Abstract
Recent reports on the simultaneous occurrence of systemic inflammation and airflow obstruction are usually based on a highly selective patient population, but their importance warrants further evaluation in the general population. The objectives were to study the interrelationship between airflow obstruction, smoking, hypertension, obesity and CRP as a marker of systemic inflammation in a randomly selected sample of the general Icelandic population (n = 939). This study comprised 758 randomly selected men and women 40 years and older living in Reykjavik, Iceland, and who were participating in the Burden of Obstructive Lung Disease (BOLD) study (81% response rate). In addition to the BOLD protocol, which included post-bronchodilator spirometry, they answered questions about general health and medication. Serum samples were taken for measurement of C-reactive protein (CRP). In the sample—245 individuals (33%) reported having hypertension. Subjects with hypertension were older, had a higher BMI and higher CRP levels. Subjects with hypertension had lower values of FEV1 than predicted (89.9 ± 18.5 vs. 94.5 ± 14.4%) (p < 0.001) and FVC (92.2 ± 15.1 vs. 95.3 ± 12.3%) (p = 0.002). These differences remained significant after adjusting for age, BMI, CRP and smoking. Hypertension and CRP levels above the median were both independently and additively associated with lower FEV1 and FVC. In addition a lower FVC% was also associated with a higher BMI (> 30 mg/m2). Use of betablocking antihypertensives was not related to lung function. Hypertension, BMI and systemic inflammation affect lung function independently of each other. All three variables have a negative effect on FVC, while hypertension and high CRP were independently associated with impaired FEV1.
Keywords: Airflow obstruction, hypertension, obesity, systemic inflammation, cytokines
INTRODUCTION
Worldwide attention has repeatedly been drawn to the fact that Chronic Obstructive Pulmonary Disease (COPD) is a major cause of mortality and morbidity (1). Simultaneously, an interest has arisen because of the association of COPD with other chronic systemic diseases like cardiovascular diseases (3), diabetes (4) and osteoporosis (5). In a recent report on almost 3,000 COPD patients undergoing pulmonary rehabilitation altogether 51% had at least one chronic comorbidity condition in addition to COPD, most frequently metabolic (systemic hypertension, diabetes and/or dyslipidaemia) and/or heart diseases (chronic heart failure and/or coronary heart disease) (6).
As early as 1995 Enright et al. (7) showed that impaired pulmonary function was related to the presence of ischemic heart disease and hypertension in 2,246 men and 2,955 women over age 64 years, even in nonsmokers and those without known causes of lung disease. Engström et al. found that the combination of hypertension and low FEV1 was associated with a markedly higher risk of cardiovascular disease and death (8), and they also revealed that lung function was inversely associated with increase in blood pressure over a period of 13 years. Those with poorer lung function in the beginning showed a greater increase in blood pressure and, in their opinion, this increase could not be explained by age alone (8).
Similar results were reported by Wu et al. in China (9). The Cardiovascular Health Study also demonstrated an association of coronary artery disease and hypertension with reduced pulmonary function (7). Wannamethee et al. found that a lower FEV1 was associated with higher rates of stroke in hypertensive men (10). The presence of comorbitity in COPD is strongly related to higher mortality (4, 11, 12).
Cigarette smoke induces oxidative stress and results in the local up-regulation of the synthesis of inflammatory cytokines. C-reactive protein (CRP) is a sensitive systemic marker of tissue damage and inflammation, produced by hepatocytes under the control of the cytokine IL-6 (13). Numerous prospective studies have demonstrated that increased CRP concentration is a strong predictor of myocardial infarction, stroke and peripheral vascular disease in apparently healthy adults (14). Several studies have also shown that patients with COPD have higher levels of CRP than controls (13, 15–17). It has been shown that CRP is inversely correlated with postbronchodilator FEV1 and reversibility (13).
In a population-based study on subjects aged 28–54 years from Reykjavik, Uppsala and Tartu we have previously described a strong association between high levels of CRP in non-atopic airway obstruction independent of both smoking and obesity (15). Recently, Fabbri and Rabe suggested that the diagnostic approach in COPD should not be restricted to the lungs and proposed to add the term chronic systemic inflammatory syndrome to characterize the frequent complex comorbidities in people with COPD (18). The aim of the investigation was to study the association between lung function, obesity, hypertension and CRP as a marker of systemic inflammation.
METHODS
Background
The Burden of Lung Disease Initiative (BOLD) is an international study on the prevalence of COPD (www.kpchr.org/boldcopd) carried out in 18 countries to date. Data from the first 12 countries to complete the study have been reported recently (19). BOLD was conducted in Reykjavík, Iceland, from December 2004–September 2005. The target population consisted of residents of Reykjavik and the surrounding suburbs, which comprise a population of about 180,000 individuals (compared to 300,000 for all of Iceland). The criteria for inclusion were an age of 40 years or older and that participants should be living in Reykjavík or its suburbs. Institutionalized individuals (due to illness or imprisonment) and people living permanently abroad were excluded from participating, in accordance with the international research protocol. The protocol was approved by the National Bioethics Committee of Iceland.
Sampling Design
Starting with the national census, all age-eligible Icelandic citizens who were registered as living in Reykjavík and surrounding suburbs and not having a permanent home address in one of the major nursing homes were identified as the target population or 35,228 men and 38,163 women. A sample was then drawn from the target population using a simple random sampling procedure, not stratified by gender (1,000 subjects). After sampling it was found that 61 individuals were not eligible for inclusion as they had either moved away (n = 18), had a wrong address in the national census (n = 7), were institutionalized (n = 30) or had passed away (n = 6). The final study group therefore consisted of 939 individuals.
Questionnaires
Questionnaires were originally written in English and then translated into Icelandic. The wording of individual questions was based partly on previous international studies and the ATS Standardized Respiratory Symptom Questionnaire (19, 20). An independent back translation into English was done and the results compared to the original by the Operations Center in Portland, Oregon, USA.
Procedures
After written informed consent was obtained subjects answered the standardized questionnaires administered by the trained interviewers, underwent pre- and postbronchodilator spirometry tests, and a blood sample was obtained.
Spirometry
FEV1 and FVC values were obtained by spirometry. Testing was conducted with the participant in a sitting position wearing a nose clip and a disposable mouthpiece. Participants were shown how to perform the maneuver by the technician before testing. After instructing the participant, a pre-bronchodilator test was carried out. A successful test session was defined as at least 3 acceptable maneuvers, with the 2 best FEV1s and the 2 best FVC’s from these maneuvers, both within 200 ml of each other. After at least 3 acceptable and 2 reproducible maneuvers, 2 puffs of bronchodilator (salbutamol) were administered, using a spacer.
After at least 15 minutes the post-bronchodilator test was performed. In accordance with the BOLD protocol individuals who had recently had a cold/infection were asked to participate later because of the effect on spirometry. BOLD uses the prediction equations for Caucasian adult men and women derived from the third United States National Health and Nutrition Examination Survey (NHANES III) as its primary reference equations for all participants (19, 22).
Hypertension
Participants answered questions in the BOLD questionnaire regarding high blood pressure. In addition we asked specific questions as to whether they had ever been diagnosed with hypertension by a doctor and if so, at what age. They were also asked if they were currently using any medications to treat hypertension and if so, what medications. Later their pharmacological treatment was coded according to the ATC drug classification system (23).
Body Mass Index
Participants were weighed in light clothing without shoes, with weight recorded to the nearest kilogram. Height was measured (without shoes) using a wall-mounted stadiometer and recorded to the nearest centimeter. BMI was calculated as weight in kilograms divided by the square of height in meters.
Blood Samples
Blood was drawn from the antecubital vein of the seated subjects. Specimens were collected in SST’s from Greiner (Kremsmuenster, Austria). Tubes were allowed to stand for 20 minutes before centrifugation for 10 minutes (3,000 rpm). After separation, the serum samples were stored at −20°C until analysis. The analyses were carried out at the Department of Clinical Biochemistry, Landspitali University Hospital, Iceland. CRP concentrations were measured on a Kone 30 analyser using a commercially available latex-enhanced immunoturbidimetric assay from Roche Diagnostic Systems (Mannheim, Germany). The lower detection limit of the assay is 0.1 mg/L. The between-day coefficient of variation was 1.1% at a concentration of 3.73 mg/L and 1.9% at a concentration of 0.68 mg/L.
Statistical analyses
All statistics were calculated with STATA software, version intercooled STATA 8.0 for Windows (Stata Corporation, College Station, Texas). The subjects were divided into four groups according to the quartile distribution of the CRP values (<0.75, 0.75–1.27, 1.27 −3.25, >3.25). Simple linear regression and the Chi square test were used in the univariate analyses, while multiple linear regression was used when performing multivariable analyses. The test for trend was carried out using a simple linear regression by transforming the variable studied from categorical to continuous. ANOVA was used when analyzing the univariate association between BMI groups and lung function. Tests for interaction were carried out to detect possible differences regarding sex, BMI, smoking and CRP in the association between lung function and hypertension, where a p-value less than 0.05 was regarded as statistically significant.
RESULTS
Participation
A total of 758 participated (response rate 81 %). According to chi-square statistics women aged 70 years and older had a lower participation rate (p < 0.05), but there were no significant differences between the responders and non-responders regarding age, smoking status or health. Self-reported diagnosis of hypertension was available from 750 subjects, all with acceptable post-dilator spirometry. Blood samples were analyzed from 746 participants.
Hypertension
Altogether 245 individuals (33%) reported that they had been diagnosed with hypertension by a doctor. Subjects with hypertension were older, had a higher BMI, were less likely to be current smokers and had higher CRP levels (Table 1). Mean age when diagnosed with hypertension was 51.2 (±13.3) years. Altogether 189 individuals were currently taking medication for hypertension and of these almost half were using monotherapy. Beta-blockers were used by 96 (30%) and 91 (28%) were taking thiazides.
Table 1.
Characteristics of subjects without and with hypertension (mean ± SD or%)
| No hypertension (n = 505) |
Hypertension (n = 245) |
p-value | |
|---|---|---|---|
| Age (years) | 53.3 ± 10.5 | 62.8 ± 11.6 | <0.0001 |
| Women (%) | 45.0 | 51.0 | 0.12 |
| BMI (kg/m2) | 27.3 ± 4.5 | 29.0 ± 5.4 | <0.0001 |
| Ex-smokers | 41.1 | 46.5 | 0.16 |
| Current smokers (%) | 21.4 | 12.2 | 0.002 |
| Pack-years (% per group) | 0.52 | ||
| 0 | 37.7 | 41.2 | |
| >0–5 | 13.3 | 13.1 | |
| >5–20 | 24.8 | 20.8 | |
| >20 | 24.2 | 24.9 | |
| CRP (mg/L) (%per group) | < 0.0001 | ||
| <0.75 | 25.5 | 21.8 | |
| 0.75–1.27 | 29.8 | 17.6 | |
| 1.27–3.25 | 23.5 | 28.4 | |
| >3.25 | 21.2 | 32.2 |
Association with Lung Function
The participants with reported hypertension had proportionally more often a predicted FEV1 % below 80% compared to the non-hypertensives, especially in the older age groups (Figure 1). They also had significantly lower FEV1 % and FVC% (Table 2). No significant association was found between lung function and the age when the participants were diagnosed having hypertension or the number of hypertensive drugs. No significant difference was found in FEV1 or FVC between hypertensive subjects that used or did not use beta-blockers.
Figure 1.
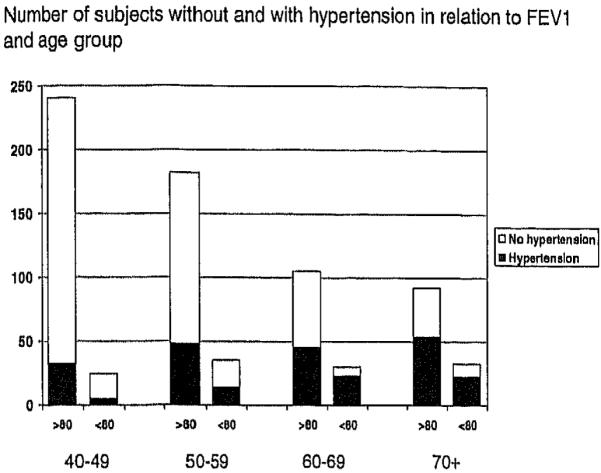
Hypertension and impaired lung function (FEV1 < 80% predicted in different age groups).
Table 2.
Lung function (mean ±SD) in relation to hypertension, BMI and CRP
| FEV1 % pred | FVC% pred | |
|---|---|---|
| Hypertension | ||
| No | 94.5 ± 14.4 | 95.3 ± 12.3 |
| Yes | 89.9 ± 18.5 | 92.2 ± 15.1 |
| p-value | 0.0002 | 0.002 |
| BMI kg/m2 | ||
| <20 (n = 15) | 83.6 ± 25.1 | 94.6 ± 19.4 |
| 20–25 (n = 201) | 94.6 ± 16.8 | 97.9 ± 13.2 |
| 25–30 (n = 330) | 94.5 ± 15.2 | 95.2 ± 12.6 |
| >30 (n = 212) | 89.8 ± 14.9 | 89.2 ± 12.8 |
| p-value | 0.0003 | < 0.0001 |
| CRP mg/L | ||
| <0.75 | 97.4 ± 15.2 | 99.0 ± 12.2 |
| 0.75–1.27 | 94.2 ± 15.6 | 95.3 ± 13.1 |
| 1.27–3.25 | 92.6 ± 14.4 | 93.4 ± 12.7 |
| >3.25 | 88.4 ± 16.8 | 89.6 ± 13.4 |
| ptrend-value | < 0.0001 | < 0.0001 |
BMI showed an inversed U-shaped association to FVC% and FEV1 % with the lowest lung function values in the underweight and obese participants (Table 2). Higher CRP values were significantly associated with lower lung function (p < 0.0001) (Table 2).
Multivariable Association between Lung Function, Hypertension. CRP and BMI
The association between hypertension and CRP and low FEV1 and FVC remained significant after adjusting for age, sex, current smoking and pack-years. BMI was negatively associated with FVC (Table 3).
Table 3.
Independent effect of hypertension, BMI and CRP on lung function shown as the difference in% predicted values (and 95% CI)
| FEV1 % pred | FVC% pred | |
|---|---|---|
| Hypertension | ||
| No | 0 | 0 |
| Yes | −3.8 (−6.3, −1.2) | −2.6 (−4.7, −0.4) |
| BMI kg/m2 | ||
| <20 | −8.6 (−17, 0.3) | −2.1 (−9.6, 5.3) |
| 20–25 | 0 | 0 |
| 25–30 | 1.2 (−1.6, 4.0) | −1.5 (−3.8, 0.9) |
| >30 | −1.8 (−5.0, 1.3) | −5.9 (−8.5, −3.2) |
| CRP mg/L | ||
| <0.75 | 0 | 0 |
| 0.75–1.27 | −3.2 (−6.4, −0.04) | −2.9 (−5.5, −0.2) |
| 1.27–3.25 | −3.7 (−6.9, −0.5) | −3,9 (−6.6, −1.2) |
| >3.25 | −6.2 (−9.5, −2.9) | −7.0 (−10, −4.2) |
The effect is adjusted for the variables in the table and age, sex, current smoking and pack-years.
The association between FVC and hypertension was significantly stronger in men than in women −4.9 (−7.7, −1.9) vs. −0.5 (−3.5, 2.7)% of predicted (p = 0.02). No significant interaction with age, smoking, CRP or BMI was found concerning the association between lung function and hypertension and. An additive effect was found between hypertension and a higher CRP (>1.27 mg/1) towards a lower FEV1. The adjusted FEV1 was was −3.3 (−5.9, −0.8)% of predicted lower if either one was present and −7.9 (−11, −4.6)% of predicted lower if both hypertension and higher CRP were present (Figure 2). Likewise, FVC was significantly lower in the presence of hypertension, higher CRP (> 1.27mg/l) and obesity (BMI > 30.0 kg/m2) (Figure 2).
Figure 2.
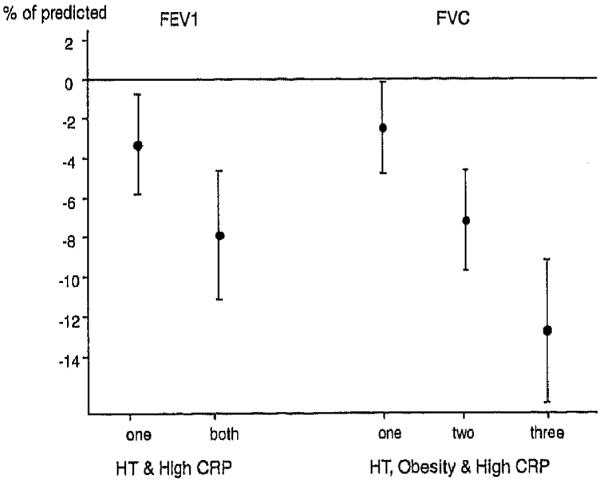
Association between lung function and hypertension, obesity (BMI > 30 kg/m2) and high CRP (above median). The associations were adjusted for age, sex, current smoking and pack-years.
DISCUSSION
The main finding of this study was that hypertension, obesity and systemic inflammation affect lung function independently of each other. All 3 variables have a negative effect on FVC, while hypertension and high CRP were independent and additive determinants of FEV1. This synergistic interaction is of clinical importance and adds to our understanding of the complex nature of lung function impairment.
The strength of this study is its use of the well standardized BOLD protocol (19) and the high response rate in a general population sample. On the other hand this study is underpowered in terms of analyzing subgroups of the general population consisting of those in the lowest BMI group with low FEV1. Also in the group of 70 + were the prevalence of hypertension is highest only few subjects have lung function impairment of less than 80% predicted. Another limitation of this study is that we only measured height and weight but not waist circumference that would have been a better index of the metabolic syndrome than is BMI.
Hypertension and lung function impairment both share smoking as a risk factor (25) and obesity in our study was also significantly associated with both higher CRP levels and lower FEV1. We have previously shown the synergistic effect of smoking and obesity for high CRP values (15), a combination that leads to markedly reduced life expectancy (25). In fact, the presence of comorbidities in COPD is invariably associated with a worse prognosis (11, 26, 27).
CRP is increased in all the major comorbidities of COPD like cardiovascular diseases, hypertension and diabetes (13, 14) but how this low-grade systemic inflammation accelerates progression of COPD is not well understood (26). Recent studies have shown that the risk associated with obesity is not homogenous but that the visceral adipose tissue compartment may be a unique pathogenic fat depot, acting as an endocrine organ that can influence the risk of developing metabolic traits (28). At least animal studies indicate that hypoperfusion and hypoxia in local adipose tissue hypoxia are partly responsible for dysregulated adipocytokine production and metabolic syndrome in obese subjects even under normoxic conditions. The ventilatory impairment associated with airflow obstruction might easily contribute to a vicious circle. It is however also possible that abdominal obesity causes hypertension, systemic inflammation, and by mechanically impairing descent of the diaphragm, a low FVC and FEV1.
The view of lung function impairment as a landmark of a systemic disease with many co-morbidities is leading to a more comprehensive approach to management. For example, a high CRP might be a novel target for treatment (26). The lipid-lowering statins have potent anti-inflammatory properties and among 418 patients followed for almost 3 years the 215 statin users showed less decline in FEV1, independent of smoking status (30) and in a retrospective 2-year follow-up of 854 patients the mortality was lowest among statin users, especially if they were also taking inhalation corticosteroids (31). In another retrospective follow-up of 3,371 vascular surgery patients those with COPD and using statins showed a much lower 10-year mortality, especially if they were using high statin doses (32).
Hypertension, BMI and systemic inflammation affect lung function independently of each other. Further studies are needed to understand the interrelationship between lung function impairment and the complex comorbidities, but our study supports the view that lung diseases such as COPD may be part of chronic systemic inflammatory syndrome.
ACKNOWLEDGMENTS
This study was funded by Landspitali University Science Fund, Astra Zeneca in Iceland and GlaxoSmithKline in Iceland.
Footnotes
Declaration of interest The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Contributor Information
Olof Birna Margretardottir, Email: olofbrn@hi.is.
Sigurdur James Thorleifsson, Email: sjth@hi.is.
Gunnar Gudmundsson, Email: ggudmund@landspitali.is.
Islelfur Olafsson, Email: isleifur@landspitali.is.
Bryndis Benediktsdottir, Email: brynben@hi.is.
Christer Janson, Email: christer.janson@medsci.uu.se.
A. Sonia Buist, Email: buists@ohsu.edu.
Thorarinn Gislason, Email: thorarig@landspitaii.is.
REFERENCES
- 1.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS, GOLD Scientific Committee Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163(5):1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 2.Mannino DM, Buist AS. Global burden of COPD: Risk factory prevalence, and future trends. Lancet. 2007 Sep 1;370(9589):765–773. doi: 10.1016/S0140-6736(07)61380-4. Review. [DOI] [PubMed] [Google Scholar]
- 3.Rutten FH, Cramer MJ, Lammers JW, Grobbee DE, Hoes AW. Heart failure and chronic obstructive pulmonary disease: An ignored combination? Eur J Heart Fail. 2006 Nov;8(7):706–711. doi: 10.1016/j.ejheart.2006.01.010. Review. [DOI] [PubMed] [Google Scholar]
- 4.Rana JS, Mittleman MA, Sheikh J, Hu FB, Manson JE, Coldltz GA, Speizer FE, Barr RG, Camargo CA., Jr. Chronic obstructive pulmonary disease, asthma, and risk of type 2 diabetes in women. Diabetes Care. 2004 Oct;27(10):2478–2484. doi: 10.2337/diacare.27.10.2478. [DOI] [PubMed] [Google Scholar]
- 5.Jørgensen NR, Schwarz P. Osteoporosis in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med. 2008 Mar;14(2):122–127. doi: 10.1097/MCP.0b013e3282f4efb6. Review. [DOI] [PubMed] [Google Scholar]
- 6.Crisafulli E, Costl S, Luppi F, Cirelll G, Cilione C, Coletti O, Fabbri LM, Clini EM. Role of comorbidities in a cohort of patients with COPD undergoing pulmonary rehabilitation. Thorax. 2008 Jun;63(6):487–492. doi: 10.1136/thx.2007.086371. [DOI] [PubMed] [Google Scholar]
- 7.Enright PL, Kronmal RA, Smith VE, Gardin JM, Schenker MB, Manolio TA. Reduced vital capacity in elderly persons with hypertension, coronary heart disease, or left ventricular hypertrophy. The Cardiovascular Health Study. Chest. 1995;107(1):28–35. doi: 10.1378/chest.107.1.28. [DOI] [PubMed] [Google Scholar]
- 8.Engström G, Hedblad B, Valind S, Janzon L. Increased incidence of myocardial infarction and stroke in hypertensive men with reduced lung function. J Hypertens. 2001 Feb;19(2):295–301. doi: 10.1097/00004872-200102000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Wu Y, Vollmer WM, Buist AS, Tsai R, Cen R, Wu X, Chen P, LI Y, Guo C, Mai J, Davis CE. Relationship between lung function and blood pressure in Chinese men and women of Beijing and Guangzhou. Int J Epidemiol. 1998 Feb;27(1):49–56. doi: 10.1093/ije/27.1.49. [DOI] [PubMed] [Google Scholar]
- 10.Wannamethee SG, Shaper AG, Ebrahim S. Respiratory function and risk of stroke. Stroke. 1995 Nov;26(11):2004–2010. doi: 10.1161/01.str.26.11.2004. [DOI] [PubMed] [Google Scholar]
- 11.Sin DD, Anthonisen NR, Soriano UB, Agusti AG. Mortality in COPD: Role of comorbidities. Eur Respir J. 2006 Dec;28(6):1245–1257. doi: 10.1183/09031936.00133805. Review. [DOI] [PubMed] [Google Scholar]
- 12.Barbara MP Foschino, Carpagnano GE, Spanevello A, Cagnazzo MG, Barnes PJ. Inflammation, oxidative stress and systemic effects in mild chronic obstructive pulmonary disease. Int J Immunopathol Pharmacol. 2007 Oct-Dec;20(4):753–763. doi: 10.1177/039463200702000411. [DOI] [PubMed] [Google Scholar]
- 13.Pepys MB, Hirschfield GM. C-reactive protein: A critical update. J Clin Invest. 2003;111(12):1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jovicić S, Ignjatović S, Dajak M, Majkić-Singh N. Analytical performance and clinical efficacy for cardiovascular risk estimation of an Olympus immunoturbidimetric high-sensitivity C-reactive protein assay. Clin Chem Lab Med. 2006;44(2):228–231. doi: 10.1515/CCLM.2006.042. [DOI] [PubMed] [Google Scholar]
- 15.Olafsdottir IS, Gislason T, Thjodleifsson B, Olafsson I, Gislason D, Jögi R, Janson C. C-reactive protein levels are increased in non-allergic but not allergic asthma: A multicentre epidemiological study. Thorax. 2005 Jun;60(6):451–454. doi: 10.1136/thx.2004.035774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broekhuizen R, Wouters EF, Creutzberg EC, Schols AM. Raised CRP levels mark metabolic and functional impairment in advanced COPD. Thorax. 2006 Jan;61(1):17–22. doi: 10.1136/thx.2005.041996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franciosi LG, Page CP, Celli BR, Cazzola M, Walker MJ, Danhof M, Rabe KF, Della Pasqua OE. Markers of disease severity in chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2006;19(3):189–199. doi: 10.1016/j.pupt.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Fabbri LM, Rabe KF. From COPD to chronic systemic inflammatory syndrome? Lancet. 2007 Sep 1;370(9589):797–799. doi: 10.1016/S0140-6736(07)61383-X. [DOI] [PubMed] [Google Scholar]
- 19.Bulst AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, Menezes AMB, Sullivan SD, Lee TA, Weiss KB, Jensen RL, Marks GB, Gulsvik A, Nizankowska-Mogilnicka E. International variation in the prevalence of COPD (the BOLD Study): A population-based prevalence study. Lancet. 2007;370:741–750. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 20.ECRH European Community Respiratory Survey II. 2004. ( http://ecrhs.org/quest.com)
- 21.Lung Health Study Questionnaire 2nd edition. 2004. ( http://bccrc.ca/download/ci/lc02_questionnaire.doc)
- 22.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999 Jan;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 23.WHO . WHO Collaborating Centre for Drug Statistics Methodology. Norwegian Institute of Public Health; Oslo, Norway: http://www.whocc.no/atcddd. [Google Scholar]
- 24.Schols AM, Slangen J, Volovics L, Wouters EF. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998 Jun;157(6 Pt 1):1791–1797. doi: 10.1164/ajrccm.157.6.9705017. [DOI] [PubMed] [Google Scholar]
- 25.Peeters A, Barendregt JJ, Willekens F, Mackenbach JP, Al Mamun A, Bonneux L, NEDCOM, the Netherlands Epidemiology and Demography Compression of Morbidity Research Group Obesity in adulthood and its consequences for life expectancy: A life-table analysis. Ann Intern Med. 2003 Jan 7;138(1):24–32. doi: 10.7326/0003-4819-138-1-200301070-00008. [DOI] [PubMed] [Google Scholar]
- 26.Fabbri LM, Luppi F, Beghé B, Rabe KF. Complex chronic comorbidities of COPD. Eur Respir J. 2008 Jan;31(1):204–212. doi: 10.1183/09031936.00114307. [DOI] [PubMed] [Google Scholar]
- 27.Gudmundsson G, Gislason T, Lindberg E, Hallin R, Ulrik CS, Brøndum E, Nieminen MM, Aine T, Bakke P, Janson C. Mortality in COPD patients discharged from hospital: The role of treatment and co-morbidity. Respir Res. 2006 Aug 16;7:109. doi: 10.1186/1465-9921-7-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Melgs JB, Cupples LA, D’Agostino RB, Sr, O’Donnell CJ. Abdominal visceral and subcutaneous adipose tissue compartments: Association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007 Jul 3;116(1):39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 29.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007 Apr;56(4):901–911. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 30.Keddissi JI, Younis WG, Chbeir EA, Daher NN, Dernaika TA, Klnasewitz GT. The use of statins and lung function in current and former smokers. Chest. 2007 Dec;132(6):1764–1771. doi: 10.1378/chest.07-0298. [DOI] [PubMed] [Google Scholar]
- 31.Søyseth V, Brekke PH, Smith P, Omland T. Statin use is associated with reduced mortality in COPD. Eur Respir J. 2007 Feb;29(2):279–283. doi: 10.1183/09031936.00106406. [DOI] [PubMed] [Google Scholar]
- 32.van Gestel YR, Hoeks SE, Sin DD, Simsek C, Welten GM, Schouten O, Stam H, Mertens FW, van Domburg RT, Poldermans D. Effect of statin therapy on mortality in patients with peripheral arterial disease and comparison of those with versus without associated chronic obstructive pulmonary disease. Am J Cardiol. 2008 Jul 15;102(2):192–196. doi: 10.1016/j.amjcard.2008.03.038. [DOI] [PubMed] [Google Scholar]


