Abstract
The exact role of arousal in central and peripheral hemodynamic responses to passive limb movement in humans is unclear but has been proposed as a potential contributor. Thus, we used a human model with no lower limb afferent feedback to determine the role of arousal on the hemodynamic response to passive leg movement. In nine people with a spinal cord injury, we compared central and peripheral hemodynamic and ventilatory responses to one-leg passive knee extension with and without visual feedback (M+VF and M-VF, respectively) as well as in a third trial with no movement or visual feedback but the perception of movement (F). Ventilation (V̇e), heart rate, stroke volume, cardiac output, mean arterial pressure, and leg blood flow (LBF) were evaluated during the three protocols. V̇e increased rapidly from baseline in M+VF (55 ± 11%), M-VF (63 ± 13%), and F (48 ± 12%) trials. Central hemodynamics (heart rate, stroke volume, cardiac output, and mean arterial pressure) were unchanged in all trials. LBF increased from baseline by 126 ± 18 ml/min in the M+VF protocol and 109 ± 23 ml/min in the M-VF protocol but was unchanged in the F protocol. Therefore, with the use of model that is devoid of afferent feedback from the legs, the results of this study reveal that, although arousal is invoked by passive movement or the thought of passive movement, as evidenced by the increase in V̇e, there is no central or peripheral hemodynamic impact of this increased neural activity. Additionally, this study revealed that a central hemodynamic response is not an obligatory component of movement-induced LBF.
Keywords: blood flow, cardiac output, exercise, afferent feedback
local vasoregulation in exercising skeletal muscle is certainly an important factor in the initiation of exercise-induced hyperemia (10), but further examination of this phenomenon reveals the importance of neural influences on muscle blood flow (24). Recently, our group (17, 22, 26) and others (19) have underlined the role of afferent signals that invoke cardioacceleration, which supports the hyperemic response to passive limb movement. Similarly, efferent signals from the brain have been documented to invoke cardiovascular responses (11, 21). Indeed, recent research in this area has revealed neural networks in the brain that are involved in centrally mediated cardiovascular activation, which do not appear to require parallel motor activation (21) and thus could play a role in the cardiovascular response to passive movement. However, it is currently not clear if “arousal,” defined as an increase in brain activity independent of motor command (4), at the onset of passive movement influences movement-induced central and peripheral hemodynamic responses.
One experimental approach to examine the role of afferent feedback on the movement-induced hyperemic response is to perform passive movement while blocking feedback from mechanoceptors and chemoreceptors in the moving limb. Indeed, our group (22) recently used an intrathecal fentanyl injection to partially block this afferent nerve traffic and identified a critical role for this feedback in both central and peripheral hemodynamic responses as well as ventilatory responses to passive limb movement. Although it would have been interesting to take advantage of this reduced afferent feedback to examine the role of efferent signals in isolation, the fentanyl block allows enough sensory perception for this to be impossible. However, subjects with a complete lesion of their spinal cord (SCI) offer a “naturally” occurring human model of a complete block of all afferent signals from the lower limbs and therefore no sensory perception from below the lesion. Thus, the SCI model affords the unique opportunity to investigate the role of arousal during passive movement in the absence of movement-induced afferent feedback, from below the lesion, without the need for a pharmacological intervention.
Consequently, we used the human SCI model to better understand the contribution of arousal on central and peripheral hemodynamic responses to passive limb movement. Specifically, we studied 1) passive limb movement with visual feedback and the perception of such movement (M+VF), 2) passive limb movement with no visual feedback but the perception of such movement (M-VF), and 3) no passive limb movement and no visual feedback but the false perception of such movement (F). Two hypotheses motivated this investigation: 1) there will be a minimal but significant arousal associated with passive limb movement or the thought of passive limb movement, as evidenced by an increase in ventilation (V̇e) and heart rate (HR) despite a lack of afferent feedback from the limb being moved and 2) arousal, although present, will not be of significant magnitude to affect peripheral hemodynamic responses to passive limb movement.
METHODS
Subjects.
Nine people with a SCI (7 men and 2 women, age: 42 ± 9 yr, weight: 74 ± 10 kg, height: 180 ± 12 cm) participated in this study. All subjects had clinically confirmed complete lesions between the 6th (T-6) and 12th thoracic vertebra (T-12; American Spinal Injury Association class A) (16). The level of lesion was also tested in the laboratory by neurological exam to confirm the absence of feedback (cutaneous pinprick and cold perception) on the lumbar region and legs and the maintenance of such sensations on the upper torso and arms. The time postinjury was 16 ± 13 yr (range: 2–36 yr), and, based on the Ashworth test (1), at the time of evaluation none of the subjects exhibited muscle spasticity. However, two of the subjects were taking Baclofene to avoid spasticity. None of the subjects were smokers, and most were physically active, performing endurance-type exercise 6.5 ± 3.5 h/wk. Descriptive characteristics of the subjects are shown in Table 1. All procedures conformed with the standards set by the Declaration of Helsinki, and the Institutional Review Boards of the University of Utah and Salt Lake City Veterans Affairs Medical Center approved the study. Written informed consent was obtained by all subjects before their participation. All protocols were performed in a thermoneutral environment (22°C). Subjects reported to the laboratory in the fasted state and had not performed exercise within the past 24 h.
Table 1.
Subject characteristics
| Subjects | Mean | SE | Range |
|---|---|---|---|
| Age, yr | 42 | 9 | 28–54 |
| Mass, kg | 74 | 10 | 63–94 |
| Height, m | 1.80 | 0.12 | 1.62–1.90 |
| Thigh mass, kg | 3.1 | 0.3 | 2.0–4.3 |
| American Spinal Injury Association grade | A | A–A | |
| Time postinjury, yr | 16 | 13 | 2–36 |
| Quadriceps spasticity Ashworth scale (from 0 to 4) | 0 | 0 | |
| Glucose, mg/dl | 97 | 11 | 73–162 |
| Cholesterol, mg/dl | 160 | 8 | 129–186 |
| HDL, mg/dl | 43 | 5 | 28–63 |
| LDL, mg/dl | 98 | 5 | 86–129 |
| Triglycerides, mg/dl | 96 | 15 | 56–152 |
| Hemoglobin, g/dl | 14.6 | 0.3 | 13.2–15.4 |
| White blood cells, ×103 cells/μl | 4.4 | 0.7 | 4.1–6.7 |
| Neutrophils, ×103 cells/μl | 2.6 | 0.3 | 1.91–3.49 |
| Lymphocytes, ×103 cells/μl | 1.7 | 0.2 | 1.06–2.52 |
| Monocytes, ×103 cells/μl | 0.43 | 0.03 | 0.32–0.56 |
Data are presented as means ± SE. All subjects had a spinal cord injury between T-6 and T12. The American Spinal Injury Association (16) score was used to classify the severity of the lesion, where A = sensory and motor complete. The quadriceps spasticity Ashworth scale (1) was used to classify the severity of muscular spasms during passive movements, where 0 = no spasms.
Passive movement protocols.
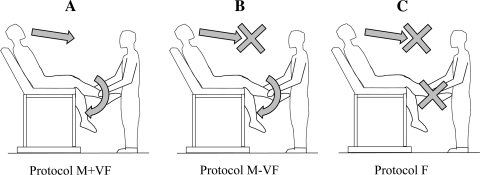
To avoid the potentially confounding effect of vestibular feedback and to avoid possible contractions of upper body accessory muscles, subjects were comfortably positioned on a contoured adjustable chair designed for 20 min before the start of data collection and remained in this position throughout the study. The first protocol consisted of 30 s of resting baseline followed by 2 min of passive knee extension with visual feedback (M+VF protocol; Fig. 1A). Passive exercise was achieved by a member of the research team moving the subject's lower leg through the range of motion of 90° (knee joint angle) at 1 Hz (throughout the protocol the control leg was supported in fully extended position). Real-time feedback was provided to the researcher moving the limb by a position sensor, ensuring a consistent range of motion, and a metronome was used to maintain cadence. The M-VF protocol (Fig. 1B) was identical to the M+VF protocol except there was no longer any visual feedback. This lack of visual feedback was achieved by drawing a curtain across the subject at waist level, obscuring the legs and the researcher in charge of limb movement. In the F protocol (Fig. 1C), which was also performed behind the curtain, the subject's leg was not actually moved; however, researchers provided other feedback (chair movement that was typically associated with leg movement, the metronome, etc.) to provide the perception of limb movement. Thus, in all three protocols, subjects were under the impression that their leg was being passively moved, and, in each scenario, they were instructed to think that they were experiencing passive limb movement. Each protocol was separated by 10 min of rest, and the order of the M-VF and F protocols were balanced.
Fig. 1.
Schematic representation of the three experimental protocols. The first protocol consisted of passive limb movement with visual feedback (M+VF; A). The second protocol (M-VF; B) was identical to the M+VF protocol with the exception that there was no visual feedback, which was achieved by drawing a curtain across the subject at waist level. In the third protocol (F; C) there was no passive limb movement and no visual feedback, but subjects perceived that their leg was moving.
Femoral blood flow.
Measurements of arterial blood velocity and vessel diameter were taken in both legs distal to the inguinal ligament and proximal to the deep superficial femoral bifurcation with Logiq-7 and Logiq-e ultrasound systems (General Electric Medical Systems, Milwaukee, WI). The ultrasound systems were equipped with 12- to 14-MHz linear array transducers. Artery diameter was determined at a 90° angle along the central axis of the scanned area. Blood velocity was measured using the same probes using a frequency of 5 MHz. Measurements of blood velocity were obtained with the probe positioned to maintain an insonation angle of 60° or less, and the sample volume was centered and maximized according to vessel size. Arterial diameter was measured, and second-by-second blood velocity (angle corrected and intensity weighted) was automatically calculated using Logiq-7 and Logiq-e software. Combining arterial diameter and mean blood velocity, blood flow was calculated as follows: blood flow = mean blood velociy × π(vessel diameter/2)2 × 60, where blood flow is in milliliters per minute. In our hands, the femoral blood flow response to passive limb movement was both robust and reproducible, with a coefficient of variation of <10%.
Central hemodynamics.
HR, stroke volume (SV), cardiac output (CO), and mean arterial pressure (MAP) were determined using a Finometer (Finapres Medical Systems, Amsterdam, The Netherlands). SV was estimated using the Modelflow algorithm (Beatscope version 1.1a, Finapres Medical Systems) (6). CO was then calculated as the product of HR and SV. The same method has been documented to accurately track CO during exercise (2, 20). Vascular conductance was calculated as leg blood flow × MAP−1.
V̇e and metabolic responses.
Throughout each protocol, V̇e, O2 consumption (V̇o2), and CO2 production (V̇co2) were measured using a metabolic cart (TrueOne 2400, Parvo Medics, Salt Lake City, UT).
Thigh mass.
Thigh volume was calculated as previously described (15) based on thigh circumference (three sites: distal, middle, and proximal), thigh length, and thigh skinfold measurements. The thigh volume in liters was then converted into kilograms of mass (thigh volume × body density) (9, 14).
Data acquisition.
During each protocol, HR, SV, CO, MAP, ECG, and knee joint angle underwent analog-to-digital conversion and were simultaneously acquired (200 Hz) by commercially available data-acquisition software (AcqKnowledge, Biopac Systems, Goleta, CA).
Data collection and analysis.
The data acquisition software allowed the second-by-second analysis of HR, SV, CO, and MAP throughout the protocols. Mean blood velocity was analyzed with this time resolution on Doppler ultrasound systems (GE Logiq-7 and Logiq-e) for 30 s at rest and the first 60 s of movement; however, 12-s averages were then used from 60 to 120 s of movement. A 3-s rolling average was applied to the data for HR, SV, CO, MAP, blood flow, and vascular conductance. For this study and in other our investigations (12, 18, 22, 23), we compared data that were averaged across 3, 5, 10, and 15 s. The 3-s averages provided the best balance between the quality and real variance of the signal. V̇e was measured breath by breath and averaged second by second; V̇o2 and V̇co2 were assessed with 20-s averaging throughout the protocols. Repeated t-tests were used in an exploratory manner to determine when exercise HR, SV, CO, MAP, blood flow, femoral vascular conductance, and V̇e differed from the 30 s of baseline measurement. Due to the exploratory nature of these statistical analyses, there were no post hoc corrections made for multiple comparisons, an approach used previously (17). Repeated-measures ANOVA was also used to determine significant differences between the M+VF, M-VF, and F conditions. After these analyses, where indicated, a Tukey post hoc test was used to determine which of the trials was different. Significance was set at an α-level of 0.05, and data are presented as means ± SE throughout.
RESULTS
Subject characteristics.
All subjects took part in the three experimental protocols (M+VF, M-VF, and F) without problems or discomfort, and none experienced spastic muscle contractions during the passive movement (Table 1). In all subjects, neurological exam confirmed no feedback from the lumbar region or legs, whereas such sensations were intact in the upper torso and arms. A questionnaire completed at the end of the study revealed that all subjects had the same perception of limb movement in the M+VF, M-VF, and F protocols and were thus unable to discern M-VF and F protocols.
V̇e and metabolic responses.
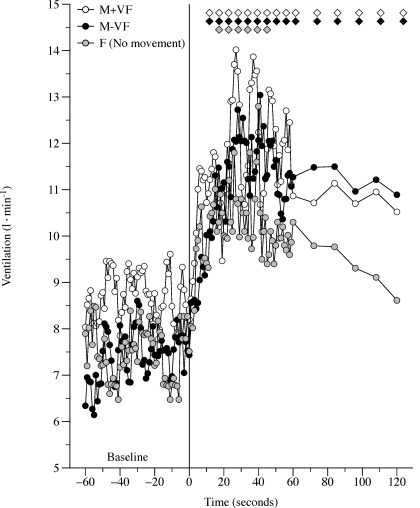
Pulmonary V̇e over time during each of the three protocols (M+VF, M-VF, and F) is shown in Fig. 2. Each protocol resulted in a rapid and significant increase in V̇e (range: 50–63%). Temporal resolution of the average response was blurred somewhat, as with all variables (Figs. 2–4). There were no differences in terms of individual maximal changes in V̇e among M+VF, M-VF, and F conditions (Table 2). Resting V̇co2 was similar in the three protocols (∼0.20 l/min, P = 0.7), and average V̇co2 within 30–50 s of limb movement, or the perception of movement, was not significantly increased from baseline (M+VF: 0.24 ± 0.05 l/min, M-VF: 0.22 ± 0.07 l/min, and F: 0.24 ± 0.04 l/min, all P > 0.7). Similarly, resting V̇o2 was similar before the three conditions (∼0.22 l/min, P = 0.8) and did not significantly increase during M+VF (0.29 ± 0.05 l/min), M-VF (0.30 ± 0.07 l/min), and F (0.31 ± 0.08 l/min) protocols (all P > 0.7).
Fig. 2.
Ventilatory responses to passive movement in the M+VF, M-VF, and F protocols. Note that the responses shown are the average responses of all subjects; therefore, the maximal peak values are underestimated. Diamonds indicate significant differences from the 60-s baseline average. Due to the multiple trials, variance in the data was omitted for clarity.
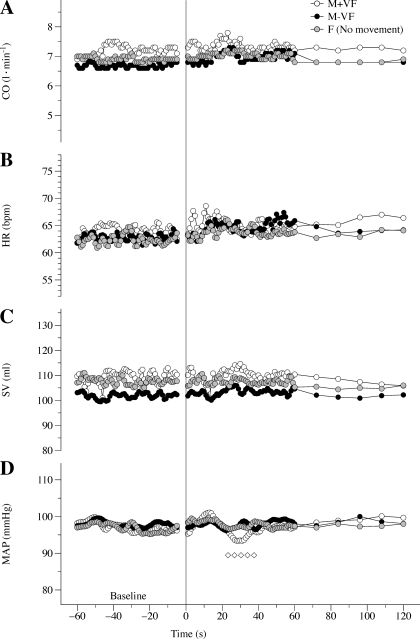
Fig. 3.
Central hemodynamic responses to passive movement in the M+VF, M-VF, and F protocols. A: cardiac output (CO) over time. B: heart rate [HR; in beats/min (bpm)] over time. C: stroke volume (SV) over time. D: mean arterial pressure (MAP) over time. Note that the responses shown are the average responses of all subjects; therefore, the maximal peak values are underestimated. Diamonds indicate significant differences from the 60-s baseline average. Due to the multiple trials, variance in the data was omitted for clarity.
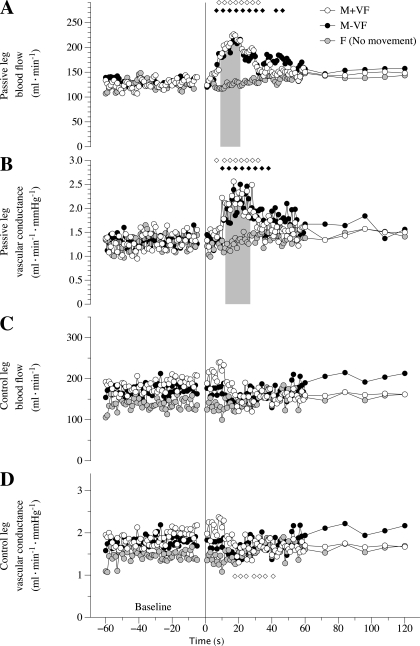
Fig. 4.
Peripheral hemodynamic responses to passive movement in the M+VF, M-VF, and F protocols. A: passive leg blood flow over time. B: passive leg vascular conductance over time. C: control leg blood flow over time. D: control leg vascular conductance over time. Note that the responses shown are the average responses of all subjects; therefore, the maximal peak values are underestimated. Diamonds indicate significant differences from the 60-s baseline average. The shaded bars in A and B indicate significantly lower values in the F protocol with respect to both the M+VF and M-VF protocols. Due to the multiple trials, variance in the data was omitted for clarity.
Table 2.
Rest and maximum central and peripheral hemodynamic reponses as well as ventilation responses in response to passive movement in the M+VF, M-VF, and F protocols
| Rest | Maximum | Absolute Change From Rest | |
|---|---|---|---|
| Ventilation, l/min | |||
| M+VF | 9 ± 0.1 | 14 ± 0.2* (45) | 5 |
| M-VF | 8 ± 0.1 | 13 ± 0.1* (43) | 5 |
| F | 8 ± 0.1 | 12 ± 0.2* (40) | 4 |
| Cardiac output, l/min | |||
| M+VF | 7.2 ± 0.1 | 7.8 ± 0.1 | 0.6 |
| M-VF | 6.7 ± 0.1 | 7.3 ± 0.2 | 0.6 |
| F | 6.9 ± 0.1 | 7.2 ± 0.1 | 0.3 |
| Heart rate, beats/min | |||
| M+VF | 64 ± 0.3 | 69 ± 0.3 | 5 |
| M-VF | 63 ± 0.2 | 67 ± 0.2 | 4 |
| F | 62 ± 0.3 | 66 ± 0.3 | 4 |
| Stroke volume, ml | |||
| M+VF | 109 ± 0.8 | 115 ± 0.9 | 6 |
| M-VF | 102 ± 0.4 | 106 ± 0.5 | 4 |
| F | 108 ± 0.5 | 111 ± 0.7 | 3 |
| Mean arterial pressure, mmHg | |||
| M+VF | 97 ± 0.4 | 93 ± 0.6* (29) | −4 |
| M-VF | 98 ± 0.3 | 96 ± 0.5 | −2 |
| F | 97 ± 0.4 | 96 ± 0.5 | −1 |
| Passive leg blood flowm ml/min | |||
| M+VF | 127 ± 5.6 | 253 ± 25* (17) | 126 |
| M-VF | 131 ± 3.6 | 240 ± 31* (17) | 109 |
| F | 124 ± 4.9 | 156 ± 23† | 32 |
| Passive leg vascular conductance, ml·min−1·mmHg−1 | |||
| M+VF | 1.3 ± 0.1 | 2.6 ± 0.2* (17) | 1.3 |
| M-VF | 1.3 ± 0.1 | 2.5 ± 0.2* (21) | 1.2 |
| F | 1.3 ± 0.1 | 1.5 ± 0.2† | 0.3 |
| Control leg blood flow, ml/min | |||
| M+VF | 183 ± 4.7 | 240 ± 75 | 57 |
| M-VF | 172 ± 4.1 | 210 ± 90 | 38 |
| F | 138 ± 4.5 | 184 ± 68 | 46 |
| Control leg vascular conductance, ml·min−1·mmHg−1 | |||
| M+VF | 1.9 ± 0.1 | 1.3 ± 0.2* (20) | −0.6 |
| M-VF | 1.8 ± 0.1 | 1.4 ± 0.3 | −0.4 |
| F | 1.5 ± 0.1 | 1.3 ± 0.2 | −0.2 |
Data are means ± SE. The M+VF protocol consisted of passive limb movement with visual feedback. The M-VF protocol was identical to the M+VF protocol with the exception that there was no visual feedback. In the F protocol, there was no passive limb movement and no visual feedback, but subjects perceived that their leg was moving.
Maximum values were significantly different from baseline;
significant difference between exercise conditions. The times required to reach the maximum value (if significantly different from baseline) are shown parentheses.
Central hemodynamic responses.
Baseline HR, SV, and CO were unchanged in all three protocols as a consequence of passive movement (M+VF and M-VF protocols) or no movement (F protocol; Fig. 3). Only in the M+VF protocol was there a short and transient fall in MAP from 25 to 38 s of passive movement. In terms of identifying a maximal response, this transient fall in MAP during the M+VF protocol was the only significant peak in the central hemodynamic responses to passive limb movement.
Peripheral hemodynamic responses.
In the passively moved leg, there was a significant increase in both blood flow and vascular conductance above baseline in the M+VF and M-VF protocols from approximately the 8th to 30th sec of limb movement (Fig. 4). During the F protocol, leg blood flow and vascular conductance did not change from baseline. Indeed, there was a significantly greater blood flow and vascular conductance response in the M+VF and M-VF protocols compared with the F protocol between ∼9 and ∼25 s of passive movement (Fig. 4A, shaded bar). Leg blood flow and vascular conductance in the control leg (Fig. 4, C and D) were similar and invariant in all three conditions, with the only exception being a transient fall in vascular conductance compared with baseline in the M+VF protocol for seconds 19–33 of passive movement. However, this change was not significantly different from the M-VF and F protocols. In terms of maximal responses, in the passively moved leg, the M+VF and M-VF protocols produced significantly greater peak responses in both blood flow and vascular conductance compared with the F protocol, which, essentially, had no discernable change and therefore no real peak (Table 2).
DISCUSSION
This study examined the contribution of arousal to central and peripheral hemodynamic responses associated with the onset of passive limb movement. To this end, in subjects with a complete lesion of their spinal cord, we compared HR, SV, CO, MAP, leg blood flow, leg vascular conductance, and V̇e responses to M+VF, M-VF, and F protocols (Fig. 1). The major finding of this study is that, using a model where there is neither afferent feedback nor voluntary muscular contraction, arousal can be clearly documented to be present by the ventilatory response to passive movement in all three conditions, but this phenomenon does not play a significant role in either central or peripheral hemodynamic responses. Additionally, as there was a significant increase in leg blood flow and leg vascular conductance with leg movement, despite the lack of afferent feedback, this study highlights the peripheral vasodilatory factors that can function without a significant concomitant central hemodynamic response. Therefore, in combination, these findings provide evidence that passive limb movement-induced hyperemia need not be significantly influenced by the descending neural signal that originates from higher brain centers (arousal). Additionally, the results of this study reveal that a central hemodynamic response is not an obligatory component of the movement-induced increase in leg blood flow and highlight that the stimuli responsible must, in this case, be peripheral in origin.
Evidence of arousal.
By experimental design, the present subjects all lacked afferent feedback from the lower limbs due to a SCI, and, despite this condition, previous investigations (8) have documented an immediate ventilatory response at the onset of electrically induced muscular contractions in this population. The only explanation for this observation is that it is the descending neural signal originating in the cortex that drives this transitory response (7). Other studies have revealed that the magnitude and onset kinetics of the ventilatory response are clearly linked to the arousal (3, 5) and not predominantly caused by changes in V̇o2 and V̇co2 (13). Moreover, the offset kinetics of ventilation may be influenced by the combined effect of a fall in arousal and chemoreceptor feedback, but this will require further study. Therefore, monitoring this particular response in people with a SCI during passive movement provides an index of the magnitude of arousal. Indeed, in an elegant study, Bell et al. (4) revealed that, in intact humans, afferent feedback from the muscle bed stimulates V̇e at the onset of passive movement. Interestingly, when a cognitive task was superimposed on the passive movement, the V̇e response was inactivated, indicating that arousal, and not solely afferent feedback, plays a role in this ventilatory response. However, unlike the present study, Bell et al. (4) did not focus on the cardiovascular responses to the passive movement. In the present study, the increase in V̇e despite the SCI, across not only the two trials with movement (M+VF and M-VF protocols) but, most importantly, when the subjects only perceived that their leg was moving but it was not (F protocol), provides evidence of a significant and similar arousal in all three conditions.
Central hemodynamics: the role of afferent signals and arousal.
It has been well documented that in the intact human, passive limb movement induces a significant, albeit transient, increase in central hemodynamic variables (HR, SV, CO, and MAP) (17, 22). In contrast, in the present subjects with a SCI, central hemodynamics did not significantly differ from baseline on the initiation of passive limb movement (Fig. 3). Although even more clear here, this annulling of the central hemodynamic response to limb movement was similar to our previously published findings of an attenuated response (33% reduced) with the partial pharmacological blockade (fentanyl) of type III and IV afferents in able-bodied volunteers (22). Therefore, in combination with our previous work (22), these data not only highlight the importance of afferent signals from the passively moved limb in the instigation of a central hemodynamic response but also provide the opportunity to document that arousal (which was evident in the ventilatory response of these subjects) does not appear to play a significant role in terms of central cardiovascular responses to passive limb movement.
Peripheral hemodynamics: the role of afferent signals and arousal.
It has also been well documented that, in the intact human, passive limb movement induces a transitory increase in peripheral hemodynamic variables (leg blood flow and vascular conductance) (17, 22, 23). This was again the case in the present subjects with a SCI, who also exhibited a significant transient increase in both leg blood flow and leg vascular conductance as a consequence of passive limb movement (Fig. 4). Although in the intact physiological system there is significant evidence that multifactorial neural control, involving regions of the cerebral cortex and signals from peripheral afferent receptors, play an important role in the regulation of blood flow to the brain (24, 25), such regulation of skeletal muscle blood flow is not so clear. In the present study, which was performed in people with no neural afferent and efferent transmission to the lower limbs, the very similar peripheral hemodynamic responses in both the M+VF and M-VF trials, in which the leg was actually passively moved, a factor that was absent in the motionless F trial, confirms that neural control is not obligatory for movement-induced hyperemia. Additionally, these findings highlight the importance of the local mechanical stimuli in this movement-induced peripheral hemodynamic response (10).
Integration of central and peripheral hemodynamic responses to limb movement.
At the onset of exercise, a complex combination of both peripheral and central hemodynamic factors contribute to the increase in blood flow to active skeletal muscle. Recently, our group (12, 18, 22, 23) has used passive movement as a reductionist model in which exercise-induced hyperemia can be studied without the increased complexity of altered muscle metabolism. To better understand the integration of mechanical and neurological factors that contribute to the immediate increase in blood flow to a moving limb, we (12, 22) studied heart transplant recipients with denervated hearts and young subjects with a partial block of afferent nerve fibers. These studies revealed that passive movement-induced hyperemia in the intact human is associated with a significant central hemodynamic response (∼5–24%), which, in turn, supports and sustains the elevated limb blood flow (12, 22).
Due to the already discussed failure of arousal to induce a measureable central hemodynamic effect during passive limb movement and the SCI-induced absence of neurological feedback from the limb, a central hemodynamic response was not significantly invoked in the present subjects (Fig. 3). Despite the absence of this central hemodynamic response, there was a clear and significant transient increase in leg blood flow and vascular conductance (Fig. 4). However, it should be noted that, in agreement with our other recent work (12), where there was a greatly attenuated central hemodynamic response in heart transplant recipients, the magnitude of the hyperemic response in people with a SCI was limited (∼90% increase) compared with our previously published data from healthy subjects in a supine position of ∼285% (22). Moreover, even when normalized for the somewhat reduced thigh muscle mass, the magnitude of hyperemia was ∼38 ml·min−1·kg−1 in the present subjects with a SCI compared with ∼190 ml·min−1·kg−1 in able-bodied subjects (23), implying that this attenuated hyperemic response was not just a consequence of reduced limb volume. The explanation for this difference remains unclear, but the possibility that this attenuated hyperemia may be the consequence of peripheral vascular dysfunction of the lower paralyzed limbs cannot be ruled out. However, this was not the focus of the present study, which was to better understand the mechanisms responsible for limb movement-induced hyperemia. The careful assessment of central and peripheral responses to such limb movement in people with an SCI may have important implications for rehabilitative medicine in this population.
Conclusions.
This study documented that passive limb movement is associated with an increase in arousal, as evidenced by the significant increase in V̇e. However, this clearly documented increase in arousal did not contribute to either central or peripheral hemodynamic responses to passive limb movement. Additionally, the results of this study reveal that a central hemodynamic response is not an obligatory component of movement-induced leg blood flow.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grant HL-09183 and University of Utah College of Health and Center for Rehabilitative Research Grant CRR-F 2008-04.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.V. and J.D.T. performed experiments; M.V., M.K.A., J.M., J.D.T., and A.S.F. analyzed data; M.V. and R.S.R. interpreted results of experiments; M.V. and M.K.A. prepared figures; M.V., M.K.A., J.M., J.D.T., and A.S.F. drafted manuscript; M.V. and R.S.R. edited and revised manuscript; M.K.A. and R.S.R. conception and design of research; R.S.R. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors greatly appreciate the time and effort of the subjects that participated in this study.
REFERENCES
- 1. Ashworth B. Preliminary trial of carisoprodol in multiple sclerosis. Practitioner 192: 540–542, 1964 [PubMed] [Google Scholar]
- 2. Azabji Kenfack M, Lador F, Licker M, Moia C, Tam E, Capelli C, Morel D, Ferretti G. Cardiac output by Modelflow method from intra-arterial and fingertip pulse pressure profiles. Clin Sci 106: 365–369, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Bell HJ, Duffin J. CO2 does not affect passive exercise ventilatory decline. J Appl Physiol 95: 322–329, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Bell HJ, Duffin J. Respiratory response to passive limb movement is suppressed by a cognitive task. J Appl Physiol 97: 2112–2120, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Bell HJ, Feenstra W, Duffin J. The initial phase of exercise hyperpnoea in humans is depressed during a cognitive task. Exp Physiol 90: 357–365, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Bogert LW, van Lieshout JJ. Non-invasive pulsatile arterial pressure and stroke volume changes from the human finger. Exp Physiol 90: 437–446, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Brice AG, Forster HV, Pan LG, Funahashi A, Hoffman MD, Murphy CL, Lowry TF. Is the hyperpnea of muscular contractions critically dependent on spinal afferents? J Appl Physiol 64: 226–233, 1988 [DOI] [PubMed] [Google Scholar]
- 8. Brown DR, Forster HV, Pan LG, Brice AG, Murphy CL, Lowry TF, Gutting SM, Funahashi A, Hoffman M, Powers S. Ventilatory response of spinal cord-lesioned subjects to electrically induced exercise. J Appl Physiol 68: 2312–2321, 1990 [DOI] [PubMed] [Google Scholar]
- 9. Bulbulian R, Johnson RE, Gruber JJ, Darabos B. Body composition in paraplegic male athletes. Med Sci Sports Exerc 19: 195–201, 1987 [PubMed] [Google Scholar]
- 10. Clifford PS, Tschakovsky ME. Rapid vascular responses to muscle contraction. Exerc Sport Sci Rev 36: 25–29, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Gregg ME, James JE, Matyas TA, Thorsteinsson EB. Hemodynamic profile of stress-induced anticipation and recovery. Int J Psychophysiol 34: 147–162, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Hayman MA, Nativi JN, Stehlik J, McDaniel J, Fjeldstad AS, Ives SJ, Wray DW, Bader F, Gilbert EM, Richardson RS. Understanding exercise-induced hyperemia: central and peripheral hemodynamic responses to passive limb movement in heart transplant recipients. Am J Physiol Heart Circ Physiol 299: H1653–H1659, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Horner RL, Rivera MP, Kozar LF, Phillipson EA. The ventilatory response to arousal from sleep is not fully explained by differences in CO2 levels between sleep and wakefulness. J Physiol 534: 881–890, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jackson AS. Research design and analysis of data procedures for predicting body density. Med Sci Sports Exerc 16: 616–622, 1984 [PubMed] [Google Scholar]
- 15. Jones PR, Pearson J. Anthropometric determination of leg fat and muscle plus bone volumes in young male and female adults. J Physiol 204: 63P–66P, 1969 [PubMed] [Google Scholar]
- 16. Maynard FM, Jr, Bracken MB, Creasey G, Ditunno JF, Jr, Donovan WH, Ducker TB, Garber SL, Marino RJ, Stover SL, Tator CH, Waters RL, Wilberger JE, Young W. International standards for neurological and functional classification of spinal cord injury. American Spinal Injury Association. Spinal Cord 35: 266–274, 1997 [DOI] [PubMed] [Google Scholar]
- 17. McDaniel J, Fjeldstad AS, Ives S, Hayman M, Kithas P, Richardson RS. Central and peripheral contributors to skeletal muscle hyperemia: response to passive limb movement. J Appl Physiol 108: 76–84, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McDaniel J, Hayman MA, Ives S, Fjeldstad AS, Trinity JD, Wray DW, Richardson RS. Attenuated exercise-induced hyperemia with age: mechanistic insight from passive limb movement. J Physiol 588: 4507–4517, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nobrega AC, Araujo CG. Heart rate transient at the onset of active and passive dynamic exercise. Med Sci Sports Exerc 25: 37–41, 1993 [PubMed] [Google Scholar]
- 20. Tam E, Azabji Kenfack M, Cautero M, Lador F, Antonutto G, di Prampero PE, Ferretti G, Capelli C. Correction of cardiac output obtained by Modelflow from finger pulse pressure profiles with a respiratory method in humans. Clin Sci 106: 371–376, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Trinder J, Padula M, Berlowitz D, Kleiman J, Breen S, Rochford P, Worsnop C, Thompson B, Pierce R. Cardiac and respiratory activity at arousal from sleep under controlled ventilation conditions. J Appl Physiol 90: 1455–1463, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Trinity JD, Amann M, McDaniel J, Fjeldstad AS, Barrett-O'Keefe Z, Runnels S, Morgan DE, Wray DW, Richardson RS. Limb movement-induced hyperemia has a central hemodynamic component: evidence from a neural blockade study. Am J Physiol Heart Circ Physiol 299: H1693–H1700, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Trinity JD, McDaniel J, Venturelli M, Fjeldstad AS, Ives SJ, Witman MA, Barrett-O'Keefe Z, Amann M, Wray DW, Richardson RS. Impact of body position on central and peripheral hemodynamic contributions to movement-induced hyperemia: implications for rehabilitative medicine. Am J Physiol Heart Circ Physiol 300: H1885–H1889, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vianna LC, Araujo CG, Fisher JP. Influence of central command and muscle afferent activation on anterior cerebral artery blood velocity responses to calf exercise in humans. J Appl Physiol 107: 1113–1120, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Williamson JW, Fadel PJ, Mitchell JH. New insights into central cardiovascular control during exercise in humans: a central command update. Exp Physiol 91: 51–58, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Wray DW, Donato AJ, Uberoi A, Merlone JP, Richardson RS. Onset exercise hyperaemia in humans: partitioning the contributors. J Physiol 565: 1053–1060, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]