Abstract
Exercise training (EX) induces increases in coronary transport capacity through adaptations in the coronary microcirculation including increased arteriolar diameters and/or densities and changes in the vasomotor reactivity of coronary resistance arteries. In large animals, EX increases capillary exchange capacity through angiogenesis of new capillaries at a rate matched to EX-induced cardiac hypertrophy so that capillary density remains normal. However, after EX coronary capillary exchange area is greater (i.e., capillary permeability surface area product is greater) at any given blood flow because of altered coronary vascular resistance and matching of exchange surface area and blood flow distribution. The improved coronary capillary blood flow distribution appears to be the result of structural changes in the coronary tree and alterations in vasoreactivity of coronary resistance arteries. EX also alters vasomotor reactivity of conduit coronary arteries in that after EX, α-adrenergic receptor responsiveness is blunted. Of interest, α- and β-adrenergic tone appears to be maintained in the coronary microcirculation in the presence of lower circulating catecholamine levels because of increased receptor responsiveness to adrenergic stimulation. EX also alters other vasomotor control processes of coronary resistance vessels. For example, coronary arterioles exhibit increased myogenic tone after EX, likely because of a calcium-dependent PKC signaling-mediated alteration in voltage-gated calcium channel activity in response to stretch. Conversely, EX augments endothelium-dependent vasodilation throughout the coronary arteriolar network and in the conduit arteries in coronary artery disease (CAD). The enhanced endothelium-dependent dilation appears to result from increased nitric oxide bioavailability because of changes in nitric oxide synthase expression/activity and decreased oxidant stress. EX also decreases extravascular compressive forces in the myocardium at rest and at comparable levels of exercise, mainly because of decreases in heart rate and duration of systole. EX does not stimulate growth of coronary collateral vessels in the normal heart. However, if exercise produces ischemia, which would be absent or minimal under resting conditions, there is evidence that collateral growth can be enhanced. While there is evidence that EX can decrease the progression of atherosclerotic lesions or even induce the regression of atherosclerotic lesions in humans, the evidence of this is not strong due to the fact that most prospective trials conducted to date have included other lifestyle changes and treatment strategies by necessity. The literature from large animal models of CAD also presents a cloudy picture concerning whether EX can induce the regression of or slow the progression of atherosclerotic lesions. Thus, while evidence from research using humans with CAD and animal models of CAD indicates that EX increases endothelium-dependent dilation throughout the coronary vascular tree, evidence that EX reverses or slows the progression of lesion development in CAD is not conclusive at this time. This suggests that the beneficial effects of EX in CAD may not be the result of direct effects on the coronary artery wall. If this suggestion is true, it is important to determine the mechanisms involved in these beneficial effects.
Keywords: atherosclerosis, coronary blood flow, endothelium, physical activity, smooth muscle
this article is part of a collection on Cardiovascular Response to Exercise. Other articles appearing in this collection, as well as a full archive of all collections, can be found online at http://ajpheart.physiology.org/.
Although there is no evidence that coronary blood flow (CBF) capacity limits aerobic metabolism in the normal heart, dynamic exercise training (EX) in normal subjects increases coronary transport capacity through increases in CBF capacity and capillary exchange capacity. Training-induced coronary vascular adaptations can be divided into functional (alterations in vasomotor control) and structural adaptations (angiogenesis and vascular remodeling) (21, 84, 85, 87). Functional adaptations in normal subjects include changes in neurohumoral control of vascular resistance and changes in vasomotor reactivity of coronary resistance arteries. Although EX does not increase the oxygen carrying capacity of blood (28), myocardial oxygen extraction slightly increases following EX (155, 168). Much of the interest in the effects of EX on the coronary circulation results from the well-established effectiveness of physical activity/EX in the prevention (10, 162) and in the treatment of coronary artery disease (CAD) (161). While the mechanisms involved in EX-induced adaptations in the normal coronary circulation likely contribute to adaptations responsible for the effectiveness of EX in the prevention and treatment of CAD, it is apparent that additional mechanisms contribute because the beneficial effects of EX in CAD cannot be fully explained by the effects seen in normal subjects. The purpose of this review is to summarize and discuss what is known about the effects of EX on the coronary circulation in health and disease. We begin by discussing vascular adaptations seen in normal subjects and animals and then extend the discussion to the effects seen in the presence of atherosclerosis and CAD.
It is important to keep in mind that EX also increases maximal cardiac output, which is the result of cardiac adaptations in left ventricular (LV) mass and diameter produced by eccentric myocardial hypertrophy. Also, EX decreases myocardial oxygen demand because of decreases in heart rate and LV work. CBF per gram of myocardium is decreased in EX subjects because heart rate is decreased (5, 29, 68, 110, 168) and myocardial contractility, LV systolic wall stress, and ventricular work per gram of myocardium are minimally affected at rest and at a given level of submaximal exercise (24, 43, 109, 120, 141, 145, 174). These cardiac adaptations can also influence CBF capacity through a decrease in the extravascular compressive forces acting on the intramural coronary microvessels. Thus bradycardia contributes to the increased CBF capacity after EX because the bradycardia-mediated reduction in systolic time decreases impedance to CBF produced by systolic compression of the intramural coronary vessels (40). While these cardiac adaptations to EX are important, because of space limitations our primary focus in this discussion will be on coronary vascular adaptations induced by EX.
Structural Vascular Adaptations in the Normal Heart
Epicardial arteries.
Results from early experiments using corrosion casts of the coronary vasculature of young rodents indicated that EX induces an increase in the volume of the coronary vasculature (154, 157) because of increased conduit artery size (15, 62, 82, 99, 180). For example, 60 min of swimming per day increased coronary artery luminal area in young male rats with cardiac hypertrophy, but rats that exercised only twice each week and did not develop cardiac hypertrophy did not exhibit this adaptation (99). Increases in coronary artery size related to cardiac hypertrophy have also been reported in EX dogs (180) and monkeys (82). EX also appears to increase the size of human epicardial coronary arteries since echocardiographic and MRI measures (80, 81, 128, 185) indicate increases in the cross-sectional area of proximal coronary arteries. These increases in artery size were proportional to increases in LV mass in elite athletes compared with healthy sedentary individuals (128, 185). Windecker et al. (177) reported that 5 mo of EX resulted in a 28% increase in LV mass and a 23% increase in combined cross-sectional area of the left main and right coronary arteries measured with quantitative angiography. In contrast, Haskell et al. (62) reported no difference in angiographically measured cross-sectional areas of right coronary artery, left main, and left anterior descending coronary artery (LAD) of ultra-distance runners and sedentary subjects under basal resting conditions. However, nitroglycerin-induced increases in coronary cross-sectional area were positively correlated with aerobic exercise capacity. Hildick-Smith et al. (71) reported that nitroglycerin produced significantly greater dilation of the LAD in athletes than in sedentary men. Kozakova et al. (80) also reported a twofold greater dipyridamole-induced dilation of the left main coronary artery in athletes than in control subjects. Thus the results indicate that EX causes growth of epicardial arteries in proportion to the degree of exercise-induced cardiac hypertrophy. Simultaneously, conduit coronary artery tone during basal resting conditions is greater in EX individuals, so that conduit artery vasodilator capacity is greater in EX humans (62, 71, 177).
Resistance vessels.
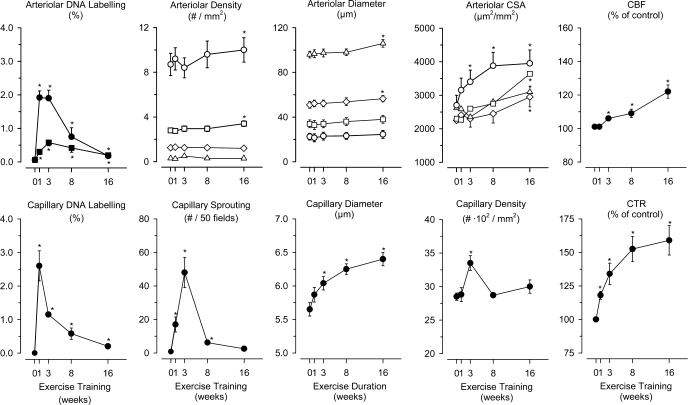
The number of coronary arterioles per square millimeters (numerical density) was reported to be 40–60% greater in treadmill EX than in sedentary swine (20, 174). White et al. (173) measured EX-induced adaptations of arterioles and found that total cross-sectional area (μm2 arterioles per mm2 of myocardium) of arterioles in the diameter range of 20–120 μm significantly increased by 16 wk of EX, with a greater increase in total area in arterioles of 20–40 μm in diameter (40–60%) than in arterioles of 40–120 μm in diameter (15–30%) (Fig. 1). Interestingly, in 20–40-μm-diameter arterioles, the increase primarily resulted from an increase in the number of arterioles, whereas in 40–120-μm-diameter arterioles, an increase in arteriolar diameter was the major cause of increased total cross-sectional area (173).
Fig. 1.
Effects of exercise training (EX) in swine on DNA labeling in arterioles [top, left: smooth muscle cells (■) and endothelial cells (●)], arteriolar density [top, second panel: 20–30-μm-diameter (○), 31–40-μm-diameter (□), 41–70-μm-diameter (◊), and 71–120-μm-diameter (▵) arterioles], arteriolar diameter [top, third panel: 20–30-μm-diameter (○), 31–40-μm-diameter (□), 41–70-μm-diameter (◊), and 71–120-μm-diameter (▵) arterioles], total arteriolar cross-sectional area [CSA; top, fourth panel: 20–30-μm-diameter (○), 31–40-μm-diameter (□), 41–70-μm-diameter (◊), and 71–120-μm-diameter (▵) arterioles], coronary blood flow (CBF; top, right) are shown. DNA labeling of capillaries (bottom, left), sprouting of new capillaries (bottom, second panel), capillary diameter (bottom, third panel), capillary density (bottom, fourth panel), and coronary transport reserve (CTR; bottom, right) is shown. Data are from White et al. (173), presented as means ± SE for 5 groups, with 6 animals in each group (0, 1, 3, 8 and 16 wk). *P < 0.05 different from the 0-wk time point (sedentary swine). See text for further explanation. Modified from Duncker and Bache (39) with permission from the American Physiological Society.
Capillary numerical density.
Early studies suggested an increase in capillary numerical density in young guinea pigs (130, 131) and dogs (163) following treadmill EX. Also, capillary density was found to be greater in wild than in domesticated rabbits (169, 170). In contrast, capillary-to-fiber ratio did not increase in young guinea pigs subjected to running (55) or in adult guinea pigs subjected to swimming (47). Rats from 3 to 18 mo of age, trained by either treadmill exercise or swimming, exhibit increased capillary densities with training (1, 9, 12, 26, 73, 98, 99, 107, 112, 158, 164, 167). However, in older rats angiogenesis is matched to cardiac hypertrophy so that EX does not alter capillary density (12, 73, 164) [see detailed review (39)].
Studies using dogs (94, 180, 181) and swine (20) also report no change in capillary-to-fiber ratio (20, 94) or capillary density (20, 94, 180, 181) following treadmill EX [see Table 2 in (39)]. Interestingly, endothelial cell division in pig coronary circulation was increased as was capillary sprouting at 1, 3, and 8 wk of EX but were no longer different from control after 16 wk of EX (Fig. 1). Capillary growth exceeded myocyte growth at 3 wk, but by 8 wk of EX, capillary density had returned to control (173). Thus, during the training program capillary angiogenesis occurred and temporarily exceeded the increase in LV mass, but with prolonged EX, angiogenesis was matched to LV hypertrophy.
Summary.
EX increases epicardial coronary artery diameter and arteriolar densities and/or diameters, providing a morphometric basis for increased CBF capacity in EX animals (Fig. 1). Although there is evidence that EX increases myocardial capillary density in young rats, larger animals subjected to EX exhibit capillary density matched to cardiac hypertrophy; i.e., capillary density is maintained in the normal range. This matching of capillary angiogenesis to the degree of myocardial hypertrophy is in contrast to pathological forms of myocardial hypertrophy (due to hypertension or aortic stenosis) where capillary rarefaction often occurs (2).
Adaptations of Neurohumoral Control
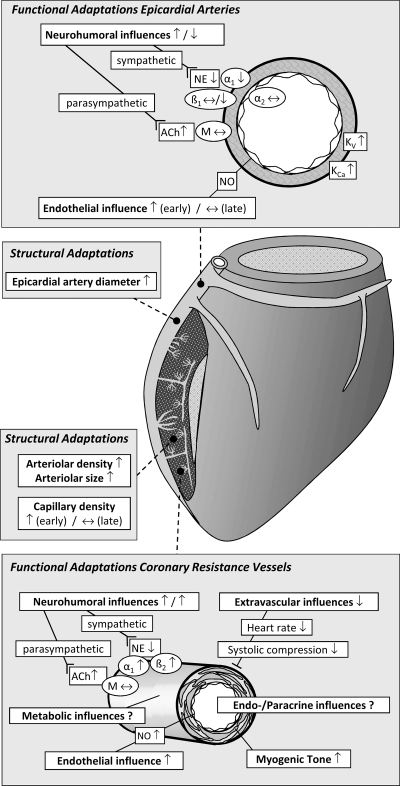
Altered neurohumoral control of the coronary vascular resistance can result from changes in the following: 1) central autonomic activity, 2) the number and/or affinity of vascular receptors, or 3) receptor/second messenger signaling in vascular cells (186) (Figs. 2 and 3). EX increases resting parasympathetic tone to the heart, but myocardial muscarinic receptor density and sensitivity appear to be unchanged (4, 44) or slightly decreased (14, 176) after EX. Also, circulating levels of catecholamines are lower in EX humans and animals (11, 133, 146), particularly when comparing similar absolute levels of submaximal exercise. These combined results suggest that EX results in increased parasympathetic and decreased sympathetic activity to the heart (11, 133, 146). Although there is little information concerning the effects of EX on adrenergic or muscarinic receptor density/sensitivity in coronary vasculature, myocardial β-adrenergic receptor density and sensitivity are reported to be unchanged (58, 137, 176) or slightly decreased (4) after EX, whereas α-adrenergic receptor density has been reported to be either increased (44) or decreased (176) in the myocardium after EX.
Fig. 2.
Summary of the structural and functional coronary microcirculatory adaptations to chronic EX in normal subjects. ACh, acetylcholine; M, muscarinic receptor; NE, norepinephrine; α1, α1-adrenergic receptor; β1, β1-adrenergic receptor; β2, β2-adrenergic receptor; Kv, voltage-gated K+ channel; KCa, Ca2+-activated K+ channel; NO, nitric oxide. Modified from Duncker and Bache (39) with permission of the American Physiological Society.
Fig. 3.
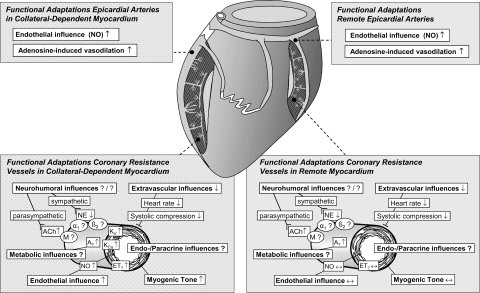
Summary of the structural and functional coronary adaptations to chronic EX in the collateral circulation. A, adenosine receptor; ET, endothelin receptor. Modified from Duncker and Bache (39) with permission of the American Physiological Society.
Conduit coronary arteries.
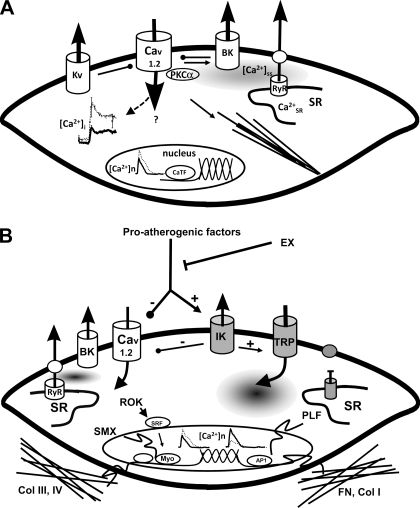
Bove and Dewey (15) reported that EX blunted the vasoconstrictor response of the proximal coronary arteries to α1-adrenergic receptor stimulation with phenylephrine but not to serotonin in closed-chest, sedated adult dogs. Similarly, vasoconstrictor responses to norepinephrine and phenylephrine were blunted in conduit coronary arteries of EX dogs (136) and swine (123). Stehno-Bittel et al. (152, 153) proposed that the decreased vasoconstrictor reactivity is the result of EX-induced decreases of intracellular calcium (Cai2+) in coronary vascular smooth muscle. Interestingly, vasoconstrictor responses of proximal coronary arteries to KCl or prostaglandin F2α are not altered by EX (123, 136). As summarized in Fig. 4A, there appear to be a number of mechanisms for the altered vasoconstrictor responses due to remodeling of control of Cai2+ in coronary vascular smooth muscle of conduit arteries. Conversely, endothelium-dependent vasodilation produced by α2-adrenergic receptor stimulation was not altered in coronary arteries from EX dogs (123). Finally, blunting of the β-adrenergic vasodilator response to isoproterenol was observed in coronary arteries of EX dogs (136) but not in EX swine (123).
Fig. 4.
A: model of EX-induced adaptations of coronary smooth muscle (CSM) in normal subjects (boldface indicates those elements altered by EX). The left center illustrates decreased intracellular calcium concentration ([Ca2+]i) response to selective agonists (e.g., endothelin), which produces a reduced Ca2+-dependent activation of contraction. This decreased [Ca2+]i occurs despite an increased Ca2+-influx through L-type Ca2+ channels (Cav1.2), which is buffered by a non-sarcoplasmic reticulum (SR), non-Na+/Ca2+ exchanger mechanisms. Nuclear Ca2+ responses ([Ca2+]n) are similarly reduced by EX, which may affect Ca2+-dependent transcription factors (CaTF, e.g., cAMP response element-binding protein and nuclear factor of activated T cell) and target gene expression. On the top right note that EX increases spontaneous, slow-Ca2+ release from the SR into the subsarcolemmal space ([Ca2+]ss), which may contribute to the increased activation of large-conductance Ca2+-activated (BK) K+ channels by EX. In addition, Kv channels are also activated by EX. In arteriolar CSM, Ca2+-dependent PKC (e.g., PKCα) signaling enhances Cav1.2, leading to activation of contractile filaments and enhanced myogenic tone. Together, these changes result in an increase in the gain of the vasomotor contractile system and a more stable mature CSM phenotype. B: proposed model illustrating the manner in which EX may interact with proatherogenic factors, thereby decreasing lesion progression and/or stimulating lesion regression of atherosclerosis through regulation of CSM phenotype in coronary artery disease (CAD; gray, atherogenic effects). Proatherogenic factors (such as PDGF-BB, TNF-α, leptin) upregulate intermediate conductance Ca2+-activated K+ channels (KCa3.1, IK) and voltage-independent Ca2+ channels (e.g., TRP) while suppressing Cav1.2 and BK (KCa1.1) channels. CAD also disrupts SR Ca2+ release and extrusion and increases [Ca2+]n. As shown on the right, this ion channel profile switch enhances CSM synthesis of fibronectin (FN) and collagen I (Col I), leading to a more proinflammatory matrix composition and synthesis of proliferative genes (PLF). EX adaptations described in A produce a stable, noninflammatory phenotype and increased expression of collagens III and IV (Col III, IV), producing a noninflammatory matrix. Note that there are a number of similarities and differences in the effects of EX on CSM in normal and diseased states. RyR, ryanodine receptor; ROK, Rho-associated protein kinase; SMX, smooth muscle-specific gene expression; Myo, myocardin; AP1, activator protein-1.
Coronary resistance vessels.
Important evidence that EX may alter adrenergically mediated vasomotor function in coronary resistance arteries came from experiments that demonstrated that 4 to 5 wk of EX resulted in a blunted phentolamine-induced increase in diastolic CBF during exercise (101). Subsequent studies from the same laboratory found that α-adrenergic blockade with phentolamine resulted in significantly greater (53), smaller (37), or, in the presence of β-blockade, similar (37) increases in mean CBF during submaximal exercise in EX dogs compared with sedentary animals. In open-chest dogs, α1-adrenoceptor blockade caused a slightly larger increase in mean CBF in EX than in sedentary animals (85). Thus current literature suggests that after EX, α-adrenergic tone is maintained or slightly increased in coronary resistance vessels. These findings are consistent with an interpretation of increased adrenergic sensitivity after EX in the presence of lower circulating levels of catecholamines (11, 133, 146). This interpretation is supported by observations that both peripheral (97, 108) and coronary (36, 53) resistance vessels exhibit greater constriction in response to α1-adrenergic stimulation after EX. The sustained α-adrenergic tone may contribute to enhanced myocardial oxygen extraction after EX (155, 168).
There are reports that EX and sedentary dogs exhibit similar decreases in CBF in response to nonselective β-adrenoceptor blockade (53, 102), β1-selective adrenoceptor blockade (53, 102), or β2-selective adrenoceptor blockade (37) during submaximal exercise. A maintained β-adrenergic vasodilator influence in the presence of lower circulating catecholamines is consistent with the reported increase in β2-adrenergic receptor responsiveness of coronary resistance vessels following EX (36, 60).
There is no evidence for altered parasympathetic control of coronary resistance vessel tone after EX. Muscarinic receptor blockade did not change CBF in exercising dogs before or after EX (53).
Summary.
Available data indicate that in the coronary microcirculation of EX subjects, there is a maintained or slightly increased α-adrenergic and maintained β-adrenergic tone during submaximal exercise. Maintenance of adrenergic tone in the presence of lower circulating catecholamine levels is consistent with the observed increased receptor responsiveness to adrenergic stimulation. Conversely, α- and β-adrenergic responsiveness seems to be reduced in large conduit coronary arteries of EX animals, which together with the lower catecholamine levels circulating in the blood, likely translates into lower α- and β-adrenergic influences in EX subjects. Finally, there is no evidence for altered parasympathetic control of coronary conduit and resistance vessel tone after EX.
Intrinsic Coronary Vascular Control
Endothelial control of conduit arteries.
Wang et al. (172) reported enhanced conduit coronary artery vasodilation in response to acetylcholine and reactive hyperemia in chronically instrumented dogs after 2 h/day of treadmill EX for only 7 days. This training had no effect on LV mass, heart rate, or CBF either at rest or during exercise. The enhanced dilation was abolished by the inhibition of nitric oxide (NO) synthase with nitro-l-arginine, and the dilation induced by nitroglycerine was not affected by EX. In contrast, endothelium-dependent relaxation (EDD) of proximal arteries was not increased following longer periods of EX (>10 wk) in dogs (136), swine (124), or rats (127) irrespective of whether EDD was stimulated in response to α2-adrenergic stimulation, substance P, vasoactive intestinal peptide, bradykinin, or acetylcholine. EX had little (123) to no (124) effect on the vasodilator response of the proximal coronary arteries to the endothelium-independent vasodilator nitroprusside in swine. Taken together, these observations suggest that during chronic EX, progressive outward remodeling of the epicardial arteries occurs so that shear stress levels and hence endothelial NO synthase expression normalize (86, 93), thereby allowing EDD responses to return toward baseline levels (86, 95, 124, 136).
Endothelial control of microcirculation.
EX of dogs for 8 wk was reported to enhance serotonin-induced increases in CBF measured in closed-chest anesthetized dogs, suggesting augmented EDD (15). Similarly, Muller et al. (115) reported enhanced bradykinin-induced EDD in coronary arterioles (64–157 μm in diameter) isolated from EX swine. The treatment of the arterioles with NG-monomethyl-l-arginine inhibited bradykinin-induced vasodilation to a greater extent in the EX group and eliminated the difference between the two groups, suggesting that EX enhances NO production (115). The observation that cytosolic copper/zinc superoxide dismutase (SOD-1) was upregulated (139) suggests that the increased EDD responses were, at least in part, the result of a decreased quenching of NO by superoxide. The vasodilator response to sodium nitroprusside was not different between sedentary and EX swine (115), suggesting that EX principally increased NO production. Consistent with this interpretation, Laughlin et al. (93) reported an increased endothelial NO synthase content in the coronary arterioles of EX swine.
Myogenic tone and smooth muscle responsiveness to constrictors.
Although active changes in diameter of coronary arterioles (75–150 μm in diameter) in response to 10-mmHg increments of intraluminal pressure were similar in arterioles from EX and sedentary swine, enhanced constriction was observed in coronary arterioles of EX swine for pressures above 40 mmHg (115). Subsequent work revealed that the enhanced tone was due to altered calcium-dependent PKC signaling in the coronary vascular smooth muscle cells (79) and enhanced voltage-gated calcium currents in large arterioles through L-type calcium channels (Fig. 4) (17). This increase of basal myogenic tone in arterioles appears to be specific for stretch-mediated contractions because neither the receptor-mediated vasoconstriction in response to endothelin-1 or acetylcholine nor the vasoconstriction produced by direct voltage-gated calcium channel activation by the L-type calcium channel agonist Bay K 8644 or by K+ were modified by EX (91). The results from coronary vascular smooth muscle in epicardial arteries from EX animals suggest that the adaptation may involve increased activity of voltage-gated K+ (Kv) and calcium-activated K+ (KCa) channels or adaptations at the level of the sarcoplasmic reticulum (19, 63, 87). However, it is uncertain whether mechanisms involved in coronary vascular smooth muscle of epicardial arteries also occur in coronary vascular smooth muscle of coronary arterioles.
Metabolic control.
Although adenosine does not play an essential role in the regulation of CBF under conditions of normal arterial inflow (3, 72, 165), the maximal adenosine-induced increase in CBF is reported to be greater after EX in both dogs (36, 85, 94) and miniature swine (92) in vivo. Also, von Restorff et al. (168) and Stone (155) reported a slight increase in myocardial oxygen extraction in EX dogs, suggesting an altered local control of CBF. EX has also been reported to result in slightly lower values of CBF per gram of myocardium both at rest and during submaximal exercise (68, 168). However, CBF at similar levels of cardiac work is not changed by EX (53, 68, 101, 155, 168), suggesting that EX has a minimal effect on the coupling between myocardial metabolism and CBF.
Heaps et al. (64) found that Kv or KCa channel function in arterioles from remote, normally perfused myocardium in hearts with a chronic coronary artery occlusion were not altered following EX. In contrast, EX enhanced Kv or KCa channel activity in porcine large conductance arteries, which could serve to facilitate downstream metabolic vasodilation (Fig. 4) (18). The effects of EX on arteriolar K+ channel function in the normal heart remain to be determined.
Summary.
EX produces a sustained augmentation of EDD in normal coronary microcirculation but apparently only transiently in conduit coronary arteries. This enhanced endothelium responsiveness appears to be principally due to increased NO bioavailability. Available data also indicate that EX alters the intrinsic vasomotor properties of coronary resistance vessels. Arterioles exhibit an increased myogenic tone, which appears specific for stretch-induced contractions, as vasoconstrictor responses to various agonists are unchanged. The mechanisms underlying the increased myogenic tone likely include a calcium-dependent PKC signaling-mediated alteration in voltage-gated calcium channel activity in response to stretch. It is currently unclear whether metabolic control mechanisms, including adenosine and K+ channel activity, in coronary resistance vessels are altered by EX.
Integrated Coronary Vascular Adaptations
The significance of the structural and functional adaptations of the various segments within the coronary vascular tree is demonstrated in the improved myocardial oxygen transport capacity apparent after EX. Current literature indicates that maximal CBF and capillary diffusion capacity are both increased by EX.
Maximum CBF.
Experiments designed to determine the effects of EX on CBF capacity have reported either no change (5, 16, 20, 25, 30, 100, 143, 155, 183) or an increase in CBF capacity after EX (6, 23, 36, 85, 89, 92, 94, 103, 129, 151, 173). Differing results may be caused by several factors including differences in species, age, and sex of the experimental animals and the duration, intensity, and type of the EX. Based on our review of the literature, we consider the most important factor to be the method used to assess CBF capacity as previously discussed in detail (39, 84, 85, 87, 90, 92). In studies where maximal vasodilation was demonstrated under tightly controlled hemodynamic conditions and where the efficacy of the training program was documented, CBF capacity was consistently reported to be increased following EX in swine (92, 173), dogs (85, 94), and rats (23) [for details, see Table 3 in (39)]. There have also been a number of clinical studies that assessed CBF capacity using echo-Doppler or positron emission tomography. These clinical studies also provide a mixed view of effects of EX on CBF capacity, with some reporting increases (54, 71, 80, 81, 177) and others no change (59, 67, 76, 132). Similar to the animal studies, assurance of maximal vasodilation, control of hemodynamic variables and documentation of training effectiveness make it difficult to evaluate the quality of the CBF capacity measurements in these clinical studies. Importantly, it is common to use standard doses of adenosine with cut-off values below normal values in clinical studies to estimate what is referred to as coronary reserve (70). However, as summarized by Heusch (70), adenosine is not simply a coronary vasodilator but also has cardioprotective, algesic, and antihypertrophy properties. Thus, in these clinical studies of coronary vasodilator reserve, maximal adenosine vasodilation is generally not demonstrated, so that while these measures provide valuable prognostic information, they may not rigorously estimate true coronary flow reserve. The weight of the currently available evidence suggests that EX increases maximal CBF per gram of myocardium when 1) CBF capacity is measured rigorously and 2) when the exercise program is of sufficient intensity to produce a training effect.
Capillary diffusion capacity.
Capillary exchange capacity, measured as the permeability surface area product, is determined by capillary permeability and total capillary surface area available for exchange. Although capillary numerical density is not increased by EX, increased capillary permeability surface area product could still result from the optimization of CBF distribution, effectively increasing the capillary surface area so that the maximal capillary exchange capacity becomes available for exchange. Laughlin and associates reported that EX caused an increase in coronary capillary permeability surface area product in dogs (85, 88, 94) and miniature swine (92). When morphometric measurements of capillarization and capillary permeability surface area product were examined in the same hearts, EX was found to increase coronary capillary permeability surface area product with no change in capillary density (92, 94). In the maximally vasodilated bed, capillary permeability surface area product is a function of blood flow, possibly because the increased CBF is associated with the recruitment of more capillary exchange area (92, 94). White et al. (174) also demonstrated that EX increased CBF capacity and capillary permeability surface area product with no net change in capillary density. However, as summarized in Fig. 1, White et al. reported increases in capillary diameters and increases in arteriolar densities, two adaptations that could decrease capillary transit time and improve matching of capillary blood flow and exchange capacity. Although a higher capillary permeability surface area product in trained animals could be due to the higher maximal flow rates, this is unlikely because a higher capillary permeability surface area product was also observed when sedentary and EX animals were compared at similar flow rates (92, 94, 126). Thus EX changes the coronary vascular resistance distribution, thereby increasing the effective capillary surface area and capillary permeability surface area product without a change in coronary capillary numerical density.
These effects of EX on capillary exchange may contribute to the decreased coronary sinus oxygen content and increased percent oxygen extraction reported by von Restorff et al. (168) and Stone (155) in EX dogs, although Stone (155) reported there was no statistically significant difference in absolute arteriovenous oxygen content difference after training. These studies reveal that after EX at any given myocardial oxygen consumption rate, oxygen extraction was greater and coronary sinus oxygen content was less, demonstrating that the coronary circulation was providing the required oxygen with less blood flow after training (155, 168). It is likely that these EX-induced changes are, in part, the result of changes in the distribution of CBF through the coronary capillary bed, resulting in improved oxygen extraction.
Summary.
Available evidence indicates that both an increase in maximum CBF and an increase in coronary capillary permeability surface area product contribute to the enhanced capacity and reserve to deliver oxygen to the myocardium in EX subjects.
EX, CAD, and the Coronary Collateral Circulation
Although EX does not provide immunity to atherosclerosis (34, 113), EX has emerged as an intervention for the primary and secondary prevention of CAD (49, 50, 87, 106, 160–162). The mechanisms proposed to contribute to the beneficial effects of EX include regression of atherosclerosis, improvement of endothelial function, formation of collaterals (arteriogenesis), and development of new vessels (angiogenesis/vasculogenesis) (87, 106). For an overview of the effects of EX in patients with CAD and the potential mechanisms, the reader is referred to several review articles (49, 50, 106, 134). Here we briefly summarize evidence concerning the effects of EX on coronary lesion progression/regression, endothelial and coronary vascular smooth muscle cell function in CAD, and the collateral circulation and coronary microcirculation within collateral-dependent myocardium.
Effects of exercise on coronary plaque/lesion progression/regression in CAD.
It is well established that EX stimulates the enlargement of conduit coronary arteries in normal humans and animals (80, 81, 90, 128, 180, 185). There is also evidence that EX reduces the development, and/or causes the regression, of atherosclerotic lesions in coronary arteries of animal models of disease including mice, rabbits, pigs, and nonhuman primates.
Okabe et al. (121) reported that 20 min of swimming exercise 3 times/wk for 8 and 16 wk in apolipoprotein E-deficient mice fed a high-fat diet exhibited less fatty streak formation and lesser fibrofatty plaques in the root of the aorta than nonexercised mice. Using this same mouse model, this group (122, 150) also reported that the decreased lesion progression in swim EX mice was due to increased antioxidant effects mediated through the NO system. In contrast, Young et al. (184) reported that swim training of apolipoprotein E-deficient mice did not alter atherosclerotic lesion development (Oil Red O staining) or oxidant load in the aorta. Consistent with the results from the Okabe group, Yang et al. (182) reported that treadmill exercise blunted the development of lipid deposition and expression of inflammatory mediators in the endothelium of the aorta of rabbits on a high-fat diet without modifying blood lipid contents. Endothelium-dependent relaxation of the atherosclerotic aortas was also improved by EX in these rabbits (182).
Link et al. (104) reported less CAD in left coronary arteries and fewer total atheromas in the arterial tree of male and female pigs trained for 22 mo. In contrast, recent results of experiments on pigs in early stage disease demonstrated that EX did not appear to retard the progression or reverse coronary atherosclerosis (166). Kramsch et al. (82) reported that exercise decreased CAD in monkeys, but a recent study by Williams et al. (175) found that wheel running in adult male monkeys did not appear to retard the progression or reverse CAD as measured with angiographic measures of lesion area. While their results indicated that exercise did not inhibit the progression of CAD or improve vascular lesions, their results also indicated that exercise improved measures of cardiovascular health (175). We and others have demonstrated in a number of porcine models of CAD that EX has beneficial effects on endothelial function/phenotype, as reflected in an increased EDD and altered expression of endothelial genes toward a more atheroprotective phenotype (159, 171, 179).
The current literature contains several randomized human trials, which angiographically evaluated the effects of exercise on coronary lesion progression/regression in CAD (56, 61, 125, 147). While these trials provide important insight into whether or not exercise has a direct effect on lesion size in CAD, these data must be carefully interpreted since the studies included exercise as one component of lifestyle and/or medical intervention. Ornish et al. (125) included exercise in combination with low-fat diet as part of the lifestyle interventions in the Lifestyle Heart Trial and found that the percent stenosis decreased (regressed) in the coronary arteries of the treatment group but increased (progressed) in the control group. The Heidelberg Regression Study (56, 147) reported the results of angiographic studies and documented the ability of regular, leisure time exercise to retard the progression of and/or reverse CAD. In the Hambrecht et al. study (56), CAD patients were prospectively randomized either to an intervention group that participated in regular exercise (n = 29) or to a control group (n = 33) receiving usual care and no supervised exercise. Thirty minutes of daily exercise was recommended for both groups, but the intervention group also received two 60-min supervised exercise sessions each week. Energy expenditure in leisure-time physical activity was estimated from standardized questionnaires and from participation in group exercise sessions. After one year of participation, repeat coronary angiography was performed and coronary lesions were measured by digital image processing. Patients in the exercise intervention group exhibited an increase in oxygen uptake of 7% (P < 0.001) at ventilatory threshold and of 14% at peak exercise (P < 0.05). Both indexes of cardiorespiratory fitness were decreased in patients in the control group. The exercise intervention group exhibited regression of CAD in 8 patients (28%), the progression of disease in 3 patients (10%), and no change in coronary morphology in 18 patients (62%), whereas in the control group progression was observed in 45%, no change in 49%, and regression of disease in 6%. When the patient groups were combined into groups according to progression/no change/regression of CAD, the lowest level of leisure time physical activity was noted in patients with progression of disease (1,022 ± 142 kcal/wk) as opposed to patients with no change (1,533 ± 122 kcal/wk) or regression of disease (2,204 ± 237 kcal/wk) (P < 0.005) (56). Thus these seminal experiments suggested that if a CAD patient could sustain 2,204 kcal/wk in leisure-time physical activity for one year, they would induce regression of CAD lesions (56). Similar results were obtained from the Stanford Coronary Risk Intervention Project (61) in which patients were assigned to a risk reduction group or usual care group. The risk reduction group exhibited 47% less lesion progression after one year than the usual care group. Of interest, the results indicated that the change in treadmill exercise performance was the best predictor of the change in coronary stenosis in this data set (61). It does not appear that the question of whether or not EX can cause regression or slower growth of coronary atherosclerotic lesions has been addressed in human subjects since the mid-1990s. Indeed, in a recent review article on this topic, Gielen et al. (49) state that “Surprisingly, not a single study has addressed exercise-mediated changes in plaque volume by intravascular ultrasound thus far. . . . ”
There is evidence that EX may have a greater effect on CAD lesion progression/regression following treatment with percutaneous transluminal coronary angiography and/or placement of intravascular stents. Belardinelli et al. (8) divided 118 patients who received percutaneous transluminal coronary angioplasty and/or stent treatments into two groups: EX patients, who performed three supervised exercise bouts (at 60% peak oxygen consumption), and control patients (mild normal activity of life). Follow-up studies were performed 6 mo after group assignment. They found that the EX patients exhibited improved functional capacity and quality of life relative to controls with fewer coronary events and lower hospital readmissions. Whereas the number of patients exhibiting restenosis was not affected by EX, the extent of restenosis was lower in the EX patients in both the balloon-dilated and stented segments. In three EX patients, regression of CAD was seen and no control subjects exhibited regression of CAD. Furthermore, the number of new lesions in epicardial coronary arteries not undergoing angioplasty was lower in the EX patients. In related experiments, Fleenor and Bowles (45) recently reported that EX inhibits coronary artery lesion development and alters the extracellular matrix composition of the coronary neointima formed following percutaneous transluminal coronary angioplasty in Yucatan miniswine. EX significantly decreased lesion size and neointima proliferation in the LAD and attenuated type I collagen expression. These beneficial effects of EX were not observed in the left circumflex coronary artery. Total collagen was increased and fibronectin was decreased by EX in the neointima of both LAD and left circumflex coronary arteries. The authors concluded that EX following percutaneous transluminal coronary angioplasty may increase event-free survival rates by decreasing coronary lesion size and altering the composition of the extracellular matrix of the wall of the coronary arteries (45). EX also appeared to reduce vascular disease in the peri-stent and non-stent regions of coronary arteries treated with percutaneous transluminal coronary angioplasty and stents in Ossabaw pigs, a model of type 2 diabetes (42).
In summary, the current literature paints a mixed picture concerning whether or not EX limits progression or produces regression of atherosclerotic lesions. Most data in large mammals do not indicate that exercise alters lesion progression/regression, but there is evidence of the beneficial effects of EX in coronary arteries that have been treated with percutaneous transluminal coronary angioplasty and/or intravascular stents. If our conclusion is correct that the current literature suggests that EX does not limit lesion development or lead to CAD plaque regression except following interventional procedures (percutaneous transluminal coronary angioplasty or stent placement), it is not clear why this is the case. It is possible that coronary hemodynamics are altered by percutaneous transluminal coronary angioplasty and/or stenting and related catheter laboratory procedures so that exercise bouts generate more beneficial mechanical signals in the walls of these arteries. If this is true, the mechanisms await discovery. Perhaps the most compelling evidence of a beneficial effect of exercise on lesion regression, in the absence of interventional procedures, is from human subjects. However, the reported changes in percent stenosis in these studies are quite small and therefore likely of little functional significance (56, 61, 125, 147). In this regard, Linke et al. (106) concluded that “the almost negligible amount of regression is unlikely to account for the tremendous relief in symptoms of CAD and the improvement of myocardial perfusion in patients undergoing exercise training.” Linke et al. (106) went on to conclude that the beneficial effects of EX may be largely attributed to EX-induced improvement in EDD and/or the overall endothelial function in the coronary circulation.
Finally, exercise-induced alterations in atherosclerotic lesion composition represent a compelling potential mechanism for reduced coronary heart disease mortality in physically active individuals. Human clinical trials report a reduction in coronary heart disease mortality as the most consistent effect of physical activity. Acute coronary syndromes and sudden death are most often associated with rupture of complex, vulnerable plaques that are otherwise clinically benign, i.e., <70% luminal stenosis, and asymptomatic (27). Lakka et al. (83) demonstrated an inverse relationship between chronic physical activity and the incidence of first acute myocardial infarction, even in individuals with normal stress EKGs consistent with the concept that physical activity may shift a “vulnerable” thin-cap atheroma to a more stable lesion less prone to rupture. Experimental evidence in animal models provides some support for this concept. EX in mice (75, 116) has been shown to reduce plaque rupture and increase cap thickness and collagen content, the latter likely due to lower matrix metalloproteinase (MMP-9) and increased tissue inhibitor of metalloproteinases. Similar beneficial changes in lesion composition have also been shown in postangioplasty restenosis in swine (45).
EX may alter lesion progression in atherosclerosis by changing coronary smooth muscle phenotype.
Smooth muscle plays an important role in both the initiation and progression of atherosclerotic lesions (148, 149). There is also growing evidence that EX can modify coronary vascular smooth muscle cell phenotypic modulation in atherosclerosis through altered ion channel regulation of Cai2+ (17–19, 171). Clearly these EX-induced changes in the control of Cai2+ would be expected to decrease the tendency for coronary vasospasm as well. Also, as summarized in Fig. 4B, there is evidence that EX interacts with proatherogenic factors, thereby decreasing lesion progression and/or regression through its effects on shear stress stimulated release of NO, alterations in transmural pressure and/or altered circulating factors. It is clear that more work is needed to fully understand the effects or EX on coronary vascular smooth muscle cell and its role in atherogenesis.
EX increases EDD in hyperlipidemia, atherosclerosis, and CAD.
EX has been shown to improve endothelial function in normal coronary (115, 172) and peripheral arteries (35, 74, 111), which is, at least in part, due to increased NO production through endothelial NO synthase (93, 95, 96, 115, 178) and increased expression of SOD (139, 140). Also, Hambrecht et al. (57) reported that EX increased EDD of conduit and resistance coronary arteries of CAD patients (57). Furthermore, EX appears to preserve EDD of coronary arteries of female (179) and male hypercholesterolemic pigs (159) in early stage CAD. Indeed, the endothelial dysfunction produced by a high-fat diet as well as the effects of EX in preserving endothelial function in coronary arteries are generally similar in male and female pigs (159, 179). EX attenuated the deleterious effects of hypercholesterolemia on endothelial function in coronary arteries, in part, by increased NO bioavailability (perhaps due to increased SOD content) and by the removal of a prostanoid vasoconstrictor, but EX did not appear to alter the role of non-cyclooxygenase, non-NO synthase factors such as endothelium-derived hyperpolarizing factor (159, 179). Thus most available information indicates that in animal models of CAD and in patients with CAD, EX increases EDD in the conduit arteries and in arterioles (49, 87, 105, 106, 134).
Effects of EX on collateral arteries.
A comparison of collateral CBF between sedentary and EX animals where collateral CBF is measured as retrograde flow from a cannulated collateral dependent coronary artery (22, 33, 143) or with radioactive microspheres (32, 38, 77, 78, 142, 144) provides exclusively negative results; i.e., collateral flow in EX animals is the same as sedentary values. Knight and Stone (77) measured collateral CBF in the same chronically instrumented dogs before and after EX and found that collateral CBF was increased by EX. An advantage of this study (77) was that measuring collateral CBF before and after EX allowed an adjustment for interanimal differences in the native collateral bed of the dogs. Cohen (32) also observed an increase in collateral CBF in chronically instrumented dogs following EX. Importantly, Cohen (32) found that the chronic instrumentation procedure stimulated the growth of coronary collaterals independent of EX, as he reported a similar increase in collateral CBF in sedentary animals maintained sedentary while the EX dogs trained. Thus the available literature indicates that EX does not enhance native collateral blood flow in the normal heart.
Effects of EX on collateral arteries: coronary stenosis or occlusion.
A pivotal study by Eckstein (41) reported that EX of dogs with a chronic coronary artery stenosis increased collateral formation. Eckstein (41) measured retrograde blood flow from the cannulated collateral-dependent artery and found that EX produced the greatest increase in this measure of collateral CBF when imposed on a dog with a mild stenosis, which resulted in minimal collateral formation in the sedentary animals. These results suggested that if exercise bouts produce myocardial ischemia, it is more effective in stimulating collateral vessel growth. However, many angiographic studies of collateralization in CAD patients have reported that EX did not improve angiographically detectable collateralization (48, 119). Angiographic measures of collateralization are limited to the measurement of the size of relatively large coronary collaterals and do not assess collateral blood flow. Circumventing this limitation of angiography, Belardinelli et al. (7) used thallium uptake to measure collateral-dependent myocardial perfusion in patients with ischemic cardiomyopathy and reported that 8 wk of moderate EX increased collateral CBF. The current literature indicates that EX does not appear to stimulate collateral formation in the coronary circulation of normal hearts, whereas evidence exists to suggest that if exercise bouts produce or aggravate myocardial ischemia in a region of myocardium distal to a coronary lesion (stenosis), then EX is effective in stimulating collateral vessel growth.
Another model of coronary disease that has been used to explore the hypothesis that EX can increase growth of coronary collaterals is the gradual coronary artery occlusion model. Most commonly, ameroid constrictors have been applied on a coronary artery, and as the ameroid material absorbs water, it gradually occludes the coronary artery over a period of 3 to 5 wk. Use of this model of CAD has also yielded equivocal results relative to the effects of EX on collateralization. Heaton et al. (66) and Neill and Oxendine (118) examined the effects of EX on collateral CBF in dogs and reported no effects of EX on collateral CBF 6–8 wk after placement of an ameroid constrictor on a coronary artery. In contrast to these results in dogs, CBF was ∼70% of normal in the collateral-dependent region of pigs that were EX, compared with ∼55% in sedentary pigs with an ameroid coronary occluder in place (13, 138).
Vasomotor control of coronary resistance vessels in collateral-dependent myocardium.
As discussed in Effects of EX on collateral arteries: coronary stenosis or occlusion, the literature does not consistently indicate that EX results in enhanced growth of collateral arteries in models of CAD or in patients with CAD. The literature is more consistent in reference to the question of whether or not EX has beneficial effects on the vasomotor reactivity of collateral arteries and/or vasomotor control of resistance in collateral-dependent myocardium. The majority of experiments relating to this question have used the chronic ameroid occlusion model. For example, examination of the effects of EX on epicardial arteries within the collateral-dependent region of EX hearts revealed improved EDD (51) and improved adenosine-induced vasodilation (65) compared with sedentary pigs with ameroid occlusions. EX also improved bradykinin-induced EDD in arterioles isolated from the collateral-dependent myocardium (52), and VEGF165-induced EDD was reported to be enhanced in collateral-dependent arterioles (46). Fogarty et al. (46) also reported that the enhanced VEGF-induced EDD was mediated principally via increased NO bioavailability. Another important observation of these studies was that EX did not improve bradykinin-induced EDD of arterioles isolated from the normally perfused region of collateralized hearts (52) in contrast to results from coronary arterioles isolated from normal EX pigs (115). Griffin et al. (51) proposed that the effects of EX on coronary arteriolar vasomotor reactivity are modified in the nonoccluded arterial beds of chronically coronary occluded hearts (52). Thus the coronary arteries located in the nonoccluded zones of hearts cannot be assumed to be “control” vessels.
It is interesting that EX appears to increase myogenic tone in arterioles isolated from collateral-dependent zones of this model of CAD (64) in a manner similar to that reported for arterioles from normal EX hearts (114). EX is also reported to enhance endothelin-1-mediated contractile responses in collateral-dependent resistance arteries (∼150 μm) that may be mediated by increased PKC-mediated coronary smooth muscle calcium sensitization (135). Further evidence of EX effects on coronary vascular smooth muscle in collateral-dependent arteries is provided by the report of augmented vasodilator responses through Kv channel activity (64).
Summary.
There is no doubt that epidemiologic studies consistently indicate that there is an inverse relationship between CAD and regular physical activity and/or cardiorespiratory fitness (49, 106, 161). However, the current evidence does not firmly support the hypothesis that these beneficial effects of exercise result from a blunted progression and/or an increased regression of coronary lesions. The strongest evidence supporting this hypothesis is from human studies, but these results may reflect the overall effects of lifestyle changes, of which exercise was but one component. In general, the current evidence indicates that EX improves endothelial phenotype and function in coronary arteries of humans and animal models with CAD, and that EX has beneficial effects on coronary vascular smooth muscle in diseased coronary arteries.
Coronary collateral growth does not appear to be increased by EX in normal subjects. However, there is evidence that collateral growth can be enhanced by EX if exercise produces or increases myocardial ischemia. Nevertheless, the concept that exercise-induced ischemia stimulates collateral vessel growth is not supported by studies in which β-adrenergic blockade was administered to minimize the occurrence of myocardial ischemia during gradual coronary occlusion with an ameroid constrictor. Thus propranolol had no effect on the rate of collateral formation in either swine (156) or dogs (31, 117), suggesting that other factors such as the pressure gradient between vascular beds, which determines the shear stress on the collateral vessel endothelium, may actually be more important than ischemia in EX-induced stimulation of growth of collateral vessels.
Resistance arteries isolated from the collateral-dependent region of hearts from EX pigs exhibit increased basal tone and increased vasodilator influences, increased NO production, and increased K+ channel activity (Fig. 3). Coronary resistance arteries isolated from the hearts of pigs with atherosclerotic CAD exhibit similar adaptations to EX (69). It seems reasonable to propose that these arteriolar adaptations to EX may provide a greater capacity of the coronary microcirculation to sustain CBF to myocardium at risk of ischemia. Indeed, Linke et al. (103) proposed that the effects of EX on endothelium and EDD may be greater in importance than any other known effect of EX on the coronary circulation.
Conclusions and Future Perspectives
In the normal heart, EX produces a variety of structural adaptations in the coronary circulation, including 1) increased conduit artery diameters, 2) increased arteriolar densities and diameters, and 3) maintained coronary capillary numerical density commensurate with the degree of cardiac hypertrophy. These changes likely underlie the increased CBF per gram of myocardium and increased diffusion capacity in EX hearts.
EX also induces functional adaptations in coronary circulation, including 1) an increased EDD (transient in conduit arteries, sustained in resistance vessels) that is principally NO mediated, 2) altered α-adrenergic influence (reduced in conduit vessels and increased in resistance vessels), and 3) a change in local control of resistance vessels. Coronary arterioles exhibit increased myogenic tone after EX.
In animal models of coronary artery obstruction and CAD patients, if exercise bouts produce or increase ischemia, there is evidence that collateral growth can be enhanced. In CAD patients and animal models of CAD, the evidence indicates that EX increases EDD in conduit arteries and in the arterial tree. However, the evidence that EX reverses or slows the progression of lesion development in CAD is not conclusive.
Notwithstanding these major advances in our understanding of coronary vascular adaptation to EX in health and CAD, several research questions remain outstanding. For example, the molecular mechanisms underlying the structural and functional adaptations to EX in the coronary vascular tree of the normal heart remain incompletely understood and await further elucidation. Also, the mechanism by which EX induces/enhances collateral formation in hearts with a coronary artery stenosis remains incompletely understood. Moreover, there seems to be no explanation for the lack of effect of EX on atherosclerosis progression/regression, despite the apparent beneficial effects of EX on endothelial function in animal models of CAD. Finally, we do not fully understand the mechanism underlying the clinical benefit of EX in patients with CAD. Answering these questions will be a major challenge in years to come.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-52490 and Netherlands Heart Foundation Grant 2000T038.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.H.L., D.K.B., and D.J.D. conception and design of research; M.H.L. and D.J.D. prepared figures; M.H.L. drafted manuscript; M.H.L., D.K.B., and D.J.D. edited and revised manuscript; M.H.L., D.K.B., and D.J.D. approved final version of manuscript; D.K.B. interpreted results of experiments; D.J.D. analyzed data.
REFERENCES
- 1. Anversa P, Levicky V, Beghi C, McDonald SL, Kikkawa Y. Morphometry of exercise-induced right ventricular hypertrophy in the rat. Circ Res 52: 57–64, 1983 [DOI] [PubMed] [Google Scholar]
- 2. Bache RJ. Effects of hypertrophy on the cornary circulation. Prog Cardiovasc Dis 30: 403–440, 1988 [DOI] [PubMed] [Google Scholar]
- 3. Bache RJ, Dai XZ, Schwartz JS, Homans DC. Role of adenosine in coronary vasodilation during exercise. Circ Res 62: 846–853, 1988 [DOI] [PubMed] [Google Scholar]
- 4. Barbier J, Reland S, Ville N, Rannou-Bekono F, Wong S, Carre F. The effects of exercise training on myocardial adrenergic and muscarinic receptors. Clin Auton Res 16: 61–65, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Barnard RJ, Duncan HW, Baldwin KM, Grimditch G, Buckberg DD. Effects of intensive exercise training on myocardial performance and coronary blood flow. J Appl Physiol 49: 444–449, 1980 [DOI] [PubMed] [Google Scholar]
- 6. Baur TS, Brodowicz GR, Lamb DR. Indomethacin suppresses the coronary flow response to hypoxia in exercise trained and sedentary rats. Cardiovasc Res 24: 733–736, 1990 [DOI] [PubMed] [Google Scholar]
- 7. Belardinelli R, Georgiou D, Ginzton L, Cianci G, Purcaro A. Effects of moderate exercise training on thallium uptake and contractile response to low-dose dobutamine of dysfunctional myocardium in patients with ischemic cardiomyopathy. Circulation 97: 553–561, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Belardinelli R, Paolini I, Cianci G, Piva R, Georgiou D, Purcaro A. Exercise training intervention after coronary angioplasty: the ETICA trial. J Am Coll Cardiol 37: 1891–1900, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Bell RD, Rasmussen RL. Exercise and the myocardial capillary-fiber ratio during growth. Growth 38: 237–244, 1974 [PubMed] [Google Scholar]
- 10. Blair SN, Church TS. The fitness, obesity, and health equation: is physical activity the common denominator? JAMA 292: 1232–1234, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Blomqvist CG, Saltin B. Cardiovascular adaptations to physical training. Annu Rev Physiol 45: 169–189, 1983 [DOI] [PubMed] [Google Scholar]
- 12. Bloor CM, Leon AS. Interaction of age and exercise on the heart and its blood supply. Lab Invest 22: 160–165, 1970 [PubMed] [Google Scholar]
- 13. Bloor CM, White FC, Sanders TM. Effects of exercise on collateral development in myocardial ischemia in pigs. J Appl Physiol 56: 656–665, 1984 [DOI] [PubMed] [Google Scholar]
- 14. Bolter CP, Hughson RL, Critz JB. Intrinsic rate and cholinergic sensitivity of isolated atria from trained and sedentary rats. Proc Soc Exp Biol Med 144: 364–367, 1973 [DOI] [PubMed] [Google Scholar]
- 15. Bove AA, Dewey JD. Proximal coronary vasomotor reactivity after exercise training in dogs. Circulation 71: 620–625, 1985 [DOI] [PubMed] [Google Scholar]
- 16. Bove AA, Hultgren PB, Ritzer TF, Carey RA. Myocardial blood flow and hemodynamic responses to exercise training in dogs. J Appl Physiol 46: 571–578, 1979 [DOI] [PubMed] [Google Scholar]
- 17. Bowles DK, Hu Q, Laughlin MH, Sturek M. Exercise training increases L-type calcium current density in coronary smooth muscle. Am J Physiol Heart Circ Physiol 275: H2159–H2169, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Bowles DK, Laughlin MH, Sturek M. Exercise training increases K+-channel contribution to regulation of coronary arterial tone. J Appl Physiol 84: 1225–1233, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Bowles DK, Wamhoff BR. Coronary smooth muscle adaptation to exercise: does it play a role in cardioprotection? Acta Physiol Scand 178: 117–121, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Breisch EA, White FC, Nimmo LE, McKirnan MD, Bloor CM. Exercise-induced cardiac hypertrophy: a correlation of blood flow and microvasculature. J Appl Physiol 60: 1259–1267, 1986 [DOI] [PubMed] [Google Scholar]
- 21. Brown MD. Exercise and coronary vascular remodelling in the healthy heart. Exp Physiol 88: 645–658, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Burt JJ, Jackson R. The effects of physical exercise on the coronary collateral circulation of dogs. J Sports Med Phys Fitness 5: 203–206, 1965 [PubMed] [Google Scholar]
- 23. Buttrick PM, Levite HA, Schaible TF, Ciambrone G, Scheuer J. Early increases in coronary vascular reserve in exercised rats are independent of cardiac hypertrophy. J Appl Physiol 59: 1861–1865, 1985 [DOI] [PubMed] [Google Scholar]
- 24. Buttrick PM, Scheuer J. Physiologic, biochemical, and coronary adaptation to exercise conditioning. Cardiol Clin 5: 259–270, 1987 [PubMed] [Google Scholar]
- 25. Carey RA, Santamore WP, Michele JJ, Bove AA. Effects of endurance training on coronary resistance in dogs. Med Sci Sports Exerc 15: 355–359, 1983 [PubMed] [Google Scholar]
- 26. Carlsson S, Ljungqvist A, Tornling G, Unge G. The myocardial capillary vasculature in repeated physical exercise. Acta Pathol Microbiol Scand A 86: 117–119, 1978 [DOI] [PubMed] [Google Scholar]
- 27. Casscells W, Naghavi M, Willerson JT. Vulnerable atherosclerotic plaque: a multifocal disease. Circulation 107: 2072–2075, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Cerretelli P, Di Prampero PE. Gas exchange in exercise. In: Handbook of Physiology. The Respiratory System, Gas Exchange. Bethesda, MD: Am. Physiol. Soc., 1987, sect. 3, vol. IV, chapt. 16, p. 297–340 [Google Scholar]
- 29. Clausen JP, Larsen OA, Trap-Jensen J. Physical training in the management of coronary artery disease. Circulation 40: 143–154, 1969 [DOI] [PubMed] [Google Scholar]
- 30. Cohen MV. Coronary vascular reserve in the greyhound with left ventricular hypertrophy. Cardiovasc Res 20: 182–194, 1986 [DOI] [PubMed] [Google Scholar]
- 31. Cohen MV. Lack of effect of propranolol on canine coronary collateral development during progressive coronary stenosis and occlusion. Cardiovasc Res 27: 249–254, 1993 [DOI] [PubMed] [Google Scholar]
- 32. Cohen MV. Training in dogs with normal coronary arteries: lack of effect on collateral development. Cardiovasc Res 24: 121–128, 1990 [DOI] [PubMed] [Google Scholar]
- 33. Cohen MV, Yipintsoi T, Malhotra A, Penpargkul S, Scheuer J. Effect of exercise on collateral development in dogs with normal coronary arteries. J Appl Physiol 45: 797–805, 1978 [DOI] [PubMed] [Google Scholar]
- 34. Currens JH, White PD. Half a century of running. Clinical, physiologic and autopsy findings in the case of Clarence DeMar (“Mr Marathon”). N Engl J Med 265: 988–993, 1961 [DOI] [PubMed] [Google Scholar]
- 35. Delp MD, McAllister RM, Laughlin MH. Exercise training alters endothelium-dependent vasoreactivity of rat abdominal aorta. J Appl Physiol 75: 1354–1363, 1993 [DOI] [PubMed] [Google Scholar]
- 36. DiCarlo SE, Blair RW, Bishop VS, Stone HL. Daily exercise enhances coronary resistance vessel sensitivity to pharmacological activation. J Appl Physiol 66: 421–428, 1989 [DOI] [PubMed] [Google Scholar]
- 37. DiCarlo SE, Blair RW, Bishop VS, Stone HL. Role of beta 2-adrenergic receptors on coronary resistance during exercise. J Appl Physiol 64: 2287–2293, 1988 [DOI] [PubMed] [Google Scholar]
- 38. Dodd-o JM, Gwirtz PA. Cardiac response to acute coronary artery occlusion in exercise-trained dogs. Med Sci Sports Exerc 24: 1245–1251, 1992 [PubMed] [Google Scholar]
- 39. Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev 88: 1009–1086, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Duncker DJ, Van Zon NS, Crampton M, Herrlinger S, Homans DC, Bache RJ. Coronary pressure-flow relationship and exercise: contributions of heart rate, contractility, and alpha 1-adrenergic tone. Am J Physiol Heart Circ Physiol 266: H795–H810, 1994 [DOI] [PubMed] [Google Scholar]
- 41. Eckstein RW. Effect of exercise and coronary artery narrowing on coronary collateral circulation. Circ Res 5: 230–235, 1957 [DOI] [PubMed] [Google Scholar]
- 42. Edwards JM, Neeb ZP, Alloosh MA, Long X, Bratz IN, Peller CR, Byrd JP, Kumar S, Obukhov AG, Sturek M. Exercise training decreases store-operated Ca2+ entry associated with metabolic syndrome and coronary atherosclerosis. Cardiovasc Res 85: 631–640, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ehsani AA, Hagberg JM, Hickson RC. Rapid changes in left ventricular dimensions and mass in response to physical conditioning and deconditioning. Am J Cardiol 42: 52–56, 1978 [DOI] [PubMed] [Google Scholar]
- 44. Favret F, Henderson KK, Clancy RL, Richalet JP, Gonzalez NC. Exercise training alters the effect of chronic hypoxia on myocardial adrenergic and muscarinic receptor number. J Appl Physiol 91: 1283–1288, 2001 [DOI] [PubMed] [Google Scholar]
- 45. Fleenor BS, Bowles DK. Exercise training decreases the size and alters the composition of the neointima in a porcine model of percutaneous transluminal coronary angioplasty (PTCA). J Appl Physiol 107: 937–945, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fogarty JA, Muller-Delp JM, Delp MD, Mattox ML, Laughlin MH, Parker JL. Exercise training enhances vasodilation responses to vascular endothelial growth factor in porcine coronary arterioles exposed to chronic coronary occlusion. Circulation 109: 664–670, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Frank A. Experimentelle herzhypertrophie. Z Ges Exp Med 115, 1950 [Google Scholar]
- 48. Franklin BA. Exercise training and coronary collateral circulation. Med Sci Sports Exerc 23: 648–653, 1991 [PubMed] [Google Scholar]
- 49. Gielen S, Schuler G, Adams V. Cardiovascular effects of exercise training: molecular mechanisms. Circulation 122: 1221–1238, 2010 [DOI] [PubMed] [Google Scholar]
- 50. Gielen S, Schuler G, Hambrecht R. Exercise training in coronary artery disease and coronary vasomotion. Circulation 103: e1–e6, 2001 [DOI] [PubMed] [Google Scholar]
- 51. Griffin KL, Laughlin MH, Parker JL. Exercise training improves endothelium-mediated vasorelaxation after chronic coronary occlusion. J Appl Physiol 87: 1948–1956, 1999 [DOI] [PubMed] [Google Scholar]
- 52. Griffin KL, Woodman CR, Price EM, Laughlin MH, Parker JL. Endothelium-mediated relaxation of porcine collateral-dependent arterioles is improved by exercise training. Circulation 104: 1393–1398, 2001 [DOI] [PubMed] [Google Scholar]
- 53. Gwirtz PA, Stone HL. Coronary vascular response to adrenergic stimulation in exercise-conditioned dogs. J Appl Physiol 57: 315–320, 1984 [DOI] [PubMed] [Google Scholar]
- 54. Hagg U, Wandt B, Bergstrom G, Volkmann R, Gan Lm. Physical exercise capacity is associated with coronary and peripheral vascular function in healthy young adults. Am J Physiol Heart Circ Physiol 289: H1627–H1634, 2005 [DOI] [PubMed] [Google Scholar]
- 55. Hakkila J. Studies on the myocardial capillary concentration in cardiac hypertrophy due to training; an experimental study with guinea pigs. Ann Med Exp Biol Fenn 33: 1–82, 1955 [PubMed] [Google Scholar]
- 56. Hambrecht R, Niebauer J, Marburger C, Grunze M, Kalberer B, Hauer K, Schlierf G, Kubler W, Schuler G. Various intensities of leisure time physical activity in patients with coronary artery disease: effects on cardiorespiratory fitness and progression of coronary atherosclerotic lesions. J Am Coll Cardiol 22: 468–477, 1993 [DOI] [PubMed] [Google Scholar]
- 57. Hambrecht R, Wolf A, Gielen S, Linke A, Hofer J, Erbs S, Schoene N, Schuler G. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med 342: 454–460, 2000 [DOI] [PubMed] [Google Scholar]
- 58. Hammond HK, White FC, Brunton LL, Longhurst JC. Association of decreased myocardial beta-receptors and chronotropic response to isoproterenol and exercise in pigs following chronic dynamic exercise. Circ Res 60: 720–726, 1987 [DOI] [PubMed] [Google Scholar]
- 59. Hannukainen JC, Janatuinen T, Toikka JO, Jarvisalo MJ, Heinonen OJ, Kapanen J, Nagren K, Nuutila P, Kujala UM, Kaprio J, Knuuti J, Kalliokoski KK. Myocardial and peripheral vascular functional adapation to exercise training. Scand J Med Sci Sports 17: 139–147, 2007 [DOI] [PubMed] [Google Scholar]
- 60. Harri MN. Physical training under the influence of beta-blockade in rats. II. Effects on vascular reactivity. Eur J Appl Physiol Occup Physiol 42: 151–157, 1979 [DOI] [PubMed] [Google Scholar]
- 61. Haskell W, Alderman E, Fair J, Maron D, Mackey S, Superko H, Williams P, Johnstone I, Champagne M, Krauss R. Effects of intensive multiple risk factor reduction on coronary atherosclerosis and clinical cardiac events in men and women with coronary artery disease. The Stanford Coronary Risk Intervention Project (SCRIP). Circulation 89: 975–990, 1994 [DOI] [PubMed] [Google Scholar]
- 62. Haskell WL, Sims C, Myll J, Bortz WM, Goar FG, Alderman EL. Coronary artery size and dilating capacity in ultradistance runners. Circulation 87: 1076–1082, 1993 [DOI] [PubMed] [Google Scholar]
- 63. Heaps C, Bowles DK, Sturek M, Laughlin MH, Parker JL. Enhanced L-type Ca2+ channel current density in coronary smooth muscle of exercise-trained pigs is compensated to limit myoplasmic free Ca2+ accumulation. J Physiol 528: 435–445, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Heaps CL, Mattox ML, Kelly KA, Meininger CJ, Parker JL. Exercise training increases basal tone in arterioles distal to chronic coronary occlusion. Am J Physiol Heart Circ Physiol 290: H1128–H1135, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Heaps CL, Sturek M, Rapps JA, Laughlin MH, Parker JL. Exercise training restores adenosine-induced relaxation in coronary arteries distal to chronic occlusion. Am J Physiol Heart Circ Physiol 278: H1984–H1992, 2000 [DOI] [PubMed] [Google Scholar]
- 66. Heaton WH, Marr KC, Capurro NL, Goldstein RE, Epstein SE. Beneficial effect of physical training on blood flow to myocardium perfused by chronic collaterals in the exercising dog. Circulation 27: 575–581, 1978 [DOI] [PubMed] [Google Scholar]
- 67. Heinonen I, Nesterov SV, Liukko K, Kemppainen J, Någren K, Luotolahti M, Virsu P, Oikonen V, Nuutila P, Kujala UM, Kainulainen H, Boushel R, Knuuti J, Kalliokoski KK. Myocardial blood flow and adenosine A2A receptor density in endurance athletes and untrained men. J Physiol 586: 5193–5202, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Heiss HW, Barmeyer J, Wink J, Hell G, Cerny FJ, Keul J, Reindell H. Studies on the regulation of myocardial blood flow in man. I. Training effects on blood flow and metabolism of the healthy heart at rest and during standardized heavy exercise. Basic Res Cardiol 71: 658–675, 1976 [DOI] [PubMed] [Google Scholar]
- 69. Henderson KK, Turk JR, Rush JW, Laughlin MH. Endothelial function in coronary arterioles from pigs with early-stage coronary disease induced by high-fat, high-cholesterol diet: effect of exercise. J Appl Physiol 97: 1159–1168, 2004 [DOI] [PubMed] [Google Scholar]
- 70. Heusch G. Adenosine and maximum coronary vasodilation in humans: myth and misconceptions in the assessment of coronary reserve. Basic Res Cardiol 105: 1–5, 2010 [DOI] [PubMed] [Google Scholar]
- 71. Hildick-Smith DJ, Johnson PJ, Wisbey CR, Winter EM, Shapiro LM. Coronary flow reserve is supranormal in endurance athletes: an adenosine transthoracic echocardiographic study. Heart 84: 383–389, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ishibashi Y, Duncker DJ, Zhang J, Bache RJ. ATP-sensitive K+ channels, adenosine, and nitric oxide-mediated mechanisms account for coronary vasodilation during exercise. Circ Res 82: 346–359, 1998 [DOI] [PubMed] [Google Scholar]
- 73. Jacobs TB, Bell RD, McClements JD. Exercise, age and the development of the myocardial vasculature. Growth 48: 148–157, 1984 [PubMed] [Google Scholar]
- 74. Johnson LR, Rush JW, Turk JR, Price EM, Laughlin MH. Short-term exercise training increases ACh-induced relaxation and eNOS protein in porcine pulmonary arteries. J Appl Physiol 90: 1102–1110, 2001 [DOI] [PubMed] [Google Scholar]
- 75. Kadoglou NP, Kostomitsopoulos N, Kapelouzou A, Moustardas P, Katsimpoulas M, Giagini A, Dede E, Boudoulas H, Konstantinides S, Karayannacos PE, Liapis CD. Effects of exercise training on the severity and composition of atherosclerotic plaque in apoE-deficient mice. J Vasc Res 48: 347–356, 2011 [DOI] [PubMed] [Google Scholar]
- 76. Kjaer A, Meyer C, Wachtell K, Olsen MH, Ibsen H, Opie L, Holm S, Hesse B. Positron emission tomographic evaluation of regulation of myocardial perfusion in physiological (elite athletes) and pathological (systemic hypertension) left ventricular hypertrophy. Am J Cardiol 96: 1692–1698, 2005 [DOI] [PubMed] [Google Scholar]
- 77. Knight DR, Stone HL. Alteration of ischemic cardiac function in normal heart by daily exercise. J Appl Physiol 55: 52–60, 1983 [DOI] [PubMed] [Google Scholar]
- 78. Koerner JE, Terjung RL. Effect of physical training on coronary collateral circulation of the rat. J Appl Physiol 52: 376–387, 1982 [DOI] [PubMed] [Google Scholar]
- 79. Korzick DH, Laughlin MH, Bowles DK. Alterations in PKC signaling underlie enhanced myogenic tone in exercise-trained porcine coronary resistance arteries. J Appl Physiol 96: 1425–1432, 2004 [DOI] [PubMed] [Google Scholar]
- 80. Kozakova M, Galetta F, Gregorini L, Bigalli G, Franzoni F, Giusti C, Palombo C. Coronary vasodilator capacity and epicardial vessel remodeling in physiological and hypertensive hypertrophy. Hypertension 36: 343–349, 2000 [DOI] [PubMed] [Google Scholar]
- 81. Kozakova M, Paterni M, Bartolomucci F, Morizzo C, Rossi G, Galetta F, Palombo C. Epicardial coronary artery size in hypertensive and physiologic left ventricular hypertrophy. Am J Hypertens 20: 279–284, 2007 [DOI] [PubMed] [Google Scholar]
- 82. Kramsch DM, Aspen AJ, Abramowitz BM, Kreimendahl T, Hood WB., Jr Reduction of coronary atherosclerosis by moderate conditioning exercise in monkeys on an atherogenic diet. N Engl J Med 305: 1483–1489, 1981 [DOI] [PubMed] [Google Scholar]
- 83. Lakka TA, Venalainen JM, Rauramaa R, Salonen R, Tuomilehto J, JT Relation of leisure-time physical activity and cardiorespiratory fitness to the risk of acute myocardial infarction. N Engl J Med 330: 1549–1554, 1994 [DOI] [PubMed] [Google Scholar]
- 84. Laughlin MH. Coronary transport reserve in normal dogs. J Appl Physiol 57: 551–561, 1984 [DOI] [PubMed] [Google Scholar]
- 85. Laughlin MH. Effects of exercise training on coronary transport capacity. J Appl Physiol 58: 468–476, 1985 [DOI] [PubMed] [Google Scholar]
- 86. Laughlin MH. Endothelium-mediated control of coronary vascular tone after chronic exercise training. Med Sci Sports Exerc 27: 1135–1144, 1995 [PubMed] [Google Scholar]
- 87. Laughlin MH. Joseph B. Wolfe Memorial lecture. Physical activity in prevention and treatment of coronary disease: the battle line is in exercise vascular cell biology. Med Sci Sports Exerc 36: 352–362, 2004 [DOI] [PubMed] [Google Scholar]
- 88. Laughlin MH, Diana JN. Myocardial transcapillary exchange in the hypertrophied heart of the dog. Am J Physiol 229: 838–846, 1975 [DOI] [PubMed] [Google Scholar]
- 89. Laughlin MH, Diana JN, Tipton CM. Effects of exercise training on coronary reactive hyperemia and blood flow in the dog. J Appl Physiol 45: 604–610, 1978 [DOI] [PubMed] [Google Scholar]
- 90. Laughlin MH, Korthuis RJ, Duncker DJ, Bache RJ. Control of blood flow to cardiac and skeletal muscle during exercise. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD: Am. Physiol. Soc., 1996, sect. 12, chapt. 16, p. 705–769 [Google Scholar]
- 91. Laughlin MH, Muller JM. Vasoconstrictor responses of coronary resistance arteries in exercise-trained pigs. J Appl Physiol 84: 884–889, 1998 [DOI] [PubMed] [Google Scholar]
- 92. Laughlin MH, Overholser KA, Bhatte MJ. Exercise training increases coronary transport reserve in miniature swine. J Appl Physiol 67: 1140–1149, 1989 [DOI] [PubMed] [Google Scholar]
- 93. Laughlin MH, Pollock JS, Amann JF, Hollis ML, Woodman CR, Price EM. Training induces nonuniform increases in eNOS content along the coronary arterial tree. J Appl Physiol 90: 501–510, 2001 [DOI] [PubMed] [Google Scholar]
- 94. Laughlin MH, Tomanek RJ. Myocardial capillarity and maximal capillary diffusion capacity in exercise-trained dogs. J Appl Physiol 63: 1481–1486, 1987 [DOI] [PubMed] [Google Scholar]
- 95. Laughlin MH, Turk JR, Schrage WG, Woodman CR, Price EM. Influence of coronary artery diameter on eNOS protein content. Am J Physiol Heart Circ Physiol 284: H1307–H1312, 2003 [DOI] [PubMed] [Google Scholar]
- 96. Laughlin MH, Welshons WV, Sturek M, Rush JW, Turk JR, Taylor JA, Judy BM, Henderson KK, Ganjam VK. Gender, exercise training, and eNOS expression in porcine skeletal muscle arteries. J Appl Physiol 95: 250–264, 2003 [DOI] [PubMed] [Google Scholar]
- 97. LeBlanc J, Boulay M, Dulac S, Jobin M, Labrie A, Rousseau-Migneron S. Metabolic and cardiovascular responses to norepinephrine in trained and nontrained human subjects. J Appl Physiol 42: 166–173, 1977 [DOI] [PubMed] [Google Scholar]
- 98. Leon AS, Bloor CM. The effect of complete and partial deconditioning on exercise-induced cardiovascular changes in the rat. Adv Cardiol 18: 81–92, 1976 [DOI] [PubMed] [Google Scholar]
- 99. Leon AS, Bloor CM. Effects of exercise and its cessation on the heart and its blood supply. J Appl Physiol 24: 485–490, 1968 [DOI] [PubMed] [Google Scholar]
- 100. Liang IY, Hamra M, Stone HL. Maximum coronary blood flow and minimum coronary resistance in exercise-trained dogs. J Appl Physiol 56: 641–647, 1984 [DOI] [PubMed] [Google Scholar]
- 101. Liang IY, Stone HL. Changes in diastolic coronary resistance during submaximal exercise in conditioned dogs. J Appl Physiol 54: 1057–1062, 1983 [DOI] [PubMed] [Google Scholar]
- 102. Liang IY, Stone HL, Gwirtz PA. Effect of beta 1-receptor blockade on coronary resistance in partially trained dogs. Med Sci Sports Exerc 19: 382–388, 1987 [PubMed] [Google Scholar]
- 103. Liang IY, Stone HL. Effect of exercise conditioning on coronary resistance. J Appl Physiol 53: 631–636, 1982 [DOI] [PubMed] [Google Scholar]
- 104. Link RP, Pedersoli WM, Safanie AH. Effect of exercise on development of atherosclerosis in swine. Atherosclerosis 15: 107–122, 1972 [DOI] [PubMed] [Google Scholar]
- 105. Linke A, Adams V, Schulze PC, Erbs S, Gielen S, Fiehn E, Mobius-Winkler S, Schubert A, Schuler G, Hambrecht R. Antioxidative effects of exercise training in patients with chronic heart failure: increase in radical scavenger enzyme activity in skeletal muscle. Circulation 111: 1763–1770, 2005 [DOI] [PubMed] [Google Scholar]
- 106. Linke A, Erbs S, Hambrecht R. Exercise and the coronary circulation—alterations and adaptations in coronary artery disease. Prog Cardiovasc Dis 48: 270–284, 2006 [DOI] [PubMed] [Google Scholar]
- 107. Ljungqvist A, Unge G. Capillary proliferative activity in myocardium and skeletal muscle of exercised rats. J Appl Physiol 43: 306–307, 1977 [DOI] [PubMed] [Google Scholar]
- 108. Mann SJ, Krakoff LR, Felton K, Yeager K. Cardiovascular responses to infused epinephrine: effect of the state of physical conditioning. J Cardiovasc Pharmacol 6: 339–343, 1984 [DOI] [PubMed] [Google Scholar]
- 109. Maron BJ. Structural features of the athlete heart as defined by echocardiography. J Am Coll Cardiol 7: 190–203, 1986 [DOI] [PubMed] [Google Scholar]
- 110. Martin WH, 3rd, Spina RJ, Korte E, Ogawa T. Effects of chronic and acute exercise on cardiovascular beta-adrenergic responses. J Appl Physiol 71: 1523–1528, 1991 [DOI] [PubMed] [Google Scholar]
- 111. McAllister RM, Laughlin MH. Short-term exercise training alters responses of porcine femoral and brachial arteries. J Appl Physiol 82: 1438–1444, 1997 [DOI] [PubMed] [Google Scholar]
- 112. McElroy CL, Gissen SA, Fishbein MC. Exercise-induced reduction in myocardial infarct size after coronary artery occlusion in the rat. Circulation 57: 958–962, 1978 [DOI] [PubMed] [Google Scholar]
- 113. Mohlenkamp S, Bose D, Mahabadi AA, Heusch G, Erbel R. On the paradox of exercise: coronary atherosclerosis in an apparently healthy marathon runner. Nat Clin Pract Cardiovasc Med 4: 396–401, 2007 [DOI] [PubMed] [Google Scholar]
- 114. Muller JM, Myers PR, Laughlin MH. Exercise training alters myogenic responses in porcine coronary resistance arteries. J Appl Physiol 75: 2677–2682, 1993 [DOI] [PubMed] [Google Scholar]
- 115. Muller JM, Myers PR, Laughlin MH. Vasodilator responses of coronary resistance arteries of exercise- trained pigs. Circulation 89: 2308–2314, 1994 [DOI] [PubMed] [Google Scholar]
- 116. Napoli C, Williams-Ignarro S, de Nigris F, Lerman LO, D'Armiento FP, Crimi E, Byrns RE, Casamassimi A, Lanza A, Gombos F, Sica V, Ignarro LJ. Physical training and metabolic supplementation reduce spontaneous atherosclerotic plaque rupture and prolong survival in hypercholesterolemic mice. Proc Natl Acad Sci USA 103: 10479–10484, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Neill WA, Nakornchai V, Oxendine J, Paul MS. Effect of beta-adrenergic suppression by propranolol on coronary collateral development in response to chronic coronary ischemia in dogs. Circulation 59: 280–285, 1979 [DOI] [PubMed] [Google Scholar]
- 118. Neill WA, Oxendine JM. Exercise can promote coronary collateral development without improving perfusion of ischemic myocardium. Circulation 60: 1513–1519, 1979 [DOI] [PubMed] [Google Scholar]
- 119. Niebaurer J, Hambrecht R, Marburger C, Hauer K, Velich T, von Hodenberg E, Schlierf G, Kubler W, Schuler G. Impact of intensive physical exercise and low-fat diet on collateral vessel formation in stable angina pectoris and angiographically confirmed coronary artery disease. Am J Cardiol 76: 771–775, 1995 [DOI] [PubMed] [Google Scholar]
- 120. Nutter DO, Fuller EO. The role of isolated cardiac muscle preparations in the study of training effects on the heart. Med Sci Sports 9: 239–245, 1977 [PubMed] [Google Scholar]
- 121. Okabe T, Kishimoto C, Murayama T, Yokode M, Kita T. Effects of exercise on the development of atherosclerosis in apolipoprotein E-deficient mice. Exp Clin Cardiol 11, 276–279, 2006 [PMC free article] [PubMed] [Google Scholar]
- 122. Okabe TA, Shimada K, Hattori M, Murayama T, Yokode M, Kita T, Kishimoto C. Swimming reduces the severity of atherosclerosis in apolipoprotein E deficient mice by antioxidant effects. Cardiovasc Res 74: 537–545, 2007 [DOI] [PubMed] [Google Scholar]
- 123. Oltman CL, Parker JL, Adams HR, Laughlin MH. Effects of exercise training on vasomotor reactivity of porcine coronary arteries. Am J Physiol Heart Circ Physiol 263: H372–H382, 1992 [DOI] [PubMed] [Google Scholar]
- 124. Oltman CL, Parker JL, Laughlin MH. Endothelium-dependent vasodilation of proximal coronary arteries from exercise-trained pigs. J Appl Physiol 79: 33–40, 1995 [DOI] [PubMed] [Google Scholar]
- 125. Ornish D, Brown SE, Scherwitz LW, Billings JH, Armstrong WT, Ports TA, McLanahan SM, Kirkeeide RL, Brand RJ, Gould KL. Can lifestyle changes reverse coronary heart disease? The Lifestyle Heart Trial. Lancet 336: 129–133, 1990 [DOI] [PubMed] [Google Scholar]
- 126. Overholser KA, Bhatte MJ, Laughlin MH. Modeling the effect of flow heterogeneity on coronary permeability-surface area. J Appl Physiol 71: 758–769, 1991 [DOI] [PubMed] [Google Scholar]
- 127. Parker JL, Mattox ML, Laughlin MH. Contractile responsiveness of coronary arteries from exercise-trained rats. J Appl Physiol 83: 434–443, 1997 [DOI] [PubMed] [Google Scholar]
- 128. Pelliccia A, Spataro A, Granata M, Biffi A, Caselli G, Alabiso A. Coronary arteries in physiological hypertrophy: echocardiographic evidence of increased proximal size in elite athletes. Int J Sports Med 11: 120–126, 1990 [DOI] [PubMed] [Google Scholar]
- 129. Penpargkul S, Scheuer J. The effect of physical training upon the mechanical and metabolic performance of the rat heart. J Clin Invest 49: 1859–1868, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Petren T, Sjöstrand T, Sylven B. Der influss des trainings auf die haufigkeit der capillaren in herz- und skeltenmuskulatur. Arbeitsphysiologie 9: 376–386, 1930 [Google Scholar]
- 131. Petren T, Sylven B. Weitere untersuchungen uber den Einfluss des Trainings auf die Kapillarisierung der Herzmuskulator. Morphol Jahrb 80: 439–444, 1937 [Google Scholar]
- 132. Radvan J, Choudhury L, Sheridan DJ, Camici PG. Comparison of coronary vasodilator reserve in elite rowing athletes versus hypertrophic cardiomyopathy. Am J Cardiol 80: 1621–1623, 1997 [DOI] [PubMed] [Google Scholar]
- 133. Raven PB, Rohm-Young D, Blomqvist CG. Physical fitness and cardiovascular response to lower body negative pressure. J Appl Physiol 56: 138–144, 1984 [DOI] [PubMed] [Google Scholar]
- 134. Ribeiro F, Alves AJ, Duarte JA, Oliveira J. Is exercise training an effective therapy targeting endothelial dysfunction and vascular wall inflammation? Int J Cardiol 141: 214–221, 2010 [DOI] [PubMed] [Google Scholar]
- 135. Robles JC, Sturek M, Parker JL, Heaps CL. Ca2+ sensitization and PKC contribute to exercise training-enhanced contractility in porcine collateral-dependent coronary arteries. Am J Physiol Heart Circ Physiol 300: H1201–H1209, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Rogers PJ, Miller TD, Bauer BA, Brum JM, Bove AA, Vanhoutte PM. Exercise training and responsiveness of isolated coronary arteries. J Appl Physiol 71: 2346–2351, 1991 [DOI] [PubMed] [Google Scholar]
- 137. Roth DA, White CD, Podolin DA, Mazzeo RS. Alterations in myocardial signal transduction due to aging and chronic dynamic exercise. J Appl Physiol 84: 177–184, 1998 [DOI] [PubMed] [Google Scholar]
- 138. Roth DM, White FC, Nichols ML, Dobbs SL, Longhurst JC, Bloor CM. Effect of long-term exercise on regional myocardial function and coronary collateral development after gradual coronary artery occlusion in pigs. Circulation 82: 1778–1789, 1990 [DOI] [PubMed] [Google Scholar]
- 139. Rush JW, Laughlin MH, Woodman CR, Price EM. SOD-1 expression in pig coronary arterioles is increased by exercise training. Am J Physiol Heart Circ Physiol 279: H2068–H2076, 2000 [DOI] [PubMed] [Google Scholar]
- 140. Rush JW, Turk JR, Laughlin MH. Exercise training regulates SOD-1 and oxidative stress in porcine aortic endothelium. Am J Physiol Heart Circ Physiol 284: H1378–H1387, 2003 [DOI] [PubMed] [Google Scholar]
- 141. Sable DL, Brammell HL, Sheehan MW, Nies AS, Gerber J, Horwitz LD. Attenuation of exercise conditioning by beta-adrenergic blockade. Circulation 65: 679–684, 1982 [DOI] [PubMed] [Google Scholar]
- 142. Sanders M, White FC, Peterson TM, Bloor CM. Effects of endurance exercise on coronary collateral blood flow in miniature swine. Am J Physiol Heart Circ Physiol 234: H614–H619, 1978 [DOI] [PubMed] [Google Scholar]
- 143. Scheel KW, Ingram LA, Wilson JL. Effects of exercise on the coronary and collateral vasculature of beagles with and without coronary occlusion. Circ Res 48: 523–530, 1981 [DOI] [PubMed] [Google Scholar]
- 144. Scheffer MG, Verdouw PD. Decreased incidence of ventricular fibrillation after an acute coronary artery ligation in exercised pigs. Basic Res Cardiol 78: 298–309, 1983 [DOI] [PubMed] [Google Scholar]
- 145. Scheuer J, Stezoski SW. Effect of physical training on the mechanical and metabolic response of the rat heart to hypoxia. Circ Res 30: 418–429, 1972 [DOI] [PubMed] [Google Scholar]
- 146. Scheuer J, Tipton CM. Cardiovascular adaptations to physical training. Annu Rev Physiol 39: 221–251, 1977 [DOI] [PubMed] [Google Scholar]
- 147. Schuler G, Hambrecht R, Schlierf G, Neibauer J, Hauer K, Neumann J, Hoberg E, Drinkmann A, Bacher F, Grunze M. Regular physical exercise and low-fat diet. Effects on progression of coronary artery disease. Circulation 86: 1–11, 1992 [DOI] [PubMed] [Google Scholar]
- 148. Schwartz SM. Smooth muscle migration in atherosclerosis and restenosis. J Clin Invest 100: S87–S89, 1997 [PubMed] [Google Scholar]
- 149. Schwartz SM, deBlois D, O'Brien ER. The intima: soil for atherosclerosis and restenosis. Circ Res 77: 445–465, 1995 [DOI] [PubMed] [Google Scholar]
- 150. Shimada K, Kishimoto C, Okabe Ta Hattori M, Murayama T, Yokode M, Kita T. Exercise training reduces severity of atherosclerosis in apolipoprotein e knockout mice via nitric oxide. Circ J 71: 1147–1151, 2007 [DOI] [PubMed] [Google Scholar]
- 151. Spear KL, Koerner JE, Terjung RL. Coronary blood flow in physically trained rats. Cardiovasc Res 12: 135–143, 1978 [DOI] [PubMed] [Google Scholar]
- 152. Stehno-Bittel L, Laughlin MH, Sturek M. Exercise training alters Ca release from coronary smooth muscle sarcoplasmic reticulum. Am J Physiol Heart Circ Physiol 259: H643–H647, 1990 [DOI] [PubMed] [Google Scholar]
- 153. Stehno-Bittel L, Laughlin MH, Sturek M. Exercise training depletes sarcoplasmic reticulum calcium in coronary smooth muscle. J Appl Physiol 71: 1764–1773, 1991 [DOI] [PubMed] [Google Scholar]
- 154. Stevenson JA, Feleki V, Rechnitzer P, Beaton JR. Effect of exercise on coronary tree size in the rat. Circ Res 15: 265–269, 1964 [DOI] [PubMed] [Google Scholar]
- 155. Stone HL. Coronary flow, myocardial oxygen consumption, and exercise training in dogs. J Appl Physiol 49: 759–768, 1980 [DOI] [PubMed] [Google Scholar]
- 156. Symons JD, Pitsillides KF, Longhurst JC. Chronic reduction of myocardial ischemia does not attenuate coronary collateral development in miniswine. Circulation 86: 660–671, 1992 [DOI] [PubMed] [Google Scholar]
- 157. Tepperman J, Pearlman D. Effects of exercise and anemia on coronary arteries of small animals as revealed by the corrosion-cast technique. Circ Res 9: 576–584, 1961 [DOI] [PubMed] [Google Scholar]
- 158. Thomas DP. Effects of acute and chronic exercise on myocardial ultrastructure. Med Sci Sports Exerc 17: 546–553, 1985 [PubMed] [Google Scholar]
- 159. Thompson MA, Henderson KK, Woodman CR, Turk JR, Rush JW, Price E, Laughlin MH. Exercise preserves endothelium-dependent relaxation in coronary arteries of hypercholesterolemic male pigs. J Appl Physiol 96: 1114–1126, 2004 [DOI] [PubMed] [Google Scholar]
- 160. Thompson PD. Exercise for patients with coronary artery and/or coronary heart disease. In: Exercise and Sports Cardiology, edited by Thompson PD. New York: McGraw-Hill, 2001, chapt. 16, p. 354–370 [Google Scholar]
- 161. Thompson PD. Exercise prescription and proscription for patients with coronary artery disease. Circulation 112: 2354–2363, 2005 [DOI] [PubMed] [Google Scholar]
- 162. Thompson PD, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH, Berra K, Blair SN, Costa F, Franklin B, Fletcher GF, Gordon NF, Pate RR, Rodriguez BL, Yancey AK, Wenger NK. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the council on clinical cardiology (subcommittee on exercise, rehabilitation, and prevention) and the council on nutrition, physical activity, and metabolism (subcommittee on physical activity). Arterioscler Thromb Vasc Biol 23: E42–E49, 2003 [Google Scholar]
- 163. Thörner W. Trainingsversuche an Hunden. III. Histologische Beobachtungen und Herz- und Skeletmuskeln. Arbeitphysiologie 8: 359–370, 1935 [Google Scholar]
- 164. Tomanek RJ. Effects of age and exercise on the extent of the myocardial capillary bed. Anat Rec 167: 55–62, 1970 [DOI] [PubMed] [Google Scholar]
- 165. Tune JD, Richmond KN, Gorman MW, Olsson RA, Feigl EO. Adenosine is not responsible for local metabolic control of coronary blood flow in dogs during exercise. Am J Physiol Heart Circ Physiol 278: H74–H84, 2000 [DOI] [PubMed] [Google Scholar]
- 166. Turk JR, Laughlin MH. Physical activity and atherosclerosis: which animal model?. Can J Appl Physiol 29: 657–683, 2004 [DOI] [PubMed] [Google Scholar]
- 167. Unge G, Carlsson S, Ljungqvist A, Tornling G, Adolfsson J. The proliferative activity of myocardial capillary wall cells in variously aged swimming-exercised rats. Acta Pathol Microbiol Scand A 87: 15–17, 1979 [DOI] [PubMed] [Google Scholar]
- 168. von Restorff W, Holtz J, Bassenge E. Exercise induced augmentation of myocardial oxygen extraction in spite of normal coronary dilatory capacity in dogs. Pflügers Arch 372: 181–185, 1977 [DOI] [PubMed] [Google Scholar]
- 169. Wachtlova M, Rakusan K, Poupa O. The coronary terminal vascular bed in the heart of the hare (Lepus europeus) and the rabbit (Oryctolagus domesticus). Physiol Bohemoslov 14: 328–331, 1965 [PubMed] [Google Scholar]
- 170. Wachtlova M, Rakusan K, Roth Z, Poupa O. The terminal vascular bed of the myocardium in the wild rat (Rattus norvegicus) and the laboratory rat (Rattus norvegicus lab.). Physiol Bohemoslov 16: 548–554, 1967 [PubMed] [Google Scholar]
- 171. Wamhoff BR, Bowles DK, Dietz NJ, Hu Q, Sturek M. Exercise training attenuates coronary smooth muscle phenotypic modulation and nuclear Ca2+ signaling. Am J Physiol Heart Circ Physiol 283: H2397–H2410, 2002 [DOI] [PubMed] [Google Scholar]
- 172. Wang J, Wolin MS, Hintze TH. Chronic exercise enhances endothelium-mediated dilation of epicardial coronary artery in conscious dogs. Circ Res 73: 829–838, 1993 [DOI] [PubMed] [Google Scholar]
- 173. White FC, Bloor CM, McKirnan MD, Carroll SM. Exercise training in swine promotes growth of arteriolar bed and capillary angiogenesis in heart. J Appl Physiol 85: 1160–1168, 1998 [DOI] [PubMed] [Google Scholar]
- 174. White FC, McKirnan MD, Breisch EA, Guth BD, Liu YM, Bloor CM. Adaptation of the left ventricle to exercise-induced hypertrophy. J Appl Physiol 62: 1097–1110, 1987 [DOI] [PubMed] [Google Scholar]
- 175. Williams JK, Kaplan JR, Suparto IH, Fox JL, Manuck SB. Effects of exercise on cardiovascular outcomes in monkeys with risk factors for coronary heart disease. Arterioscler Thromb Vasc Biol 23: 864–871, 2003 [DOI] [PubMed] [Google Scholar]
- 176. Williams RS. Role of receptor mechanisms in the adaptive response to habitual exercise. Am J Cardiol 55: 68D–73D, 1985 [DOI] [PubMed] [Google Scholar]
- 177. Windecker S, Allemann Y, Billinger M, Pohl T, Hutter D, Orsucci T, Blaga L, Meier B, Seiler C. Effect of endurance training on coronary artery size and function in healthy men: an invasive followup study. Am J Physiol Heart Circ Physiol 282: H2216–H2223, 2002 [DOI] [PubMed] [Google Scholar]
- 178. Woodman CR, Muller JM, Laughlin MH, Price EM. Induction of nitric oxide synthase mRNA in coronary resistance arteries isolated from exercise-trained pigs. Am J Physiol Heart Circ Physiol 273: H2575–H2579, 1997 [DOI] [PubMed] [Google Scholar]
- 179. Woodman CR, Turk JR, Rush JW, Laughlin MH. Exercise attenuates the effects of hypercholesterolemia on endothelium-dependent relaxation in female porcine coronary arteries. J Appl Physiol 96: 1105–1113, 2004 [DOI] [PubMed] [Google Scholar]
- 180. Wyatt HL, Mitchell JH. Influences of physical conditioning and deconditioning on coronary vasculature of dogs. J Appl Physiol 45: 619–625, 1978 [DOI] [PubMed] [Google Scholar]
- 181. Wyatt HL, Mitchell JH. Influences of physical training on the heart of dogs. Circ Res 35: 883–889, 1974 [DOI] [PubMed] [Google Scholar]
- 182. Yang AL, Jen CJ, Chen HI. Effects of high-cholesterol diet and parallel exercise training on the vascular function of rabbit aortas: a time course study. J Appl Physiol 95: 1194–1200, 2003 [DOI] [PubMed] [Google Scholar]
- 183. Yipintsoi T, Rosenkrantz J, Codini MA, Scheuer J. Myocardial blood flow responses to acute hypoxia and volume loading in physically trained rats. Cardiovasc Res 14: 50–57, 1980 [DOI] [PubMed] [Google Scholar]
- 184. Young CG, Knight CA, Vickers KC, Westbrook D, Madamanchi NR, Runge MS, Ischiropoulos H, Ballinger SW. Differential effects of exercise on aortic mitochondria. Am J Physiol Heart Circ Physiol 288: H1683–H1689, 2005 [DOI] [PubMed] [Google Scholar]
- 185. Zandrino F, Molinari G, Smeraldi A, Odaglia G, Masperone MA, Sardanelli F. Magnetic resonance imaging of athlete's heart: myocardial mass, left ventricular function, and cross-sectional area of the coronary arteries. Eur Radiol 10: 319–325, 2000 [DOI] [PubMed] [Google Scholar]
- 186. Zanesco A, Antunes E. Effects of exercise training on the cardiovascular system: Pharmacological approaches. Pharmacol Ther 114: 307–317, 2007 [DOI] [PubMed] [Google Scholar]