Abstract
Effective arterial elastance(EA) is a measure of the net arterial load imposed on the heart that integrates the effects of heart rate(HR), peripheral vascular resistance(PVR), and total arterial compliance(TAC) and is a modulator of cardiac performance. To what extent the change in EA during exercise impacts on cardiac performance and aerobic capacity is unknown. We examined EA and its relationship with cardiovascular performance in 352 healthy subjects. Subjects underwent rest and exercise gated scans to measure cardiac volumes and to derive EA[end-systolic pressure/stroke volume index(SV)], PVR[MAP/(SV*HR)], and TAC(SV/pulse pressure). EA varied with exercise intensity: the ΔEA between rest and peak exercise along with its determinants, differed among individuals and ranged from −44% to +149%, and was independent of age and sex. Individuals were separated into 3 groups based on their ΔEAI. Individuals with the largest increase in ΔEA(group 3;ΔEA≥0.98 mmHg.m2/ml) had the smallest reduction in PVR, the greatest reduction in TAC and a similar increase in HR vs. group 1(ΔEA<0.22 mmHg.m2/ml). Furthermore, group 3 had a reduction in end-diastolic volume, and a blunted increase in SV(80%), and cardiac output(27%), during exercise vs. group 1. Despite limitations in the Frank-Starling mechanism and cardiac function, peak aerobic capacity did not differ by group because arterial-venous oxygen difference was greater in group 3 vs. 1. Thus the change in arterial load during exercise has important effects on the Frank-Starling mechanism and cardiac performance but not on exercise capacity. These findings provide interesting insights into the dynamic cardiovascular alterations during exercise.
Keywords: arterial elastance, cardiovascular performance
the arterial system is increasingly recognized as an important modulator of cardiac performance (6), and a potent predictor of cardiovascular (CV) outcomes (30, 52). The arterial system imposes a load on the heart, both at rest and during exercise. A higher arterial load increases the heart's energetic costs to eject a given amount of blood (10, 24).
Effective arterial elastance (EA) characterizes the net arterial load that is imposed on the heart (51). EA was originally derived from a 3-element Windkessel model, which characterizes the arterial system in terms of resistive and pulsatile components (51). Operationally, EA is defined as end systolic arterial pressure/ stroke volume index. A higher EA reflects a greater arterial load imposed on the left ventricle (LV) and is directly related to heart rate (HR) and peripheral vascular resistance (which is determined, in large part, by the small arteries), and is inversely related to total arterial compliance (which is determined, in large part, by the central elastic arteries) (11). At rest, the resistive component is the dominant determinant of EA (11). During exercise both arterial resistance and compliance usually decrease, and the relative contribution of the pulsatile component to EA increases, so that by 80% of peak exercise the resistive and the pulsatile components provide nearly equal contributions to EA(37). In various studies, EA has been shown to increase (34, 37), decline (3) or remain unchanged (16) during exercise. The pattern of change in EA may vary with age, sex and the intensity of exercise (37, 45). Such marked inter-individual differences in the change in EA during exercise likely reflect the specific details of the population under study and varying combinations in the determinants of EA (resistance, compliance and heart rate) among study subjects during exercise (21, 36). How the exercise-induced change in EA relates to a change in LV filling and ejection has not been established, nor has its relationship with aerobic capacity, a clinically important marker of overall health and mortality (33) . We therefore evaluated whether the variability in the change in EA during exercise is associated with a distinct pattern of change in arterial and cardiac function during exercise that may have important effects on exercise capacity.
METHODS
Study population.
The study population consisted of community dwelling volunteers with a broad age-range from the Baltimore Longitudinal Study of Aging (46), who underwent multi-gated blood pool scans at rest and during exercise. All subjects in the current investigation had a resting LV ejection fraction (EF) greater than 50% without wall motion abnormalities at rest or during exercise. All subjects were free of CV disease as determined by detailed history and physical examination, normal resting and treadmill exercise electrocardiograms, and absence of perfusion abnormality on thallium scintigraphy during treadmill stress testing in men over 40 years, and in women over 50 years of age. From baseline SBP values, 35% of the study population presented with hypertension based on the JNC 7 guidelines (14). However, no subject was taking any cardiac or antihypertensive medications. The study was approved by the institutional review boards of the National Institute on Aging and Johns Hopkins University. All subjects provided written informed consent to participate.
Evaluations.
All subjects underwent an upright seated bicycle graded exercise test. Pedal speed was maintained constant at 60 rpm, and workloads were increased by increments of 25W every 3 min until exhaustion. Maximum workload (MWL) was defined as the maximum wattage attained during the exercise test. Systolic and diastolic blood pressures (BP) were measured with cuff sphygmomanometry at seated upright rest, and during the final minute of each stage of exercise. End-systolic pressure (ESP) was noninvasively approximated by 0.9 (25) Despite the validation of the estimation of ESP as 0.9 × SBP (13, 25), some studies have suggested that the multiplier value is closer to 0.73 (39). When we repeated the analyses using ESP estimated as 0.73 × SBP the results were not affected; thus, only values derived from ESP estimated as 0.9 × SBP are shown. Noninvasive measurements of cardiac volumes at rest and during each stage of exercise were determined with multi-gated blood technetium 99m pool scans as previously described (21). This method has been validated both at rest (18) and during exercise (47). Adequate scaling of physiological measures for body size is essential for correct interpretation, and often the relationship between body size and physiological function may not be linear, a major assumption for the ratiometrically scaling approach. The allometric scaling approach accounts for this potential nonlinear relationship by normalizing physiological measures using exponential powers that linearize the relationship. Thus all cardiac volumes were ratiometrically or allometrically scaled to body surface area (5), yielding their respective volume indexes: end-diastolic volume index (EDVI), end-systolic volume index (ESVI), and stroke volume index (SVI). Of note, as the types of scaling for body size did not affect the findings, only the values of the ratiometrically scaled parameters are shown. Cardiac index (CI; l/min/m2) was calculated as SVI × HR. Peripheral vascular resistance index (PVRI; dyne.sec/cm5/m2) was calculated as mean arterial pressure (MAP; mmHg) × 80/CI, with MAP calculated as (1/3 × systolic BP + 2/3 × diastolic BP). To convert from Wood units to dyn·s·cm−5 MAP must be multiplied by 80. A crude estimate of total arterial compliance index (TACI) was calculated as SVI/pulse pressure (PP) (37). Ejection fraction (EF) was calculated as SVI/EDVI, and in the absence of Vo is inversely related to EAI/ELVI [EAI/ELVI = (1-EF)/EF]. Peak oxygen consumption (VO2peak) was measured using the Medgraphics CPX-D system (Medgraphics, St Paul, Minnesota, USA). Measurement of VO2peak was introduced in this study in 1986, and data on VO2peak were only available on a subset (49%) of the cohort. Peak arterio-venous oxygen extraction was calculated from the Fick equation (VO2peak/CI).Effective arterial elastance index (EAI) was calculated as ESP/SVI (25), which was previously shown to closely approximate the arterial load directly measured invasively from aortic input impedance and arterial compliance data (25). LV end-systolic elastance, a measure of LV chamber performance was calculated as ELVI = ESP/ESV-Vo (51) wherein V0, the volume-axis intercept of the end-systolic pressure volume relationship, was assumed to be zero, as previously reported (15). Stroke work index was used as an indirect measure of LV systolic performance (4), and was calculated as SWI = SVIxMAP. To adjust for the known effects of chamber size on ELVI and SWI (5), EDVI was added as a covariate in the statistical models, and parameter estimates were used as the EDVI adjusted values for ELVI and SWI. The interaction between the heart and arterial system was depicted by the arterial-ventricular coupling ratio (EAI/ELVI) = ESVI/SVI (9). Of note, when Vo is assumed to be negligible, EAI/ELVI is related to EF (15). The changes from rest to peak exercise in EAI (ΔEAI), and in other arterial and cardiac variables, were calculated by subtracting resting from peak values.
Statistical analysis.
All analyses were performed using the statistical packages SPSS version 13 (SPSS Inc, Chicago, Illinois). Grouped data are reported as mean ± SD unless otherwise stated.
ΔEAI for the entire population exhibited a Gaussian distribution (Fig. 1A). One analytical approach was to compare the relationship of ΔEAI in three groups stratified by the lowest, highest, and middle two quartiles to other arterial and cardiac variables. The descriptive characteristics of the three groups, stratified by ΔEAI, were compared using analysis of variance (ANOVA) or chi-square. The arterial and cardiac parameters measured at rest, at peak exercise, and their change from rest to peak exercise were compared among the 3 groups by ANOVA after adjusting for age, sex and maximal workload, and by a repeated measures ANOVA across relative workloads of 25%, 50%, and 100%of maximum, with an EAI group by workload interaction term.. The analyses were also adjusted for multiple comparisons using Bonferroni's method. We also examined the relationship between ΔEAI and CV performance by BP group (normotensive vs. hypertensives) and the relationship between a hypertensive SBP response to the exercise stress test and CV performance. A hypertensive response to exercise was defined as an SBP>220 mmHg for men and >190 mmHg for women (26). A two-tailed P < 0.05 was required for significance. A second analytical approach was to examine the bivariate relationships among the changes from rest to peak exercise in the arterial and cardiac variables as continuous functions in the entire cohort via Pearson's correlation coefficients, and with linear regression analyses that were adjusted for age, sex and maximal workload. To evaluate the contributions of TACI, PVRI and HR to EAI, multiple regression analysis was performed, and the standardized regression coefficient and the semi-partial correlations were calculated as an index of contribution to EAI.
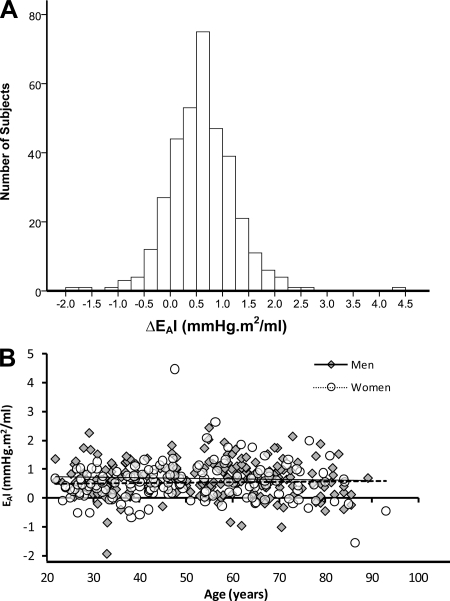
Fig. 1.
The distribution of the change in EAI during exercise A: The frequency distribution of the change in effective arterial elastance index (ΔEAI) from rest to peak exercise. B: The relationship of ΔEAI to age in men (closed symbols) and women (open symbols).
RESULTS
The descriptive characteristics of the 3 groups in the resting state are shown in Table 1. The 3 EAI groups did not differ significantly in age, sex, or body size.
Table 1.
Descriptive characteristics of the study population in the resting state
| EAI |
||||
|---|---|---|---|---|
| Total Cohort | Group 1 | Group 2 | Group 3 | |
| n | 352 | 88 | 176 | 88 |
| Age, years | 53 ± 17 | 54 ± 19 | 51 ± 17 | 53 ± 15 |
| Sex, % men | 56 | 50 | 53 | 66 |
| Height, cm | 171 ± 10 | 170 ± 9 | 171 ± 10 | 172 ± 10 |
| Weight, kg | 74 ± 14 | 72 ± 15 | 73 ± 15 | 77 ± 13 |
| Body mass index, kg/m2 | 25 ± 4 | 25 ± 4 | 25 ± 4 | 26 ± 4 |
| Body surface area, m2 | 1.85 ± 0.21 | 1.83 ± 0.22 | 1.84 ± 0.21 | 1.89 ± 0.20 |
| Maximal workload, watts | 127 ± 42 | 118 ± 41 | 128 ± 40 | 134 ± 45* |
| Aerobic capacity, ml·kg−1·min−1 | 23.8 ± 7.2 | 23.6 ± 7.4 | 23.8 ± 7.1 | 24.1 ± 8.0 |
Values are means ± SD, or percentage. Effective arterial elastance index (EAI) groups are defined according to quartiles of ΔEAI: EAI group 1, lowest quartile (ΔEAI < 0.22 mmHg·m2−1·ml−1); EAI group 2, middle two quartiles (0.22 ≤ ΔEAI < 0.98 mmHg·m2−1·ml−1); and EAI group 3, highest quartile (ΔEAI ≥ 0.98 mmHg·m2−1·ml−1).
P < 0.05 compared with EAI group 1 by ANOVA followed by Bonferroni correction.
ΔEAI and its determinants during graded exercise.
During exercise, the observed changes in EAI ranged from a 44% decline to a 149% increase (Fig. 1A). In ∼17% of individuals, EAI was lower at peak exercise than at rest (i.e., ΔEAI ≤ 0 mmHg.m2/ml). Conversely, in 19% of individuals, EAI increased by 50% or more from baseline to peak exercise. The interaction term between age and sex was for ΔEAI was nonsignificant (Fig. 1B). Furthermore, ΔEAI was also not associated with body composition. The change in EAI from rest to peak exercise (ΔEAI) was stratified into three groups: EAI group 1, included individuals whose ΔEAI was in the lowest quartile i.e., ΔEAI < 0.22 mmHg.m2/ml, (n = 88); EAI group 2 included individuals whose ΔEAI was in the middle two quartiles i.e., 0.22 ≤ ΔEAI < 0.98 mmHg.m2/ml (n = 176); and EAI group 3 included individuals whose ΔEAI was in the highest quartile i.e., ΔEAI ≥ 0.98 mmHg.m2/ml (n = 88). Fig. 2A shows that the 3 groups differed not only in their ΔEAI, but dramatically differed in the pattern of change in EAI as the intensity of exercise increased. Group 1, i.e., the group with the lowest ΔEAI during exercise, had the highest EAI at rest, associated with a higher resting HR, a lower TACI, and a nonsignificant trend for a higher PVRI (Fig. 2).
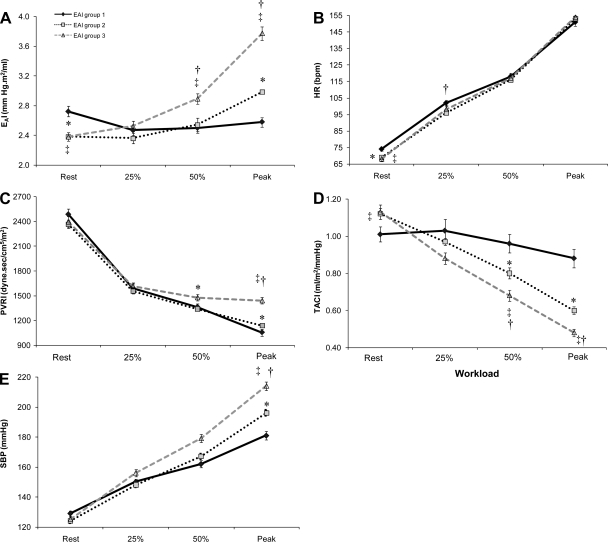
Fig. 2.
The change in EAI and its components across relative exercise workloads. Comparisons of EAI(A), heart rate (HR: B), peripheral vascular resistance (PVRI: C), total arterial compliance (TACI: D), and systolic blood pressure (SBP: E) among the 3 EAI groups at rest, at 25% and 50% of peak, and at peak exercise (data shown as means ± SEM).*P < 0.05 EAI group 1 vs. 2, ‡P < 0.05 EAI group 1 vs. 3, †P < 0.05 EAI group 2 vs. 3, adjusted for age, sex and maximal workload.
At rest, the components of EAI, namely TACI (β= −0.25; semi-partial correlations = −0.04) PVRI (β = 0.86; semi-partial correlations = 0.44) and HR (β = 0.54; semi-partial correlations = 0.20) significantly (P < 0.01) accounted for 98% of the variance in EAI. Similarly, at peak exercise TACI (β = −0.33; semi-partial correlations = −0.10), PVRI (β = 0.93; semi-partial correlations = 0.57), and HR (β = 0.52; semi-partial correlations = 0.18) significantly (P < 0.01) accounted for 90% of the variance in EAI. When considered as a continuous variable across the entire cohort, ΔEAI was positively associated with ΔHR (r = 0.12, P < 0.05), ΔPVRI(r = 0.41, P < 0.01), and inversely associated with ΔTACI(r = −0.46, P < 0.01). But the manner and magnitude in which these EAI determinants (PVRI, TACI, and HR) change from rest to max effort differs according to the relative intensity of the exercise workload and among the three EAI groups. HR changes uniformly during exercise in all three groups (Fig. 2B). Thus group differences in HR acceleration do not account for the group differences in EAI during vigorous exercise. Changes in PVRI and TACI during exercise differed by EAI group (Figures 2C & 2D) (significant EAI group by workload interaction term) whereby EAI group 1 had a blunted reduction in TACI, and a greater reduction in PVRI compared with EAI group 3. Further, despite similar SBP values at rest, EAI group 1 exhibited a smaller increase in SBP during exercise, and EAI group 3 a greater SBP response to exercise such that, SBP at peak exercise was significantly higher in EAI group 3 > groups 2 > group 1 (Fig. 2E).
It should be noted that the above comparisons among the 3 groups, and the associations with ΔEAI persisted even after adjusting for age, sex and maximal workload (Fig. 2; Tables 2 and 3). Further, similar results were found when we removed the hypertensive individuals from the analyses (see online supplement). The only significant EAI group by BP group (normotensive vs. hypertensive) interaction was found for ΔEAI whereby hypertensive individuals in EAI group 1 had a smaller ΔEAI response, whereas hypertensive individuals in EAI group 3 had a greater ΔEAI response compared with EAI group 3.
Table 2.
Comparison of resting cardiovascular parameters assessed among the three EAI groups
| EAI |
|||
|---|---|---|---|
| Group 1 | Group 2 | Group 3 | |
| EAI, mmHg. m2/ml | 2.72 ± 0.66 | 2.38 ± 0.53*** | 2.38 ± 0.54*** |
| Blood pressure, mmHg | |||
| Systolic | 129 ± 17.7 | 124 ± 16.1 | 125 ± 16.8 |
| Diastolic | 81 ± 11.1 | 78 ± 9.5* | 79 ± 10.5 |
| Pressure, mmHg | |||
| End systolic | 116 ± 16 | 111 ± 15 | 113 ± 15 |
| Mean arterial | 97 ± 12 | 93 ± 11** | 95 ± 11 |
| Pulse | 48 ± 14 | 46 ± 12 | 46 ± 13 |
| Peripheral vascular resistance index, dyn·s/cm5/m2 | 2,486 ± 629 | 2,361 ± 592 | 2,394 ± 596 |
| Total arterial compliance index, ml/m2/mmHg | 1.01 ± 0.34 | 1.12 ± 0.37 | 1.13 ± 0.36* |
| Heart rate, beats/min | 74 ± 11 | 69 ± 10*** | 68 ± 11*** |
| Cardiac index, l/min/m2 | 3.2 ± 0.7 | 3.3 ± 0.7 | 3.3 ± 0.7 |
| Volume index, ml/m2 | |||
| Stroke | 45 ± 9 | 48 ± 9** | 49 ± 10** |
| End-diastolc | 69 ± 14 | 74 ± 13* | 74 ± 14* |
| End-systolic | 25 ± 7 | 26 ± 7 | 25 ± 8 |
| Ejection fraction, % | 64 ± 7 | 65 ± 7 | 67 ± 7* |
| End-systolic elastance index, mmHg·m2/ml# | 5.2 ± 1.1 | 4.7 ± 1.1** | 5.1 ± 1.1† |
| Stroke work index, mmHg·ml/m2# | 5,151 ± 875 | 5,471 ± 873* | 5,449 ± 876 |
| Arterial-ventricular coupling ratio | 0.57 ± 0.18 | 0.54 ± 0.15 | 0.52 ± 0.17* |
Values are means ± SD. Statistics are adjusted for age, sex, and maximal workload. However, similar statistical findings are present for the unadjusted comparisons.
P < 0.05,
P < 0.01,
P < 0.001, compared with EAI group 1;
P < 0.05, compared with EAI group 2 by ANOVA followed by Bonferroni correction;
end-diastolic volume index-adjusted values.
Table 3.
Comparisons of the change in cardiovascular parameters from rest to peak exercise assessed among the three EAI groups
| EAI group 1 |
|||
|---|---|---|---|
| Group 1 | Group 2 | Group 3 | |
| ΔArterial elastance index, mmHg. m2/ml | −0.14 ± 0.37 | 0.60 ± 0.20*** | 1.40 ± 0.49***††† |
| Blood pressure, mmHg | |||
| ΔSystolic | 52 ± 26 | 73 ± 19*** | 88 ± 22***††† |
| ΔDiastolic | 12 ± 16 | 12 ± 15 | 19 ± 17**†† |
| Pressure, mmHg | |||
| ΔEnd systolic | 47 ± 24 | 65 ± 17*** | 79 ± 20***††† |
| ΔMean arterial | 25 ± 13 | 32 ± 13*** | 42 ± 14***††† |
| ΔPulse | 41 ± 32 | 60 ± 23*** | 70 ± 25*** |
| ΔPeripheral vascular resistance index (dyne.sec/cm5/m2) | −1459 ± 490 | −1220 ± 478*** | −949 ± 515***††† |
| ΔTotal arterial compliance index (ml/m2/mmHg) | −0.13 ± 0.51 | −0.52 ± 0.30*** | −0.67 ± 0.33***†† |
| ΔHeart rate, beats/min | 78 ± 23 | 84 ± 22 | 86 ± 24 |
| ΔCardiac index (l/min/m2) | 6.52 ± 1.91 | 5.99 ± 1.87*** | 4.76 ± 1.83***††† |
| ΔStroke volume index (ml/m2) | 20 ± 8 | 13 ± 7*** | 4 ± 8***††† |
| Volume index, ml/ m2 | |||
| ΔEnd-diastolc | 14 ± 11 | 3 ± 9*** | −4 ± 9***††† |
| ΔEnd-systolic | −7 ± 10 | −10 ± 8 | −8 ± 8 |
| ΔEjection fraction (%) | 15 ± 10 | 15 ± 8 | 10 ± 9***††† |
| ΔEnd-systolic elastance index (mmHg·m2/ml)# | 14 ± 18 | 12 ± 16 | 7 ± 17*† |
| ΔStroke work index (mmHg·ml/m2)# | 2942 ± 1433 | 3192 ± 1269 | 3215 ± 1372 |
| ΔArterial-ventricular coupling ratio | −0.29 ± 0.21 | −0.28 ± 0.17 | −0.19 ± .018***††† |
Values are means ± SD. Changes (Δ) in variables are calculated by subtracting basal from postexercise values. Statistics are adjusted for age, sex, and maximal workload. However, similar statistical findings are present for the unadjusted comparisons.
P < 0.05,
P < 0.01,
P < 0.001 compared with EAI group 1;
P < 0.05,
P < 0.01.
P < 0.001 compared with EAI group 2 by ANOVA followed by Bonferroni correction.
EDVI adjusted values.
Cardiac volumes at rest and during exercise.
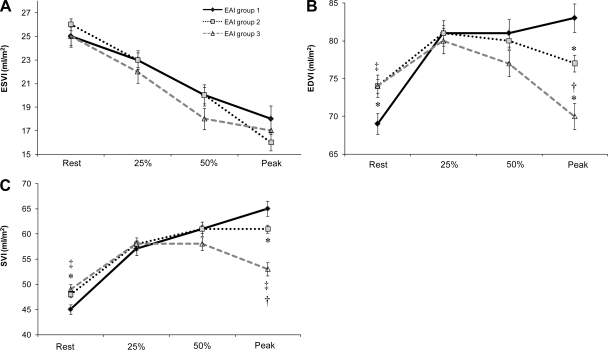
Resting EDVI and SVI were lower in group 1 vs. the other two groups whereas resting ESVI did not differ among the 3 groups (Fig. 3). The ΔESVI during exercise did not parallel ΔEAI, whereas the change in EDVI and SVI across the relative workloads differed by EAI group (Fig. 3)(significant EAI group by workload interaction term). However, despite an initial increase in EDVI and SVI in all three groups EAI group 3 did not utilize the Frank-Starling mechanism above 25% of peak effort and thus no further increase in SVI was noted. Thus the 3 groups exhibited marked differences from rest to peak exercise in ΔEDVI (20% in group 1, 4% group 2, −5% group 3) and in ΔSVI (44%, 27%, 8% respectively). An unexpected finding is that the relationship of EDVI to exercise intensity was an inverse mirror image of the relationship of ΔEAI with exercise intensity (Fig. 2A). Indeed ΔEAI, expressed as a continuous function of EDVI in the entire cohort, was significantly and inversely correlated with ΔEDVI (r = −0.58, P < 0.01). Of note, these relationships were independent of age, sex and maximal workload. In addition, similar results were found when we examined normotensive and hypertensive individuals separately (see online supplement).
Fig. 3.
The change in cardiac volumes across relative exercise workloads Comparisons of ESVI(A), EDVI(B), and SVI(C), among the 3 EAI groups at rest, at 25% and 50% of peak, and at peak exercise (data shown as means ± SEM). *P < 0.05 vs. EAI group 1 vs. 2, ‡P < 0.05 EAI group 1 vs. 3, †P < 0.05 EAI group 2 vs. 3, adjusted for age, sex and maximal workload.
Cardiac performance at rest and during exercise.
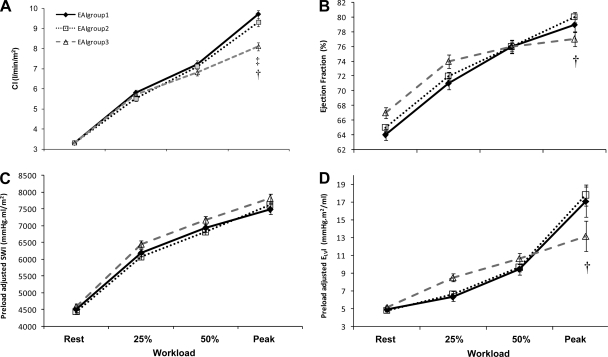
Resting CI did not differ among the 3 groups, but CI significantly higher in group 1 vs. group 3 at peak exercise (Fig. 4A). Further ΔCI as a continuous function among all subjects was inversely associated with ΔEAI (r=−0.37, P < 0.05).
Fig. 4.
The change in cardiac performance across relative exercise workloads. Comparisons of cardiac index (CI: A), ejection fraction (EF: B), EDVI adjusted values of stroke work (SWI: C), and end-systolic elastance (ELVI: D) among the 3 EAI groups at rest, at 25% and 50% of peak, and at peak exercise (data shown as means ± SEM). *P < 0.05 vs. EAI group 1 vs. 2, ‡P < 0.05 EAI group 1 vs. 3, †P < 0.05 EAI group 2 vs. 3, adjusted for age, sex and MWL.
At rest, group 3 had the highest EF; note, however, that at peak exercise group 3 had the lowest EF (Fig. 4B). ΔEF was inversely associated with ΔEAI (r = −0.20, P < 0.05) and thus differed between groups. Because EF is influenced by the loading conditions of the ventricle, we also examined SWI and ELVI, measures of LV pump performance; since both SWI and ELVI are affected by chamber size, we therefore adjusted SWI and ELVI for EDVI (a surrogate marker of chamber in size) (5, 40) in the ANCOVA (Fig. 4C and 4D). At rest, neither EDVI adjusted statistically for SWI nor ELVI differed between EAI groups. . Further SWI did not differ among groups during rest or at peak exercise. In contrast, EAI group 3 demonstrated a blunted increase in ELVI from 50% of peak to peak exercise compared with EAI groups 1 and 2. A significant interaction between EAI group and exercise workload for ELVI was noted which further suggests that EAI group 3 demonstrated a deficit in peak LV performance (i.e., limited Frank-Starling mechanism leading to reduced SVI and CI)(Fig. 4D). The above relationships persisted when we examined normotensives and hypertensives separately (see online supplementation).
In spite of a lower peak exercise CI in group 3 (Fig. 4A), peak aerobic capacity did not differ between EAI groups because calculated peak arterial-venous oxygen extraction which was highest in EAI group 3 (12.06 ± 3.5 ml/100ml), vs. group 1 (9.58 ± 2.4 ml/100ml), and vs. group 2 (10.02 ± 2.5 ml/100ml), and compensated for the lower peak CI. Further, the differences in arterial-venous oxygen difference between EAI groups were not due to differences in hemoglobin concentrations (EAI group 1 = 14.3 ± 1.3; EAI group 2 = 14.3 ± 1.3; EAI group 3 = 14.5 ± 1.3). These results were also observed when normotensives and hypertensives were analyzed separately (data not shown).
DISCUSSION
Effective arterial elastance is a measure of the net arterial load that is imposed on the LV and correlates well with aortic input impedance assessed invasively (25). In the current study, we found that the changes in EAI during exercise in a large cohort of men and women of a broad age range encompassed a spectrum of values, with 17% of the cohort experiencing a decline in EAI during exercise, and the rest showing varying increases up to 149%. Further the change in EAI during exercise was accompanied by a distinct pattern of change in cardiovascular dynamics.
The arterial load has an important effect on LV pump function (20), whereby a higher resting EAI increases the myocardial energetic costs to provide a given SVI (10, 24). Those which have examined EAI during exercise in humans have reported that EAI declines (3), remains the same (16), or increases (34, 37). The mechanisms behind the diverse change in EAI with exercise, reflects the manner and magnitude of the changes in the components of EAI, namely TACI, and PVRI, which vary within an individual and as a function of exercise intensity. Further, the variable changes in EAI during exercise may also reflect subclinical arterial disease, such as insufficient vasodilatory response due to autonomic or endothelial dysfunction. Our study is the first to examine the exercise-induced changes in EAI and the concomitant patterns of exercise induced-changes in ventricular volumes and function. We found a striking inverse relationship between ΔEAI and the recruitment of EDVI, and the enhancement of SVI and CI with exercise, suggesting that individuals expressing a large increase in EAI during exercise demonstrate a blunted utilization of the Frank-Starling mechanism, irrespective of age, sex, and body size.
Patterns of arterial alterations.
In this study ΔEAI was not associated with age or sex, which differs from previous studies. Park et al. (38) found that older hypertensive women (59 ± 8 yrs) expressed a greater change in EAI from rest to 75W compared with older (58 ± 9 yrs) hypertensive men. Further, Najjar et al. (34) found that during exercise EAI was similar in young and older normotensive men, but was higher in older vs. younger normotensive women. These differences may be related to our inclusion of normotensive (65%) and hypertensives. Indeed when we separated our cohort into BP groups we find that ΔEAI is associated with age and sex (data not shown). Further, Park et al. (38) examined the change in EAI in the supine position and only reported data up to 75watts of exercise, which may further explain the differences between studies, as our figures show.
EAI was originally derived from a 3-element Windkessel model which characterizes the hemodynamics of the arterial system as TACI, PVRI along with characteristic impedance (51). TACI provides information about the viscoelastic properties of the arteries. Several methods have been used to estimate TACI derived from the Windkessel model (28), and we direct readers to Westerhof et al. (53) for a detail review. The ratio of SV/PP (which is used in this study) is a simple index of TACI and has a physiological basis in the 2-element Windkessel model (12, 48–49). Theoretically, if we assume the periphery is closed, an increase in pressure (PP) resulting from a single stroke volume (SV), TACI relates to compliance as SV/PP. However, because part of the SV outflow leaves the arterial system through the microcirculation while cardiac ejection occurs the SV/PP ratio can overestimates TACI by as much as 60% (43). Thus, to reproduce the 2-element Windkessel model, only the part of blood ejected within the time of peak pressure should be measured. Despite this overestimation, close relationships have been noted between the SV/PP ratio and more direct measures of Windkessel compliance i.e., the area (r = 0.98 and r = 0.58) (12, 43), and the PP method (r = 0.97, and 0.80) (23, 43). Further, Resnick et al. (41) demonstrated that the SV/PP ratio was correlated with both capacitive (reflecting the TACI component associated with the conduit, capacitive function of larger arteries) and oscillatory (depicting the reflective component of TACI) compliance of the arterial tree using a modified Windkessel model (r = 0.92 and 0.68, respectively). However, despite this finding, the SV/PP ratio does not fully take into account the effects of characteristic impedance. It has therefore been suggested that SV/PP is an index of normal/abnormal compliance rather than a calculation or estimation of TACI (12).
Previous studies (11, 37, 42) have demonstrated that the resistive component is the dominant determinant of EAI, at rest and that during exercise the contribution of the pulsatile component (TACI) to EAI gradually increases. These results were confirmed in our study whereby SVRI accounted for most of the variance in EAI at rest (44%; β = 0.86) and peak exercise (57%; β = 0.93). With the contribution of TACI to EAI increasing from rest (4%; β = −0.25) to peak exercise (10%; β = −0.33). However, the contribution of HR to EAI both at rest (20%; β = 0.54) and peak exercise (18%; β = 0.52) was similar. Furthermore, the variance in EAI at rest (r2 = 0.98) and peak exercise (r2 = 0.90) were almost entirely accounted for by TACI, PVRI and HR. However, as with ΔEAI, the manner and magnitude by which the components of ΔEAI change during exercise differ among individuals and by the intensity of exercise and within a given individual, and therefore cannot be predicted a priori. This was highlighted in EAI group 1 (minimal change in ΔEAI) in which a blunted reduction in TACI, and the largest reduction in PVRI was noted. The larger reduction in PVRI during exercise in EAI group 1, denotes an augmented peripheral vasodilatation possibly due to an enhanced endothelial reactivity or reduced sympathetic tone. Further, the ability to preserve TACI during exercise in this group, in spite of the exercise-induced increase in BP (and SVI) suggests an attenuated reduction in arterial distensibility. In EAI group 3, the greater reduction in TACI than group 1 could reflect an increased vascular smooth muscle cell tone which would limit the vasodilatory response of central aorta and increase arterial impedance (32), and thus the greater BP response noted in EAI group 3. However, all groups had a similar HR response to exercise, and therefore HR was unlikely to be the cause of the varied TACI response to exercise. In contrast, at rest HR was lower in EAI group 3 than group 1, and therefore may have contributed to the difference in TACI at rest between groups (27). Further, in EAI group 3, the excessive increase in MAP than group 1, a key determinant of arterial stiffness, may have contributed to the increased arterial load because at higher MAP the less compliant collagen fibers predominate in the maintenance of vessel-wall stresses (2). In contrast, at lower MAP (noted in EAI group 1) the compliant elastin fibers are predominately recruited which may contribute to lower arterial stiffness and reduced arterial load.
Some of the components of ΔEAI were themselves related to each other, whereby a greater preservation of TACI during exercise is associated with a greater reduction in PVRI (and a smaller increase in MAP and PP). This suggests that the tandem changes in resistance and compliance appear to be linked, and raises the possibility of a cross-talk between central and peripheral arteries. However, TACI is influenced, in part, by the compliance of the resistive vessels, and the resistive vessels have compliance properties. Further, central arterial compliance is also influenced by the timing and magnitude of the reflective waves, which are not fully accounted for by TACI (originally from the Windkessel model). Further, given that both parameters are derived noninvasively from pressure and flow, more direct and independent measurements of resistance and compliance are required to further explore this interesting theoretical interaction.
Patterns of ventricular alterations.
Another striking and novel finding of the present study is that the changes in EAI during exercise were also coupled to correlated patterns of change in LV preload during exercise. Further, changes in LV preload were also associated with changes in PVRI and TACI suggesting that the cross-talk between the large and small arteries may extend to involve the heart. Indeed, Guyton postulated that cardiovascular performance is simply regulated by the rate of blood flow into the heart from the systemic circulation (22). In our study individuals with a larger ΔEAI (i.e., those with a higher ΔTACI, and a smaller ΔPVRI) have a negative ΔEDVI and a smaller positive ΔSVI. This suggests that these individuals have a blunted reliance on the Frank-Starling mechanism to augment SVI during exercise. Further, the pattern of change in ΔEDVI mimicked the pattern of change in the TACI to PVRI relationship. Similarly, individuals with the largest EDVI at rest, and also at peak exercise, expressed the highest TACI/PVRI ratio. Individuals in EAI group 3 were able to initially augment EDVI from rest to 25% of MWL, which may be attributed to the mobilization of blood in the extremities to the central circulation thereby increasing venous return.. However, at 50% of MWL the EDVI began to decline, corresponding to the higher absolute EAI values, which may be attributed to an elevated PVRI, inadequate venous return (from the higher hydraulic load and higher peripheral resistance), increased LV diastolic stiffness (thereby impairing LV filling) (1), abnormal venous tone (thereby impairing endothelial function), or a combination of these factors. In line with our results, Borlaug at al. (7) found that blunted ΔPVRI was associated with attenuated increase of EDVI with exercise in heart failure patients with a preserved EF (HFpEF). Previous work (21, 50) had reported that the utilization of the Frank-Starling mechanism during exercise to simply ensure that SVI during exercise did not decline with age. In contrast, in our study, individuals with a smaller ΔEAI who exhibited greater utilization of the Frank-Starling mechanism (EAI group 1) during exercise, also achieved a higher SVI (and a higher CI) independent of age, sex or MWL. Thus, our findings provide novel insights in that they establish, for the first time, a link between ΔEAI and the utilization of the Frank-Starling mechanism to increase SVI.
The deficit in cardiac performance (blunted preload and CI response) in EAI group 3 did not translate into a deficit in peak aerobic capacity. Since VO2peak is determined by the product of CI and arterial-venous oxygen extraction, the similar VO2peak because arterial-venous oxygen extraction was increased in EAI group 3 vs. 1, which was not due to differences in hemoglobin concentrations between groups. Thus, the arterial and cardiac alterations during exercise also appear to be linked to adaptations in peripheral oxygen extraction. The signaling pathways that underlie this adaptive response require further study.
Interestingly, Borlaug et al. (8) recently identified evidence of global impairment in cardiovascular reserve function in HFpEF patients including a blunted contractile, endothelial, and vascular (EAI and PVRI) reserve. Further, it has been suggested that the depressed reserve responses correlated with a reduced exercise capacity in HFpEF patients (8). Unlike our study where the blunted increase in EAI was accompanied by a greater reduction in PVRI, the HFpEF patients exhibited a blunted response in both EAI and PVRI, which may suggest that they are no longer able to compensate for the limited EAI response by reducing PVRI to maintain adequate LV filling. Indeed ΔEDVI was smaller in HFpEF patients compared with healthy controls (8).
Finally, the patterns that were observed during exercise are also linked to distinct (albeit more modest) arterial and ventricular patterns in the resting state. At rest, individuals EAI group 1 exhibited higher EAI, and a lower TACI, EDVI and SVI. However, these are the same individuals who, at peak exercise, have a lower EAI, and a higher TACI, EDVI and SVI. This would suggest a more compliant system during exercise but not at rest in EAI group 1 and vice versa in EAI group 3. Acute shifts in compliance from rest to exercise are related to the arterial structure and vasoreactivity (e.g., sympathetic activity, prostacyclins or nitric oxide bioavailability), and mechanical forces (e.g., sheer circumferential stress, and HR), and how these change during exercise and interact with arterial structure determines the compliance of the artery during exercise. Unfortunately we are unable to determine the underlying mechanisms that alter the compliance of the artery from rest to peak exercise and contribute to the differences noted between groups.
The effects of hypertension and a hypertensive response to exercise.
We examined the effects of hypertension on the relationship between ΔEAI and the CV performance by categorizing individuals into normotensives and hypertensives groups (14), and by categorizing individuals based on their BP response during the exercise stress (26). Individuals who normotensive and hypertensive at rest had similar arterial and cardiac responses to exercise. The only significant EAI group by BP group interaction was found for ΔEAI whereby hypertensive individuals in EAI group 1 had a smaller ΔEAI response, whereas hypertensive individuals in EAI group 3 had a greater ΔEAI response. These data illustrate that the findings of the study are not due the inclusion of individuals with hypertension.
Several studies (19, 31, 44) have shown that a BP response to exercise can predict the development of hypertension and target organ damage. Individuals with a hypertensive response to exercise had a greater ΔEAI. With the exception of the change in ELVI during exercise, no other hemodynamic differences were noted during exercise between BP response groups. However, differences were noted at rest and peak exercise; individuals with a hypertensive response to exercise had a higher EAI, SVRI, and BP, and a lower TACI both at rest and at peak exercise compared with those with a normotensive BP response. This illustrates that examining the change in SBP alone during exercise does not fully reveal alterations in CV hemodynamic during exercise that are clearly evident when the ΔEAI response is examined. Future research is required to determine whether the change in EAI during exercise could predict future CV events.
Study limitations.
Some limitations of the study are worth noting. Because of the noninvasive nature of this study, some of the arterial and LV parameters were calculated using brachial rather than central pressures. Because of BP amplification across the arterial tree, brachial BP is known to overestimate central pressures (35); the magnitude of this overestimation is inversely related to age, and is markedly augmented during exercise (45). Although the estimation of ESP as 0.9 × brachial SBP has been shown to closely estimate central ESP (25), this was only assessed in the resting state. Because Vo (volume-axis intercept of the end-systolic pressure volume relationship) was not measured and assumed to be zero, ELVI should not be considered an absolute measure of LV contractility but only an approximation of that. Further, arterial compliance was not directly assessed; instead the ratio of SVI/PP was used as a crude estimate of total system arterial compliance (17). This index, which has been shown to be a predictor of cardiovascular events (17) has well known limitations (17, 29). However, the ratio of SVI/PP is a practical and feasible measure to acquire during exercise, particularly since this study was largely conducted prior to the availability of modern devices that facilitate the assessment of arterial stiffness during exercise (45). Finally, because the calculation of PVRI and TACI were measured indirectly through changes in pressure and volumes, caution should be applied to the interpretation of any cross talk between large and small arteries. More direct and independent assessment is required to identify whether there is any cross talk between small and large arteries involved in the change in EAI during exercise.
Conclusions
This is the first study to demonstrate the effects of graded exercise on the changes in EAI and the corresponding changes in cardiovascular dynamics and to show the heterogeneity in EAI among individuals across a range of exercise intensities. The changes in EAI from rest to peak exercise reflect a complex interaction among changes in arterial and cardiac function that appear to be linked to adaptive features in the peripheral cardiovascular system. Because some of the components of ΔEAI, at least at rest, have been shown to be predictors of outcomes, in future studies it would be worthwhile to examine whether the specific patterns of change in arterial load and the coupled patterns of change in LV response confer any clinical or prognostic information.
GRANTS
This work was supported by contract N01AG82109 (Gary Gerstenblith), and, in part, by the Intramural Research Program of the NIH, National Institute on Aging. VM is currently supported from the State Department of Health, Czech Republic (grant NS 10497-3/2009).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: P.D.C. analyzed data; P.D.C., V.M., S.P.S., G.G., L.C.B., L.F., J.L.F., E.G.L., and S.S.N. interpreted results of experiments; P.D.C. prepared figures; P.D.C., V.M., J.L.F., E.G.L., and S.S.N. drafted manuscript; P.D.C., V.M., S.P.S., G.G., L.C.B., L.F., J.L.F., E.G.L., and S.S.N. edited and revised manuscript; P.D.C., V.M., S.P.S., G.G., L.C.B., L.F., J.L.F., E.G.L., and S.S.N. approved final version of manuscript; J.L.F., E.G.L., and S.S.N. conception and design of research; J.L.F. performed experiments.
*Present address of J. L. Fleg: Division of Cardiovascular Diseases, National Heart, Lung, and Blood Institute, Bethesda, Maryland. ″ Present address of S. S. Najjar: Division of Cardiology, Washington Hospital Center.
ACKNOWLEDGMENTS
The authors thank Susan Townsend, RN, John Clulow, ARRT, CNMT, and Terry Frank, CNMT, MR, for their efforts in exercise testing, acquisition and measurement of the cardiac volumes, and Denis Muller, MS, for statistical guidance.
Present address of J. L. Fleg: Division of Cardiovascular Diseases, National Heart, Lung, and Blood Institute, Bethesda, Maryland.
Present address of S. S. Najjar: Division of Cardiology, Washington Hospital Center.
REFERENCES
- 1. Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, Thomas JD, Palmer D, Levine BD. Effect of Aging and Physical Activity on Left Ventricular Compliance. Circulation 110: 1799–1805, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Armentano RL, Levenson J, Barra JG, Fischer EI, Breitbart GJ, Pichel RH, Simon A. Assessment of elastin and collagen contribution to aortic elasticity in conscious dogs. Am J Physiol Heart Circ Physiol 260: H1870–1877, 1991 [DOI] [PubMed] [Google Scholar]
- 3. Asanoi H, Kameyama T, Ishizaka S, Miyagi K, Sasayama S. Ventriculoarterial coupling during exercise in normal human subjects. Int J Cardiol 36: 177–186, 1992 [DOI] [PubMed] [Google Scholar]
- 4. Baicu CF, Zile MR, Aurigemma GP, Gaasch WH. Left Ventricular Systolic Performance, Function, and Contractility in Patients With Diastolic Heart Failure. 111: 2306–2312, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Belcher P, Boerboom LE, Olinger GN. Standardization of end-systolic pressure-volume relation in the dog. Am J Physiol Heart Circ Physiol 249: H547–553, 1985 [DOI] [PubMed] [Google Scholar]
- 6. Borlaug BA, Melenovsky V, Redfield MM, Kessler K, Chang HJ, Abraham TP, Kass DA. Impact of Arterial Load and Loading Sequence on Left Ventricular Tissue Velocities in Humans. J Am Coll Cardiol 50: 1570–1577, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC, Kass DA. Impaired Chronotropic and Vasodilator Reserves Limit Exercise Capacity in Patients With Heart Failure and a Preserved Ejection Fraction. Circulation 114: 2138–2147, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD, Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol 56: 845–854, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chantler PD, Lakatta EG, Najjar SS. Arterial-Ventricular Coupling: Mechanistic Insights into Cardiovascular Performance at Rest and During Exercise. J Appl Physiol 1342–1351, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chantler PD, Melenovsky V, Schulman SP, Gerstenblith G, Becker LC, Ferrucci L, Fleg JL, Lakatta EG, Najjar SS. The sex-specific impact of systolic hypertension and systolic blood pressure on arterial-ventricular coupling at rest and during exercise. Am J Physiol Heart Circ Physiol 295: H145–153, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chemla D, Antony I, Lecarpentier Y, Nitenberg A. Contribution of systemic vascular resistance and total arterial compliance to effective arterial elastance in humans. Am J Physiol Heart Circ Physiol 285: H614–620, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Chemla D, Hebert JL, Coirault C, Zamani K, Suard I, Colin P, Lecarpentier Y. Total arterial compliance estimated by stroke volume-to-aortic pulse pressure ratio in humans. Am J of Phys 274: H500–505, 1998 [DOI] [PubMed] [Google Scholar]
- 13. Chen CH, Fetics B, Nevo E, Rochitte CE, Chiou KR, Ding PA, Kawaguchi M, Kass DA. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol 38: 2028–2034, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT, Roccella EJ. Committee tNHBPEPC Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure Hypertension 42: 1206–1252, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Cohen-Solal A, Caviezel B, Himbert D, Gourgon R. Left ventricular-arterial coupling in systemic hypertension: analysis by means of arterial effective and left ventricular elastances. J Hypertens 12: 591–600, 1994 [PubMed] [Google Scholar]
- 16. Cohen-Solal A, Faraggi M, Czitrom D, Le Guludec D, Delahaye N, Gourgon R. Left ventricular-arterial system coupling at peak exercise in dilated nonischemic cardiomyopathy. Chest 113: 870–877, 1998 [DOI] [PubMed] [Google Scholar]
- 17. de Simone G, Roman MJ, Koren MJ, Mensah GA, Ganau A, Devereux RB. Stroke Volume/Pulse Pressure Ratio and Cardiovascular Risk in Arterial Hypertension. Hypertension 33: 800–805, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Dehmer GJ, Firth BG, Lewis SE, Willerson JT, Hillis LD. Direct measurement of cardiac output by gated equilibrium blood pool scintigraphy: validation of scintigraphic volume measurements by a nongeometric technique. Am J Cardiol 47: 1061–1067, 1981 [DOI] [PubMed] [Google Scholar]
- 19. Dlin RA, Hanne N, Silverberg DS, Bar-Or O. Follow-up of normotensive men with exaggerated blood pressure response to exercise Am Heart J 106: 316–320, 1983 [DOI] [PubMed] [Google Scholar]
- 20. Elzinga G, Westerhof N. Pressure and flow generated by the left ventricle against different impedances. Circ Res 32: 178–186, 1973 [DOI] [PubMed] [Google Scholar]
- 21. Fleg JL, O'Connor F, Gerstenblith G, Becker LC, Clulow J, Schulman SP, Lakatta EG. Impact of age on the cardiovascular response to dynamic upright exercise in healthy men and women. J Appl Physiol 78: 890–900, 1995 [DOI] [PubMed] [Google Scholar]
- 22. Guyton AC. Determination of cardiac output by equating venous return curves with cardiac response curves. Physiol Rev 35: 123–129, 1955 [DOI] [PubMed] [Google Scholar]
- 23. Haluska BA, Jeffriess L, Brown J, Carlier S, Marwick TH. A comparison of methods for assessing total arterial compliance. J Hum Hypertens 24: 254–262, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation 107: 714–720, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Kelly RP, Ting CT, Yang TM, Liu CP, Maughan WL, Chang MS, Kass DA. Effective arterial elastance as index of arterial vascular load in humans. Circulation 86: 513–521, 1992 [DOI] [PubMed] [Google Scholar]
- 26. Lauer MS, Levy D, Anderson KM, Plehn JF. Is there a relationship between exercise systolic blood pressure response and left ventricular mass? The Framingham Heart Study Ann Intern Med 116: 203–210, 1992 [DOI] [PubMed] [Google Scholar]
- 27. Liang YL, Gatzka CD, Du XJ, Cameron JD, Kingwell BA. EFFECTS OF HEART RATE ON ARTERIAL COMPLIANCE IN MEN. Clin and Exper Pharm and Phys 26: 342–346, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Liu Z, Brin KP, Yin FC. Estimation of total arterial compliance: an improved method and evaluation of current methods. Am J Physiol Heart Circ Physiol 251: H588–600, 1986 [DOI] [PubMed] [Google Scholar]
- 29. Liu Z, Brin KP, Yin FC. Estimation of total arterial compliance: an improved method and evaluation of current methods. Am J Physiol Heart Circ Physiol 251: H588–600, 1986 [DOI] [PubMed] [Google Scholar]
- 30. Mattace-Raso FUS, van der Cammen TJM, Hofman A, van Popele NM, Bos ML, Schalekamp MADH, Asmar R, Reneman RS, Hoeks APG, Breteler MMB, Witteman JCM. Arterial Stiffness and Risk of Coronary Heart Disease and Stroke: The Rotterdam Study. Circulation 113: 657–663, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Matthews CE, Pate RR, Jackson KL, Ward DS, Macera CA, Kohl HW, Blair SN. Exaggerated blood pressure response to dynamic exercise and risk of future hypertension. J Clin Epidemiol 51: 29–35, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Murgo JP, Westerhof N, Giolma JP, Altobelli SA. Effects of exercise on aortic input impedance and pressure wave forms in normal humans. Circ Res 48: 334–343, 1981 [DOI] [PubMed] [Google Scholar]
- 33. Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise Capacity and Mortality among Men Referred for Exercise Testing. New Eng J of Med 346: 793–801, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Najjar SS, Schulman SP, Gerstenblith G, Fleg JL, Kass DA, O′Connor F, Becker LC, Lakatta EG. Age and gender affect ventricular-vascular coupling during aerobic exercise. J Am Coll Cardiol 44: 611–617, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Nichols WW, O'Rourke MF, editor. McDonalds Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. New York: Hodder Arnold, 2005, p. 616 [Google Scholar]
- 36. Ogawa T, Spina RJ, Martin WH, Kohrt WM, 3rd, Schechtman KB, Holloszy JO, Ehsani AA. Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation 86: 494–503, 1992 [DOI] [PubMed] [Google Scholar]
- 37. Otsuki T, Maeda S, Iemitsu M, Saito Y, Tanimura Y, Ajisaka R, Miyauchi T. Contribution of systemic arterial compliance and systemic vascular resistance to effective arterial elastance changes during exercise in humans. Acta Physiol 188: 15–20, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Park S, Ha JW, Shim Y, Choi EY, Kim JM, Ahn JA, Lee SW, Rim SJ, Chung N. Gender-related difference in arterial elastance during exercise in patients with hypertension. Hypertension 51: 1163–1169, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Pauca AL, O′Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension 38: 932–937, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation 112: 2254–2262, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Resnick LM, Militianu D, Cunnings AJ, Pipe JG, Evelhoch JL, Soulen RL, Lester MA. Pulse waveform analysis of arterial compliance: relation to other techniques, age, and metabolic variables. Am J Hypertens 13: 1243–1249, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Segers P, Stergiopulos N, Westerhof N. Relation of effective arterial elastance to arterial system properties. Am J Physiol Heart Circ Physiol 282: H1041–1046, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Segers P, Verdonck P, Deryck Y, Brimioulle S, Naeije R, Carlier S, Stergiopulos N. Pulse pressure method and the area method for the estimation of total arterial compliance in dogs: sensitivity to wave reflection intensity. Ann Biomed Eng 27: 480–485, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Sharabi YBCR, Hanin A, Martonovitch G, Grossman E. The significance of hypertensive response to exercise as a predictor of hypertension and cardiovascular disease. J of Hum Hyper 15: 353–356, 2001 [DOI] [PubMed] [Google Scholar]
- 45. Sharman JE, McEniery CM, Campbell RI, Coombes JS, Wilkinson IB, Cockcroft JR. The effect of exercise on large artery haemodynamics in healthy young men. Euro J of Clin Inves 35: 738–744, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Shock NG, RC, Andres RA, Costa Paul, Lakatta T, Jr, Edward G, Tobin Jordan D. Normal Human Aging: The Baltimore Longitudinal Study of Aging(1984). Washington, DC: U. S. Government Printing Office; Publication no. 84-2450.14., 1984, p. 425 [Google Scholar]
- 47. Sorensen SG, Ritchie JL, Caldwell JH, Hamilton GW, Kennedy JW. Serial exercise radionuclide angiography. Validation of count-derived changes in cardiac output and quantitation of maximal exercise ventricular volume change after nitroglycerin and propranolol in normal men Circulation 61: 600–609, 1980 [DOI] [PubMed] [Google Scholar]
- 48. Stergiopulos N, Meister JJ, Westerhof N. Evaluation of methods for estimation of total arterial compliance. Am J Physiol Heart Circ Physiol 268: H1540–1548, 1995 [DOI] [PubMed] [Google Scholar]
- 49. Stergiopulos N, Segers P, Westerhof N. Use of pulse pressure method for estimating total arterial compliance in vivo. Am J Physiol Heart Circ Physiol 276: H424–428, 1999 [DOI] [PubMed] [Google Scholar]
- 50. Stratton JR, Levy WC, Cerqueira MD, Schwartz RS, Abrass IB. Cardiovascular responses to exercise. Effects of aging and exercise training in healthy men Circulation 89: 1648–1655, 1994 [DOI] [PubMed] [Google Scholar]
- 51. Sunagawa K, Maughan WL, Burkhoff D, Sagawa K. Left ventricular interaction with arterial load studied in isolated canine ventricle. Am J Physiol Heart Circ Physiol 245: H773–780, 1983 [DOI] [PubMed] [Google Scholar]
- 52. Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A. for the Health A BCS Elevated Aortic Pulse Wave Velocity, a Marker of Arterial Stiffness, Predicts Cardiovascular Events in Well-Functioning Older Adults Circulation 111: 3384–3390, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Westerhof N, Lankhaar JW, Westerhof BE. The arterial Windkessel. Med Biol Eng Comput 47: 131–141, 2009 [DOI] [PubMed] [Google Scholar]