Abstract
Intracellular polyamine levels are highly regulated by the activity of ornithine decarboxylase (ODC), which catalyzes the first rate-limiting reaction in polyamine biosynthesis, producing putrescine, which is subsequently converted to spermidine and spermine. We have shown that polyamines regulate proliferation, migration, and apoptosis in intestinal epithelial cells. Polyamines regulate key signaling events at the level of the EGFR and Src. However, the precise mechanism of action of polyamines is unknown. In the present study, we demonstrate that ODC localizes in lamellipodia and in adhesion plaques during cell spreading. Spermine regulates EGF-induced migration by modulating the interaction of the EGFR with Src. The EGFR interacted with integrin β3, Src, and focal adhesion kinase (FAK). Active Src (pY418-Src) localized with FAK during spreading and migration. Spermine prevented EGF-induced binding of the EGFR with integrin β3, Src, and FAK. Activation of Src and FAK was necessary for EGF-induced migration in HEK293 cells. EGFR-mediated Src activation in live HEK293 cells using a FRET based Src reporter showed that polyamine depletion significantly increased Src kinase activity. In vitro binding studies showed that spermine directly binds Src, and preferentially interacts with the SH2 domain of Src. The physical interaction between Src and the EGFR was severely attenuated by spermine. Therefore, spermine acts as a molecular switch in regulating EGFR-Src coupling both physically and functionally. Upon activation of the EGFR, integrin β3, FAK and Src are recruited to EGFR leading to the trans-activation of both the EGFR and Src and to the Src-mediated phosphorylation of FAK. The activation of FAK induced Rho-GTPases and subsequently migration. This is the first study to define mechanistically how polyamines modulate Src function at the molecular level.
Keywords: Polyamines, Migration, Ornithine Decarboxylase (ODC), Lamellipodia
Introduction
The polyamines, spermidine and spermine, and their precursor, putrescine, are found in virtually all cells of higher eukaryotes [1] and are intimately involved in, and required for, cell growth and proliferation [2, 3]. Intracellular polyamine levels are highly regulated and depend primarily on the activity of ornithine decarboxylase (ODC), which catalyses the first rate-limiting step in polyamine biosynthesis, the decarboxylation of ornithine to form the diamine putrescine [4]. An increase in ODC activity is one of the earliest events associated with the induction of cellular proliferation, and depletion of polyamines by DL-α-difluoromethyl- ornithine (DFMO), a specific and irreversible inhibitor of ODC, attenuates trophic responses in tissues and cultured cells [5–7]. DFMO has been used to inhibit ODC and deplete cells of polyamines in many studies, and has no effects except those caused by the inhibition of ODC and the subsequent decrease in intracellular polyamines [8, 9]. The inhibitory effects of DFMO on cell proliferation, migration and apoptosis are prevented by the addition of exogenous polyamines [9–11]. We have shown that ECM-mediated signaling increased the autophosphorylation of EGFR, Src, and FAK in a polyamine dependent manner and regulated migration and apoptosis in IEC-6 cells [12,13]. Furthermore, the activation of EGFR, Src, and integrin β3 by DFMO were immediate and were instantaneously prevented by the addition of putrescine along with DFMO [14]. Based on these results, we predicted that polyamines might regulate interactions among these proteins at the proximity of the plasma membrane.
Cell migration is an essential event in cell growth and cancer metastasis [15]. Epidermal growth factor (EGF), a ligand for EGFR, promotes cancer cell migration and metastasis by activating multiple downstream protein kinases, such as c-Src [16], focal adhesion kinase [17], and p21-activated kinase [18]. The c-Src protein is composed of an N-terminal myristylation sequence that directs the association of proteins with the plasma membrane, a unique region where the greatest sequence divergence among family members occurs, a Src-homology-3 (SH3) domain and an SH2 domain that mediate protein-protein interactions, a kinase domain and a C-terminal regulatory domain [19]. Src is located in the cytoplasm, at cellular sites of integrin clustering (the so-called focal adhesions in fibroblasts) and at cadherin-mediated cell-cell adhesions in epithelial cells. Both the EGFR and c-Src are over-expressed in a variety of human tumors, including breast cancer, suggesting that these tyrosine kinases may functionally interact and contribute to the progression of the disease [20, 21]. FAK, a non-receptor tyrosine kinase, is activated and autophosphorylated at Y397 by binding to integrin complexes [22]. C-Src kinase then is recruited to the FAK complex by binding at phosphorylated Y397 through its SH2 domain [23]. Upon recruitment at Y397, Src phosphorylates multiple tyrosine residues and fully activates FAK and thereby, downstream signaling [24]. Although, EGF initiates signal transduction between the EGFR and FAK, evidence for the direct interaction between these proteins is lacking. We have shown that fibronectin-induced integrin activation is associated with EGFR phosphorylation and downstream activation of ERK1/2 [14]. Since the inhibition of Src by PP2 decreased the phosphorylation of the EGFR and integrin β3, it appears that the activation of the EGFR and integrin β3 is linked by a common mechanism involving Src.
Polyamines are reported to be involved in many actions of EGF. For instance, DFMO can prevent the inhibition of parietal cell acid secretion by EGF [25]. Polyamines inhibit EGFR tyrosine kinase activity in A-431 cells [26]. In a colon cancer cell line, Caco-2, polyamine uptake is stimulated by EGF and inhibited by genistein, a tyrosine phosphorylation inhibitor [27]. The overexpression of ODC is oncogenic in transfected NIH/3T3 cells [28]. In L6 cells and fetal bovine myoblasts, EGF stimulates polyamine biosynthesis, suggesting that the biosynthesis of polyamines is important for the early events mediated by EGFR [29]. In this study, we identified a specific polyamine-protein interaction, which is responsible for the polyamine effect on Src signaling in response to EGF. This is the first study to define mechanistically how polyamines modulate Src function at the cellular and molecular level.
Materials and Methods
Cell Lines, Vectors and Cloning
HEK293 cells were grown in DMEM-F12 (Invitrogen) supplemented with 10% FBS. For FRET imaging, HEK293 cells were transfected with pcDNA3 (containing CFP-Src-YFP, a kind gift from Dr. Shu Chien at the University of California-San Diego, La Jolla, California), and positive colonies were selected using 400 mg/ml G418 and confirmed by fluorescence microscopy. For pull-down and co-immunoprecipitation studies, HEK293 cells were transfected with a lentiviral expression system encoding V5-tagged EGFR (tag at the C-terminus), and the positive colonies were selected using 2.0 mg/ml puromycin. Various domains of c-Src (mouse neuronal) were subjected to PCR with complimentary overhangs created by building appropriate 5′ extensions into the primers according to the manufacturer’s instructions (Novagen). The PCR products were purified (Qiagen PCR purification kit) and treated with Ligation-independent cloning (LIC)-qualified T4 DNA polymerase in the presence of dATP to generate pTRIEX-4 and pET41 vector specific overhangs (Novagen). Clones were selected and sequenced for conformation. pTRIEX-4 has an N-terminal his6- and an S-tag. pET41 has an N-terminal GST-, his6- and S-tag. The EGFR cDNA sequence was originally cloned in pLenti6-V5-D-TOPO [30] but was separated from the V5 epitope by a stop codon. To remove the stop codon and place the EGFR sequence in-frame with the V5-epitope, an EGFR fragment from nucleotide 2704 to 3630 that lacked a stop codon and was flanked by BstEII and Xho1 restriction sites was amplified by PCR and inserted into the BstEII/XhoI digested pLenti6 V5-D-TOPO EGFR construct. The resultant pLenti6-V5+D-TOPO EGFR construct was transfected into 293FT cells (Invitrogen, Carlsbad, CA) and packaged into virus using the Virapower Lentivirus packaging system (Invitrogen, Carlsbad CA) according to the manufacturer’s instruction. HEK293 cells were then transduced with the virus at a multiplicity of infection no less than 106 cfu/mL. Stable clones were selected using blasticidin at a concentration of 5 mg/mL and maintained at a blasticidin concentration of 2 mg/mL. CA-Src, DN-Src plasmid and pUSE empty vector were purchased from Millipore (Billerica, MA).
Cell culture
The IEC-6 cell line (CRL-1592) was obtained from the American Type Culture Collection (Manassas, VA) and maintained in T-150 flasks in Dulbecco’s Minimal Essential Medium (DMEM) supplemented with 10% FBS, 10μg/ml insulin and 50μg/ml gentamicin sulfate at 37 °C and 10% CO2. Stock cells were passaged once a week and medium was changed three times a week. Prior to an experiment cells were trypsinized, counted using a Beckman Coulter counter and grown for 3 days in dialyzed FBS in control medium and were serum starved for 24 hours prior to an experiment.
Cell migration assay
This assay was carried out as described previously [31]. Six well plates containing a confluent monolayer of cells were marked in the center (along the diameter) with a marker. A wound was created perpendicular to the mark by scraping the monolayer with a gel loading micro-tip. Plates were washed, and the wound area was captured with a charged coupled device (CCD) camera system using NIH Image (Version1.62) at the intersection of the marked line at 0h and at the desired time point (7h). The wound area covered during migration was measured using Image J (Version1.36b) software. Cell migration was calculated as the wound area covered. Each experiment was carried out in triplicate and two observations were recorded from each well (n=6).
FRET Imaging Microscopy and Image Analysis
FRET imaging and image analysis were performed as described before [30]. Briefly, HEK293 cells (expressing stable CFP-Src-YFP) for FRET imaging were seeded in the 35-mm glass bottom culture dishes (MatTek) and grown for 48–72 hrs in DMEM (with 10% FBS) containing control, 5 mM DFMO, 5 mM DFMO plus 10 μM putrescine (or 5 μM each for spermidine, spermine, and putrescine), or 10 μM putrescine only (or 5 μM each for spermidine, spermine, and putrescine), and then continued to grow in serum-deprived medium containing the above compounds for another 24 hrs before the FRET assay. Prior to the FRET imaging, cells were washed twice with Hanks’ balance salt solution (HBSS) before being mounted on an Olympus microscopy system for FRET imaging. Cells were maintained in HBSS (containing the above-mentioned respective compounds) in the dark at room temperature with the addition of EGF as indicated. Images were recorded with a cooled charged-coupled device camera Hamamatsu ORCA285 (Hamamatsu, Japan) mounted on the Olympus microscope IX51 (U-Plan Fluorite 60 × 1.25 NA oil-immersion objective), and the system was controlled by SlideBook 4.1 software (Intelligent Imaging Innovations, Denver, CO) with ratio and FRET modules used to obtain and analyze the FRET images. Excitation light was provided by a 300W Xenon lamp and attenuated with a neutral density filter with 50% light transmission. Images were captured using a JP4 CFP/YFP filter set (Chroma, Brattleboro, VT) including a 430/25-nm (25-nm band-pass centered at 430-nm) excitation filter, a double dichroic beam splitter (86002v2bs), and two emission filters (470/30-nm for CFP and 535/30-nm for FRET) alternated by a high-speed filter-changer Lambda 10-3 (Sutter Instruments, Novato, CA). Time-lapse images were acquired with 4 × 4 binning mode, 200–400 ms exposure time, and 1-min intervals to reduce photo bleaching of the fluorophores. Acquired fluorescent images were background-subtracted, and multiple regions of interest (ROIs) on the cell periphery were selected for quantitative data analysis (~20–30 ROIs per cell, and 4–6 cells per condition were averaged). The emission ratio images (CFP/FRET) were generated at different time points on a pixel-by-pixel basis by the SlideBook ratio module and normalized through dividing all ratios by the emission ratio right before the addition of reagents, thereby setting the basal emission ratio to 1, as formulated below,
The ratio (R) was normalized as Rt/R0, where Rt is the ratio at time point t and R0 is the ratio at time point = 0 (right before the addition of first test compound). The cell images are presented in pseudocolor to highlight the changes in the ratio of CFP/FRET fluorescence intensity.
ELISA Assay
Different amounts of the GST, or GST-Src (100 μl) in TBS were applied to duplicate wells and incubated for 1 hr at 22°C. The plates were washed three times with 200 μl/well of TBS containing 0.1% Tween 20 (TBST), followed by blocking with 200 μl of TBST containing 1% BSA at 22°C for 1 hr. After decanting the blocking solution, 100 μl of BSA-conjugated spermine in TBST was added and incubated with the immobilized GST or GST-Src for 3 h at 22°C (or at 4°C overnight). The plates were washed three times with TBST, and then blocked again with 200 μl of TBST containing 1% BSA at room temperature for 20 min. 100 μl/well of the Primary Antibody (anti-Spermine antibody; 1:10,000 dilution in TBST/1% BSA) was added and incubated for 1 hour at 22°C. Plates were washed three times with TBST, followed by incubation with 100 μl/well of the secondary antibody (goat anti-rabbit HRP-conjugated, 1:20,000 dilution in TBST/1% BSA) for 1 hour at 22°C. After three washes with TBST, 100 μl of the 1-Step™ Slow TMB-ELISA substrate was added to each well, and incubated at room temperature in the dark for 5–30 minutes. The reaction was stopped by adding 100 μl of 2 M sulfuric acid (H2SO4) to each well. The absorbance of each well was measured at 450 nm using a microplate reader (Molecular Devices, CA).
Pair-wise Binding Assay
For pair-wise binding between the SH-2 domain of His-S-Src or GST-His-S-Src and BSA-conjugated spermine, lysis buffer (200 μl) was added into a 1.5 ml microfuge tube containing 5 mg of BSA-spermine. Purified His-S-Src SH domain (20 mg) or GST fusion proteins (0–40 mg) were added and mixed at 22°C for 30 min. To this mix, 20 ml S-beads or glutathione beads were added and mixed for 16 h at 4°C. The mixture was washed 3 times with lysis buffer followed by centrifugation at 3000xg for 1 minute. 15 μl of 5X-sample buffer was added to the tubes containing complexes and subjected to western blot analysis. Blots were probed using anti-spermine serum (1:2000; Chemicon). For pair-wise binding between GST or GST-Src SH2 domain and EGFR-V5 in the presence of BSA-conjugated spermine (BSA-Spermine), lysis buffer (200 μl) was added into a 1.5 ml microfuge tube containing various amounts of BSA-spermine. Purified GST or GST-Src-SH2 (5 mg) were added and mixed at 22°C for 30 min, followed by the addition of purified the EGFR-V5 (200ng), and the mixture was incubated for another 2 hrs. Then, to this mixture, 20 ml glutathione beads were added and mixed for 16 h at 4°C. The mixture was washed 3 times and processed for western blot analysis as described above. Blots were probed using anti-V5 IgG (1:5000).
Western Blotting
Protein samples were separated by SDS-PAGE and transferred to PVDF (polyvinylidene difluoride) membranes. The membranes were then blocked with either 5% bovine serum albumin (BSA) or blocking grade non-fat dry milk made in tris-buffered saline containing 0.1% Tween 20. Appropriate primary and secondary antibodies were used to detect the proteins of interest by enhanced chemiluminescence detection reagents.
Immunocytochemistry
Immunolocalization studies of proteins were done as described previously [32,33]. Cells seeded on poly-L-lysine-coated glass coverslips placed in 24-well plates were allowed to attach and spread. Cells were fixed with 3.7% para-formaldehyde for 15 minutes, washed twice with DPBS, permeabilized with 0.1% Triton X-100 for 10 minutes, and washed again with PBS. Blocking was carried out with 2% BSA for 20 minutes followed by a two hour incubation with the appropriate primary antibody. The coverslips were washed with PBS, followed by incubation with an appropriate fluorescent dye-conjugated secondary antibody. The coverslips were mounted on glass slides and photographed using a Nikon Diaphot inverted fluorescence microscope with appropriate filters.
Pull-down Assay
Pull-down assays were performed as described before [34–36]. Briefly, HEK293 cells overexpressing the EGFR-V5 were lysed in lysis buffer (PBS/0.2% Triton X100 + protease inhibitors). Cell lysates were mixed at 4°C for 15 min followed by centrifugation at 16,000xg for 10 min at 4°C. GST or GST-Src-SH2 domain or -SH3 domain were added and mixed with the clear supernatant and incubated for 30–60 min. at 4°C followed by additional incubation for 2h with glutathione Sepharose beads (20 μl). Thereafter, the mixtures were spun at 800xg for 2 min, and the beads were washed three times with the same lysis buffer before the proteins were eluted from beads with Laemmli sample buffer (containing 2.5% β-mercaptoethanol). Eluates were separated on 4–15% gel and immunoblotted with anti-V5 IgG.
Co-immunoprecipitation
Confluent serum starved IEC-6 cells left untreated or treated with EGF were exposed to 10 μM spermine for 5–15 min. and washed with DPBS. Cell lysates were subjected to immunoprecipitation using EGFR antibody at 4°C. Immunoprecipitates were washed twice with TBST, and the proteins were eluted from beads with Laemmli sample buffer. Eluates were analyzed by western blotting to detect the levels of Src, integrin β3, EGFR, pY-EGFR, and FAK proteins. The HEK293 cells overexpressing EGFR-V5 were lysed in lysis buffer containing 15 μM polyamines consisting of 5 μM each of spermidine, spermine, and putrescine for 10 min at 4°C. The lysate was centrifuged (16,000g, 10 min, 4°C), and the clear supernatant was used to immunoprecipitated the EGFR using immobilized anti-V5 IgG beads (or non-immune) in a 15 ml tube. Tube contents were mixed at 4°C for 16h and washed 3 times with lysis buffer (3000 g, 1 min each). The beads were transferred to a Spin Column (Pierce Chem. Co) and washed with lysis buffer (100 mM Tris-0.2% TX-100) followed by10 mM Tris-0.2% TX-100, and eluted the antigen using 100 μl of elution buffer (Glycine 100 mM, pH 2.2 containing 0.2% TX-100). The pH of the eluate was neutralized using 10 ml of 1M Tris-HCl, pH 8.8 and subjected to western blotting. Blots were probed using anti-Src and V5 IgG.
Data Analysis and Statistics
Results are presented as means ± SEMs for the FRET, ELISA, and migration studies. Western blots and examples of immunocytochemistry shown are representative of at least three observation. Statistical analysis was performed using Student’s t-test. A value of P < 0.05, or P < 0.01 was considered significant.
Results
Role of Src and Spermine in EGF-induced Migration
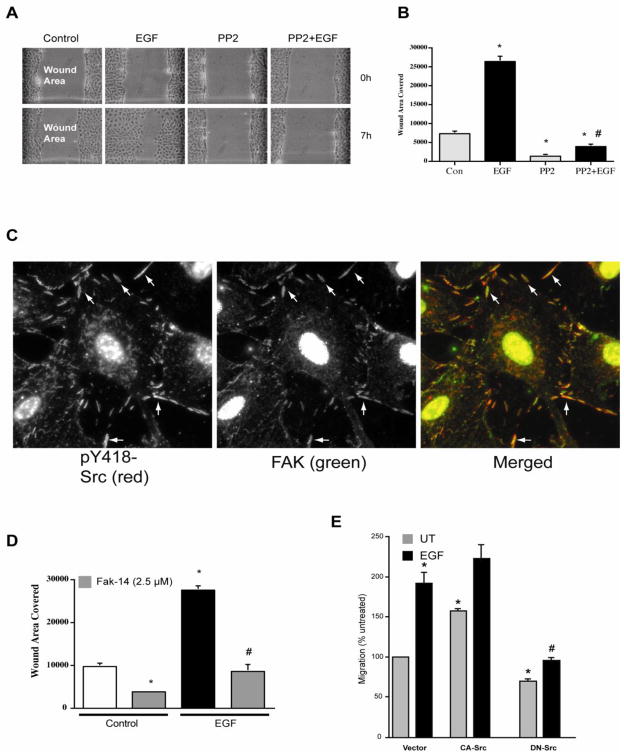
Our earlier results suggested that the observed effects of polyamines on migration and apoptosis might be mediated through the interaction of polyamines with either integrin-β3, the EGFR, or Src [12–14]. We have also shown that EGF increased the phosphorylation of Src (pY418-Src) and, thereby, its activation in IEC-6 cells [14]. EGF significantly decreased the wound width compared to that seen in untreated monolayers (Fig. 1A). Furthermore, inhibition of Src by PP2 almost completely prevented EGF-induced migration and also inhibited basal migration (Fig. 1 A and B). Although, PP2 had no effect on EGF-induced ERK1/2 activation [14], it completely prevented EGF-induced migration suggesting that Src activity is essential for migration and that MEK/ERK may regulate downstream processes activated by Src. Activated Src strongly localized with focal adhesion kinase (FAK) during spreading of these cells (Fig. 1C). The inhibition of focal adhesion kinase activity by F14 significantly inhibited EGF-induced migration (Fig. 1D). Since pharmacological inhibitors may have non-specific effects on other kinases, we examined EGF-induced migration in IEC-6 cells transfected with constitutively active (CA) or dominant negative (DN) Src. IEC-6 cells lines stably expressing CA-Src and DN-Src were characterized and reported in a previous study by our laboratory [37]. Consistent with observations reported in Fig. 1B, EGF increased migration in cells transfected with empty vector. Cells expressing CA-Src increased basal migration, which was further increased in response to EGF (Fig. 1E). Conversely, expression of DN-Src blocked basal and EGF-induced migration, suggesting that Src plays a critical role in the regulation of migration.
Fig. 1. Role of Src in the EGF-induced migration.
A, confluent monolayers of IEC-6 cells were wounded with a gel loading tip in the center of the plates, washed and left untreated or treated with 10 nM EGF in the presence and absence of 10 μM PP2. B, migration was calculated as described in the methods. Values are means ± SEMs of triplicates. * Significantly different compared to UT group. C, following attachment and spreading of cells, coverslips were washed, fixed, permeabilized and processed for immunolocalization for the pY418-Src and FAK as described in the methods. Representative images from three observations are shown. D, confluent monolayers of IEC-6 cells were wounded with a gel loading tip in the center of the plates, washed and left untreated or treated with 10 nM EGF in the presence and absence of 2.5μM FAK14 (FAK inhibitor). Migration was calculated as described in the methods. Values are means ± SEMs of triplicates. * Significantly different compared to UT group. E, Confluent monolayers of IEC-6 cells stably expressing empty vector, constitutively active (CA), and dominant negative (DN) Src were wounded with a gel loading tip in the center of the plates, washed and left untreated (UT) or treated with 10 nM EGF. Migration was expressed as percent untreated. Values are means ± SEMs of triplicates. * Significantly different compared to UT group, # significantly different compared to cells expressing empty vector and treated with EGF.
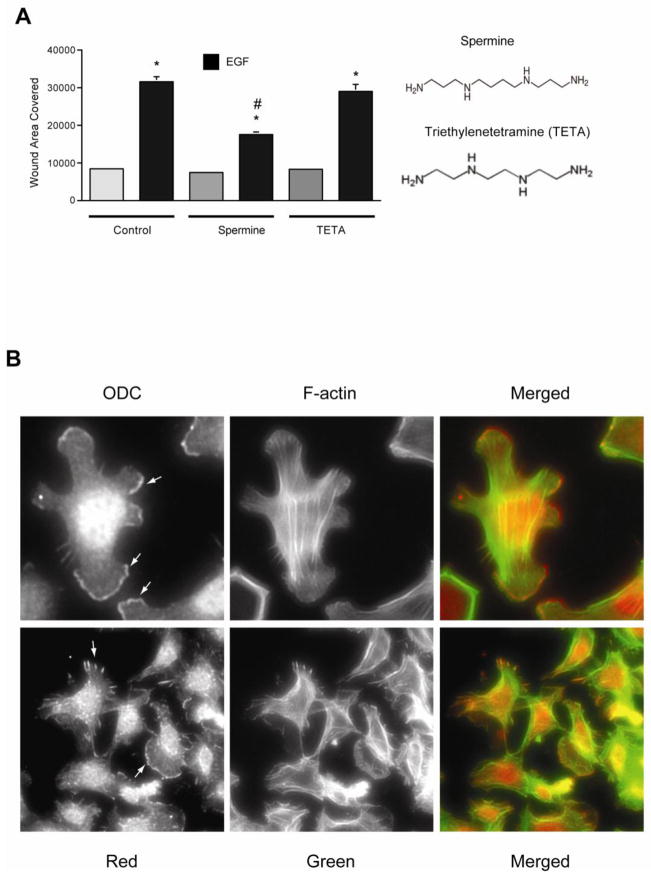
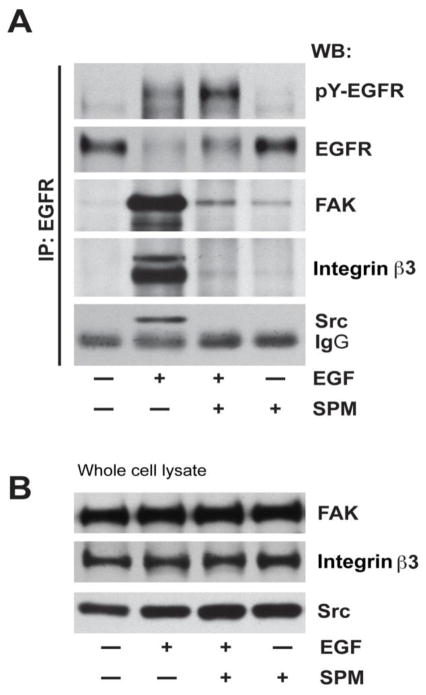
Since spermine prevented the activation of Src by EGF and DFMO and eliminated the protection against apoptosis conferred by EGF and DFMO in IEC-6 cells [14], we examined the effect of spermine on EGF-induced migration to understand the early signaling events regulated by polyamines. EGF increased migration, measured as wound healing, about 3 fold compared to the untreated monolayer (Fig. 2A). Addition of 5μM spermine significantly decreased EGF-induced migration (Fig. 2A). The specificity of the effect of spermine was confirmed by using triethylenetetramine (TETA), which has the same number of amine groups but a shorter carbon skeleton. Unlike spermine, TETA failed to block EGF-induced migration (Fig. 2A). We also confirmed that 5μM spermine had no apoptotic effects on these cells (data not shown). Our recent study demonstrated that Src activation is essential for the attachment and spreading of cells [12], and results in figure 2B show that cells formed large lamellipodia, F-actin stress fibers, and focal plaques during spreading. In these cells, ODC localized in the lamellipodia as well as with the focal plaques (Fig. 2B, arrows), suggesting the involvement of polyamines during attachment and spreading. These data suggest that Src plays a key role in regulating downstream signal transmission during migration and also suggest the possibility that spermine modulates Src activation. We therefore, examined the binding of Src with the EGFR in cells treated with EGF for various time intervals in the presence and absence of spermine. EGF activated the EGFR and binding of Src to the EGFR was detected at 10 min (Fig. 3A). EGFR activation was evident by increased phosphorylation (pY-EGFR), which was associated with decreased EGFR levels due to internalization and degradation. Spermine prevented the binding of the EGFR with Src at 10 minutes (Fig. 3A) without affecting Src expression (Fig. 3B). Furthermore, spermine decreased EGF-induced internalization and degradation of EGFR and consequently led to the accumulation of pY-EGFR. We reported earlier that EGF rapidly increased the phosphorylation of EGFR, integrin β3, Src, and FAK in IEC-6 cells [12, 14]. Therefore, we determined binding of integrin β3 and FAK with the EGFR in cells treated with EGF. Both FAK and integrin β3 binding with the EGFR increased in response to EGF. However, total FAK and integrin β3 protein levels did not change (Fig. 3B). Spermine inhibited the association of EGFR with FAK and integrin β3 indicating that spermine binds either Src or integrin β3, and modulates their activities and the activities of the EGFR associated binding partners including FAK and Src.
Fig. 2. Spermine inhibits EGF-induced migration.
A, confluent monolayers of IEC-6 cells were wounded with a gel loading tip in the center of the plates, washed and left untreated or treated with 10 nM EGF in the presence and absence of 10 μM of spermine or triethylenetetramine (TETA). Migration was calculated as described in the methods. Values are means ± SEMs of triplicates. * Significantly different compared to UT group. B, serum starved IEC-6 cells were plated in DMEM containing dFBS and were allowed to attach and spread. Cells were washed, fixed and processed for localization of ODC and f-actin as described in methods. A cropped image of a cell showing ODC localization in lamellipodia is shown (upper panel), A group of cells showing ODC and F-actin in lamellipodia and focal plaques (lower panel). Representative images from three experiments are shown.
Fig. 3. Spermine prevents interaction of EGFR with integrin β3, Src, and FAK.
Confluent monolayers of IEC-6 cells were washed and left untreated or treated with 10 nM EGF in the presence and absence of 10 μM of spermine for 10 min. A, Monolayers were washed and lysates were subjected to immunoprecipitation using anti-EGFR antibody. Membranes were probed for the detection of integrin β3, Src, and FAK. Membranes were striped and probed for pY-EGFR and total-EGFR using specific antibodies. Blots shown are representative of three observations. B, Whole cell lysates were analyzed by western blot using FAK, integrin β3 and Src-specific antibodies. Representative blots from three experiments are shown.
Polyamine depletion augments EGF-induced Src activation in live HEK293 cells
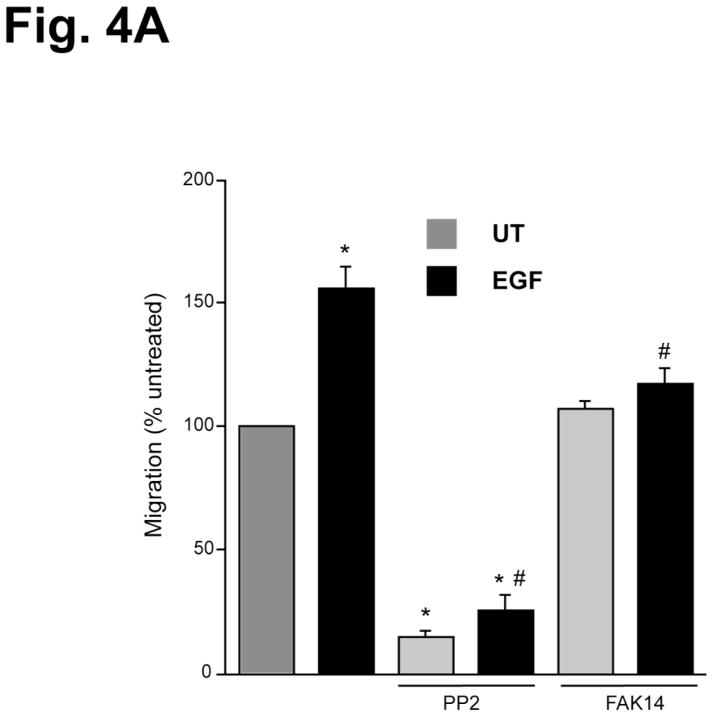
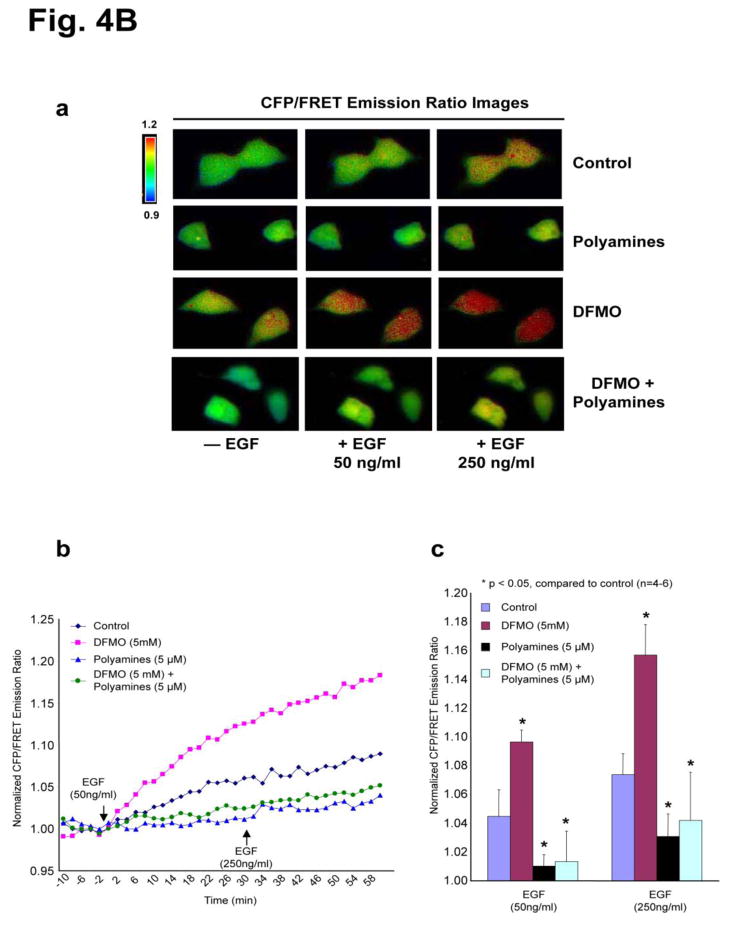
To demonstrate that EGF promotes cell migration via a Src and FAK-dependent manner; we tested the role of these protein kinases in EGF-induced migration in HEK293 cells. EGF significantly increased migration of HEK293 cells and pharmacologic inhibition of Src kinase (using PP2) blocked basal and EGF-induced migration (Fig. 4A), which is similar to IEC-6 cells. Furthermore, inhibition of FAK (using FAK14) had no effect on basal migration, but blocked EGF-induced migration, suggesting that EGF-induced migration requires activation of Src and FAK (Fig. 4A). Upon confirming the role of Src and FAK in EGF-induced migration of HEK293 cells, we used a fluorescence resonance energy transfer (FRET) based reporter for Src kinase activity in HEK293 cells [34] as a tool to determine the effects of polyamines on Src activity. This Src reporter is composed of CFP at the N-terminus, the SH2 domain of c-Src, a Src substrate peptide, and YFP at the C-terminus. The proximity of the N and C termini of the SH2 domain allows the juxtaposition of CFP and YFP to yield a high FRET. Upon Src phosphorylation, the substrate peptide can bind to the phospho-peptide-binding pocket of the SH2 domain and separate YFP from CFP, thus decreasing FRET [34]. Phosphorylation of the Src reporter by activated endogenous Src enhances CFP emission at the expense of YFP emission, leading to an increased cyan-to-yellow emission ratio (CFP/FRET or CFP/YFP). Because of the low efficiency of transfection in IEC-6 cells, HEK293 cells were transfected with the Src reporter and used in this study. EGF induced a 5–10% emission ratio change in a dose-dependent manner [Fig. 4B, panels a–c], indicating Src phosphorylation in real time in live cells. Polyamine depletion by DFMO treatment elicited a 10–20% emission ratio change, demonstrating a significant increase in EGF-induced Src activity compared to the control (Fig. 4B, panels a–c). However, the phosphorylation of Src in response to EGF was attenuated when cells were grown with exogenous polyamines or DFMO plus exogenous polyamines (Fig. 4B, panels a–c). It is noteworthy that DFMO-treated cells demonstrated a higher basal level emission ratio than cells grown in control or DFMO plus exogenous polyamines containing media (Fig. 4B, panels a–c).
Fig. 4. Polyamines prevent Src activation in live cells.
A, confluent monolayers of HEK293 cells were wounded with a gel loading tip in the center of the plates, washed and left untreated or treated with 10 nM EGF in the presence and absence of 10 μM PP2 and 10 μM FAK14 for 20h. Migration was calculated as described in the methods. Values are means ± SEMs of triplicates. * Significantly different compared to UT group, and # significantly different compared to cells treated with EGF. B, panel a, the CFP/FRET emission ratio images in response to EGF in HEK293 cells overexpressing a Src reporter pretreated with DFMO (5 mM) and/or polyamines (5 mM each for spermidine, spermine, and putrescine) for 3–4 days before the FRET imaging. panel b, the time courses of normalized CFP/FRET emission ratio of the Src reporter. The arrows indicate the addition of EGF (first arrow: 50ng/ml, the second arrow: 250ng/ml). panel c, the bar graphs of the normalized CFP/FRET emission ratio at the time point (20 min) after EGF addition (n=4–6).
Spermine interacts directly with Src and associates preferentially with the SH2 domain
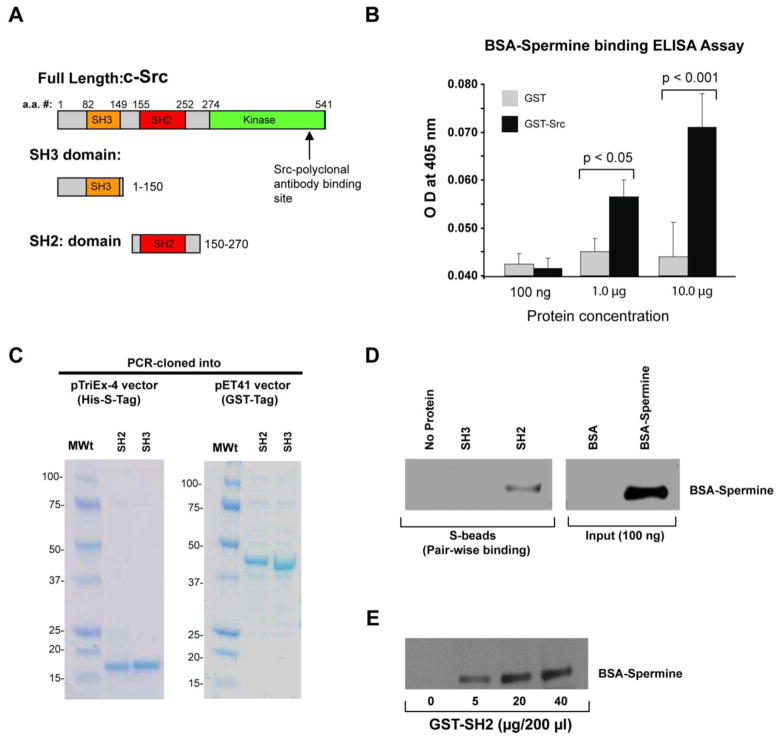
Src is a 60 kDa protein comprised of SH2, SH3, and catalytic (kinase) domains (Fig. 5A). The domain structure accounts for its ability to interact with various effector molecules and regulate signaling pathways. The SH2 domain is reported to interact with FAK [38] and EGFR [39], while the SH3 domain has been shown to bind to integrin [40]. Given that polyamines can affect Src kinase activity, we tested whether polyamines could physically interact with Src. GST-Src fusion protein was purified as previously reported [35], and the purified GST-Src was recognized specifically by anti-Src monoclonal and polyclonal antibodies in dot blots (data not shown). An ELISA-based pair-wise binding assay was performed to detect the interaction between GST-Src and BSA-conjugated spermine (BSA-spermine). Anti-spermine antibody was generated using BSA-spermine as an immunogen. Therefore, we used the same BSA-Spermine for the in vitro binding and pull down assays. Pair-wise binding detects interactions between two individually purified proteins [36]. In cells, precursor putrescine is rapidly converted to spermidine and subsequently to spermine. Therefore, we used spermine in binding assays. Our data showed a dose dependent binding of GST-Src with BSA-spermine against BSA as the blank (Fig. 5B). Furthermore, we sought to define the domain(s) of Src involved in the interaction with spermine. In order to determine the precise location of the spermine-binding site on Src, we cloned the SH2 and SH3 domains of Src in pTriEx-4 and pET-41 vectors (Fig. 5C). The fusion proteins were expressed and purified from bacteria, and we found that both the SH3 (amino acids 1–150) and the SH2 (amino acids 150–270) domains were quite stable (Fig. 5C). Purified SH2- and SH3-domain containing fusion proteins were incubated with BSA-Spermine in pair-wise binding assays. The SH2-domain bound directly to spermine, while the SH3-domain lacked spermine binding (Fig. 5D). BSA alone did not bind to either the SH2- or SH3- domain. Dose-dependent binding (0–40 μg) of GST-SH2 with BSA-spermine was observed, and maximal binding was seen with 20 μg of GST-SH2 (Fig. 5E). These observations suggest that spermine binds directly and preferentially to the SH2 domain of c-Src.
Fig. 5. Spermine interacts directly with Src and associates preferentially with the SH2 domain.
A, various domains of mouse neuronal c-Src B, ELISA-based pair-wise binding of GST-Src with BSA-conjugated spermine (n=3). BSA-conjugated spermine was incubated with GST or GST-Src immobilized in the ELISA wells, followed by the incubation with anti-spermine serum. The absorbance was measured at 450 nm. C, PCR-cloned His-S-tagged or GST-tagged Src domain proteins resolved by SDS-PAGE. D, pair-wise binding between His-S-SH2 and HisS-SH3 domains of Src with BSA-spermine. The mixture was pulled down using S-beads, and blotted with anti-spermine serum. Representative blots from three independent observations are shown. E, pair-wise binding between BSA-spermine and different doses of GST-His-S-SH2 domain of Src. The mixture was pulled down using glutathione beads, and blotted with anti-spermine serum. A representative blot from three independent observations is shown.
Physical association of EGFR with the Src-SH2 domain is inhibited by spermine
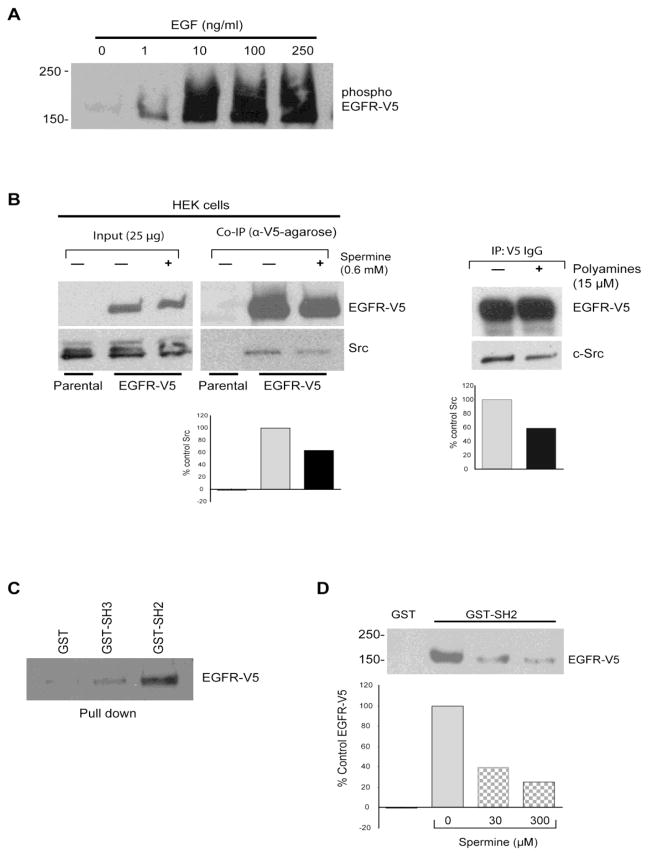
Accumulated evidence suggests that the mutual interaction between activated EGFR and c-Src is critical to many EGFR-mediated cellular functions [20, 21]. Based on results shown in figure 5, it appears that polyamines could disrupt both physical and functional coupling of EGFR and Src kinase. To test this, we generated a HEK293 cell line that stably overexpressed V5-tagged EGFR in a lentiviral expression system (supplemental Fig. S1A, left panel) [41] and determined EGFR expression in HEK293 cell and its phosphorylation by EGF. EGF increased EGFR phosphorylation in a dose-dependent fashion (Fig. 6A), which is similar to IEC-6 cells. Immunoprecipitation of EGFR from HEK293-EGFR-V5 cells that express endogenous Src incubated in the presence or absence of spermine showed a significant reduction in the amount of endogenous Src that co-immunoprecipitated with V5-tagged EGFR protein when cells were exposed to spermine (Fig. 6B). A similar decrease in Src binding with EGFR was evident when the EGFR-V5 cell extract was incubated in the presence of 15 μM polyamines (5μM each of putrescine, spermidine, and spermine) (Fig. 6B Right panel). We purified V5-tagged EGFR (supplemental Fig. S1A, right panel) and confirmed its identity by MALDI TOF/Mass Spec (supplemental Fig. S1B). GST or GST-SH2 and GST-SH3 domains of Src proteins were employed to pull-down EGFR-V5. We found that SH2 domain of Src binds to the EGFR with the highest affinity (Fig. 6C), consistent with the previous report [39]. Next, we performed pairwise binding between purified EGFR-V5 and the purified GST-SH2 domain of Src in the presence of exogenous spermine. Our data demonstrated that spermine exhibited a dose-dependent inhibitory effect on the direct interaction between EGFR and the SH2-domain of Src (Fig. 6D). These results clearly demonstrate that EGFR binds preferentially to the SH2-domain of Src kinase, and that this binding is inhibited by spermine. It is clear from this study that spermine binds the SH2-domain and modulates its interaction with its binding partners.
Fig. 6. Physical association of the EGFR with the Src-SH2 domain is inhibited by polyamines.
A, EGFR phosphorylation in response to EGF in HEK293 cells overexpressing V5-tagged EGFR. The cells were subjected to EGF treatment for 30 min at 37°C before being lysed in the lysis buffer. The overexpressed EGFR-V5 was immunoprecipitated with anti-V5 IgG, and immunoblotted with anti-pY-HRP (for phosphor-EGFR-V5). A representative blot from three independent observations is shown. B, co-immunoprecipitation of endogenous Src and over-expressed EGFR in HEK293 cells in the presence or absence of 0.6 mM spermine or 15 μM polyamines (consisting of 5 μM each of spermidine, spermine, and putrescine). The cells were lysed in lysis buffer containing the above-mentioned compounds before being subjected to co-IP. Representative blots with densitometry from three independent observations are shown. C, interaction between the EGFR and various domains of Src. EGFRs-V5 over-expressed in HEK293 cells were subjected to pull-down assay using GST-Src fusion proteins, and probed with anti-V5 IgG. Representative blots from three independent observations are shown. D, pairwise binding between purified EGFR-V5 (200 ng) and the GST-Src SH2 domain (5 μg) in the presence of polyamines (i.e., spermine conjugated to BSA; 0–300 μM). A representative blot and densitometry are shown from three independent observations.
Discussion
There is agreement that the effects of polyamines depend on their positive charges. Putrescine, spermidine and spermine contain two, three, or four amine groups, respectively, with pK values above 9, so they are polycations at physiological pH and strongly bind to negatively charged macromolecules, particularly nucleic acids and proteins. The fact that these are not point charges, but are fixed along a flexible carbon chain, allows polyamines to interact with macromolecules in structurally specific ways. Spermine, for example, binds to phosphate groups of the DNA helix, occupying the small groove and stabilizing the helix by binding the two strands together [42, 43]. At physiologic pH, spermine stimulation is seen at NR1/NR2B subunits of NMDA receptors [44]. Ishihara and Ihara showed that spermine and spermidine blocked the strong inward rectifier K (+) channel Kir2.1 [45]. Thus, direct interaction of polyamines with proteins modulates functional properties. The interaction of spermidine/spermine acetyltransferase (SSAT) with α9β1 integrin at the receptor level caused a localized inhibition in the levels of polyamines and augmented potassium efflux and enhanced migration [46]. These studies indicate that polyamines affect global as well as localized effects on migration [47].
At this juncture it’s important to discuss the concept of “polyamine depletion”. DFMO is a perfect suicide inhibitor of ODC. Many investigators have shown that its only effect is to block ODC, and that the biological effects are all caused by a reduction in cellular polyamines. We know this because in each instance supplying exogenous putrescine, spermidine, or spermine along with DFMO prevented the biological effect of DFMO. Following DFMO treatment, putrescine disappears almost immediately, spermidine within 24h, and spermine decreases to 40–50% of normal after 4 days [8,9]. We have described several instances where DFMO produces effects within an hour or two, NF-κB [48] and STAT-3 [49] activation, respectively, and within a few minutes, EGFR and Src phosphorylation [14]. The cells still contain large amounts of polyamines, yet these early events are also prevented by exogenous polyamines and are due to polyamine depletion. We believe that in these instances, it is the free accessible, biologically active pool that is being depleted. Almost all cellular polyamines are bound to macromolecules due to their strong positive charges, and most are present in granules or in the nucleus. This bound pool may well be important for certain cell functions, but newly synthesized, free polyamines appear to be essential as well. This is best illustrated by the many experiments showing that administration of growth factors, trophic hormones, serum, and damaging agents cause ODC activity to increase from barely detectable to maximum level within 3h in cultured cells and in the appropriate tissues in vivo [5–7]. We believe that immediately upon exposure to DFMO, putrescine production ceases and any remainder is rapidly converted to spermidine and spermine and bound.
Over the years, we have demonstrated that polyamine depletion inhibits proliferation, apoptosis and migration of IEC-6 cells [10, 12, 14, 37, 50]. All of the terminal effects of polyamine depletion are regulated at the level of MEK/ERK and Src pathways, which converge on EGFR and integrin β3. We have shown that polyamine depletion achieved by growing cells in the presence of 5 mM DFMO for 4 days significantly increases autophosphorylation of Src, integrin β3, and EGFR without affecting the levels of these proteins [37]. In addition, activation of integrin β3 trans-activated the EGFR and induced ERK1/2 activity in these cells. Furthermore, inhibition of ODC by DFMO significantly increased the phosphorylation of the EGFR, integrin β3, and Src within 30 min, which was prevented by the addition of putrescine along with DFMO [14]. Thus, the EFGR and integrin β3 respond to instantaneous as well as prolonged changes in the intracellular polyamine content. Since both the EGFR and integrin β3 activated Src [14, 37], and since Src also caused the reciprocal activation of both receptors, we predicted that polyamines might regulate Src by modulating the interaction between Src, integrinβ3 and the EGFR.
The protective effects of polyamine depletion on apoptosis are due to the constitutive activation of antiapoptotic pathways. However, it is intriguing that the same pathways reported to increase migration, had inhibitory effects on migration in polyamine depleted cells. The regulation of migration involves dynamic remodeling of the F-actin cytoskeletal structure, and FAK is essential for the Tiam1-mediated activation of Rho-GTPases during migration [12]. We presume that constitutive activation of Src in polyamine-depleted cells may prevent dynamic turnover of FAK during migration. Therefore, it appears that the spatio-temporal changes in the levels of polyamines regulate the turn over of Src-mediated FAK activity and, thereby, migration. Cell migration involves the coordinated activation and deactivation of signaling proteins allowing the dynamic organization of F-actin, extension and retraction of laemilipodia, and focal adhesions. Thus, all of these processes are transient and spatial.
The pharmacological inhibition of Src and expression of dominant-negative Src decreased basal as well as EGF-induced migration (Fig. 1). In addition, basal migration increased in cells expressing constitutively active Src (Fig. 1E) suggesting that Src is essential for migration in response to EGF and to migration in general. 10μM spermine significantly inhibited EGF-induced migration while TETA did not (Fig. 2A). Compared to spermine, TETA contains a shorter carbon backbone with the same number of cationic charges (Fig.2A). These observations indicate that these effects of spermine are specific. Activated Src (pY418) localized with FAK during cell spreading, and the inhibition of FAK by Fak-14 significantly decreased basal as well as EGF-induced migration. Furthermore, EGF increased migration of HEK293 cells and pharmacologic inhibition of Src inhibited basal and EGF-induced migration (Fig. 4A). Inhibition of FAK by FAK14 had no effect on basal migration but blocked EGF-induced migration of HEK293 cells. These results further confirmed the roles of Src and FAK in migration (Fig. 1D, 4A). ODC protein was found precisely localized at the leading edges of lamellipodia during spreading and at the focal contact points in some cells indicating the involvement of newly synthesized polyamines in these processes (Fig. 2B).
Phosphorylation of Tyr 418 in the kinase domain activates Src and augments its interaction with other signaling intermediates including focal adhesion kinase [51]. FAK activity is vital for the development of tisssues and regulates diverse cellular processes, including cell adhesion, migration, polarity, proliferation, and survival [52]. This range of functions is evidence that FAK plays a fundamental role in integrating signals from integrin and growth factor receptors to regulate the cellular responses mentioned above [51, 52].
Immunoprecipitation of EGFR confirmed the binding of activated EGFR with Src and integrin β3. Spermine almost completely prevented the interaction of EGFR with Src and integrin β3 (Fig. 3A). FAK was associated with phosphorylated pY-EGFR and spermine prevented this interaction (Fig. 3A). Although, growth factors activate FAK, direct binding of FAK with the activated EGFR has not been demonstrated. However, it is predicted that additional adaptor proteins might be involved in this process. The FRET based Src reporter assay also confirmed that polyamine depletion increased Src activation, which was also prevented by polyamines (Fig. 4B, panels a–c). Immunoprecipitation of the EGFR from HEK cells transfected with V5-EGFR showed association of Src with the EGFR (Fig. 6B). Furthermore, the presence of polyamines decreased the association between the EGFR and Src (Fig. 6B). EGFR-V5 pull down by GST-SH2 fusion protein, the decreased association of GST-SH2 with the EGFR in the presence of polyamines, and the binding of spermine with SH2 domain of Src suggest that polyamines mediate the physical interaction between Src and the EGFR (Figs. 5 and 6). Activated EGF receptors form heteromeric complexes with multiple signaling and bridging molecules via SH2-Tyr (p) interactions [53–55]. It has been shown that the SH2 domain of the Src protein binds activated EGFR, and endogenous pp60c-src tightly associates with tyrosine-phosphorylated EGFR [39]. These findings and other reports demonstrated that the c-Src SH2 domain is required for EGFR-mediated signal cascades [56] suggesting the direct interaction between the EGFR and c-Src in vivo. Previously, we have demonstrated that polyamine depletion leads to increased phosphorylation of the EGFR at tyrosine 1173, which is localized in the intracellular domain, and of tyrosine 845, which is a putative phosphorylation site for Src, suggesting that under basal conditions polyamines bind Src and prevent its interaction with the EGFR [53–55]. Thus, increased EGFR activation and thereby ERK1/2 phosphorylation in polyamine depleted cells might result from increased binding of Src to the EGFR.
These observations provide direct evidence for the interaction of the EGFR and Src and the prevention of that interaction by polyamines, suggesting that polyamines modulate the interaction of the SH2 domain of Src with the EGFR. We examined the binding of spermine with the purified GST-SH2 and GST-SH3 domains of Src. It is clear from the results that the SH2 domain specifically binds spermine in a dose dependent fashion (Fig. 6).
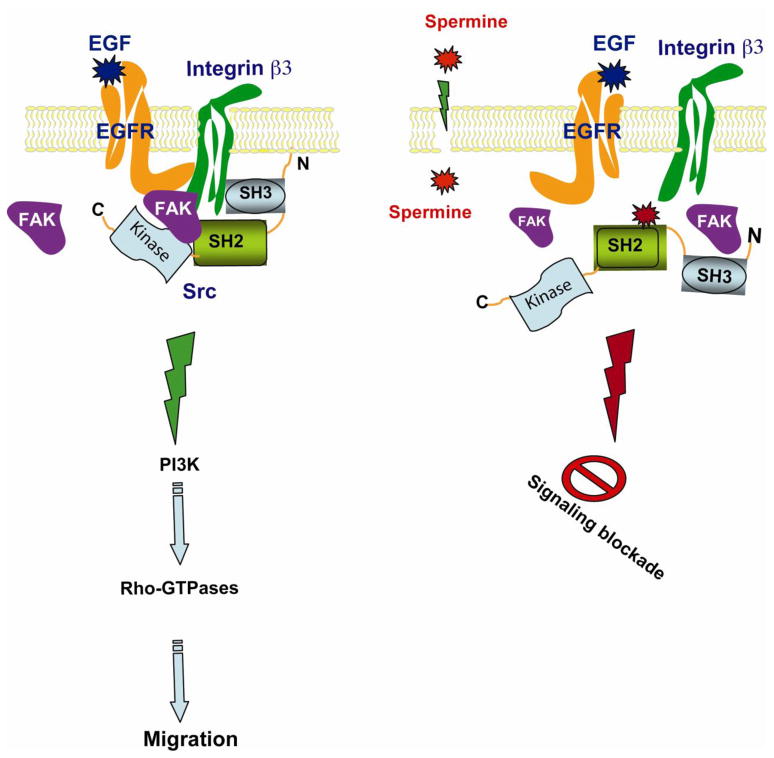
Our results show that the activation of the EGFR increases trans-phosphorylation of integrin β3 allowing the recruitment of Src, which activates both the EGFR and integrin β3, amplifying the signaling cascade leading to the activation of downstream targets regulating migration. Src and FAK activate the PI-3 kinase/Akt signaling axis, which in turn recruits Tiam1 and other actin binding proteins leading to increased activation of Rho-GTPases and cell migration. Our study demonstrates for the first time that spermine regulates EGF-induced migration by preventing the formation of the scaffold consisting of EGFR, integrin β3, FAK and Src (Fig.7). The single most important finding in this study is the demonstration of the mechanism by which spermine regulates the EGFR/integrin β3/Src/FAK scaffold in intestinal epithelial cells. Spermine binds the SH2 domain of Src and prevents its interaction with activated EGFR and integrin β3 and subsequently the phosphorylation of FAK.
Fig. 7. A proposed model depicting the mechanism by which polyamines modulate EGFR-mediated signaling.
EGF induced autophosphorylation of the EGFR recruits integrin β3, FAK and Src via the SH2 domain and, thereby, forms a macromolecular signaling complex. As a result, EGFR phosphorylates Src and enhances autophosphorylation of FAK. Activated FAK and Src induce downstream signaling cascades, which include PI3K, and Rho-GTPases. These pathways regulate migration. Polyamines bind to the SH2 domain of Src, thus preventing the physical interaction of EGFR with integrin β3 and the Src SH2 domain, and attenuates the Src-mediated FAK activation and thereby Rho-GTPases, essential for spreading and migration.
The assembly and disassembly of focal adhesions (dynamic turnover) is essential for migration. In the absence of polyamines static focal adhesions form due to the continual activation of Src which inhibits migration. However, spermine binds the SH2 domain of Src and prevents its association with the scaffold structure allowing the disassembly of focal adhesions and the formation of lamellipodia. Excess spermine in the vicinity of the scaffold mimics the effects of the Src inhibitor, PP2. Thus, free intracellular spermine alters the interaction of proteins and, thereby, acts as a molecular switch turning on and off the signaling cascade required for migration.
Supplementary Material
Highlights.
EGF transactivates integrin β3, recruits Src and FAK during migration
Spermine inhibits the EGFR interaction with Src and prevents migration
Spermine binds the SH2 domain of Src and prevents EGF-induced migration
Acknowledgments
This publication was made possible by grants DK-052784 and DK-16505 (L.R.J.), and DK058545 and DK074996 (A.P.N.) from the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Health. This work was also supported by the Thomas A. Gerwin endowment (L.R.J.).
We thank Dr. Shu Chien (University of California-San Diego, La Jolla, CA) for kindly providing CFP-Src-YFP construct and Dr. Alexander Levitzki (The Hebrew University of Jerusalem, Jerusalem, Israel) for GST-Src construct.
The abbreviations used are
- ODC
ornithine decarboxylase
- DFMO
DL-α-difluoromethyl ornithine
- FRET
fluorescence resonance energy transfer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pegg AE, McCann PP. Am J Physiol. 1982;243:C212–C221. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- 2.Pegg AE. Biochem J. 1986;234:249–262. doi: 10.1042/bj2340249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tabor CW, Tabor H. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- 4.Russell DH. Adv Enzyme Regul. 1983;21:201–222. doi: 10.1016/0065-2571(83)90015-8. [DOI] [PubMed] [Google Scholar]
- 5.Wang JY, Johnson LR. Am J Physiol. 1990;258:G78–G85. doi: 10.1152/ajpgi.1990.258.1.G78. [DOI] [PubMed] [Google Scholar]
- 6.Wang JY, Johnson LR. Am J Physiol. 1990;259:G584–G592. doi: 10.1152/ajpgi.1990.259.4.G584. [DOI] [PubMed] [Google Scholar]
- 7.Wang JY, McCormack SA, Viar MJ, Wang H, Tzen CY, Scott RE, Johnson LR. Am J Physiol. 1993;265:G331–G338. doi: 10.1152/ajpgi.1993.265.2.G331. [DOI] [PubMed] [Google Scholar]
- 8.Russell DH. Drug Metab Rev. 1985;16:1–88. doi: 10.3109/03602538508991430. [DOI] [PubMed] [Google Scholar]
- 9.Pegg AE, McGovern KA, Wiest L. Biochem J. 1987;241:305–307. doi: 10.1042/bj2410305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ray RM, McCormack SA, Covington C, Viar MJ, Zheng Y, Johnson LRJ. Biol Chem. 2003;278:13039–13046. doi: 10.1074/jbc.M208741200. [DOI] [PubMed] [Google Scholar]
- 11.Schipper RG, Penning LC, Verhofstad AA. Semin Cancer Biol. 2000;10:55–68. doi: 10.1006/scbi.2000.0308. [DOI] [PubMed] [Google Scholar]
- 12.Elias BC, Bhattacharya S, Ray RM, Johnson LR. Cell Adh Migr. 2010;300:164–175. doi: 10.4161/cam.4.3.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ray RM, Viar MJ, McCormack SA, Johnson LR. Am J Physiol Cell Physiol. 2001;281:C475–C485. doi: 10.1152/ajpcell.2001.281.2.C475. [DOI] [PubMed] [Google Scholar]
- 14.Ray RM, Bhattacharya S, Johnson LR. Cell Signal. 2007;19:2519–2527. doi: 10.1016/j.cellsig.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedl P, Wolf K. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 16.Goi T, Shipitsin M, Lu Z, Foster DA, Klinz SG, Feig LA. EMBO J. 2000;17:623–630. doi: 10.1093/emboj/19.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, Schlaepfer DD. Nat Cell Biol. 2000;2:249–256. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- 18.Bokoch GM. Annu Rev Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- 19.Parsons SJ, Parsons JT. Oncogene. 2004;23:7906–7909. doi: 10.1038/sj.onc.1208160. [DOI] [PubMed] [Google Scholar]
- 20.Ishizawar R, Parsons SJ. Cancer Cell. 2004;6:209–214. doi: 10.1016/j.ccr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Biscardi JS, Ishizawar RC, Silva CM, Parsons SJ. Breast Cancer Res. 2000;2:203–210. doi: 10.1186/bcr55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlaepfer DD, Mitra SK. Curr Opin Genet Dev. 2004;14:92–101. doi: 10.1016/j.gde.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Eide BL, Turck CW, Escobedo JA. Mol Cell Biol. 1995;15:2819–2827. doi: 10.1128/mcb.15.5.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlaepfer DD, Hanks SK, Hunter T, van der Geer P. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- 25.Wojciechowski K, Trzeciak L, Konturek SJ, Ostrowski J. Regul Pept. 1995;56:1–8. doi: 10.1016/0167-0115(95)00121-q. [DOI] [PubMed] [Google Scholar]
- 26.Faaland CA, Laskin JD, Thomas TJ. Cell Growth Differ. 1995;121:115–121. [PubMed] [Google Scholar]
- 27.Milovic V, Deubner C, Zeuzem S, Piiper A, Caspary WF, Stein J. Biochem Biophys Res Commun. 1995;206:962–968. doi: 10.1006/bbrc.1995.1136. [DOI] [PubMed] [Google Scholar]
- 28.Moshier JA, Malecka-Panas E, Geng H, Dosescu J, Tureaud J, Skunca M, Majumdar AP. Cancer Res. 1995;55:5358–5365. [PubMed] [Google Scholar]
- 29.Blachowski S, Motyl T, Grzelkowska K, Kasterka M, Orzechowski A, Interewicz B. Int J Biochem. 1994;26:891–897. doi: 10.1016/0020-711x(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 30.Li C, Krishnamurthy PC, Penmatsa H, Marrs KL, Wang XQ, Zaccolo M, Jalink K, Li M, Nelson DJ, Schuetz JD, Naren AP. Cell. 2007;131:940–951. doi: 10.1016/j.cell.2007.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ray RM, Vaidya RJ, Johnson LR. Cell Motil Cytoskeleton. 2007;64:143–156. doi: 10.1002/cm.20172. [DOI] [PubMed] [Google Scholar]
- 32.Ray RM, Guo H, Patel M, Jin S, Bhattacharya S, Johnson LR. Am J Physiol Gastrointest Liver Physiol. 2007;292:G983–G995. doi: 10.1152/ajpgi.00356.2006. [DOI] [PubMed] [Google Scholar]
- 33.Ray RM, Viar MJ, Yuan Q, Johnson LR. Am J Physiol Cell Physiol. 2000;278:C480–C489. doi: 10.1152/ajpcell.2000.278.3.C480. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Botvinick EL, Zhao Y, Berns MW, Usami S, Tsien RY, Chien S. Nature. 2005;434:1040. doi: 10.1038/nature03469. [DOI] [PubMed] [Google Scholar]
- 35.Karni R, Mizrachi S, Reiss-Sklan E, Gazit A, Livnah O, Levitzki A. FEBS Lett. 2003;537:47–52. doi: 10.1016/s0014-5793(03)00069-3. [DOI] [PubMed] [Google Scholar]
- 36.Naren AP. Methods Mol Med. 2002;70:175–186. doi: 10.1385/1-59259-187-6:175. [DOI] [PubMed] [Google Scholar]
- 37.Bhattacharya S, Ray RM, Johnson LR. Biochem J. 2006;397:437–447. doi: 10.1042/BJ20060256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitra SK, Hanson DA, Schlaepfer DD. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 39.Luttrell DK, Lee A, Lansing TJ, Crosby RM, Jung KD, Willard D, Luther M, Rodriguez M, Berman J, Gilmer TM. Proc Natl Acad Sci U S A. 1994;91:83–87. doi: 10.1073/pnas.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arias-Salgado EG, Lizano S, Sarkar S, Brugge JS, Ginsberg MH, Shattil SJ. Proc Natl Acad Sci U S A. 2003;100:13298–13302. doi: 10.1073/pnas.2336149100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sato M, Vaughan MB, Girard L, Peyton M, Lee W, Shames DS, Ramirez RD, Sunaga N, Gazdar AF, Shay JW, Minna JD. Cancer Res. 2006;66:2116–2128. doi: 10.1158/0008-5472.CAN-05-2521. [DOI] [PubMed] [Google Scholar]
- 42.Marquet R, Houssier C. Biochem Pharmacol. 1988;37:1857–1858. doi: 10.1016/0006-2952(88)90481-9. [DOI] [PubMed] [Google Scholar]
- 43.McLean MJ, Wells RD. Biochim Biophys Acta. 1988;950:243–254. doi: 10.1016/0167-4781(88)90120-0. [DOI] [PubMed] [Google Scholar]
- 44.Han X, Tomitori H, Mizuno S, Higashi K, Füll C, Fukiwake T, Terui Y, Leewanich P, Nishimura K, Toida T, Williams K, Kashiwagi K, Igarashi K. J Neurochem. 2008;107:1566–1577. doi: 10.1111/j.1471-4159.2008.05729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ishihara K, Ihara T. J Physiol. 2004;556:61–78. doi: 10.1113/jphysiol.2003.055434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Hart GW, Jin T, McCloskey DE, Pegg AE, Sheppard D. Proc Natl Acad Sci USA. 2008;105:7188–7193. doi: 10.1073/pnas.0708044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Makitie LT, Kanerva K, Andersson LC. Exp Cell Res. 2009;315:1008–1014. doi: 10.1016/j.yexcr.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 48.Pfeffer LM, Yang CH, Murti A, McCormack SA, Viar MJ, Ray RM, Johnson LR. J Biol Chem. 2001;276:45909–45913. doi: 10.1074/jbc.M108097200. [DOI] [PubMed] [Google Scholar]
- 49.Pfeffer LM, Yang CH, Pfeffer SR, Murti A, McCormack SA, Johnson LR. Am J Physiol Cell Physiol. 2000;278:C331–C335. doi: 10.1152/ajpcell.2000.278.2.C331. [DOI] [PubMed] [Google Scholar]
- 50.Ray RM, Zimmerman BJ, McCormack SA, Patel TB, Johnson LR. Am J Physiol Cell Physiol. 1999;276:C684–691. doi: 10.1152/ajpcell.1999.276.3.C684. [DOI] [PubMed] [Google Scholar]
- 51.Lietha D, Cai X, Ceccarelli DF, Li Y, Schaller MD, Eck MJ. Cell. 2007;129:1177–1187. doi: 10.1016/j.cell.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frame MC, Patel H, Serrels B, Lietha D, Eck MJ. Nat Rev Mol Cell Biol. 2010;11:802–814. doi: 10.1038/nrm2996. [DOI] [PubMed] [Google Scholar]
- 53.Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW. Exp Cell Res. 2003;284:31–53. doi: 10.1016/s0014-4827(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 54.Zwick E, Hackel PO, Prenzel N, Ullrich A. Trends Pharmacol Sci. 1999;20:4008–4012. doi: 10.1016/s0165-6147(99)01373-5. [DOI] [PubMed] [Google Scholar]
- 55.Carraway KL, 3rd, Cantley LC. Cell. 1994;78:5–8. doi: 10.1016/0092-8674(94)90564-9. [DOI] [PubMed] [Google Scholar]
- 56.Maa MC, Leu TH, McCarley DJ, Schatzman RC, Parsons SJ. Proc Natl Acad Sci U SA. 1995;92:6981– 6985. doi: 10.1073/pnas.92.15.6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.