Abstract
Atherosclerosis is a chronic inflammatory disorder that is characterized by the accumulation of modified lipoproteins in the arterial intima. C1q and mannan-binding lectin (MBL) are not only recognition components involved in activation of inflammation via the complement cascade, but they are also able to directly modulate phagocyte activation. Studies in C1q−/− and MBL−/− mice suggest that these molecules play a protective role in the early atherosclerotic lesion in the absence of, or prior to, expression of other complement components. However, in later stages, complement activation becomes an inappropriate inflammatory response, contributing to disease pathology. Therefore, to investigate possible molecular interactions of C1q and MBL in atherosclerotic lesions, we examined the influence of C1q and MBL in the clearance of native and modified lipoproteins by human monocytes and monocyte-derived macrophages. Both C1q and MBL are shown to bind and enhance the monocyte/monocyte-derived macrophage clearance of modified forms of low-density lipoprotein (LDL), including oxidized LDL and acetylated LDL, but not native LDL. Modified forms of LDL activate the classical complement pathway, but no lectin pathway activation was detected. Interestingly, monocytes that ingested modified LDL in the presence of C1q or MBL upregulated surface CD80 and CD31, as well as CCL2 chemokine gene expression. However, C1q and MBL also significantly reduced levels of free cholesterol accumulation in monocytes and human monocyte-derived macrophages that ingested oxidized LDL, while enhancing high-density lipoprotein–specific cholesterol efflux from these cells. These results suggest a novel pathway in which C1q and MBL influence removal and metabolism of atherogenic forms of LDL in the early stages of atherosclerosis.
Atherosclerosis is now widely accepted to be a chronic inflammatory disease. Cells and proteins of the immune system are found in all stages of atherosclerosis, including the early stage fatty streaks (sites of accumulation of lipids) as well as lipid-laden macrophages known as foam cells (1). Increased accumulation of cholesterol-rich low-density lipoprotein (LDL) in the artery wall provides an initiating step in atherosclerosis. LDL can undergo oxidation (OxLDL), chemical modification (acetylated LDL, or AcLDL), or enzymatic modification in the intima. Although the mechanisms for this are not precisely known, enzymes capable of LDL modification, such as lipoxygenases, myeloperoxidase, inducible NO synthase, and NADPH oxidases are present in human atherosclerotic lesions (2). These modified lipids have been shown to activate endothelial cells and provide signals leading to recruitment of leukocytes to the atheroma by upregulation of adhesion molecules and release of chemokines, such as CCL2 (3). In this environment, infiltrating monocytes differentiate into macrophages, with concomitant upregulation of many pattern recognition receptors, including scavenger receptors (4). Scavenger receptors recognize a broad range of molecular patterns, including OxLDL (particularly scavenger receptor class A and CD36), leading to uptake via receptor-mediated endocytosis (5).
In atherosclerosis, the balance between macrophage uptake and efflux of cholesterol is disrupted, leading to the accumulation of free cholesterol in the cell and the formation of foam cells (6). Studies also suggest the initial recognition step, and compartmental location of cholesterol within the phagocytic cell may also be important in determining the metabolic fate of the ingested cholesterol (7). However, in later stages of disease, defective clearance of apoptotic foam cells can lead to secondary necrosis and the formation of a lipid-rich core of the atherosclerotic lesion, enclosed by a cap of smooth muscle cells and a collagen-rich matrix. Damage to the plaque by locally produced molecules, such as proinflammatory cytokines, proteases, and oxygen radicals, can cause rupture and thrombus formation, leading to acute clinical complications, such as myocardial infarction and ischemic stroke (1).
Innate immune proteins C1q and mannan-binding lectin (MBL) are recognition components in the activation of complement via the classical or lectin pathway, respectively. Recognition of pathogen-associated molecular patterns by their carboxyl-terminal globular heads leads to downstream effector functions, including release of inflammatory mediators C3a and C5a, opsonization of the activating cell/particle with C3b, and terminal pathway activation, which results in the formation of the membranolytic membrane attack complex (8). In the atherosclerotic plaque, the classical pathway can be activated by autoantibodies against oxidized lipoprotein (9) or direct activation of C1 by modified lipoproteins (10). Many complement components are associated with the atherosclerotic plaque (11), with studies in C3- or C6-deficient animal models of atherosclerosis suggesting that complement terminal pathway activation plays an important role in the progression and maturation of atherosclerotic lesions (12, 13). However, studies in C1q-deficient mice suggest that C1q may play an atheroprotective role in the early stages of disease. Using a mouse model of atherosclerosis, C1q−/− LDLR−/− had increased accumulation of apoptotic cells and a greater aortic root lesion size compared with the C1q-sufficient LDLR−/− animals (14). These studies suggest that C1q-mediated removal of apoptotic cells may be important in containing the size and complexity of early atherosclerotic lesions. Interestingly, in this model, C5b-9 deposition was not completely abolished in the absence of C1q, suggesting involvement of the lectin and/or alternative pathways of complement activation. Additionally, in humans, MBL deficiency has been correlated with severity of atherosclerotic disease (15). Recent studies in which bone marrow transplantation from MBL-A– and MLA-C–deficient mice into LDLR−/− animals resulted in poor outcome relative to MBL-sufficient transplanted cells have highlighted the controlling role of myeloid-derived MBL expression in the development of atherosclerosis (16). However, the molecular mechanisms by which locally produced C1q and MBL exert their protective effects have not yet been identified.
Beyond their role in the activation of complement, C1q and MBL are also members of the defense collagen family of proteins, which also includes the class A scavenger receptors, so-called for their direct role in the induction of protective immune “defense” responses and their unique macromolecular structure, which contains a substantial collagen-like domain (17). Studies in our laboratory and in those of others have demonstrated that defense collagens are activation ligands for phagocytosis, rapidly triggering enhanced phagocytic activity when bound to the particle to be ingested, or presented to the phagocyte in a multivalent manner, such as when immobilized on a surface (18). Because C1q has been reported to directly bind modified forms of LDL (10), and given that C1q and MBL are present in atherosclerotic lesions, we investigated whether MBL was also capable of pattern recognition of modified lipoproteins, and whether the presence of C1q and MBL modulates macrophage clearance and metabolism of these atherogenic agents.
Materials and Methods
Materials
1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) was obtained from Molecular Probes (Invitrogen, Eugene, OR). Human LDL and their matched-lot modified forms, AcLDL and OxLDL, were purchased from Intracel (Frederick, MD) and used within specified expiration dates. Human high-density lipoprotein (HDL) was purchased from Calbiochem (EMD Chemicals, Darmstadt, Germany). Recombinant human M-CSF was purchased from PeproTech (Rocky Hill, NJ). C1q was isolated from plasma-derived normal human serum (NHS) by ion-exchange chromatography followed by size-exclusion chromatography according to the method of Tenner et al. (19) and modified as described (20). The C1q preparations used were fully active, as determined by hemolytic titration, and homogeneous, as assessed by SDS-PAGE. Protein concentration was determined using an extinction coefficient (E1%) at 280 nm of 6.82 for C1q (21). During the purification of C1q, serum depleted of C1q (C1qD) was collected after passage of plasma-derived serum over the ion-exchange resin and stored at −70°C until use. Serum depleted of both C1q and MBL (C1qD-MBLD) was prepared by passing C1qD over a column of mannan-agarose (Sigma-Aldrich, St. Louis, MO) that had been equilibrated into veronal buffer containing magnesium and calcium. Recombinant or human plasma MBLs were purified as described (22).
Cells
Human peripheral blood monocytes were isolated by counterflow elutriation using a modification of the technique of Lionetti et al. (23) as described (24). Greater than 90% of the cells in each preparation were monocytes (CD11b/CD14 positive, CD3 negative) according to flow cytometry analysis. All blood samples were collected in accordance with the guidelines and approval of the University of California Irvine Institutional Review Board. Freshly isolated monocytes were used immediately, or cultured for 7 d in RPMI 1640 media supplemented with 10% FCS (ThermoFisher-Hyclone, Milford, MA), 25ng/ml M-CSF, 1% L-glutamine, 1% penicillin/streptomycin to generate human monocyte-derived macrophages (HMDM), with 50% volume exchange with fresh media every 3–5 d.
Binding assay
Immulon 2HB plates (ThermoFisher-Hyclone) were coated with lipoproteins or BSA control protein at 50 μg protein/ml in PBS, overnight at room temperature. Remaining sites were blocked with PBS containing 5% milk for 1 h at 37°C. Dilutions of purified C1q or MBL in PBS/1% milk were incubated for 2 h at room temperature. Wells were washed with PBS/0.05% Tween 20, prior to addition of 1H11 (25) monoclonal anti-C1q (0.5 μg/ml) or 1173 polyclonal anti-MBL (26) (2 μg/ml) in PBS/1% milk and incubation for 90 min at room temperature. Wells were washed and HRP-conjugated secondary Ab (1/2000 dilution; Jackson Immuno-Research Laboratories, West Grove, PA) was added for 30 min at room temperature. The binding assay was developed by the addition of substrate o-phenylenediamine (OPD) (Sigma-Aldrich). Binding of C1q or MBL was assessed by measurement of the average absorbance of duplicate sample wells at 405 nm.
Complement activation assay
Immulon 2HB plates were coated with lipoproteins, BSA, or mannan (50 μg/ml; Sigma-Aldrich) as described above. Remaining sites were blocked with PBS/1% BSA for 1 h at 37°C. Dilutions of C1q (0.01–10 μg/ml) or MBL (10 μg/ml) were made into serum depleted of C1q and MBL as a source of other complement components (1/10 in gelatin veronal buffer containing magnesium and calcium), and incubated for 30 min at 37°C to allow for complement activation to occur. Wells were washed with PBS/0.05% Tween 20 prior to incubation with monoclonal anti-C3d (1/1000 dilution; Quidel, San Diego, CA) for 1 h at room temperature. Wells were washed and HRP-conjugated secondary Ab (1/1000 dilution; Jackson ImmunoResearch Laboratories) was added for 45 min at room temperature. The assay was developed by the addition of substrate OPD, and C3b deposition was assessed by measurement of the average absorbance at 405 nm of duplicate sample wells.
Lipoprotein uptake assay
LDL, AcLDL, and OxLDL were labeled with DiI as follows: 0.5 mg (protein) LDL or modified LDL were incubated in 1 ml PBS with 150 μg DiI for 16 h, 37°C under sterile conditions. Unconjugated dye was removed by buffer exchange of DiI-labeled lipoproteins into sterile PBS using a PD-10 desalting column (GE Healthcare, Piscataway, NJ). Protein concentration of each preparation was determined by BCA assay (Thermo-Fisher-Pierce) according to the manufacturer’s instructions. DiI-labeled lipoproteins were stored at 4°C in the dark and used within 3 wk of preparation. Freshly elutriated monocytes, or macrophages harvested using Versene/EDTA (Invitrogen), were resuspended at 1 × 106 cells/ml in phagocytosis buffer (RPMI 1640, 25 mM HEPES, 5 mM MgCl2) were added to Labtek chambers (Nunc, Rochester, NY), centrifuged at 70 × g for 3 min and cultured for 30 min at 37°C in 5% CO2 with amounts of DiI-labeled lipoproteins (1–10 μg protein/ml) as described in the figure legends. Where indicated, DiI-labeled lipoproteins were preincubated with C1q (75 μg/ml) or MBL (10 μg/ml) for 10 mins 37°C prior to being added directly to the cells. In certain experiments NHS, human serum depleted of C1q and MBL (C1qD-MBLD), or depleted serum reconstituted with 75 μg/ml C1q or 10 μg/ml MBL (C1qD-MBLD plus C1q; C1qD-MBLD plus MBL), was added to the cells at a final concentration of 10%. After incubation, cells were harvested from wells using 0.25% trypsin-EDTA (Invitrogen), and ingestion of DiI-labeled lipoproteins was analyzed by flow cytometry using the FACSCalibur (BD Biosciences, San Jose, CA). In some experiments, confocal analysis of the uptake of 10 μg protein/ml DiI-labeled lipoprotein by HMDM was performed using the Zeiss LSM 710 confocal microscope. For these experiments, HMDM were counterstained with FITC-labeled phalloidin, which recognizes actin, and with the nuclear stain DAPI (Invitrogen), according to the manufacturer’s instructions.
Cholesterol quantification
Human monocytes were added to 12-well tissue-culture plates, 1 × 106 cells/well, in X-VIVO 15 media (BioWhittaker, Walkersville, MD) with 1% L-glutamine and 1% penicillin/streptomycin. Plates were centrifuged at 70 × g for 3 min. OxLDL at 10 μg protein/ml was added to wells in the presence or absence of 75 μg/ml C1q for 2–24 h. Cells were harvested with 0.25% trypsin, extracted with chloroform/isopropanol/Triton X-100 (7:11:0.1) using a microhomogenizer, and levels of free cholesterol were then measured by enzymatic colorimetric assay using a cholesterol quantitation kit (BioVision, Mountain View, CA) as described in detail in the provided manufacturer’s protocol.
Modulation of gene expression by C1q
Human monocytes or HMDM were added to 8-well Labtek chamber slides, 1.25 × 105 cells/well, in X-VIVO 15 media, which was supplemented with 1% L-glutamine and 1% penicillin/streptomycin. Slides were centrifuged at 70 × g for 3 min. OxLDL at 10 μg protein/ml was added to wells in the presence or absence of 75 μg/ml C1q or 10 μg/ml MBL. After 2 h, RNA was harvested from cells remaining in the wells using an Illustra RNA extraction kit (GE Healthcare), and cDNA was synthesized by RT-PCR using the Moloney murine leukemia virus reverse transcriptase kit (Invitrogen) according to the manufacturer’s instructions. Levels of mRNA for GAPDH, ATP-binding cassette (ABC)-A1, ABC-G1, and MCP-1/CCL2 were analyzed by real-time quantitative RT-PCR (iCycler; Bio-Rad, Hercules, CA) using probes designed in-house (Supplemental Table 1) and synthesized by Integrated DNA Technologies (San Diego, CA). The mRNA expression levels were normalized to GAPDH as an internal standard, and they were expressed as fold difference from monocytes/HMDM in the absence of lipoprotein.
HDL-specific cholesterol efflux assays
Freshly elutriated human monocytes or HMDM (day 7) were labeled with 1 μCi/ml [3H]cholesterol (PerkinElmer, Boston, MA) for 24 h in RPMI 1640 containing 10% FCS. Cells were washed twice with PBS and equilibrated for 2 h in RPMI 1640 containing 0.2% fatty acid-free BSA (Sigma-Aldrich) at 37°C. Cells were washed into fresh RPMI 1640 containing 0.2% fatty acid-free BSA and plated at 1 × 106 cells/ml in 24-well plates, and OxLDL was then added to specific wells at 10 μg protein/ml in the presence or absence of 75 μg/ml C1q for 2 h before HDL (50 μg protein/ml) was added to wells for a further 2–24 h incubation. Cholesterol efflux was determined by measuring radioactivity in the culture medium of wells and in cell lysates, which were extracted in 0.2 N NaOH for 2 h at room temperature, by liquid scintillation counting. The percentage of cholesterol efflux was calculated from the measured counts per minute as follows: [100% × (cpmmedium)/(cpmmedium+cells)]. HDL-specific efflux was calculated as the % efflux in the presence of HDL − % efflux in identical samples cultured without addition of HDL.
Results
C1q and MBL bind modified forms of LDL
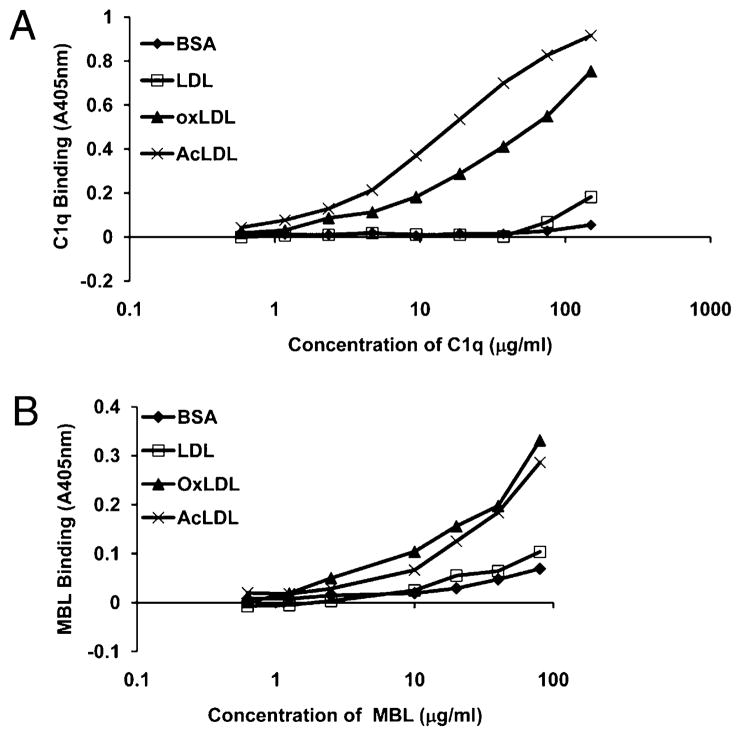
C1q binding to modified forms of LDL has previously been reported (10). To confirm this in our system, and to investigate binding of MBL, a plate binding assay was developed. LDL-, OxLDL-, AcLDL-, or BSA (control)-coated wells were incubated with dilutions of purified C1q (0–150 μg/ml) or MBL (0–80 μg/ml), and binding was assessed by Ab detection. To confirm that equivalent levels of unmodified/modified LDL were coated on the plate, levels of lipoprotein binding were assessed in parallel wells by the addition of anti–apolipoprotein B-100 (data not shown). As was previously reported, C1q bound to OxLDL, and in addition we observed binding to AcLDL, but not to unmodified LDL or BSA control, as measured by A405 nm (Fig. 1). Binding was dose-dependent on the concentration of C1q (Fig. 1A). Similar to C1q, MBL also bound to modified forms of LDL, but not to LDL alone (Fig. 1B).
FIGURE 1.
C1q and MBL bind modified forms of LDL. LDL, OxLDL, AcLDL, or BSA control protein were immobilized on a plate and incubated with 0.5–150 μg/ml C1q (A) or 0.5–80 μg/ml MBL (B). Binding was assessed by ELISA using the OPD substrate with signal absorbance measured at 405 nm. Data are from a single experiment, performed in duplicate wells, representative of three individual experiments.
Modified forms of LDL activate the classical, but not lectin, complement pathway
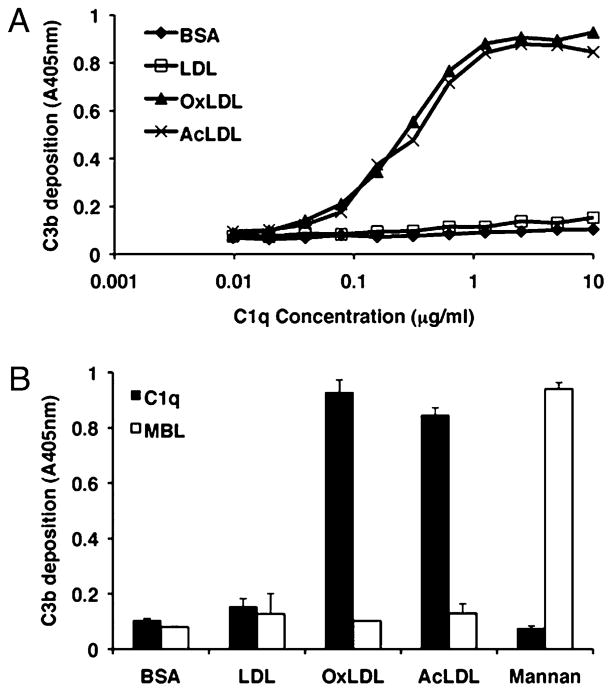
C1q binding to modified forms of LDL has previously been shown to activate the classical complement pathway (10). Because we have shown that MBL also binds modified forms of LDL, we investigated the ability of modified forms to activate the lectin complement pathway, using an ELISA-based plate assay to measure deposition of complement activation product C3b. Similar to previous reports, we show that OxLDL and AcLDL but not unmodified LDL are able to activate the classical pathway of complement, leading to deposition of C3b (Fig. 2A). Interestingly, although MBL also binds modified forms of LDL (Fig. 1B), this recognition step did not lead to activation of the lectin complement pathway (Fig. 2B). In this assay, MBL was shown to be able to activate the lectin pathway via recognition of mannan, a known lectin pathway activator.
FIGURE 2.
Modified forms of LDL activate complement via the classical pathway but not the lectin pathway. LDL, OxLDL, AcLDL, BSA (control protein), or mannan (control lectin pathway activator) were immobilized on a plate and incubated with 0.01–10 μg/ml C1q (A) or 20 μg/ml C1q or MBL (B) in C1qD-MBLD. Complement activation of the classical (A, B) or lectin pathway (B) was assessed by measuring deposition of C3b by ELISA using the OPD substrate, with signal absorbance measured at 405 nm. Data are from single experiments, performed in duplicate wells, representative of three individual experiments.
C1q and MBL enhance ingestion of modified LDL by monocytes and macrophages
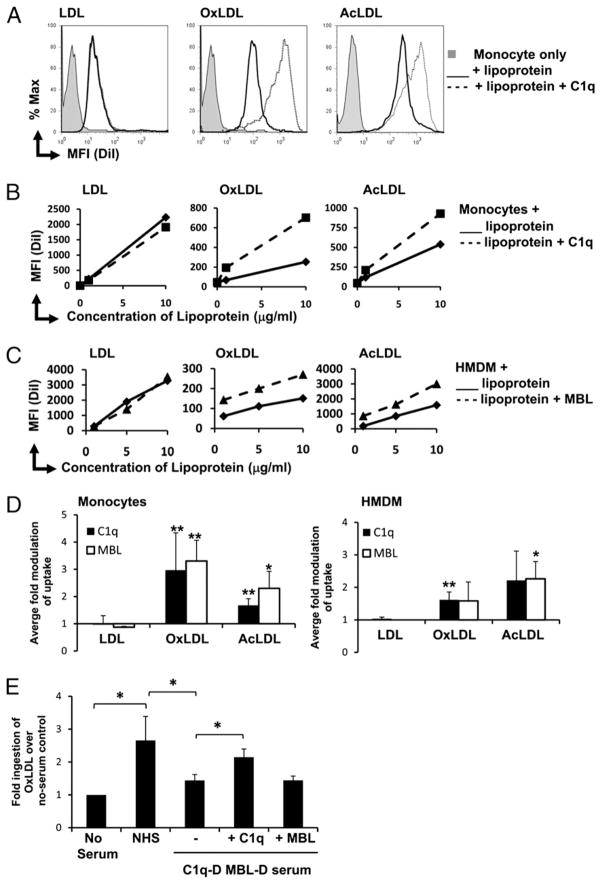
Defense collagens have been shown to enhance the phagocytosis of various particles, including targets coated with IgG/complement and apoptotic cells (17), mediated by their collagen-like domain. To assess their role in clearance of atherogenic lipoproteins, DiI-labeled LDL, OxLDL, and AcLDL were incubated with human monocytes and HMDM in the presence or absence of purified C1q or MBL and lipoprotein uptake was assessed by flow cytometry. C1q enhanced monocyte clearance of modified LDL (OxLDL and AcLDL), but not unmodified LDL (Fig. 3A). This enhancement of modified LDL uptake was seen at both 1 and 10 μg of protein/ml (Fig. 3B) and was dose-responsive to C1q concentration (data not shown), with maximal C1q-mediated clearance at physiological levels of C1q (75 μg/ml). Similar C1q-mediated enhancements were seen in HMDM, in cells from a range of donors and multiple batches of lipoprotein (Fig. 3D). Purified MBL also enhanced monocyte and HMDM clearance of OxLDL and AcLDL (Fig. 3C, 3D and data not shown). Enhanced clearance of OxLDL was dose responsive to MBL concentration (data not shown). Differences in mean fluorescence intensity (MFI) between modified forms of lipoproteins are due to variation in DiI-labeling efficiency rather than reflective of relative ingested amounts. Addition of NHS also enhanced clearance of OxLDL, but this enhancement was not seen with C1qD-MBLD (Fig. 3E). Reconstitution of C1qD-MBLD serum with 75 μg/ml C1q (approximate normal plasma level (27)) restored the ability of C1qD-MBLD serum to enhance clearance of OxLDL. However, no enhancement of uptake was observed with the reconstitution of 10 μg/ml MBL to C1qD-MBLD serum (approximate high-normal plasma level (28)). Ingestion of DiI-labeled modified LDL into vesicular compartments was confirmed by confocal microscopy (Supplemental Fig. 1).
FIGURE 3.
C1q and MBL enhance ingestion of modified LDL by human monocytes and macrophages. Lipoproteins LDL, OxLDL, and AcLDL were labeled with DiI and incubated with phagocytes in the presence or absence of C1q/MBL at 37°C, for 30 min, and the MFI of ingestion was determined by flow cytometry. A, Representative histograms of monocyte ingestion of 10 μg protein/ml lipoprotein in the absence (solid line) or presence (dotted line) of 75 μg/ml C1q. Shaded histograms represent monocyte only control. B, Measurement of MFI of LDL, OxLDL, and AcLDL clearance by monocytes over a range of lipoprotein doses in the presence and absence of C1q (75 μg/ml). C, Measurement of MFI of LDL, OxLDL, and AcLDL clearance by HMDM over a range of lipoprotein doses in the presence and absence of MBL (10 μg/ml). D, Average fold modulation of MFI of clearance of LDL, OxLDL, and AcLDL (10 μg of protein/ml) by monocytes and HMDM by C1q (75 μg/ml) or MBL (10 μg/ml) compared with levels ingested in the absence of C1q or MBL. Data are the mean ± SD. *p < 0.05; **p <0.005, two-tailed Student t test (n > 3). E, Fold modulation of MFI of clearance of DiI-OxLDL (10 μg protein/ml) by monocytes in the presence of NHS or C1qD-MBLD with or without C1q (75 μg/ml) or MBL (10 μg/ml), compared with no serum control. Data are the mean ± SD. *p < 0.05, two-tailed Student t test (n = 3). Data in A–C are from single experiments and are representative of results obtained from different donors in at least three individual experiments.
C1q and MBL modulate monocyte activation and chemokine responses during the clearance of OxLDL
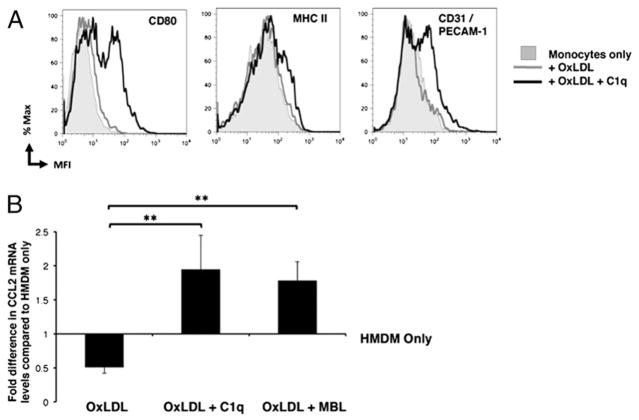
Given that C1q has also been shown to modulate gene expression and function in phagocytic cells (29), we examined the effects of C1q on monocyte responses during lipoprotein clearance. Surface expression of cellular activation markers was measured by flow cytometry in monocytes that had ingested OxLDL in the presence or absence of C1q and cultured a further 24 h (Fig. 4A). Although uptake of OxLDL alone had no effect on levels of costimulatory molecule CD80, HLA-DR/MHC class II, or adhesion molecule CD31/PECAM-1, the presence of C1q enhanced surface expression levels of these molecules in monocytes from three individual donors. These molecules also remained elevated after 48 h of culture (data not shown). Because chemokines play an important role in the recruitment of monocytes to the vascular lesion in atherosclerosis, we also investigated the effect of C1q and MBL on MCP-1/CCL2 gene expression by quantitative RT-PCR (Fig. 4B). Ingestion of 10 μg of protein/ml OxLDL reduced gene expression levels of MCP-1/CCL2 in HMDM after 2 h. However, the presence of C1q or MBL on OxLDL actually significantly enhanced gene expression levels above levels in OxLDL-treated HMDM and also levels in HMDM alone. Similar enhancements by C1q of MCP-1/CCL2 gene expression in response to OxLDL were also seen in human monocytes at 2 h (data not shown).
FIGURE 4.
C1q modulates monocyte cellular responses during the clearance of OxLDL. A, Levels of surface molecules/activation markers were measured by flow cytometry in human monocytes (shaded histogram) after 24 h incubation with 10 μg protein/ml OxLDL in the absence (gray line) or presence (black line) of 75 μg/ml C1q. Data are histograms from a single experiment, representative of two experiments. B, Analysis of mRNA levels of MCP-1/CCL2 gene expression was measured by quantitative RT-PCR of HMDM only, or incubated with OxLDL (10 μg protein/ml) in the absence or presence of 75 μg/ml C1q or 10 μg/ml MBL for 2 h. Data are expressed as average fold modulation of mRNA levels of HMDM only ± SD (n = 3).
C1q and MBL modulate cholesterol accumulation in monocytes and HMDM during ingestion of OxLDL
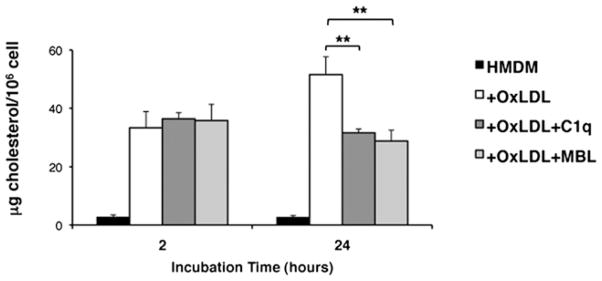
Because we have shown that C1q enhances the uptake of modified lipoproteins (Fig. 3), we measured cholesterol accumulation in HMDM incubated with OxLDL in the presence or absence of C1q or MBL (Fig. 5). As expected, HMDM that had ingested OxLDL had enhanced levels of free cholesterol compared with those cultured in the absence of lipoprotein. At 2 h, no difference was seen in free cholesterol levels in HMDM treated with OxLDL alone or in the presence of C1q or MBL. However, after 24 h, coincubation of HMDM with OxLDL and either C1q or MBL significantly reduced free cholesterol levels in HMDM that had ingested OxLDL. Similar results were obtained with human monocytes at 2 and 24 h (data not shown).
FIGURE 5.
C1q modulates cholesterol accumulation in HMDM. Levels of free cholesterol were measured by colorimetric assay in HMDM after 2 or 24 h incubation with OxLDL (10 μg protein/ml) in the absence or presence of C1q (75 μg/ml) or MBL (10 μg/ml). Data are the average ± SD of three experiments, each performed in duplicate.
C1q and MBL enhance cholesterol efflux in OxLDL-loaded monocytes and macrophages
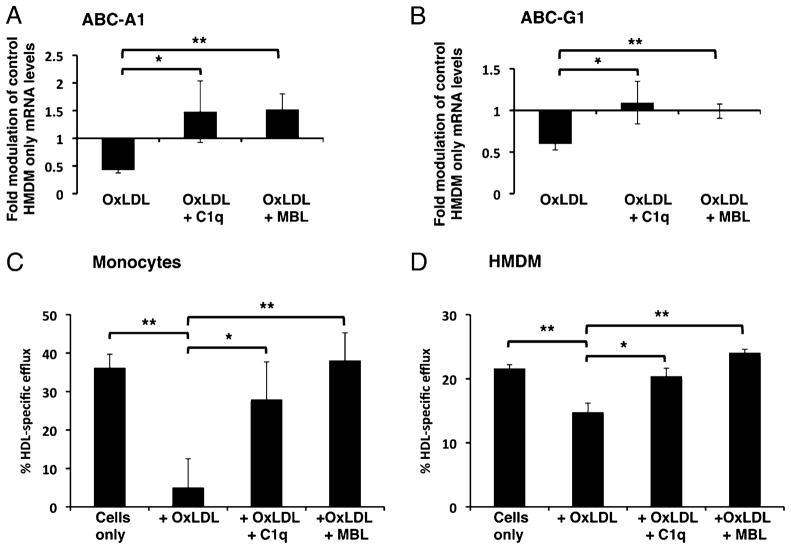
To investigate the mechanism of the C1q-mediated reduction in cholesterol accumulation in HMDM and monocytes that ingested OxLDL, we investigated the effect of C1q on genes associated with cholesterol efflux, ABC transporter molecules ABC-A1 and ABC-G1, by quantitative RT-PCR. Incubation of C1q or MBL bound to OxLDL with human monocytes for 2 h significantly enhanced mRNA levels of cholesterol transporter genes ABC-A1 and ABC-G1 in both human monocytes and HMDM above levels seen with OxLDL only (Fig. 6A, 6B and data not shown). To identify whether the increased gene expression of cholesterol transporter molecules was associated with an effect on cholesterol efflux, we assessed the effect of C1q (75 μg/ml) or MBL (10 μg/ml) on cholesterol efflux in [3H]cholesterol-loaded human monocytes and HMDM. Our results show that, similar to the literature (30), OxLDL suppressed HDL-specific cholesterol efflux from HMDM and monocytes (Fig. 6C, 6D). Soluble C1q alone did not significantly enhance levels of HDL-specific efflux from HMDM or monocytes (data not shown). However, the presence of either C1q or MBL on OxLDL restored/sustained HDL-specific efflux levels similar to those of control [3H]cholesterol-loaded macrophages in the absence of OxLDL (Fig. 6C, 6D). C1q was also seen to enhance apolipoprotein A1-specific efflux in human monocytes and HMDM (data not shown).
FIGURE 6.
C1q enhances cholesterol efflux in human monocytes and HMDM. A and B, Analysis of mRNA levels of ABC cholesterol transporter genes involved in cholesterol efflux were measured by quantitative RT-PCR of HMDM incubated with OxLDL (10 μg protein/ml) in the absence or presence of 75 μg/ml C1q or 10 μg/ml MBL for 2 h. Data are expressed as average fold modulation of mRNA levels over HMDM control cells ± SD (n = 3). Levels of HDL-specific efflux were measured in [3H]cholesterol-loaded monocytes (C) or HMDM (D) cultured with OxLDL (10 μg protein/ml) in the presence or absence of C1q (75 μg/ml) or MBL (10 μg/ml) for 24 h. Data are expressed as average ± SD for HDL-specific efflux from three individual experiments. *p < 0.05; **p < 0.01, two-tailed Student t test.
Discussion
These studies present data supporting a novel role for innate immune proteins C1q and MBL during the clearance of modified lipoproteins and cholesterol metabolism by human monocytes and HMDM. In this study, we show that both C1q and MBL can directly bind modified lipoproteins, independent of Ab (Fig. 1). Studies have already shown that the collagenous domains of the macrophage scavenger receptor mediate binding specificities for polyanionic ligands, including AcLDL (31), although the isolated collagenous domain of C1q, which shares similar specificity for polyanions, did not bind AcLDL. We were also unable to detect binding to modified LDL using isolated fragments of C1q collagenous domain or the C1q globular head domain (data not shown), suggesting that the intact molecule is required for recognition or that multivalent binding by hexameric heads is required for high-avidity binding. Similarly, in a recent report, C1q was shown to bind another form of modified LDL, enzyme-modified LDL (E-LDL), via its globular head domains, which recognize unesterified fatty acids formed by cholesterol esterase in these particles (32). Consistent with a recognition step via its globular heads, we see activation of the classical complement pathway by C1q recognition of modified, but not native, LDL as measured by C3b deposition in a modified ELISA assay (Fig. 2). Activation of the classical pathway by modified forms of LDL may therefore contribute to the inflammatory environment of the atherosclerotic region. Studies by Biró et al. (10) using purified complement components in a C1 assay showed that other forms of modified LDL, such as E-LDL, bind and activate C1 under physiological conditions. These studies also suggest that C-reactive protein is required for activation of the classical pathway by OxLDL. Because our studies used MBL- and C1q-depleted serum as a source of other complement components, C-reactive protein would also be present, and therefore the relative contributions of E-LDL versus other modified LDL particles to complement activation during the early stages of atherogenic disease remain to be established. Interestingly, in our assay, although MBL was able to bind modified lipoproteins, they did not activate complement via the lectin pathway. MBL has long been associated with atherosclerosis. In humans, MBL deficiency has been correlated with severity of atherosclerotic disease (15). Human population studies have also shown that high levels of MBL (>1 mg/l) were associated with a greatly decreased risk of myocardial infarction in hypercholesterolemic individuals (33). More recent studies have shown that MBL is abundantly present in early lesions in a mouse model of atherosclerosis. This MBL was shown to be produced locally by myeloid cells during early atherogenesis and provided direct evidence for the critical role of MBL in controlling the development of atherosclerotic lesions (16). Studies have also shown that cells of the monocyte/macrophage lineage are also a major site of C1q biosynthesis (34) and that C1q is often upregulated in response to injury (35) and can be produced in the absence of other complement components (36). C1q has also been shown to be produced locally by dendritic cells, macrophages, and macrophage foam cells in atherosclerotic lesions (37). Direct evidence for the role of C1q in atherogenesis was provided by Bhatia et al. (14), who showed that C1q reduces early development of lesions in a murine model of atherosclerosis, and suggested that this may be due to the role of C1q in the clearance of apoptotic cells (14). Collectively, these studies identify critical protective functions of these innate immune molecules beyond the activation of complement, particularly in early stages of disease.
It has been shown for other disease states that C1q may have protective effects early in disease, prior to initiation of complement activation (38). C1q and MBL are known activation ligands for phagocytosis, rapidly triggering enhanced phagocytosis of a variety of targets, including sheep erythrocytes suboptimally opsonized by IgG or complement, and apoptotic cells (17). In this study, purified C1q and MBL also enhanced ingestion of modified forms of LDL (OxLDL and AcLDL), but not unmodified LDL, similar to their binding specificities, suggesting that binding of C1q to the lipoprotein is required for the enhancement of clearance (Fig. 3). Although the mechanism of enhanced clearance is presently not known, C1q has been shown to act as a bridging molecule to enhance the uptake of other particles via its collagen-like domain, including apoptotic cells (39, 40). This domain is shared by MBL, and directed muta-genesis in that domain has disrupted the enhancement of phagocytic activity (26), suggesting that the enhanced clearance is triggered via interaction with a common phagocytic receptor. However, although several candidate receptors have been proposed, none has been definitively identified (8). Uptake assays performed using normal human serum and C1qD-MBLD as a source of complement demonstrated a significant contribution of C1q and/or MBL to lipoprotein clearance in the presence of other serum components, including downstream complement components. Addition of NHS significantly enhanced the ingestion of OxLDL by human monocytes (Fig. 3E). This enhancement of ingestion was not seen with the addition of C1qD-MBLD, suggesting that C1q and/or MBL play a role in the serum-mediated enhancement of lipoprotein clearance by these phagocytes. However, reconstitution of C1qD-MBLD with serum levels of C1q (C1qD-MBLD plus C1q), but not of MBL (C1qD-MBLD plus MBL), restored serum-mediated uptake of OxLDL. This suggests that C1q cannot only enhance OxLDL clearance via direct interaction with phagocytic cells, but also via classical pathway activation (in the presence of other complement components, such as in NHS) and C3b opsonization of modified LDL. However, the association of MBL with MBL-associated serine proteases or other complement components found in serum not only do not result in complement activation, but it also may mask the ability of MBL to enhance OxLDL clearance via direct phagocyte interactions.
The presence of C1q during modified lipoprotein clearance also modulated monocyte differentiation and cellular responses (Fig. 4). Levels of costimulatory molecule CD80 and HLA-DR/MHC class II were elevated in cells that ingested OxLDL in the presence of C1q compared with OxLDL alone, suggesting that C1q is enhancing monocyte activation and maturation toward a macrophage-like differentiation state. C1q also upregulated surface expression of the adhesion molecule CD31/PECAM-1. CD31 is expressed at varying levels on all cells of the vascular region, and it is involved in leukocyte transmigration through arterial walls (41). Similar to data from cells exposed to immobilized C1q or C1q bound to apoptotic cells (39), C1q also enhanced gene expression of chemokine CCL2/MCP-1, a molecule responsible for the recruitment of leukocytes to sites of injury. These data suggest that C1q expressed locally in atherosclerotic lesions may be providing “find-me” and activation signals, enhancing chemotaxis and migration of monocytes to the area.
Infiltrating monocytes, or macrophages in the arterial wall, can function to export cholesterol out of lesions or to process cholesterol to be excreted (42). However, excessive lipoprotein levels in the vessel wall found in atherosclerosis can overwhelm these protective mechanisms. Macrophages that ingest more cholesterol than they are able to excrete store the excess cholesterol in lipid droplets within the cell, leading to formation of macrophage foam cells, which can induce inflammatory responses and undergo apoptosis/necrosis, and thus play a key role in regulating the pathology of plaques (43). Levels of free cholesterol in monocytes were enhanced early after ingestion of OxLDL and were unaffected by the presence of C1q or MBL (2 h; Fig. 5). However, interestingly, after 24 h, cholesterol levels were significantly reduced in cells that had ingested OxLDL in the presence of C1q or MBL compared with those that had ingested OxLDL alone (Fig. 5). This is similar to a report in which adiponectin, a C1q-like protein that is secreted by adipocytes, reduced lipid accumulation in macrophages (44). mRNA levels of ATP-binding cassette transporters ABC-A1 and ABC-G1, which remove cholesterol from macrophages via efflux to HDL and its principal apolipoprotein, apolipoprotein A-1, were increased in response to C1q and MBL (Fig. 6A, 6B and data not shown) and correlated with enhanced HDL-specific efflux of [3H]cholesterol in monocytes and HMDM that had ingested OxLDL (Fig. 6C, 6D). Although the release of excess cholesterol from monocytes and macrophages is important for the prevention of foam cell formation and efficient clearance of cholesterol from the atherogenic region, efflux activities of ABC-A1 and ABC-G1 may also dampen inflammatory responses in macrophages (45).
In summary, these data describe a novel role for C1q and MBL, present in early atherosclerotic regions, in enhancing lipoprotein uptake and modulating gene expression that leads to beneficial metabolism and excretion of excess cholesterol. Because mechanisms governing the removal of modified lipoproteins and processing of cholesterol are critical to the suppression of atherosclerosis, further investigation of the molecular components of these C1q-and MBL-mediated processes should lead to novel strategies or areas for therapeutic intervention in this disease.
Supplementary Material
Acknowledgments
We thank ShuHui Chu and Elysia Chin for technical assistance. We also thank the staff of the University of California, Irvine Institute for Clinical and Translational Science for obtaining human blood for monocyte purification.
This work was supported in part by National Institutes of Health Grant AI41090 (to A.J.T.). Support for obtaining human blood products used in this study was provided in part by Public Health Service Research Grant M01RR00827.
Abbreviations used in this paper
- ABC
ATP-binding cassette
- AcLDL
acetylated low-density lipoprotein
- C1qD
serum depleted of C1q
- C1qD-MBLD
human serum depleted of C1q and mannan-binding lectin
- DiI
1,1′-dioctadecyl-3,3,3′,3′-tetrame-thylindocarbocyanine perchlorate
- E-LDL
enzyme-modified low-density lipoprotein
- HDL
high-density lipoprotein
- HMDM
human monocyte-derived macrophage
- LDL
low-density lipoprotein
- MBL
mannan-binding lectin
- MFI
mean fluorescence intensity
- NHS
normal human serum
- OPD
o-phenylenediamine
- OxLDL
oxidized low-density lipoprotein
Footnotes
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Hansson GK, Robertson AK, Söderberg-Nauclér C. Inflammation and atherosclerosis. Annu Rev Pathol. 2006;1:297–329. doi: 10.1146/annurev.pathol.1.110304.100100. [DOI] [PubMed] [Google Scholar]
- 2.Witztum JL, Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991;88:1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kume N, Cybulsky MI, Gimbrone MA., Jr Lysophosphatidylcholine, a component of atherogenic lipoproteins, induces mononuclear leukocyte adhesion molecules in cultured human and rabbit arterial endothelial cells. J Clin Invest. 1992;90:1138–1144. doi: 10.1172/JCI115932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geng Y, Kodama T, Hanson GK. Differential expression of scavenger receptor isoforms during monocyte-macrophage differentiation and foam cell formation. Arterioscler Thromb. 1994;14:798–806. doi: 10.1161/01.atv.14.5.798. [DOI] [PubMed] [Google Scholar]
- 5.Greaves DR, Gordon S. Thematic review series: the immune system and atherogenesis. Recent insights into the biology of macrophage scavenger receptors. J Lipid Res. 2005;46:11–20. doi: 10.1194/jlr.R400011-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Tabas I. Cholesterol and phospholipid metabolism in macrophages. Biochim Biophys Acta. 2000;1529:164–174. doi: 10.1016/s1388-1981(00)00146-3. [DOI] [PubMed] [Google Scholar]
- 7.Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, Sweeney M, Rong JX, Kuriakose G, Fisher EA, et al. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol. 2003;5:781–792. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- 8.Bohlson SS, Fraser DA, Tenner AJ. Complement proteins C1q and MBL are pattern recognition molecules that signal immediate and long-term protective immune functions. Mol Immunol. 2007;44:33–43. doi: 10.1016/j.molimm.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 9.Binder CJ, Chang MK, Shaw PX, Miller YI, Hartvigsen K, Dewan A, Witztum JL. Innate and acquired immunity in atherogenesis. Nat Med. 2002;8:1218–1226. doi: 10.1038/nm1102-1218. [DOI] [PubMed] [Google Scholar]
- 10.Biró A, Thielens NM, Cervenák L, Prohászka Z, Füst G, Arlaud GJ. Modified low density lipoproteins differentially bind and activate the C1 complex of complement. Mol Immunol. 2007;44:1169–1177. doi: 10.1016/j.molimm.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Yasojima K, Schwab C, McGeer EG, McGeer PL. Complement components, but not complement inhibitors, are upregulated in atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2001;21:1214–1219. doi: 10.1161/hq0701.092160. [DOI] [PubMed] [Google Scholar]
- 12.Schmiedt W, Kinscherf R, Deigner HP, Kamencic H, Nauen O, Kilo J, Oelert H, Metz J, Bhakdi S. Complement C6 deficiency protects against diet-induced atherosclerosis in rabbits. Arterioscler Thromb Vasc Biol. 1998;18:1790–1795. doi: 10.1161/01.atv.18.11.1790. [DOI] [PubMed] [Google Scholar]
- 13.Buono C, Come CE, Witztum JL, Maguire GF, Connelly PW, Carroll M, Lichtman AH. Influence of C3 deficiency on atherosclerosis. Circulation. 2002;105:3025–3031. doi: 10.1161/01.cir.0000019584.04929.83. [DOI] [PubMed] [Google Scholar]
- 14.Bhatia VK, Yun S, Leung V, Grimsditch DC, Benson GM, Botto MB, Boyle JJ, Haskard DO. Complement C1q reduces early atherosclerosis in low-density lipoprotein receptor-deficient mice. Am J Pathol. 2007;170:416–426. doi: 10.2353/ajpath.2007.060406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madsen HO, Videm V, Svejgaard A, Svennevig JL, Garred P. Association of mannose-binding-lectin deficiency with severe atherosclerosis. Lancet. 1998;352:959–960. doi: 10.1016/S0140-6736(05)61513-9. [DOI] [PubMed] [Google Scholar]
- 16.Matthijsen RA, de Winther MP, Kuipers D, van der Made I, Weber C, Herias MV, Gijbels MJJ, Buurman WA. Macrophage-specific expression of mannose-binding lectin controls atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2009;119:2188–2195. doi: 10.1161/CIRCULATIONAHA.108.830661. [DOI] [PubMed] [Google Scholar]
- 17.Fraser DA, Tenner AJ. Directing an appropriate immune response: the role of defense collagens and other soluble pattern recognition molecules. Curr Drug Targets. 2008;9:113–122. doi: 10.2174/138945008783502476. [DOI] [PubMed] [Google Scholar]
- 18.Bobak DA, Gaither TA, Frank MM, Tenner AJ. Modulation of FcR function by complement: subcomponent C1q enhances the phagocytosis of IgG-opsonized targets by human monocytes and culture-derived macrophages. J Immunol. 1987;138:1150–1156. [PubMed] [Google Scholar]
- 19.Tenner AJ, Lesavre PH, Cooper NR. Purification and radio-labeling of human C1q. J Immunol. 1981;127:648–653. [PubMed] [Google Scholar]
- 20.Young KR, Jr, Ambrus JL, Jr, Malbran A, Fauci AS, Tenner AJ. Complement subcomponent C1q stimulates Ig production by human B lymphocytes. J Immunol. 1991;146:3356–3364. [PubMed] [Google Scholar]
- 21.Reid KBM, Lowe DM, Porter RR. Isolation and characterization of C1q, a subcomponent of the first component of complement, from human and rabbit sera. Biochem J. 1972;130:749–763. doi: 10.1042/bj1300749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan SM, Chung MC, Kon OL, Thiel S, Lee SH, Lu J. Improvements on the purification of mannan-binding lectin and demonstration of its Ca2+-independent association with a C1s-like serine protease. Biochem J. 1996;319:329–332. doi: 10.1042/bj3190329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lionetti FJ, Hunt SM, Valera CR. Methods of Cell Separation. Plenum; New York: 1980. [Google Scholar]
- 24.Bobak DA, Frank MM, Tenner AJ. Characterization of C1q receptor expression on human phagocytic cells: effects of PDBu and fMLP. J Immunol. 1986;136:4604–4610. [PubMed] [Google Scholar]
- 25.Kilchherr E, V, Schumaker N, Phillips ML, Curtiss LK. Activation of the first component of human complement, C1, by monoclonal antibodies directed against different domains of subcomponent C1q. J Immunol. 1986;137:255–262. [PubMed] [Google Scholar]
- 26.Arora M, Munoz E, Tenner AJ. Identification of a site on mannan-binding lectin critical for enhancement of phagocytosis. J Biol Chem. 2001;276:43087–43094. doi: 10.1074/jbc.M105455200. [DOI] [PubMed] [Google Scholar]
- 27.Ziccardi RJ, Cooper NR. Direct demonstration and quantitation of the first complement component in human serum. Science. 1978;199:1080–1082. doi: 10.1126/science.75568. [DOI] [PubMed] [Google Scholar]
- 28.Steffensen R, Thiel S, Varming K, Jersild C, Jensenius JC. Detection of structural gene mutations and promoter polymorphisms in the mannan-binding lectin (MBL) gene by polymerase chain reaction with sequence-specific primers. J Immunol Methods. 2000;241:33–42. doi: 10.1016/s0022-1759(00)00198-8. [DOI] [PubMed] [Google Scholar]
- 29.Fraser DA, Bohlson SS, Jasinskiene N, Rawal N, Palmarini G, Ruiz S, Rochford R, Tenner AJ. C1q and MBL, components of the innate immune system, influence monocyte cytokine expression. J Leukoc Biol. 2006;80:107–116. doi: 10.1189/jlb.1105683. [DOI] [PubMed] [Google Scholar]
- 30.Dhaliwal BS, Steinbrecher UP. Cholesterol delivered to macrophages by oxidized low density lipoprotein is sequestered in lysosomes and fails to efflux normally. J Lipid Res. 2000;41:1658–1665. [PubMed] [Google Scholar]
- 31.Acton S, Resnick D, Freeman M, Ekkel Y, Ashkenas J, Krieger M. The collagenous domains of macrophage scavenger receptors and complement component C1q mediate their similar, but not identical, binding specificities for polyanionic ligands. J Biol Chem. 1993;268:3530–3537. [PubMed] [Google Scholar]
- 32.Biro A, Ling WL, Arlaud GJ. Complement protein C1q recognizes enzymatically modified low-density lipoprotein through unesterified fatty acids generated by cholesterol esterase. Biochemistry. 2010;49:2167–2176. doi: 10.1021/bi9021022. [DOI] [PubMed] [Google Scholar]
- 33.Saevarsdottir S, Oskarsson OO, Aspelund T, Eiriksdottir G, Vikingsdottir T, Gudnason V, Valdimarsson H. Mannan binding lectin as an adjunct to risk assessment for myocardial infarction in individuals with enhanced risk. J Exp Med. 2005;201:117–125. doi: 10.1084/jem.20041431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petry F, Botto M, Holtappels R, Walport MJ, Loos M. Reconstitution of the complement function in C1q-deficient (C1qa−/−) mice with wild-type bone marrow cells. J Immunol. 2001;167:4033–4037. doi: 10.4049/jimmunol.167.7.4033. [DOI] [PubMed] [Google Scholar]
- 35.Alexander JJ, Anderson AJ, Barnum SR, Stevens B, Tenner AJ. The complement cascade: Yin-Yang in neuroinflammation—neuro-protection and -degeneration. J Neurochem. 2008;107:1169–1187. doi: 10.1111/j.1471-4159.2008.05668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reboul A, Prandini MH, Bensa JC, Colomb MG. Characterization of C1q, C1s and C-1 Inh synthesized by stimulated human monocytes in vitro. FEBS Lett. 1985;190:65–68. doi: 10.1016/0014-5793(85)80428-2. [DOI] [PubMed] [Google Scholar]
- 37.Cao W, Bobryshev YV, Lord RS, Oakley RE, Lee SH, Lu J. Dendritic cells in the arterial wall express C1q: potential significance in atherogenesis. Cardiovasc Res. 2003;60:175–186. doi: 10.1016/s0008-6363(03)00345-6. [DOI] [PubMed] [Google Scholar]
- 38.Tenner AJ, Fonseca MI. The double-edged flower: roles of complement protein C1q in neurodegenerative diseases. Adv Exp Med Biol. 2006;586:153–176. doi: 10.1007/0-387-34134-X_11. [DOI] [PubMed] [Google Scholar]
- 39.Fraser DA, Laust AK, Nelson EL, Tenner AJ. C1q differentially modulates phagocytosis and cytokine responses during ingestion of apoptotic cells by human monocytes, macrophages, and dendritic cells. J Immunol. 2009;183:6175–6185. doi: 10.4049/jimmunol.0902232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogden CA, deCathelineau A, Hoffmann PR, Bratton D, Ghebrehiwet B, Fadok VA, Henson PM. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med. 2001;194:781–795. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woodfin A, Voisin MB, Nourshargh S. PECAM-1: a multi-functional molecule in inflammation and vascular biology. Arterioscler Thromb Vasc Biol. 2007;27:2514–2523. doi: 10.1161/ATVBAHA.107.151456. [DOI] [PubMed] [Google Scholar]
- 42.Brown MS, Goldstein JL. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- 43.Webb NR, Moore KJ. Macrophage-derived foam cells in atherosclerosis: lessons from murine models and implications for therapy. Curr Drug Targets. 2007;8:1249–1263. doi: 10.2174/138945007783220597. [DOI] [PubMed] [Google Scholar]
- 44.Tian L, Luo N, Klein RL, Chung BH, Garvey WT, Fu Y. Adiponectin reduces lipid accumulation in macrophage foam cells. Atherosclerosis. 2009;202:152–161. doi: 10.1016/j.atherosclerosis.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yvan-Charvet L, Wang N, Tall AR. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler Thromb Vasc Biol. 2010;30:139–143. doi: 10.1161/ATVBAHA.108.179283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.