Abstract
Tumor invasion and metastasis is a multi-step process that requires adaptation of cancer cells to conditions that they encounter during their journey to distant body sites. Understanding the molecular processes that underlie this adaptation is of exceeding importance because most cancer patients die because of metastases rather than primary tumors. In this review we assess genetically engineered mouse models (GEMMs) that have been established to investigate mechanisms of cancer invasion and metastasis.
Introduction
Tumor progression is associated with distinct histopathological changes that are indicative for prognosis. Stromalization and microvessel density increase during tumor progression and tumor cells establish heterotypic molecular interactions with stromal cells that support invasiveness. The acquired ability of tumor cells to locally invade adjacent tissues is the first important step in the invasion-metastasis cascade [1]. Two general strategies have been pursued in mice to investigate invasive tumor progression. The first strategy uses genetically engineered mouse models (GEMMs) that are suitable for the investigation of early steps in cancer invasion but infrequently give rise to far distant metastases. The second strategy uses transplantation of tumor cells or tumor tissue into host mice and is employed to study early invasiveness but also later stages such as intravasation, extravasation and colonization of tumor cells [2]. Here, we focus on GEMMs for major malignancies in humans and the progress that has been made to model human metastasis.
GEMMs as models for cancer invasion and metastasis
Tumors that appear in GEMMs as a result of the corresponding genetic manipulation often show a low incidence to develop far distant metastasis. This is also true for deletions of tumor suppressor genes that are associated with a high metastatic potential in human cancers. The long latency period makes it difficult to predict the onset of metastasis which impedes monitoring in mice despite the availability of advanced in vivo imaging techniques. Therefore, many researchers favor the use of transplantation models to study metastasis although they might neglect the impact of the immune system or skip important initial steps in the metastatic cascade such as local tissue invasion or intravasation [1,2]. However, several recently established GEMMs might represent promising new tools to investigate invasive processes and metastasis in certain cancer types (Table 1).
Table 1.
GEMMs that display invasive and metastasizing tumors (list is not complete). %: percent of mice with metastases; LN: lymph nodes; GI: gastrointestinal tract; pan.: pancreas; kid.: kidney; AG: adrenal gland; epid.: epididymis; ov.: ovary
| Mouse model | Tumor | Metastasis | % | Refs | Weblink |
|---|---|---|---|---|---|
| MMTV-rtTA/TetO-NeuNT | Breast | Lung | 92 | Moody (2002) [5] | http://www.ncbi.nlm.nih.gov/pubmed/12498714 |
| MMTV-ErbB2/MMTV-Cre/PTENflox/flox | Breast | Lung | 87.5 | Schade (2009) [37] | http://www.ncbi.nlm.nih.gov/pubmed/19435886 |
| MMTV-PyMT | Breast | LN, lung | 80–100 | Maglione (2001) [4] | http://www.ncbi.nlm.nih.gov/pubmed/11719463 |
| Wap-Cre/Cdh1flox/flox/Trp53flox/flox | Breast | LN, skin, lung, liver, GI, pan., spleen, bone | 70 | Derksen (2011) | http://www.ncbi.nlm.nih.gov/pubmed/21282721 |
| MMTV-LMW-Cyl-E/Trp53+/− | Breast | LN, lung, head | 35.2 | Akli (2007) | http://www.ncbi.nlm.nih.gov/pubmed/17671189 |
| MMTV-NeuN | Breast | Lung | 11–72 | Guy (1992) | http://www.ncbi.nlm.nih.gov/pubmed/1359541 |
| MT-Met | Breast | LN, lung, kid., heart, cecum | – | Jeffers (1998) | http://www.ncbi.nlm.nih.gov/pubmed/9826715 |
| MMTV-Wnt1 | Breast | LN, lung | – | Li (2000) | http://www.ncbi.nlm.nih.gov/pubmed/10713683 |
| PB-Cre/PTENflox/flox/Smad4flox/flox | Prostate | LN, Lung | 100 | Ding (2011) [17] | http://www.ncbi.nlm.nih.gov/pubmed/21289624 |
| Cryptdin-2-Tag | Prostate | LN, lung, liver, bone | 85 | Garabedian (1998) [12] | http://www.ncbi.nlm.nih.gov/pubmed/9860977 |
| PB-SV40T/t | Prostate | LN, lung | 67–100 | Gingrich (1996) [38] | http://www.ncbi.nlm.nih.gov/pubmed/8797572 |
| PB-Cre/PTENflox/flox | Prostate | LN, lung | 45 | Wang (2003) [13] | http://www.ncbi.nlm.nih.gov/pubmed/14522255 |
| Adeno-Cre/KRASG12D/Apcflox/flox | Colon | Liver | 20 | Hung (2010) [26] | http://www.ncbi.nlm.nih.gov/pubmed/20080688 |
| Adeno-Cre/KRASG12D/LKB1flox/flox | Lung | LN, skeleton | 61 | Ji (2007) [32] | http://www.ncbi.nlm.nih.gov/pubmed/17676035 |
| Adeno-Cre/KRASG12D/Trp53flox/flox | Lung | LN, distant organs | 50 | Jackson (2005) [39] | http://www.ncbi.nlm.nih.gov/pubmed/16288016 |
| SP-C-c-Raf/SP-C-c-Myc | Lung | LN, liver | 24 | Rapp (2009) [29] | http://www.ncbi.nlm.nih.gov/pubmed/19551151 |
| Adeno-Cre/KRASG12D/INK4a−/− | Lung | LN | 20 | Ji (2007) [32] | http://www.ncbi.nlm.nih.gov/pubmed/17676035 |
| SP-C-c-Myc | Lung | LN, liver | 17 | Rapp (2009) [29] | http://www.ncbi.nlm.nih.gov/pubmed/19551151 |
| Pdx1-Cre/KRASG12D/Trp53R172H | Pancreas | Liver, lung, LN, diaphragm, AG | 80 | Hingorani (2005) [40] | http://www.ncbi.nlm.nih.gov/pubmed/15894267 |
| Rip-VEGF-C-Tag | Pancreas | LN | 37 | Mandriota (2001) | http://www.ncbi.nlm.nih.gov/pubmed/11179212 |
| Tyr-Cre/BRAFV600E/PTENflox/flox | Melanoma | LN, lung | 100 | Dankort (2009) | http://www.ncbi.nlm.nih.gov/pubmed/19282848 |
| MT-HGF/SF | Melanoma | Liver, lung, pan., LN, epid., spleen, femur | 21 | Otsuka (1998) | http://www.ncbi.nlm.nih.gov/pubmed/9823327 |
| K14-PKC | Skin | LN | 30 | Jansen (2001) | http://www.ncbi.nlm.nih.gov/pubmed/11221859 |
| TRβPV/PV/PTEN+/− | Thyroid | Lung | 80 | Guigon (2009) | http://www.ncbi.nlm.nih.gov/pubmed/18997818 |
| TRα1−/−/TRβ−/− | Thyroid | Lung | 21 | Zhu (2010) | http://www.ncbi.nlm.nih.gov/pubmed/20062085 |
| Adeno-Cre/Trp53flox/flox/PTENflox/flox | Bladder | LN, spleen, liver, diaphragm | 60 | Puzio-Kuter (2009) | http://www.ncbi.nlm.nih.gov/pubmed/19261747 |
| CK19-Tag | Bladder | Lung | 20 | Grippo (2000) | http://www.ncbi.nlm.nih.gov/pubmed/10980120 |
| Osx1-GFP-Cre/Trp53flox/flox/Rbflox/flox | Osteosarcoma | Lung, liver, AG, spleen, kid., ov. | 40 | Berman (2008) | http://www.ncbi.nlm.nih.gov/pubmed/18697945 |
| AT-III-Tag | Liver | Lung | 10 | Dubois (1991) | http://www.ncbi.nlm.nih.gov/pubmed/1660504 |
| α-Amylase-Tag | Adipose | Liver, lung, AG, spleen, heart | – | Fox (1989) | http://www.ncbi.nlm.nih.gov/pubmed/2785714 |
Invasive and metastasizing breast cancer models
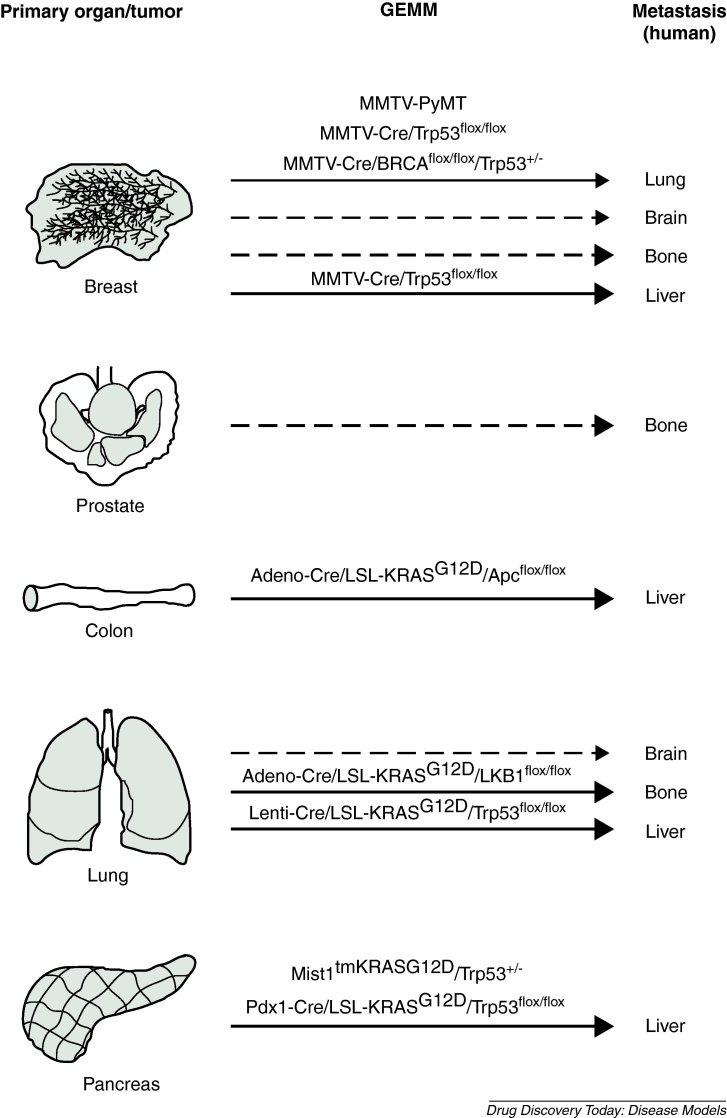
Human breast cancer frequently metastasizes to liver, bone, lung and brain. To model breast cancer in mice, potential oncogenes such as ErbB2, PyMT, Wnt1 and RAS have been expressed in mammary epithelial cells under control of the mammary gland-specific promoters MMTV and WAP (whey acidic protein). Corresponding mouse models developed breast cancers that frequently metastasized to the lung and the lymph nodes [3]. In particular, MMTV-PyMT mice displayed efficient formation of metastases with a short latency period (Fig. 1) [4]. The MMTV-PyMT transgene has been used as driver oncogene for breast cancer development in various knock-out mouse models which has revealed important gene co-operativities during breast cancer metastasis. Combined MMTV-PyMT/Akt1−/− mice showed delayed onset of breast cancer formation and compromised metastasis when compared to MMTV-PyMT mice demonstrating the importance of PI3K/Akt signaling [3]. Ablation of tumor-associated macrophages (TAMs) in MMTV-PyMT/Csf-1−/− mice had no substantial effect on tumor growth but severely compromised breast cancer invasiveness and lung metastasis [3]. Interestingly, TAMs in MMTV-PyMT mice but not tumor cells expressed epidermal growth factor (EGF). By contrast, the EGF receptor was expressed by tumor cells but not TAMs. This indicates that TAMs secrete EGF that acts on tumor cells thereby establishing a heterotypic interaction that supports invasiveness. Secretion of EGF by TAMs is stimulated by CD4+ T-cells as has been demonstrated by T-cell ablation in MMTV-PyMT/Rag1−/− and MMTV-PyMT/IL-4−/− mice [3]. These results suggest a heterotypic signaling network between innate immune cells, adaptive immune cells and tumor cells in breast cancer invasion. In addition to mammary gland-specific constitutive expression of oncogenes, doxycycline-inducible expression of TetO-NeuNT and TetO-c-Myc transgenes has been employed for the establishment of mouse models with invasive breast cancer [3]. Of these models, MMTV-rtTA/TetO-NeuNT mice developed metastasizing breast cancer with high frequency [5]. Mouse models with conditional deletion of important tumor suppressor genes in mammary epithelial cells have also been used successfully for modeling metastatic breast cancer. MMTV-Cre/Trp53flox/flox mice developed breast tumors that efficiently metastasized to lung and liver albeit after a long latency period (Fig. 1) [6]. The latency period for primary tumor formation was shorter in MMTV-Cre/Brca1flox/flox/Trp53+/− mice and metastases were detected in lymph nodes and lung (Fig. 1) [7]. No efficient bone metastasis has been reported in breast cancer mouse models.
Figure 1.
Mouse models (GEMMs) with efficient invasion and metastasis rates. The route of metastasis from primary organs (here shown as mouse organs) to distant sites is indicated by arrows. Major target organs for human metastases and corresponding GEMMs that model the human tropisms are shown. Strong arrows: most frequent sites for metastasis; weak arrows: less frequent sites for metastasis; interrupted arrows: currently no suitable mouse model available.
Invasive and metastasizing prostate cancer models
The primary target organ of human prostate cancer metastases is the bone. Several prostate cancer mouse models have been established that progress beyond the stage of prostatic intraepithelial neoplasia (PIN) and display metastasis. These models are based on conditional deletion of tumor suppressor genes in the prostatic epithelium or on epithelial-specific expression of oncogenes. In most cases, the rat probasin (PB) promoter was used for Cre recombinase or oncogene expression. To increase tissue specificity, a composite ARR2PB promoter was developed that contains two androgen responsive regions [8,9]. The TRAMP model for prostatic adenocarcinomas is based on PB promoter driven expression of SV40T/t in the prostatic epithelium and developed metastases in the lung and lymph nodes. Rarely, metastases in the bone, kidney and adrenal glands were observed in TRAMP mice [8,9]. Less aggressive prostatic carcinomas developed in the LADY mouse model that is similar to TRAMP but lacks expression of the SV40 small t antigen (therefore, LADY mice still display protein phosphatase 2A activity). Prostatic tumors in LADY mice were invasive and expressed neuroendocrine markers (a subtype of human prostate cancer develops from neuroendocrine cells) but did not metastasize [8,9]. However, additional expression of the serine protease hepsin which is highly expressed in human prostate cancer under control of the PB promoter conferred metastasis [10]. As an alternative approach, the gene for SV40T was knocked into the PSP94 (prostate secretory protein 94) genomic locus that is highly expressed in the prostate. Resulting PSP-KIMAP mice developed PINs and invasive carcinomas that metastasized to the lymph nodes, lung and liver [11]. In addition to SV40 antigens, Her-2/Neu and c-Myc was ectopically expressed in PB-Her-2/Neu, PB-c-Myc and ARR2PB-c-Myc transgenic mice which resulted in PINs and invasive adenocarcinomas. PB-c-Myc mice developed invasive adenocarcinomas later than ARR2PB-c-Myc which was attributed to a lower level of transgenic c-Myc expression [8,9]. To establish a model for neuroendocrine prostate cancer, the Cryptdin-2 promoter has been used to express SV40T in neuroendocrine cells of the prostate. The resulting prostatic tumor model developed invasive carcinomas that expressed neuroendocrine markers and metastasized predominantly to lymph nodes and liver (infrequently also to lung and bone) [12].
Conditional deletion of tumor suppressor genes in the prostate epithelium was mainly achieved with ARR2PB-Cre transgenic mice. Deletion of PTEN, a tumor suppressor gene that is frequently mutated in human prostate cancer, resulted in invasive carcinomas that metastasized to lymph nodes and lung in 45% of mice [13]. Unfortunately, no metastasis to the bone was observed indicating that the tropism of metastasising prostate cancer in this model differs from human prostate cancer. In contrast to PTEN, conditional PB-Cre-mediated deletion of Apc in the prostate resulted in invasive adenocarcinomas but no metastasis was observed [8,9]. Among compound mouse models, PTEN+/−/Nkx3.1−/− mice developed fewer PINs than Nkx3.1-proficient PTEN+/− mice that, however, progressed to invasive prostate cancer with lymph node metastases beyond one year of age [14]. Moreover, prostate-specific expression of Fgf8b in PTEN+/− mice resulted in adenocarcinoma formation by nine months of age that led to lymph node metastases [15]. Conditional inactivation of Trp53 and Rb in the prostatic epithelium resulted in prostatic adenocarcinoma formation and metastasis to lymph nodes, liver and lung [16]. Recently, compound ARR2PB-Cre/PTENflox/flox/SMAD4flox/flox mice with conditional deletion of PTEN and SMAD4 in the prostatic epithelium have been generated. These mice represent a model for invasive prostate cancer that metastasizes with 100% penetrance to lymph nodes and lung. Moreover, this model has demonstrated that TGFβ/SMAD4 signaling imposes a barrier against prostate cancer metastasis. Unfortunately, no efficient bone metastasis has been observed in this model [17].
Invasive and metastasizing colorectal cancer models
Human colorectal tumors predominantly metastasize to the liver. Several genetic and chemical mouse models have been employed to investigate intestinal cancer formation and progression. The most frequently used chemical model for the induction of colorectal tumors is the AOM/DSS model which is based on a single injection of the carcinogen AOM followed by repeated treatment of mice with Dextransulfate (DSS) in the drinking water [18]. The most commonly used genetic models exploit the strong tumor-inducing potential of Apc mutations (ApcMin, ApcΔ716, Apc1638N) upon loss of heterozygosity (LOH). These mouse models develop tumors preferentially in the small intestine but not readily in the colon [19]. Conditional inactivation of a floxed Apc allele has been alternatively employed to induce intestinal tumors. Although AOM/DSS treatment or mutation of Apc can lead to the formation of invasive carcinomas at certain frequencies, metastases have not been observed in these models. Invasiveness and tumor progression were enhanced by AOM/DSS treatment or introduction of additional mutations in compound mouse models. AOM/DSS treated CEACAM1−/− mice (CEACAM1 is a cell adhesion molecule that is downregulated in human colorectal carcinomas) develop a higher percentage of colorectal carcinomas that invade the muscularis mucosa than corresponding control animals [20]. Similarly, compound Apc1638N/CEACAM1−/−, ApcMin/PTEN+/− and ApcMin/SMAD3−/− mice were prone to the development of invasive carcinomas which highlights the importance of these pathways in intestinal cancer progression [21–23]. We have demonstrated that conditional deletion of Stat3 in intestinal epithelial cells (using the Villin-Cre transgene) of ApcMin mice promotes invasiveness and nuclear localization of β-Catenin via a CEACAM1-dependent mechanism [24]. However, no liver metastases have been detected in these models which is most likely due to premature lethality of tumor bearing mice. Evidence for liver metastases has been obtained in compound Apc1638N/pVillin-KRASG12V mice. Although metastases have not been observed histopathologically in the liver, tumor-specific pVillin-KRASG12V transgene expression was detected in liver tissue of a subset of tumor bearing mice indicating the presence of disseminated tumor cells or micrometastases [25]. More recently, a stochastic model for sporadic colorectal cancer has been established that gives rise to Cdx-2+ liver metastases with considerable efficiency [26]. This model is based on Adeno-Cre injection into the colon of LSL-KRASG12V/Apcflox/flox mice (Fig. 1). Adeno-Cre treatment deleted the floxed Apc alleles and the loxP-stop-loxP cassette in front of the oncogenic KRASG12V gene with low frequency in crypt stem cells. This protocol avoids development of colorectal tumors at high numbers therefore preventing premature lethality and allowing tumor progression and metastasis.
Invasive and metastasizing lung cancer models
Human lung cancer often metastasizes to liver, bone and brain whereas most mouse models develop adenomas or less frequently adenocarcinomas that infrequently metastasize [27]. However, a recently reported lung cancer mouse model developed liver metastases that could be backtraced to the primary tumors [28]. Transgenic approaches have used the surfactant protein C (SP-C) and Clara cell secretory protein (CCSP) promoters for oncogene or Cre recombinase expression in type II pneumocytes or non-ciliated secretory Clara cells, respectively. Expression of SV40T, c-Raf or c-Myc under control of these promoters resulted in aggressive adenocarcinomas [27]. Metastases were observed in SP-C-c-Myc single transgenic mice at lower frequency but combined expression of SP-C-c-Myc and SP-C-c-Raf in a compound double-transgenic mouse model promoted formation of metastases [29]. Other approaches used these promoters in the context of the ‘Tet-on’ system for inducible transgene expression. CCSP-rtTA/TetO7-KRASG12D, CCSP-rtTA/TetO7-BRAFV600E and CCSP-rtTA/TetO7-PIK3CAH1047R mice developed adenocarcinomas after doxycycline treatment. Lung tumor formation and progression in the first two models was accelerated in a Trp53- or INK4a-negative genetic background but no metastasis was observed [27]. In addition to oncogenic KRAS, BRAF and PIK3CA (encoding p110α of PI3K) alleles, different variants of EGF receptor originally identified in human lung cancers have been inducibly expressed. CCSP-rtTA/TetO7-EGFRDEL, CCSP-rtTA/TetO7-EGFRL858R and CCSP-rtTA/TetO7-EGFRvIII mice developed invasive adenocarcinomas but no metastases after doxycycline application [27]. Knock-in approaches were used as alternative to transgenic mice. Knock-in of the Trp53R270H allele that was identified in Li-Fraumeni patients into the endogenous Trp53 locus gave rise to aggressive lung adenocarcinomas that even metastasized (such tumors were not found in Trp53−/− mice) [30]. The most frequently used mouse model for inducible lung cancer development is the LSL-KRASG12D model that harbours an oncogenic knock-in KRASG12D allele preceded by a loxP-stop-loxP cassette. Cre induction using a Cre-transgenic mouse or a viral infection protocol with Adeno-Cre particles leads to deletion of the floxed stop cassette followed by KRASG12D expression and formation of lung adenocarcinomas [31]. LSL-KRASG12D was crossed with CCSP-Cre/PTENflox/flox mice leading to combined activation of RAS and PI3K signaling that resulted in the formation of advanced vascularized adenocarcinomas but not metastasis [27]. Instead of PTEN, deletion of tumor suppressor proteins LKB1, INK4a or Trp53 in LSL-KRASG12D led to the formation of metastasizing lung adenocarcinomas (Fig. 1) [27]. Interestingly, LSL-KRASG12D/LKB1flox/flox mice developed not only adenocarcinomas but also squamous cell carcinomas and large cell carcinomas upon treatment with Adeno-Cre particles [32]. Metastases in the lymph nodes (61% of mice) and axial skeleton (9% of mice) were observed in this model. Recently, a LSL-KRASG12D/Trp53flox/flox lung cancer model has been reported where tumor formation was induced by lentiviral delivery of Cre (Lenti-Cre) to the lung. These mice developed metastases to lymph nodes, pleura, kidney, heart, adrenal glands and liver with high frequency (Fig. 1). Because Lenti-Cre integrates randomly into the genome, the integration site was used as a unique molecular identifier to link primary tumors with related metastases which allowed the identification of metastasis-regulating genes [28].
Invasive and metastasizing pancreatic cancer models
Pancreatic cancer in humans is a very aggressive malignant disease with rapid formation of metastases in the liver. The classical mouse model for pancreatic carcinoma is the Rip-Tag model that expresses the SV40T oncogene under control of the rat insulin promoter [33]. This model was very instructive for the investigation of tumor progression, angiogenesis and metastasis and was also used in different drug treatment protocols. However, the most frequent type of pancreatic cancer is represented by pancreatic ductular adenocarcinoma (PDAC) and not islet carcinomas that develop in Rip-Tag mice. Similar to lung adenocarcinoma models, most models for PDAC rely on LSL-KRASG12D mice [31]. Deletion of the loxP-stop-loxP cassette was performed with Pdx1-Cre and p48-Cre transgenic mice which was sufficient for the formation of invasive and metastatic PDAC albeit at low frequency [34]. Preferential sites of metastasis in these models were lymph nodes, liver, lung, pleura and the neural plexus [35]. Compound Pdx1-Cre/LSL-KRASG12D or p48-Cre/LSL-KRASG12D mice with additional deletion of tumor suppressors INK4a, Trp53, SMAD4 and TGFβRII developed invasive PDAC that metastasized to many organs (Fig. 1) [34]. Moreover, knock-in of KRASG12D into the Mist1 locus (Mist1tmKRASG12D mice) that is expressed during pancreas development resulted in invasive and metastatic pancreatic cancers of different histopathologic appearance. Tumor progression and formation of liver metastasis were promoted in Mist1tmKRASG12D Trp53+/− mice [36] confirming cooperativity between KRAS and loss of Trp53 (Fig. 1). Pancreatic cancer models that are based on inducible Cre activity are EL-tTA/TRE-Cre/LSL-KRASG12V and EL-CreERT/cLGL-KRAS mice that develop invasive and invasive/metastatic PDAC, respectively [34].
Comparison and further development of metastatic mouse models
The value of GEMMs as mouse models for invasion and metastasis of cancers is influenced by several parameters that include the human relevance of introduced genetic changes to get metastasizing tumors, penetrance of metastasis and target organs of metastasizing cells. Still, some GEMMs do not satisfactorily model human metastasis and for preclinical studies, evaluation of the drug response of human cancer cells in xenograft models might be required (Table 2). However, metastasis-associated gene expression patterns obtained from human tumor samples or via in vivo selection of organ-specific metastatic tumor cell lines might be used to introduce newly identified genetic changes that improve metastasis rates in compound GEMMs. The complexity of tumor metastasis could, however, require a further refinement of experimental tools in mouse models (e.g. the application of different recombination systems to get sequential activation or deletion of genes relevant for metastasis).
Table 2.
Advantages and disadvantages of GEMMs and transplantation systems for investigation of metastasis (for review see reference [2])
| GEMMs | Allografts | Xenografts | |
|---|---|---|---|
|
Pros |
Immunocompetent environment | Immunocompetent environment | Use of human tumor cells |
| Tumors in original tissues | Wide range of metastatic target organs | Range of orthologous metastatic tropism | |
| Cre-mediated genetic manipulation of stroma/tumor cells | Timely well defined onset of metastasis | Timely well defined onset of metastasis | |
| Defined genetic background |
Short latency |
Usually short latency |
|
| Cons | Extensive genetic manipulation in mice | Limited number of suitable cell lines | Immunodeficient environment |
| Metastatic tropism often different from human tumors | Non-human system | Non-responsiveness of tumor cells to some host factors (e.g. mouse IL-6) | |
| Timely not well defined onset of metastasis | Altered behavior of human tumor cells at high passages | ||
| Long latency | |||
| Non-human system |
GEMMs and human metastatic disease
GEMMs have been established to understand the major mechanisms underlying invasion and metastasis in humans and for the evaluation of antimetastatic drug treatments. Major drawbacks are imposed by the pharmacokinetics of drugs that are not applicable to humans and the differential tropism of mouse and human tumors. Moreover, most mouse models for invasive and metastasizing cancer rely on expression or deletion of the ‘usual suspects’ of oncogenes and tumor suppressor genes. Human cancers also rely on these genes but metastasis might be more complex and affected by additional pathways that are not yet taken into account in available mouse models.
Conclusions
Syngeneic and xenograft models using human cancer cells transplanted into host mice might still provide the method of choice to investigate far distant metastasis or to apply drug treatments. At present, GEMMs are more commonly used for the investigation of tumor initiation or local invasiveness because metastasis occurs infrequently or is restricted to lymph nodes and lung. However, recently established mouse models for metastasizing prostate and lung cancer nourish the hope that GEMMs with comparable metastasis rates and tropisms to human cancers will be developed which are of preclinical relevance.
Acknowledgements
This work was supported by the Ludwig Boltzmann Gesellschaft LBG, the Austrian Science Fund FWF grant SFB F28 to RE, the FWF DK-plus grant IAI to RE and the Austrian Federal Ministry of Science and Research GENAU grant ‘Austromouse’ to EC and RE.
Section editors: Wolfgang Mikulits – Institute of Cancer Research, Comprehensive Cancer Center, Medical University of Vienna, 1090 Vienna, Austria Mario Mikula – Institute of Medical Genetics, Medical University of Vienna, 1090 Vienna, Austria.
References
- 1.Francia G. Mouse models of advanced spontaneous metastasis for experimental therapeutics. Nat. Rev. Cancer. 2011;11:135–141. doi: 10.1038/nrc3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bos P.D. Modeling metastasis in the mouse. Curr. Opin. Pharmacol. 2010;10:571–577. doi: 10.1016/j.coph.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim I.S., Baek S.H. Mouse models for breast cancer metastasis. Biochem. Biophys. Res. Commun. 2010;394:443–447. doi: 10.1016/j.bbrc.2010.03.070. [DOI] [PubMed] [Google Scholar]
- 4.Maglione J.E. Transgenic polyoma middle-T mice model premalignant mammary disease. Cancer Res. 2001;61:8298–8305. [PubMed] [Google Scholar]
- 5.Moody S.E. Conditional activation of Neu in the mammary epithelium of transgenic mice results in reversible pulmonary metastasis. Cancer Cell. 2002;2:451–461. doi: 10.1016/s1535-6108(02)00212-x. [DOI] [PubMed] [Google Scholar]
- 6.Lin S.C. Somatic mutation of p53 leads to estrogen receptor alpha-positive and -negative mouse mammary tumors with high frequency of metastasis. Cancer Res. 2004;64:3525–3532. doi: 10.1158/0008-5472.CAN-03-3524. [DOI] [PubMed] [Google Scholar]
- 7.Brodie S.G. Multiple genetic changes are associated with mammary tumorigenesis in Brca1 conditional knockout mice. Oncogene. 2001;20:7514–7523. doi: 10.1038/sj.onc.1204929. [DOI] [PubMed] [Google Scholar]
- 8.Ahmad I. Advances in mouse models of prostate cancer. Expert Rev. Mol. Med. 2008;10:e16. doi: 10.1017/S1462399408000689. [DOI] [PubMed] [Google Scholar]
- 9.Jeet V. Modeling prostate cancer: a perspective on transgenic mouse models. Cancer Metastasis Rev. 2010;29:123–142. doi: 10.1007/s10555-010-9212-9. [DOI] [PubMed] [Google Scholar]
- 10.Klezovitch O. Hepsin promotes prostate cancer progression and metastasis. Cancer Cell. 2004;6:185–195. doi: 10.1016/j.ccr.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Duan W. Knockin of SV40 Tag oncogene in a mouse adenocarcinoma of the prostate model demonstrates advantageous features over the transgenic model. Oncogene. 2005;24:1510–1524. doi: 10.1038/sj.onc.1208229. [DOI] [PubMed] [Google Scholar]
- 12.Garabedian E.M. A transgenic mouse model of metastatic prostate cancer originating from neuroendocrine cells. Proc. Natl. Acad. Sci. U.S.A. 1998;95:15382–15387. doi: 10.1073/pnas.95.26.15382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang S. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4:209–221. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- 14.Kim M.J. Cooperativity of Nkx3.1 and Pten loss of function in a mouse model of prostate carcinogenesis. Proc. Natl. Acad. Sci. U.S.A. 2002;99:2884–2889. doi: 10.1073/pnas.042688999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhong C. Cooperation between FGF8b overexpression and PTEN deficiency in prostate tumorigenesis. Cancer Res. 2006;66:2188–2194. doi: 10.1158/0008-5472.CAN-05-3440. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Z. Synergy of p53 and Rb deficiency in a conditional mouse model for metastatic prostate cancer. Cancer Res. 2006;66:7889–7898. doi: 10.1158/0008-5472.CAN-06-0486. [DOI] [PubMed] [Google Scholar]
- 17.Ding Z. SMAD4-dependent barrier constrains prostate cancer growth and metastatic progression. Nature. 2011;470:269–273. doi: 10.1038/nature09677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenberg D.W. Mouse models for the study of colon carcinogenesis. Carcinogenesis. 2009;30:183–196. doi: 10.1093/carcin/bgn267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taketo M.M. Mouse models of gastrointestinal tumors. Cancer Sci. 2006;97:355–361. doi: 10.1111/j.1349-7006.2006.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leung N. Deletion of the carcinoembryonic antigen-related cell adhesion molecule 1 (Ceacam1) gene contributes to colon tumor progression in a murine model of carcinogenesis. Oncogene. 2006;25:5527–5536. doi: 10.1038/sj.onc.1209541. [DOI] [PubMed] [Google Scholar]
- 21.Leung N. Intestinal tumor progression is promoted by decreased apoptosis and dysregulated Wnt signaling in Ceacam1−/− mice. Oncogene. 2008;27:4943–4953. doi: 10.1038/onc.2008.136. [DOI] [PubMed] [Google Scholar]
- 22.Shao J. Heterozygous disruption of the PTEN promotes intestinal neoplasia in APCmin/+ mouse: roles of osteopontin. Carcinogenesis. 2007;28:2476–2483. doi: 10.1093/carcin/bgm186. [DOI] [PubMed] [Google Scholar]
- 23.Sodir N.M. Smad3 deficiency promotes tumorigenesis in the distal colon of ApcMin/+ mice. Cancer Res. 2006;66:8430–8438. doi: 10.1158/0008-5472.CAN-06-1437. [DOI] [PubMed] [Google Scholar]
- 24.Musteanu M. Stat3 is a negative regulator of intestinal tumor progression in Apc(Min) mice. Gastroenterology. 2010;138:1003–1011. doi: 10.1053/j.gastro.2009.11.049. e1001–1005. [DOI] [PubMed] [Google Scholar]
- 25.Janssen K.P. APC and oncogenic KRAS are synergistic in enhancing Wnt signaling in intestinal tumor formation and progression. Gastroenterology. 2006;131:1096–1109. doi: 10.1053/j.gastro.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Hung K.E. Development of a mouse model for sporadic and metastatic colon tumors and its use in assessing drug treatment. Proc. Natl. Acad. Sci. U.S.A. 2010;107:1565–1570. doi: 10.1073/pnas.0908682107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Seranno S., Meuwissen R. Progress and applications of mouse models for human lung cancer. Eur. Respir. J. 2010;35:426–443. doi: 10.1183/09031936.00124709. [DOI] [PubMed] [Google Scholar]
- 28.Winslow M.M. Suppression of lung adenocarcinoma progression by Nkx2-1. Nature. 2011;473:101–104. doi: 10.1038/nature09881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rapp U.R. MYC is a metastasis gene for non-small-cell lung cancer. PLoS One. 2009;4:e6029. doi: 10.1371/journal.pone.0006029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olive K.P. Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell. 2004;119:847–860. doi: 10.1016/j.cell.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 31.DuPage M. Conditional mouse lung cancer models using adenoviral or lentiviral delivery of Cre recombinase. Nat. Protoc. 2009;4:1064–1072. doi: 10.1038/nprot.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ji H. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448:807–810. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- 33.Hanahan D. Heritable formation of pancreatic beta-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature. 1985;315:115–122. doi: 10.1038/315115a0. [DOI] [PubMed] [Google Scholar]
- 34.Grippo P.J., Tuveson D.A. Deploying mouse models of pancreatic cancer for chemoprevention studies. Cancer Prev. Res. (Phila) 2010;3:1382–1387. doi: 10.1158/1940-6207.CAPR-10-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hingorani S.R. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 36.Tuveson D.A. Mist1-KrasG12D knock-in mice develop mixed differentiation metastatic exocrine pancreatic carcinoma and hepatocellular carcinoma. Cancer Res. 2006;66:242–247. doi: 10.1158/0008-5472.CAN-05-2305. [DOI] [PubMed] [Google Scholar]
- 37.Schade B. PTEN deficiency in a luminal ErbB-2 mouse model results in dramatic acceleration of mammary tumorigenesis and metastasis. J. Biol. Chem. 2009;284:19018–19026. doi: 10.1074/jbc.M109.018937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gingrich J.R. Metastatic prostate cancer in a transgenic mouse. Cancer Res. 1996;56:4096–4102. [PubMed] [Google Scholar]
- 39.Jackson E.L. The differential effects of mutant p53 alleles on advanced murine lung cancer. Cancer Res. 2005;65:10280–10288. doi: 10.1158/0008-5472.CAN-05-2193. [DOI] [PubMed] [Google Scholar]
- 40.Hingorani S.R. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]