the present study, TES stimulated nitrite production and NO-dependent fluorescence, whereas inhibition of NOS activity attenuated TES-induced BKCa channel activity and relaxation of intact arteries. Thus, our findings clearly indicate a role for NO in mediating TES-induced, endothelium-independent coronary artery relaxation. Observation of NO-dependent effects in the absence of a functional endothelium requires expression and activity of NOS isoform(s) in CASM. We found that inhibition of NOS with l-NPA [which exhibits a 3,000- and 150-fold relative selectivity for nNOS over iNOS or eNOS, respectively (46)] completely prevented TES-stimulated NO fluorescence in myocytes from human coronary arteries. These findings suggested nNOS to be the primary source of CASM-generated NO, and subsequent immunoblot studies confirmed expression of nNOS, but not eNOS, in these cells. Thus, we propose that nNOS is an important molecular target of TES action in CASM.
The stimulatory effect of TES on BKCa channel activity could not be abolished by multiple “washing out” cycles of TES from the extracellular medium during a standard experimental time frame (20–60 min). These studies suggest that the relaxant effect of TES is less likely to involve direct interaction of steroid with channel proteins but rather is mediated by a signal transduction mechanism that amplifies and prolongs TES action. In contrast, the powerful stimulatory effect of TES on BKCa channels was inhibited by blocking activity of more downstream signals: PI3 kinase (by wortmannin) or PKG (by KT-5823), further excluding direct binding as a primary mechanism of TES action. Taken together, these findings suggest a rapid, nongenomic stimulatory action of TES that is mediated via the PI3 kinase-Akt signaling cascade, nNOS activity, and cGMP-dependent phosphorylation in CASM. To our knowledge, there are no previous reports of TES-stimulated nNOS or PI3 kinase activity in VSM; however, a recent study has provided evidence that TES, acting via an androgen receptor mechanism, stimulates eNOS activity in human aortic endothelial cells via PI3 kinase-Akt activity (Fig. 7) (44). Interestingly, 17β-estradiol, acting via estrogen receptor-α, also enhances activity of the PI3 kinase-Akt signaling system to stimulate NO production from either eNOS expressed in human endothelial cells (17) or nNOS expressed in porcine or human CASM (16). Therefore, it is possible that the effect of TES on coronary arteries could be mediated via aromatization to estrogen. However, we have demonstrated previously that dihydrotestosterone, a nonaromatizable androgen, also relaxes these arteries (8). Therefore, there are clearly direct androgen effects on these vessels independent of conversion to estrogen. In light of these findings, it appears that acute vasodilatory mechanisms of both male and female gonadal steroids, although acting via distinct receptor molecules, converge on a common signaling mechanism: NOS activity regulated by PI3 kinase/Akt-dependent phosphorylation. This NO-producing mechanism is expressed in both vascular endothelium (eNOS) and VSM (nNOS) and can account for the endothelium-dependent and -independent responses elicited by both steroids in coronary and possibly other arteries. It is likely that endothelium-independent signaling via nNOS expressed in VSM would become increasing important as endothelial dysfunction declines in aging, atherosclerosis, hypertension, or diabetes.
Although we found that TES increased the activity of PKG and, albeit to a lesser extent, PKA, our functional studies argue strongly for the importance of PKG in mediating nongenomic TES signal transduction in CASM. For example, inhibition of adenylyl cyclase did not attenuate TES-induced coronary artery relaxation, suggesting that cAMP activation of PKA or cross-activation of PKG does not play a major role in TES signaling in these arteries (14, 41). Therefore, it appears doubtful that PKA activity alone could account for the vasodilatory action of TES. In contrast, a variety of evidence indicates that PKG mediates this relaxation response, since inhibition of NOS, guanylyl cyclase, or PKG activity was very effective in attenuating tissue or cellular/molecular responses to TES in CASM. These findings are supported by a previous study demonstrating that an inhibitor of PKG activity attenuated the effect of TES on whole cell currents in VSM isolated from arteries obtained from pregnant women (3). In the present study, we also reconstituted the cellular response to TES in a cell-free, inside-out patch by application of activated/purified PKG to the cytoplasmic surface of the membrane. Thus, these biochemical and physiological studies provide consistent evidence that PKG mediates acute TES effects on CASM. Similarly, our previous findings indicated that signaling via cGMP/PKG mediates the acute, vasodilatory effect of 17β-estradiol on porcine (7, 38) and human (40) CASM as well.
In summary, our findings indicate that the nongenomic, endothelium-independent relaxation effect of TES on coronary arteries is mediated by the PI3 kinase-Akt/NO/cGMP/PKG signaling cascade, leading ultimately to stimulation of BKCa channels, repolarization of VSM cells, and arterial relaxation (see summary in Fig. 7). A limitation of the study is that, although the majority of hearts were obtained from castrated males, we were unable to completely control the prior hormonal environment of the animals. In addition, the findings do not take into account the potential effect of castration on otherwise long-term, genomic effects of androgens. For example, castration can lower potassium channel expression in VSM (47). Thus, it is possible that our findings may actually underestimate the overall importance of these proteins in mediating the vascular response to TES in vivo. Therefore, making a direct correlation between our findings from porcine arteries and human coronary arteries may not be completely straightforward; however, the potential translational relevance of these findings is supported by our detection of TES-stimulated NO production in human CASM cells. These findings provide a potential molecular signaling pathway that is consistent with clinical studies indicating that age-associated decline in androgen levels is a strong independent predictor of CVD and overall mortality in older men (23, 26).
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-080402.
DISCLOSURES
None declared.
ACKNOWLEDGMENTS
We appreciate the guidance of Dr. Abdalla El-Mowafy in the kinase assay experiments. We also acknowledge the technical assistance of Handong Ma.
Current address for V. Deenadayalu: The Oregon Clinic Pc, 1111 NE 99th Ave., Ste. 301, Portland, OR 97220.
REFERENCES
- 1. Bassil N, Alkaade S, Morley JE. The benefits and risks of testosterone replacement therapy: a review. Ther Clin Risk Manag 5: 427–448, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bowles DK, Maddali KK, Ganjam VK, Rubin LJ, Tharp DL, Turk JR, Heaps CL. Endogenous testosterone increases L-type Ca2+ channel expression in porcine coronary smooth muscle. Am J Physiol Heart Circ Physiol 287: H2091–H2098, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Cairrao E, Santos-Silva AJ, Verde I. PKG is involved in testosterone-induced vasorelaxation of human umbilical artery. Eur J Pharmacol 640: 94–101, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Ceballos G, Figueroa L, Rubio I, Gallo G, Garcia A, Martinez A, Yanez R, Perez J, Morato T, Chamorro G. Acute and nongenomic effects of testosterone on isolated and perfused rat heart. J Cardiovasc Pharmacol 33: 691–697, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Chou TM, Sudhir K, Hutchison SJ, Ko E, Amidon TM, Collins P, Chatterjee K. Testosterone induces dilation of canine coronary conductance and resistance arteries in vivo. Circulation 94: 2614–2619, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Crews JK, Khalil RA. Antagonistic effects of 17 beta-estradiol, progesterone, and testosterone on Ca2+ entry mechanisms of coronary vasoconstriction. Arterioscler Thromb Vasc Biol 19: 1034–1040, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Darkow DJ, Lu L, White RE. Estrogen relaxation of coronary artery smooth muscle is mediated by nitric oxide and cGMP. Am J Physiol Heart Circ Physiol 272: H2765–H2773, 1997 [DOI] [PubMed] [Google Scholar]
- 8. Deenadayalu VP, White RE, Stallone JN, Gao X, Garcia AJ. Testosterone relaxes coronary arteries by opening the large-conductance, calcium-activated potassium channel. Am J Physiol Heart Circ Physiol 281: H1720–H1727, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Ding AQ, Stallone JN. Testosterone-induced relaxation of rat aorta is androgen structure specific and involves K+ channel activation. J Appl Physiol 91: 2742–2750, 2001 [DOI] [PubMed] [Google Scholar]
- 10. English KM, Jones RD, Jones TH, Morice AH, Channer KS. Testosterone acts as a coronary vasodilator by a calcium antagonistic action. J Endocrinol Invest 25: 455–458, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Er F, Michels G, Brandt MC, Khan I, Haase H, Eicks M, Lindner M, Hoppe UC. Impact of testosterone on cardiac L-type calcium channels and Ca2+ sparks: acute actions antagonize chronic effects. Cell Calcium 41: 467–477, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Hamm L. Testosterone propionate in the treatment of angina pectoris. J Clin Endocrinol 2: 325–328, 1942 [Google Scholar]
- 13. Han DH, Chae MR, Jung JH, So I, Park JK, Lee SW. Effect of testosterone on potassium channel opening in human corporal smooth muscle cells. J Sex Med 5: 822–832, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Han G, Kryman JP, McMillin PJ, White RE, Carrier GO. A novel transduction mechanism mediating dopamine-induced vascular relaxation: opening of BKCa channels by cyclic AMP-induced stimulation of the cyclic GMP-dependent protein kinase. J Cardiovasc Pharmacol 34: 619–627, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Han G, Ma H, Chintala R, Fulton DJ, Barman SA, White RE. Essential role of the 90-kilodalton heat shock protein in mediating nongenomic estrogen signaling in coronary artery smooth muscle. J Pharmacol Exp Ther 329: 850–855, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Han G, Ma H, Chintala R, Miyake K, Fulton DJ, Barman SA, White RE. Nongenomic, endothelium-independent effects of estrogen on human coronary smooth muscle are mediated by type I (neuronal) NOS and PI3-kinase-Akt signaling. Am J Physiol Heart Circ Physiol 293: H314–H321, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Haynes MP, Sinha D, Russell KS, Collinge M, Fulton D, Morales-Ruiz M, Sessa WC, Bender JR. Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-kinase-Akt pathway in human endothelial cells. Circ Res 87: 677–682, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Iliescu R, Reckelhoff JF. Testosterone and vascular reactivity. Clin Sci (Lond) 111: 251–252, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Jones RD, English KM, Pugh PJ, Morice AH, Jones TH, Channer KS. Pulmonary vasodilatory action of testosterone: evidence of a calcium antagonistic action. J Cardiovasc Pharmacol 39: 814–823, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Kase H, Iwahashi K, Nakanishi S, Matsuda Y, Yamada K, Takahashi M, Murakata C, Sato A, Kaneko M. K-252 compounds, novel and potent inhibitors of protein kinase C and cyclic nucleotide-dependent protein kinases. Biochem Biophys Res Commun 142: 436–440, 1987 [DOI] [PubMed] [Google Scholar]
- 21. Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol 24: 4448–4456, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Kemp BE, Graves DJ, Benjamini E, Krebs EG. Role of multiple basic residues in determining the substrate specificity of cyclic AMP-dependent protein kinase. J Biol Chem 252: 4888–4894, 1977 [PubMed] [Google Scholar]
- 23. Khaw KT, Dowsett M, Folkerd E, Bingham S, Wareham N, Luben R, Welch A, Day N. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation 116: 2694–2701, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocr Rev 24: 313–340, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275, 1951 [PubMed] [Google Scholar]
- 26. Maggio M, Lauretani F, Ceda GP, Bandinelli S, Ling SM, Metter EJ, Artoni A, Carassale L, Cazzato A, Ceresini G, Guralnik JM, Basaria S, Valenti G, Ferrucci L. Relationship between low levels of anabolic hormones and 6-year mortality in older men: the aging in the Chianti Area (InCHIANTI) study. Arch Intern Med 167: 2249–2254, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marrachelli VG, Miranda FJ, Centeno JM, Salom JB, Torregrosa G, Jover-Mengual T, Perez AM, Moro MA, Alborch E. Role of NO-synthases and cyclooxygenases in the hyperreactivity of male rabbit carotid artery to testosterone under experimental diabetes. Pharmacol Res 61: 62–70, 2000 [DOI] [PubMed] [Google Scholar]
- 28. McNeil PL, Miyake K, Vogel SS. The endomembrane requirement for cell surface repair. Proc Natl Acad Sci USA 100: 4592–4597, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Montano LM, Calixto E, Figueroa A, Flores-Soto E, Carbajal V, Perusquia M. Relaxation of androgens on rat thoracic aorta: testosterone concentration dependent agonist/antagonist L-type Ca2+ channel activity, and 5beta-dihydrotestosterone restricted to L-type Ca2+ channel blockade. Endocrinology 149: 2517–2526, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Perusquia M, Stallone JN. Do androgens play a beneficial role in the regulation of vascular tone? Nongenomic vascular effects of testosterone metabolites. Am J Physiol Heart Circ Physiol 298: H1301–H1307, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Perusquia M, Villalon CM. Possible role of Ca2+ channels in the vasodilating effect of 5beta-dihydrotestosterone in rat aorta. Eur J Pharmacol 371: 169–178, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Rosano GM, Sheiban I, Massaro R, Pagnotta P, Marazzi G, Vitale C, Mercuro G, Volterrani M, Aversa A, Fini M. Low testosterone levels are associated with coronary artery disease in male patients with angina. Int J Impot Res 19: 176–182, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Scragg JL, Jones RD, Channer KS, Jones TH, Peers C. Testosterone is a potent inhibitor of L-type Ca2+ channels. Biochem Biophys Res Commun 318: 503–506, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Seyrek M, Yildiz O, Ulusoy HB, Yildirim V. Testosterone relaxes isolated human radial artery by potassium channel opening action. J Pharmacol Sci 103: 309–316, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Sullivan ML, Martinez CM, Gennis P, Gallagher EJ. The cardiac toxicity of anabolic steroids. Prog Cardiovasc Dis 41: 1–15, 1998 [DOI] [PubMed] [Google Scholar]
- 36. Teoh H, Quan A, Man RY. Acute impairment of relaxation by low levels of testosterone in porcine coronary arteries. Cardiovasc Res 45: 1010–1018, 2000 [DOI] [PubMed] [Google Scholar]
- 37. Tep-areenan P, Kendall DA, Randall MD. Testosterone-induced vasorelaxation in the rat mesenteric arterial bed is mediated predominantly via potassium channels. Br J Pharmacol 135: 735–740, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. White RE, Darkow DJ, Lang JL. Estrogen relaxes coronary arteries by opening BKCa channels through a cGMP-dependent mechanism. Circ Res 77: 936–942, 1995 [DOI] [PubMed] [Google Scholar]
- 39. White RE, Han G, Dimitropoulou C, Zhu S, Miyake K, Fulton D, Dave S, Barman SA. Estrogen-induced contraction of coronary arteries is mediated by superoxide generated in vascular smooth muscle. Am J Physiol Heart Circ Physiol 289: H1468–H1475, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. White RE, Han G, Maunz M, Dimitropoulou C, El-Mowafy AM, Barlow RS, Catravas JD, Snead C, Carrier GO, Zhu S, Yu X. Endothelium-independent effect of estrogen on Ca2+-activated K+ channels in human coronary artery smooth muscle cells. Cardiovasc Res 53: 650–661, 2002 [DOI] [PubMed] [Google Scholar]
- 41. White RE, Kryman JP, El-Mowafy AM, Han G, Carrier GO. cAMP-dependent vasodilators cross-activate the cGMP-dependent protein kinase to stimulate BKCa channel activity in coronary artery smooth muscle cells. Circ Res 86: 897–905, 2000 [DOI] [PubMed] [Google Scholar]
- 42. White RE, Owen MP, Stallone JN. Testosterone-induced vasorelaxation of rat mesenteric microvasculature is K+ channel- and NO-dependent, but estrogen-independent (Abstract). FASEB J 21: 972.910, 2007 [Google Scholar]
- 43. Wu FC, von Eckardstein A. Androgens and coronary artery disease. Endocr Rev 24: 183–217, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Yu J, Akishita M, Eto M, Ogawa S, Son BK, Kato S, Ouchi Y, Okabe T. Androgen receptor-dependent activation of endothelial nitric oxide synthase in vascular endothelial cells: role of phosphatidylinositol 3-kinase/akt pathway. Endocrinology 151: 1822–1828 [DOI] [PubMed] [Google Scholar]
- 45. Yue P, Chatterjee K, Beale C, Poole-Wilson PA, Collins P. Testosterone relaxes rabbit coronary arteries and aorta. Circulation 91: 1154–1160, 1995 [DOI] [PubMed] [Google Scholar]
- 46. Zhang HQ, Fast W, Marletta MA, Martasek P, Silverman RB. Potent and selective inhibition of neuronal nitric oxide synthase by N omega-propyl-L-arginine. J Med Chem 40: 3869–3870, 1997 [DOI] [PubMed] [Google Scholar]
- 47. Zhou P, Fu L, Pan Z, Ma D, Zhang Y, Qu F, Guo L, Cao J, Gao Q, Han Y. Testosterone deprivation by castration impairs expression of voltage-dependent potassium channels in rat aorta. Eur J Pharmacol 593: 87–91, 2008 [DOI] [PubMed] [Google Scholar]