Abstract
Pyrroloquinoline quinone (PQQ) is a small, redox-active molecule that serves as a cofactor for several bacterial dehydrogenases, introducing pathways for carbon utilization that confer a growth advantage. Early studies had implicated a ribosomally translated peptide as the substrate for PQQ production. This study presents a sequence and structure based analysis of the components of the pqq operon. We find the necessary components for PQQ production are present in 126 prokaryotes, most of which are Gram- negative and a number of which are pathogens. A total of five gene products, PqqA, PqqB, PqqC, PqqD and PqqE, are concluded to be obligatory for PQQ production. Three of the gene products in the pqq operon, PqqB, PqqC and PqqE, are members of large protein superfamilies. By combining evolutionary conservation patterns with information from three-dimensional structures, we are able to differentiate the gene products involved in PQQ biosynthesis from those with divergent functions. The observed persistence of a conserved gene order within analyzed operons strongly suggests a role for protein/protein interactions in the course of cofactor biosynthesis. These studies propose previously unidentified roles for several of the gene products as well as possible new targets for antibiotic design and application.
Keywords: pyrroloquinoline, quinone, pathogenicity, phylogenomic analysis, metallo-beta-lactamase, radical SAM domain, cofactorless oxidase
INTRODUCTION
Pyrroloquinoline quinone (PQQ) is a low molecular weight, redox active cofactor utilized by a number of prokaryotic dehydrogenases (1, 2). Although the cofactor is not required for bacterial survival, the presence of this molecule has been shown to enhance the rate of cell growth (3). Some prokaryotic organisms are capable of synthesizing the redox active molecule, while other species rely on the environment for their supply. The biosynthesis of PQQ is accomplished by the gene products of a specific pqq operon. In Klebsiella pneumoniae, an organism with the experimentally demonstrated ability to produce PQQ, the pqq operon comprises six genes, pqqA-F (4). (The expressed genes from K. pneumoniae and four other demonstrated PQQ produces are summarized in Figure 1 and Table S1). Genetic knockout studies show four of the six gene products (PqqA, PqqC, PqqD, and PqqE) are absolutely required for this pathway, while the role of PqqB is ambiguous (5).
Figure 1.
Domains in each K. pneumoniae Pqq proteins. All domain information is from the Pfam database, except for the Lactamase_B superfamily domain in PqqB, which comes from the Conserved Domain Database of NCBI.
The successful in vitro characterization of two gene products, PqqC and PqqE, has demonstrated first, that PqqC is a cofactorless, oxygen-activating enzyme catalyzing the final step in PQQ biosynthesis (6) and second, that PqqE is a functional radical SAM enzyme capable of catalytic reductive cleavage of SAM to methionine and 5'-deoxyadenosine (7). The putative substrate for PqqE is PqqA, a 22 amino acid peptide containing a conserved glutamate and tyrosine that provide the complement of carbon and nitrogen atoms required for PQQ synthesis (Scheme 1) (8); however, the ability of or conditions required for PqqE to functionalize PqqA have not yet been demonstrated. The roles for PqqB, PqqD and PqqF in PQQ production are even less clear. PqqD has recently been shown to interact physically with PqqE (9), though the catalytic relevance of this interaction has yet to be determined. Genetic knockout studies of PqqF, a protein with homology to zinc-dependent proteases, suggest it is not essential for PQQ production, with the implication that other cell-associated, non-specific proteases can assume its role during cofactor biogenesis (5). One of the most enigmatic gene products is PqqB with high sequence similarity to the family of metallo-β-lactamases.
Scheme 1.
The proposed cross-linking of the tyrosine and the glutamate to form PQQ.
It was determined in 1988 that PQQ is derived from a ribosomally translated peptide (10). Since that time, several other biologically active molecules produced from amino acid precursors have been discovered (11–13). Analysis of these biosynthetic pathways reveals several common gene products, including radical SAM enzymes (e.g. PqqE), metallo-β-lactamases (e.g. PqqB), small, cofactorless proteins (e.g. PqqD), and cofactorless oxygenases (e.g. PqqC) in addition to the expected peptidases (e.g. PqqF) (12–15). The common protein families and underlying structure of biosynthetic pathways suggests that elucidating the evolution of PQQ biosynthesis may be useful for the discovery of other natural products and the characterization of the pathways required for their biosynthesis. In particular, bioinformatic analysis of the growing number of genomic sequences for prokaryotic organisms has revealed orphan pathways with unknown products of potential therapeutic application (11, 16). Indeed, gene products from the pqq operon are already being used as guides for identifying such pathways (17). Determining the evolution of each gene in the PQQ biosynthetic pathway may also contribute to understanding the ubiquitous use of certain protein families in modification of peptides to form biologically active natural products.
This paper presents a bioinformatics analysis of the genes involved in PQQ biosynthesis to identify the essential, biosynthetic pqq genes and the species that contain the full complement of these genes (and, thus, are inferred to synthesize PQQ). Structural phylogenomic analyses were used to identify the sequence motifs and structural features that distinguish each biosynthetic protein from functionally divergent homologs (18). These studies serve as a guide to predict putative roles for the open reading frames within the pqq operon and to probe the contribution of conserved amino acid side chains within the gene products with demonstrated function.
METHODS
Dataset
Five bacteria with demonstrated PQQ biosynthetic capacity were selected for this study: Klebsiella pneumoniae, Methylobacterium extorquens AM1, Gluconobacter oxydans 621H, Rahnella aquatilis and Streptomyces rochei (19–23). Each of the genes in the pqq operons in these five bacteria (shown in Table 1) was used as starting points for bioinformatics analyses. pqqA–E are found in all five bacteria, while the sixth pqq gene, pqqF, is found in only two of the five species (K. pneumoniae and R. aquatilis). M. extorquens has a fused pqqC/D gene, and also an extra gene pqqG (not included in this study).
Table 1.
Reference sequences of PqqA–F from five known PQQ-forming organisms.*
|
K. pneumoniae (X58778) |
M. extorquens (NC_012808) |
G. oxydans (CP000009) |
S. rochei (AB088224) |
R. aquatilis (FJ868974) |
|
|---|---|---|---|---|---|
| PqqA | P27503 (PQQA_KLEPN) | Q49148 (PQQA_METEA) | Q9L3B4 (PQQA_GLUOX) | Q83X96 (Q83X96_STRRO) | C3VIT4 (C3VIT4_RAHAQ) |
| PqqB | B5XX61 (PQQB_KLEP3) | Q49149 (PQQB_METEA) | Q9L3B3 (PQQB_GLUOX) | Q83XA0 (Q83XA0_STRRO) | C3VIT5 (C3VIT5_RAHAQ) |
| PqqC | B5XX60 (PQQC_KLEP3) | Q49150 (PQQCD_METEA) | Q9L3B2 (PQQC_GLUOX) | Q83X97 (Q83X97_STRRO) | C3VIT6 (C3VIT6_RAHAQ) |
| PqqD | B5XX59 (PQQD_KLEP3) | Q49150 (PQQCD_METEA) | Q9L3B1 (PQQD_GLUOX) | Q83X98 (Q83X98_STRRO) | O33505 (PQQD_RAHAQ) |
| PqqE | B5XX58 (PQQE_KLEP3) | P71517 (PQQE_METEA) | Q9L3B0 (PQQE_GLUOX) | P59749 (PQQE_STRRO) | O33506 (PQQE_RAHAQ) |
| PqqF | P27508 (PQQF_KLEPN) | C5AQL6 (C5AQL6_METEA) | absent | absent | C3VIT9 (C3VIT9_RAHAQ) |
UnitProt accession number for each protein is listed. In parentheses are UnitProt identifiers.
Bioinformatics Analyses
Bioinformatics analyses were performed to characterize the taxonomic distribution of homologs, identify corresponding PFAM domains (24), and to differentiate homologs sharing the same function from those that have divergent function. Core genes were identified from these combined analyses and used to identify species with probable PQQ biosynthetic capability.
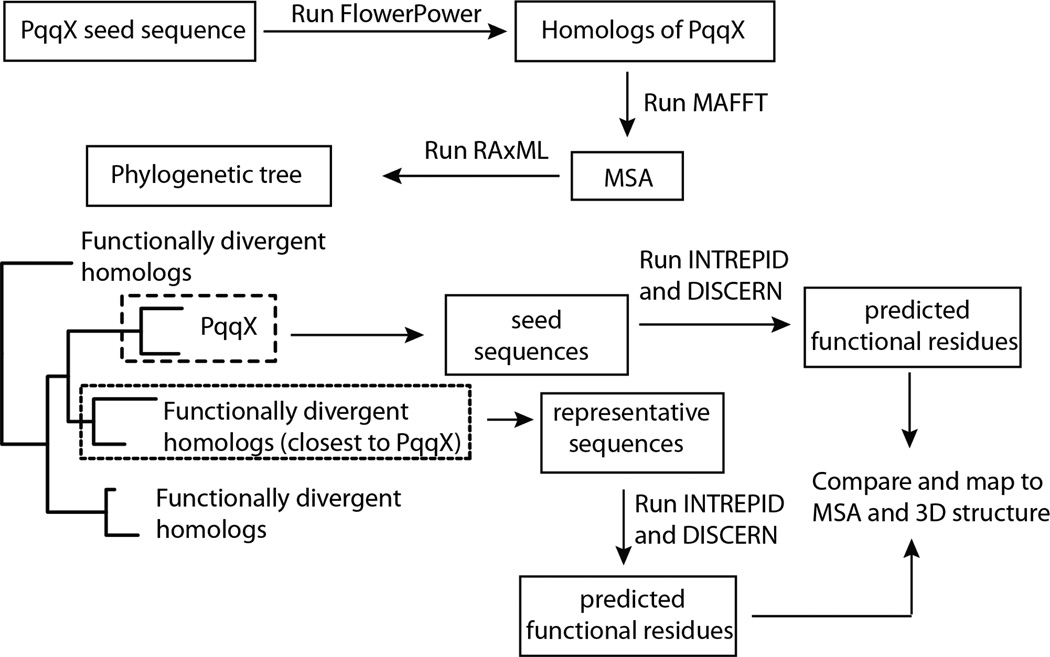
For each of the genes in the PQQ operon for these five species, we performed the following analyses: (i) homolog identification, (ii) multiple sequence alignment, (iii) phylogenetic tree construction and analysis, (iv) protein structure prediction, (v) functional site identification (see Scheme 2).
Scheme 2.
The bioinformatics analysis in this study. PqqX: a representative of Pqq proteins. MSA: multiple sequence alignment.
Homologs were retrieved from the UniProt protein database (release 2010_11) using the FlowerPower phylogenomic clustering software to select proteins sharing the same domain architecture (25). Multiple sequence alignments (MSAs) were constructed using MAFFT (26), followed by masking to remove columns with greater than 70% gap characters. Maximum likelihood trees were estimated from the masked MSAs using RAxML (27).
A solved three-dimensional structure was available for PqqC from K. pneumoniae (6); protein structures were predicted for PqqB and PqqD from K. pneumoniae using a comparative modeling approach (28, 29) with solved three-dimensional structure from other organisms as templates (PqqB from Pseudomonas putida and PqqD from Xanthamonas campestris (30)). In the case of PqqE, where no structures are yet available, the closest homolog with a solved structure (MoaA from Staphylococcus aureus, PDB ID 1TV8) was used to build a comparative model. The Phyre and Phyre2 servers (31) were used to construct comparative models for PqqB and PqqE; Modeller software (29) was used to predict the structure of PqqD.
Residues that were likely to be functionally important were identified via a combination of evolutionary conservation and structural information using the INTREPID and Discern algorithms (32, 33). INTREPID uses conservation signals over divergently related homologs organized by a phylogenetic tree to predict functional sites, while Discern uses INTREPID scores as well as information from protein three-dimensional structures. The multiple sequence alignments and phylogenetic trees were used as input to INTREPID, and the INTREPID scores and comparative models or solved structures were used as input to Discern. The Discern results are reported for PqqB–D. As the C-terminal portion of the PqqE model is not reliable, INTREPID results were reported for PqqE. These analyses identified a set of amino acids for each protein that are likely to be important functionally.
Most of the genes in the pqq operon are members of gene superfamilies including paralogs that may have diverged functionally. To identify protein residues that are diagnostic of participation in PQQ biosynthesis, we performed identical analyses on representative sequences with divergent function from the most closely related sister clade in the reconstructed phylogenies for PqqB (Figure S1), PqqC (Figure S2), PqqD (Figure S3) and PqqE (Figure S4). Residues highly ranked by either INTREPID or Discern for the biosynthetic proteins were mapped onto structures and comparative models for K. pneumoniae, and the corresponding residues in the functionally divergent homologs were plotted on structures or models for these proteins.
Additional Details on Bioinformatics Methods
HMM clustering of homologs using FlowerPower global homology clustering: all parameters default except the number of subfamily HMM scoring iterations (set to 10). MAFFT MSA construction parameters: 5 maximum iterations and default parameters. RAxML parameters: JTT+Γ model and 20 discrete γ-rate categories. The statistical support of branches was estimated using 100 bootstrap replicates. Due to the short length of the pqqA gene, standard genome annotation pipelines frequently missed pqqA (i.e., these were false negatives in gene prediction pipelines). We supplemented our standard homology detection pipeline for PqqA using translated BLAST against the whole genomes for these cases (34).
RESULTS AND DISCUSSION
Species with PQQ Biosynthetic Capability
Based on genetic knockout studies, PqqC, PqqD, and PqqE were initially considered to be the most promising core set of proteins required for inferring PQQ biosynthetic capability (20). The number of species containing different subsets of the core proteins is detailed in Figure 2. This analysis returned 126 species that include PqqC–E, of which 125 also contain PqqB (Table S2), strongly implicating PqqB as essential to PQQ biosynthesis. Due to the short length of PqqA (less than 30 amino acids), standard genome annotation pipelines failed to detect an ORF for pqqA in many cases. The standard HMM-based pipeline (in which we searched for proteins deposited in the UniProt database for other biosynthetic proteins) was supplemented with translated BLAST against whole genomes for these cases. Specifically, translated BLAST was used with each of the PqqA peptides from our five seed organisms against the whole genomes for those species for which PqqB–E proteins had been detected. This identified an additional 37 pqqA genes which had been missed by the genome annotation pipelines for those species (Table S3). To summarize, of the 126 species for which our analyses support PQQ biosynthetic capability, a total of 106 contain an identifiable PqqA (Table S2); of these species, 95 have whole genomes of which 98% (93) contain PqqA (based on either the HMM methods or translated BLAST as described in Methods). This result strongly supports the hypothesis that PQQ eg biosynthetic capability requires PqqA–E and that PqqA is the substrate for the biosynthetic pathway.
Figure 2.
Venn diagram of core PQQ biosynthesis genes (PqqC, PqqD and PqqE). Each circle represents the number of species containing the corresponding gene. pqqC homologs were found in 213 species; pqqD homologs were found in 316 species; pqqE homologs were found in 331 species. All three genes were found in 126 species.
This analysis shows that while some individual PQQ biosynthetic proteins have distant homologs outside prokaryotes, the pathway is clearly specific to prokaryotes. The ACS Paragon Plus Environment vast majority (approximately 88%) of the species predicted to be PQQ-forming are proteobacteria, with the α-, β- and γ- classes of this phylum well represented (Figure 3 and Table S2). Altogether, only six Gram-positive bacteria were found to be PQQ-forming, showing that PQQ production is more prevalent in Gram-negative than Gram-positive organisms. These results are consistent with the fact that either the enzymes or catalytic domains of enzymes requiring PQQ as a cofactor have, to date, been described in the context of a localization in the periplasm of Gram-negative bacteria (1, 35). Two species of Verrumicrobia, a recently discovered phylum that is a sister to Chylamidae, were also found to contain the homologs of PqqB–E.
Figure 3.
Distribution of PQQ synthesis proteins mapped to a phylogenetic tree based on 16s ribosomal RNA. The letters following species names indicate Pqq proteins. A–F: PqqA–PqqF. Tree nodes were collapsed if all the species have the same set of Pqq proteins (PqqA was not considered in this set as it is easily missed in gene finding procedures, and labeled with (A) if it was found in at least one species in the collapsed nodes).
While a role for bacterially-derived PQQ in mammalian homeostasis has been proposed (36), the exclusive mapping of the PQQ-generating enzymes to bacteria, together with the growth advantage conferred to selected prokaryotes by PQQ-dependent metabolism, makes the PQQ biosynthetic pathway an enticing target for the design of inhibitors for use in antibiotic cocktails. A number of the PQQ-forming species identified in this study can operate as opportunistic pathogens (Table 2), suggesting that inhibitors of the pathway may be especially well suited to a cocktail of antibiotics administered to immune-suppressed patients.
Table 2.
Potential pathogens or symbionts containing PqqB, PqqC, PqqD and PqqE, ordered by species name (see Table S2 for a complete list of predicted PQQ-synthesis species).
| Species | Pathogenicity | Set of Pqq genes |
|---|---|---|
| Acinetobacter baumannii ATCC 19606 | Opportunistic human pathogen | ABCDE |
| Acinetobacter haemolyticus ATCC 19194 | Rare human pathogen | BCDE |
| Azoarcus sp. (strain BH72) | Plant symbiont | ABCDEF |
| Bradyrhizobium japonicum | Plant symbiont | ABCDEF |
| Bradyrhizobium sp. (strain BTAil / ATCC BAA-1182) | Plant symbiont | ABCDEF |
| Burkholderia cenocepacia (strain HI2424) | Human cystic fibrosis pathogen | ABCDE |
| Burkholderia cepacia (strain J2315 / LMG 16656) | Animal and plant pathogen | ABCDE |
| Burkholderia glumae (strain BGR1) | Plant pathogen | ABCDE |
| Burkholderia multivorans (strain ATCC 17616 / 249) | Animal pathogen in Mammalia | ABCDE |
| Burkholderia phymatum (strain DSM 17167 / STM815) | Plant symbiont | ABCDE |
| Colwellia psychrerythraea (strain 34H / ATCC BAA-681) | Plant pathtogen | ABCDE |
| Cronobacter turicensis (strain DSM 18703/LMG 23827/z3032) | Neonatal pathogen | ABCDEF |
| Cupriavidus taiwanensis (strain R1 / LMG 19424) | Plant pathogen | ABCDE |
| Dinoroseobacter shibae (strain DFL 12) | Animal symbiont | ABCDEF |
| Enterobacter intermedius | Opportunistic pathogen | ABCDEF |
| Enterobacter sakazakii (strain ATCC BAA-894) | Neonatal pathogen | ABCDEF |
| Erwinia amylovora (strain ATCC 49946/CCPPB 0273/Ea273/ 27-3) | Plant pathogen | ABCDEF |
| Erwinia pyrifoliae | Plant pathogen | ABCDEF |
| Erwinia tasmaniensis (strain DSM 17950 / Et1/99) | Plant commensal | ABCDEF |
| Gluconacetobacter diazotrophicus (strain ATCC 49037/DSM 5601/PA15) | Plant symbiont | ABCDE |
| Granulibacter bethesdensis (strain ATCC BAA-1260/ CGDNIH1) | Human pathogen | ABCDE |
| Grimontia hollisae CIP 101886 | Human pathogen | BCDE |
| Klebsiella pneumoniae | Opportunistic pathogen | ABCDEF |
| Klebsiella sp. 1_1_55 | Opportunistic pathogen | BCDEF |
| Marinobacter algicola DG893 | Plant symbiont | ABCDE |
| Methylobacterium nodulans (strain ORS2060 / LMG 21967) | Plant saprophyte and symbiont | ABCDEF |
| Methylobacterium populi (strain ATCC BAA-705/NCIMB 13946/BJ001) | Plant endophyte | ABCDEF |
| Methylobacterium radiotolerans (strain ATCC 27329/DSM 1819/JCM 2831) | Plant symbiont | ABCDEF |
| Mycobacterium smegmatis (strain ATCC 700084/mc(2)155) | Commensal in Mammalia | ABCDE |
| Pantoea ananatis | Plant pathogen | ABCDEF |
| Pseudomonas aeruginosa | Opportunistic human pathogen | ABCDEF |
| Pseudomonas entomophila (strain L48) | Insect pathogen | ABCDEF |
| Pseudomonas fluorescens | Potential pathogen to birds | ABCDEF |
| Pseudomonas mendocina (strain ymp) | Rare human pathogen | ABCDEF |
| Pseudomonas savastanoi pv. savastanoi NCPPB 3335 | tumor-inducing pathogen | ABCDEF |
| Pseudomonas syringae pv. phaseolicola (strain 1448A/Race 6) | Plant symbiont | ABCDEF |
| Rahnella aquatilis | Opportunistic pathogen | ABCDEF |
| Ralstonia pickettii (strain 12D) | Opportunistic pathogen | ABCDEF |
| Rhizobium meliloti | Plant symbiont | ABCDE |
| Rhizobium sp. (strain NGR234) | Plant symbiont | ABCDEF |
| Rickettsiella grylli | Athropod pathogen | BCDEF |
| Serratia marcescens | Opportunistic human pathogen | ABCDE |
| Serratia odorifera 4Rx13 | Opportunistic pathogen | ABCDEF |
| Verminephrobacter eiseniae (strain EF01-2) | Animal endosymbiont | ABCDEF |
| Xanthomonas axonopodis pv. citri (Citrus canker) | Plant pathogen | ABCDE |
| Xanthomonas campestris pv. Campestris | Plant pathogen | ABCDE |
| Xanthomonas oryzae pv. Oryzae | Rice bacterial blight pathogen | ABCDE |
Organization and Evolution of the PQQ Operon
Of the 126 species identified here as synthesizing PQQ, 96 have gene order information, either based on whole genomes or due to the availability of contigs including all pqq genes. Of these 96, all of which contain pqqA, B, C, D, and E, 93 have no large insertions within the operon. Among these 93, 91 have the conserved gene order pqqA-B-C-D-E. In total, 96% of whole genomes having pqq operons have conserved order of the core genes with no large insertions.
Only in 17 species is pqqF clustered with pqqB–E, either in the order of pqqB-C-D-E-F or in the order of pqqF-A-B-C-D-E. In all other cases (27 species), pqqF is located remotely from the pqqA–E cluster, separated by genes unrelated to the pathway. The distance of pqqF from other pqq genes suggests that pqqF is out of the evolutionary and regulatory constraints exerted on other pqq genes. This finding also agrees with the hypothesis that the function of PqqF may be replaced by other peptidases.
The specific ordering of pqqA–E in the operon suggests that these gene products may form a catalytically relevant complex. Conservation of gene order within an operon is not characteristic of prokaryotic organisms (37, 38), and when gene order is conserved in an operon, the corresponding gene products have been shown to physically interact (39, 40). Protein-protein interactions are common in metabolic pathways, and these interactions can serve to increase the efficiency of the overall process by positioning OJ consecutively acting enzymes in proximity to one another and by protecting sensitive intermediates from degradation via direct channeling between these enzymes. In the PQQ biosynthetic pathway, protecting PqqA during the biosynthetic process would shield the peptide from proteolytic degradation before completion of the necessary modifications. The absence or remoteness of pqqF from the operon suggests that this gene product is not involved in protein-protein interactions with other members of the pathway.
The proposed interaction of pqq gene products is further supported by the fusion of pqqC and pqqD in four Methylobacterium species: M. radiotolerans, M. populi, M. extorquens, and M. chloromethanicum. In addition, we have found a potential fusion of pqqD and pqqE in Methylosinus trichosporium. The sequence is annotated as PqqE (UniProt Accession: D5QP91), but it clearly possesses a PqqD domain.
The phylogenetic profile of each pqq gene was mapped to the 16s-rRNA tree of PQQ-forming species identified in this study to infer the evolution of the PQQ biosynthetic pathway (Figure 3). As suggested by the gene locus study, PqqB, PqqC, PqqD, and PqqE appear to have evolved together in the pathway. PqqF appears to have been lost several times (e.g. in Acetobacteraceae, Burkholderia, and Acinetobacter), and presumably its function has been replaced by other peptidases in these species. Despite the limitations of bioinformatics methods for both gene identification and for reliable identification of short peptides (see above) PqqA was identified in most species (Figure 3).
Structure-Sequence Analyses of Core pqq Genes
PqqB, PqqC, PqqD, and PqqE are each found in superfamilies whose functions may have diverged from their common ancestor by gene duplication events, allowing members of the family to acquire novel functions or to partition the ancestral function (Figure 1 and Table S1). Evolutionary and structural analyses were performed to identify residues that are likely to be important functionally.
Structure-Sequence Analysis of PqqB
Initially, sequence analysis of PqqB suggested that the enzyme was a member of the metallo-β-lactamase superfamily (41). The determined three-dimensional structure of PqqB (PDB IDs 1XTO and 3JXP) has since confirmed this. Analysis of the phylogenetic tree of PqqB homologs shows that the closest functionally distinct homolog of PqqB is PhnP (Figure S1). PhnP is a phosphodiesterase that shares 26% sequence identity with PqqB (42). The comparison of PqqB to PhnP (Figure 4) shows the overall structural similarity between PhnP and PqqB; however, the ligands for one of the two metals observed to be bound in PhnP are not present in PqqB. The predicted metal ligand residues that are retained in PqqB (Asp92, His93, and His269 in PqqB from K. pneumoniae, in red in Figure 4A) are ranked 6, 1, and 3, respectively by the Discern algorithm (Table S4). The active site metals bound by PhnP are both manganese (43); however the metal ligands retained in PqqB represent a 2-His/l-carboxylate facial triad configuration, characteristic of the non-heme ferrous-binding family. While future studies with recombinant PqqB are necessary to demonstrate metal binding and function, this arrangement of ligands suggests that PqqB likely catalyzes oxidative chemistry via a conserved chemical mechanism that involves the generation of an active site FeIV=0 (44). This chemistry is precedented amongst members of the metallo-β-lactamase superfamily (45). Additionally, in the three-dimensional structure of PqqB (PDB ID 3JXP from P. putida), a zinc ion is bound several angstroms from this putative active site near the solvent interface by the motif CXCX2C (residues 19–24 in PqqB from K. pneumoniae in blue in Figure 4). This metal is conserved in both PqqB and PhnP. Studies of PhnP have shown that this zinc is not necessary for catalysis and it is proposed that the ion plays a role in maintaining the structure of the enzyme (42). The comparable location of this zinc ion in PqqB suggests that it may also play a structural role. Lastly, it can be noticed that a glycine-rich motif (GXXXGGGXPQWN residues 7–18 in PqqB from K. pneumonia) located between the proposed structural zinc ion and the putative active site of PqqB and exposed to solvent, is conserved in 80% of PqqB sequences from the 126 proposed PQQ producers, hi PhnP, this motif is less conserved, indicating that it might impart a functional specificity to PqqB. This motif has features (G repeats) common to nucleotide-binding pockets (46, 47), suggesting a possible role for a nucleotide cofactor as an electron donor in a PqqB-catalyzed activation of O2. This bioinformatics analysis indicates that PqqB may be the missing hydroxylase, performing a role in the oxidation of the tyrosine of PqqA prior to its cross-linking with glutamate (Scheme 1).
Figure 4.
Comparison of the PqqB three-dimensional structure model and PhnP structure. The spheres of Mn ions in the active site are in purple and the sphere of zinc ion is in green. Residues involved in putative structural Zn ion binding are in blue, Mn binding of the PhnP active site are in red if conserved in PqqB and in purple if not. A: model of the structure of PqqB from K. pneumoniae (UniProt accession B5XX61) using the solved structure of PqqB from P. putida (66% identity). B: structure of PhnP sequence from E. coli (UniProt accession PI6692, PDB ID 3G1P). C: The pairwise alignment of PqqB and PhnP. The numbers above columns indicate the ranking predicted by Discern for the corresponding residue of PqqB (Table S4).
Structure-Sequence Analysis of PqqC
The activity of PqqC from K. pneumoniae has been determined (6, 48). PqqC catalyzes the last step of PQQ biosynthesis: oxidation of 3a-(2-amino-2-carboxyethyl)-4,5-dioxo-4,5,6,7,8,9-hexahydroquinoline-7,9-dicarboxylic acid (AHQQ) involving transfer of 8 electrons and protons to molecular oxygen to form hydrogen peroxide/water. The closest functionally distinct homolog of PqqC identified in the phylogenetic tree is TenA (Figure S5). TenA is a thiaminase II that catalyzes the hydrolysis of 4-amino-5-aminomethyl-2-methylpyrimidine as part of a pathway for salvaging base-degraded thiamin (49). Although both proteins contain buried active sites, the reaction catalyzed by PqqC is very different from that catalyzed by TenA. The comparison of the structures of PqqC and TenA (Figure 5) shows their overall structural similarity, but the active site of PqqC is distinct from that of TenA. Discern analysis of PqqC revealed a cluster of residues in and around the active site (Table S5). In the top 20 ranked residues, four are outside the active site and could play a role in opening the active site to the substrate (in blue in Figure 5), with the remaining sixteen residues located in the active site. Only one of these residues is conserved in TenA at the corresponding position (according to the structural alignment constructed by VAST, in red in Figure 5). The residues of potential catalytic importance in PqqC (Tyr23, His24, Arg50, Gln54, Arg80, His84, Tyrl28, Glul47, Hisl54, Argl57, Tyrl75, Argl79 and Lys214 in green in Figure 5) that are not conserved in TenA likely contribute to the specificity of PqqC activity. This suggests that each enzyme in the family has evolved a distinct function highly specific to the pathway in which it is active, as evidenced by the differing active site side chains of PqqC and TenA. Residues previously shown to be important in PqqC by biochemical experiments were identified as the most likely important functional residues in our study. His84 is proposed to be a proton donor and is ranked fourth by Discern (50); Hisl54, Tyrl75 and Argl79 are proposed to form a core oxygen-binding pocket essential for oxygen activation (51) and are ranked tenth, seventh and twenty-sixth, respectively, by Discern (labeled with stars in Figure 5).
Figure 5.
Comparison of the structures of PqqC and TenA. A: Structure of PqqC (B5XX60 from K. pneumoniae, PDB ID 1OTW). The top ranked residues predicted by Discern are labeled. Residues outside the active site are labeled in blue, the residue in the active site are in red if conserved in TenA and in green if not. The residues characteristic by biochemical experiments are marked with a star. B: Structure of TenA from B. subtilis (P25052, PDB ID lyaf). Predicted functional residues are in purple. C: The pairwise structural alignment of PqqC and TenA (made by VAST). The numbers above columns indicate the ranking of catalytic residues in PqqB predicted by Discern (Table S5).
Structure-Sequence Analysis of PqqD
PqqD is a small protein (90 amino acids on average) with no detectable cofactor. In this study, the Discern algorithm was used with the PqqD protein from K. pneumoniae, and a homology model for K. pneumoniae (using PDB ID 3G2B from Xanthomonas campestris as a template with 29% sequence identity). In the case of PqqD, the global homology clustering criterion used to gather homologs resulted in no functionally divergent homologs being included in the tree (Figure S3). Thus, in this case, residues conferring functional specificity relative to functionally divergent homologs were unable to be identified. However, Discern identifies a set of positions that are clearly involved in function. The finding that conserved residues are largely constrained to an exposed, unstructured region of PqqD (Figure 6 and Table S6) suggest that the previously observed interaction of this protein with PqqE may necessitate significant restructuring of PqqD (9).
Figure 6.
Structure-sequence analysis of PqqD. A: Homology model of PqqD from K. pneumoniae (UniProt accession number B5XX59) modeled onto the three-dimensional structure of PqqD from X. campestris (PDB ID 3G2B). Residues ranked within the top ten by Discern are colored in red (Table S6). B: MSA of the five PqqD seed sequences constructed using MAFFT and displayed using Belvu. Residues ranked within the top ten by DESCERN are colored in red. KP, K. pneumoniae; ME, M. extorquens AMI; GO, G.r oxydans 621H; RA, R. aquatilis; SR, S. rochei (Table S6)
Structure-Sequence Analysis of PqqE
The closest functionally divergent homolog of PqqE is NirJ (Figure S4), sharing 26% sequence identity (using NirJ from Sulfurovum Sp., UniProt accession A6X6Z2). NirJ catalyzes a key step in the biosynthesis of heme dl. PqqE and NirJ both have been experimentally verified to be members of the radical SAM superfamily (52, 53), catalytically cleaving the universal co-substrate of this superfamily, S-adenosylmethionine, to form a highly oxidizing radical used for hydrogen abstraction on the remaining substrate (7). Members of the radical SAM superfamily coordinate a 4Fe-4S cluster using the motif CX3CX2C; the three cysteine ligands for this SAM-binding cluster were ranked within the top three evolutionarily conserved residues by INTREPID (Cys22, Cys26, and Cys29 in PqqE from K. pneumoniae) (Table S7). The GGE motif (residues 66–68 in PqqE from K. pneumoniae, ranked 10, 7, and 11 respectively by INTREPID) that is characteristic of radical SAM enzymes and serves to stabilize SAM in the active site is found in both enzymes as well (Table S7) (54, 55).
In contrast to PqqB and PqqC, little experimental evidence is available for close homologs of PqqE. Low sequence identity amongst homologs is, indeed, a common feature of the radical SAM superfamily, though nearly all of its members adopt a half to full TIM-barrel fold. The structure of radical SAM enzymes can be described as two distinct regions, each catalyzing a specific half reaction (53). The N-terminal region of superfamily members constitutes a highly conserved radical SAM core where S-adenosylmethionine is catalytically cleaved, and this region spans approximately 200 amino acids (residues 16 – 174 in PqqE). The second region (located at the C-terminus) is the location of the specific half reaction catalyzed by each enzyme of the superfamily and, consequently, is highly variable in both length and sequence identity. This variability gives rise to the low overall sequence identity observed for the radical SAM superfamily (53, 55).
Homology between the C-terminal regions of PqqE (residues 206–380 from K. pneumoniae, UniProt accession B5XX58) and NirJ is supported by a 35% sequence identity (BLAST E-value of 5 × 10−6) when comparing PqqE to a NirJ from Methanosarcina acetivorans (residues 249 – 346 from UniProt accession Q8TT64) (34); the annotation of NirJ from M acetivorans was verified by locating the ORF for this gene product within the operon encoding heme dl biosynthesis in this organism. This homology between the C-termini of PqqE and NirJ allows inferences about the function of key amino acid residues in this region of the protein. Both PqqE and NirJ have been experimentally determined to bind an additional 4Fe-4S cluster, likely located outside of the N-terminal radical SAM core. Three C-terminal cysteine residues were ranked in the top 25 by INTREPID (Cys306, Cys309, and Cys337 in PqqE from K. pneumoniae) for both PqqE and NirJ, and analysis of the MSA of PqqE and NirJ proteins indicates that these residues are the only C-terminal cysteines conserved for both proteins (Figure 7C). These residues form a CX2CX27C motif; this analysis suggests that this motif serves to coordinate the non-SAM binding 4Fe-4S cluster.
Figure 7.
Structure-sequence analysis of PqqE. A: Homology models of PqqE from K. pneumoniae (UniProt accession number B5XX58). B: Homology models of NirJ from Sulfurovum sp. (UniProt accession A6X6Z2) constructed using the Phyre2 webserver based on the three-dimensional structure of MoaA (PDB ID 1TV8). C: MSA of PqqE (UniProt accession number B5XX58) and NirJ (UniProt accession number A6X6Z2) constructed using MAFFT and displayed using Belvu. The INTREPID ranking of important functional residues is indicated by numbers above the alignment (Table S7). Colors in panels A, B, and C correspond to the following groups: blue: iron sulfur cluster ligands, red: residues located within the vicinity of the SAM-binding 4Fe-4S cluster, green: residues highly conserved in PqqE that are not present in NirJ, purple: regions where the homology is less reliable and so structural information is speculative. KP, K. pneumoniae; SS, Sulfurovum sp.
A comparative (homology) structural model for PqqE was constructed in order to identify the relative location of the two 4Fe-4S clusters. The evolutionarily closest three-dimensional structure to PqqE is of MoaA from Staphylococcus aureus with a BLAST E-value 1 × 10−6 and 23% identity and 41% positives (identical and similar amino acids) for PqqE residues 1–206. The PHYRE2 server produced a comparative model for the entire three-dimensional structure of PqqE, extending beyond the conserved N-terminus into the highly variable C-terminus. Figure 7A presents the PqqE structural model, differentiating between the region anticipated to be accurately modeled (the first 206 amino acids) (shown in grey) and the variable C-terminal region (shown in purple). A comparative model is also provided for NirJ in Figure 7B. Based on these structural models, the second 4Fe-4S cluster is located opposite the N-terminal SAM-binding 4Fe-4S cluster, across the putative hydrophobic active site channel. Also highlighted by INTREPID and the MSA of PqqE proteins were Cys319 and Cys321 that are not present in NirJ. These residues are located proximal to the CX2CX27C motif at the C-terminus of PqqE and so might be involved in a function specific to the role of PqqE in the biosynthetic pathway. Possible roles for these two residues will depend on whether these residues face the active site or solvent interface. In the former case they may act to stabilize the peptide substrate in the active site; in the latter case, they may play a role in the protein-protein interactions demonstrated with PqqD (55).
CONCLUSIONS
This work presents use a novel bioinformatics protocol toward identifying species encoding a natural products biosynthesis pathway. The most conserved proteins previously shown as essential to PQQ production -- PqqC, D, and E -- were used to identify 126 bacterial species with PQQ biosynthetic capability. Genomes encoding these three proteins were further examined to determine the presence/absence of additional pqq genes. These analyses revealed the conservation of pqqA and pqqB, substantiating a previously unclear essential role for PqqB. The ORF for pqqF appears to be frequently lost, and is assumed to be non-essential to this pathway, allowing substitution by other proteases. The results further reveal a highly conserved gene order of pqqA–E within the operon, which along with recent evidence for a PqqD/E interaction, strongly suggests a role for macromolecular complex formation in function. A detailed sequence/structure analysis was employed to distinguish homologs that share a common multi-domain architecture from those that have only partial homology, and to identify sequence motifs that are diagnostic of each of the separate biosynthetic proteins. From such a phylogenetic/structure/sequence analysis it is seen that PqqB contains a 2-His, 1-Asp active site configuration characteristic of non-heme iron monooxygenases. The presented findings may aid in the tailoring of antibiotics specifically toward the inhibition of PQQ biosynthesis, while furthering our understanding of related enzymes present in other pharmaceutically relevant biosynthetic pathways.
Supplementary Material
ACKNOWLEDGEMENT
We thank Ruchira Datta, Glen Javis, and Shailen Tuli for technical advice on different aspects of this work.
ABBREVIATIONS
- PQQ
pyrroloquinoline quinone
- MSA
Multiple Sequence Alignment
- HMM
Hidden Markov Model
- SAM
S-Adenosyl Methionine
- ORF
open reading frame
- AHQQ
3a-(2-amino-2-carboxyethyl)-4,5-dioxo-4,5,6,7,8,9-hexahydroquinoline-7,9-dicarboxylic acid
Footnotes
This work was supported by funding from the National Institutes of Health (GM039296 to J.P.K.); the National Science Foundation (0732065 to K.S.); and the Department of Energy (DE-SC0004916 to K.S.).
SUPPORTING INFORMATION AVAILABLE
Additionally included in supplemental information but not referenced in the text of this paper are the multiple sequence alignments selected from each core biosynthetic protein and its closest functionally distinct homologs (Figures S6, S7, and S8). This material is available free of charge via the internet at http://pubs.acs.org.
CONFLICT OF INTEREST DISCLOSURE
The authors declare no competing financial interest.
REFERENCES
- 1.Anthony C. Pyrroloquinoline quinone (PQQ) and quinoprotein enzymes. Antioxid. Redox. Sign. 2001;3:757–774. doi: 10.1089/15230860152664966. [DOI] [PubMed] [Google Scholar]
- 2.Goodwin PM, Anthony C. The biochemistry, physiology and genetics of PQQ and PQQ-containing enzymes. Adv. Microb. Physiol. 1998;40:1–80. doi: 10.1016/s0065-2911(08)60129-0. [DOI] [PubMed] [Google Scholar]
- 3.Sode K, Ito K, Witarto AB, Watanabe K, Yoshida H, Postma P. Increased production of recombinant pyrroloquinoline quinone (PQQ) glucose dehydrogenase by metabolically engineered Escherichia coli strain capable of PQQ biosynthesis. J. Biotech. 1996;49:239–243. doi: 10.1016/0168-1656(96)01540-4. [DOI] [PubMed] [Google Scholar]
- 4.Meulenberg JJ, Sellink E, Riegman NH, Postma PW. Nucleotide sequence and structure of the Klebsiella pneumoniae pqq operon. Mol. Gen. Genet. 1992;232:284–294. doi: 10.1007/BF00280008. [DOI] [PubMed] [Google Scholar]
- 5.Velterop JS, Sellink E, Meulenberg JJ, David S, Bulder I, Postma PW. Synthesis of pyrroloquinoline quinone in vivo and in vitro and detection of an intermediate in the biosynthetic pathway. J. Bacteriol. 1995;177:5088–5098. doi: 10.1128/jb.177.17.5088-5098.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magnusson OT, Toyama H, Saeki M, Rojas A, Reed JC, Liddington RC, Klinman JP, Schwarzenbacher R. Quinone biogenesis: Structure and mechanism of PqqC, the final catalyst in the production of pyrroloquinoline quinone. Proc. Natl. Acad. Sci. U.S.A. 2004;101:7913–7918. doi: 10.1073/pnas.0402640101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wecksler SR, Stoll S, Tran H, Magnusson OT, Wu SP, King D, Britt RD, Klinman JP. Pyrroloquinoline quinone biogenesis: demonstration that PqqE from Klebsiella pneumoniae is a radical S-adenosyl-L-methionine enzyme. Biochemistry. 2009;48:10151–10161. doi: 10.1021/bi900918b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Houck DR, Hanners JL, Unkefer CJ, van Kleef MA, Duine JA. PQQ: biosynthetic studies in Methylobacterium AM1 and Hyphomicrobium X using specific 13C labeling and NMR. Antonie van Leeuwenhoek. 1989;56:93–101. doi: 10.1007/BF00822589. [DOI] [PubMed] [Google Scholar]
- 9.Wecksler SR, Stoll S, Iavarone AT, Imsand EM, Tran H, Britt RD, Klinman JP. Interaction of PqqE and PqqD in the pyrroloquinoline quinone (PQQ) biosynthetic pathway links PqqD to the radical SAM superfamily. Chem. Commun. 2010;46:7031–7033. doi: 10.1039/c0cc00968g. [DOI] [PubMed] [Google Scholar]
- 10.Mazodier P, Biville F, Turlin E, Gasser F. Localization of a pyrroloquinoline quinone biosynthesis gene near the methanol dehydrogenase structural gene in Methylobacterium organophilum DSM 760. J. Gen. Microbiol. 1988;134:2513–2524. doi: 10.1099/00221287-134-9-2513. [DOI] [PubMed] [Google Scholar]
- 11.McClerren AL, Cooper LE, Quan C, Thomas PM, Kelleher NL, van der Donk WA. Discovery and in vitro biosynthesis of haloduracin, a two-component lantibiotic. Proc. Natl. Acad. Sci. U.S.A. 2006;103:17243–17248. doi: 10.1073/pnas.0606088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milne JC, Eliot AC, Kelleher NL, Walsh CT. ATP/GTP hydrolysis is required for oxazole and thiazole biosynthesis in the peptide antibiotic microcin B17. Biochemistry. 1998;37:13250–13261. doi: 10.1021/bi980996e. [DOI] [PubMed] [Google Scholar]
- 13.Velasquez JE, van der Donk WA. Genome mining for ribosomally synthesized natural products. Curr. Opin. Chem. Biol. 2011;15:11–21. doi: 10.1016/j.cbpa.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Makris TM, Chakrabarti M, Muenck E, Lipscomb JD. A family of diiron monooxygenases catalyzing amino acid beta-hydroxylation in antibiotic biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 2010;107:15391–15396. doi: 10.1073/pnas.1007953107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fetzner S, Steiner RA. Cofactor-independent oxidases and oxygenases. Appl. Microbiol. Biot. 2010;86:791–804. doi: 10.1007/s00253-010-2455-0. [DOI] [PubMed] [Google Scholar]
- 16.Sudek S, Haygood MG, Youssef DT, Schmidt EW. Structure of trichamide, a cyclic peptide from the bloom-forming cyanobacterium Trichodesmium erythraeum, predicted from the genome sequence. Appl. Environ. Microb. 2006;72:4382–4387. doi: 10.1128/AEM.00380-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haft DH. Bioinformatic evidence for a widely distributed, ribosomally produced electron carrier precursor, its maturation proteins, and its nicotinoprotein redox partners. BMC Genomics. 2011;12 doi: 10.1186/1471-2164-12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sjolander K. Getting started in structural phylogenomics. PLoS Comput. Biol. 2010;6:el000621. doi: 10.1371/journal.pcbi.1000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arakawa K, Sugino F, Kodama K, Ishii T, Kinashi H. Cyclization mechanism for the synthesis of macrocyclic antibiotic lankacidin in Streptomyces rochei. Chem. Biol. 2005;12:249–256. doi: 10.1016/j.chembiol.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Velterop JS, Sellink E, Meulenberg JJ, David S, Bulder I, Postma PW. Synthesis of pyrroloquinoline quinone in vivo and in vitro and detection of an intermediate in the biosynthetic pathway. J. Bacteriol. 1995;177:5088–5098. doi: 10.1128/jb.177.17.5088-5098.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toyama H, Chistoserdova L, Lidstrom ME. Sequence analysis of pqq genes required for biosynthesis of pyrroloquinoline quinone in Methylobacterium extorquens AM1 and the purification of a biosynthetic intermediate. Microbiology-UK. 1997;143:595–602. doi: 10.1099/00221287-143-2-595. [DOI] [PubMed] [Google Scholar]
- 22.Hoelscher T, Goerisch H. Knockout and overexpression of pyrroloquinoline quinone biosynthetic genes in Gluconobacter oxydans 621H. J. Bacteriol. 2006;188:7668–7676. doi: 10.1128/JB.01009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo YB, Li J, Li L, Chen F, Wu W, Wang J, Wang H. Mutations that disrupt either the pqq or the gdh gene of Rahnella aquatilis abolish the production of an antibacterial substance and result in reduced biological control of grapevine crown gall. Appl. Environ. Microb. 2009;75:6792–6803. doi: 10.1128/AEM.00902-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bateman A, Coin L, Durbin R, Finn RD, Hollich V, Griffiths-Jones S, Khanna A, Marshall M, Moxon S, Sonnhammer EL, Studholme DJ, Yeats C, Eddy SR. The Pfam protein families database. Nucleic Acids Res. 2004;32:D138–D141. doi: 10.1093/nar/gkh121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krishnamurthy N, Brown D, Sjolander K. FlowerPower: clustering proteins into domain architecture classes for phylogenomic inference of protein function. BMC Evol. Biol. 2007;7(Suppl 1):S12. doi: 10.1186/1471-2148-7-S1-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- 28.Bennett-Lovsey RM, Herbert AD, Sternberg MJ, Kelley LA. Exploring the extremes of sequence/structure space with ensemble fold recognition in the program Phyre. Proteins. 2008;70:611–625. doi: 10.1002/prot.21688. [DOI] [PubMed] [Google Scholar]
- 29.Eswar N, Webb B, Marti-Renom MA, Madhusudhan MS, Eramian D, Shen MY, Pieper U, Sali A. Comparative protein structure modeling using Modeller. Curr. Prot. Bioinformatics. 2006;5(Unit 56) doi: 10.1002/0471250953.bi0506s15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsai TY, Yang CY, Shih HL, Wang AH, Chou SH. Xanthomonas campestris PqqD in the pyrroloquinoline quinone biosynthesis operon adopts a novel saddle-like fold that possibly serves as a PQQ carrier. Proteins. 2009;76:1042–1048. doi: 10.1002/prot.22461. [DOI] [PubMed] [Google Scholar]
- 31.Kelley LA, Sternberg MJ. Protein structure prediction on the Web: a case study using the Phyre server. Nat. Protoc. 2009;4:363–371. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- 32.Sankararaman S, Sjolander K. INTREPID-Information-theoretic TREe traversal for Protein functional site identification. Bioinformatics. 2008;24:2445–2452. doi: 10.1093/bioinformatics/btn474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sankararaman S, Sha F, Kirsch JF, Jordan MI, Sjolander K. Active site prediction using evolutionary and structural information. Bioinformatics. 2010;26:617–624. doi: 10.1093/bioinformatics/btq008. PMCID: PMC2828116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller Q, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acid Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duine JA. Quinoproteins: enzymes containing the quinonoid cofactor pyrroloquinoline quinone, topaquinone, or tryptophan-tryptophan quinone. Eur. J. Biochem./FEBS. 1991;200:271–284. doi: 10.1111/j.1432-1033.1991.tb16183.x. [DOI] [PubMed] [Google Scholar]
- 36.Virdi NS, Price D, Ellison J, Yadalam S, Nigam S. Use of GDH-PQQ glucose meter systems in patients receiving maltose-containing therapies. Diabetologia. 2010;53 [Google Scholar]
- 37.Mushegian AR, Koonin EV. Gene order is not conserved in bacterial evolution. Trends Genet. 1996;12:289–290. doi: 10.1016/0168-9525(96)20006-x. [DOI] [PubMed] [Google Scholar]
- 38.Omelchendo MV, Makarova KS, Wolf YI, Rogozin IB, Koonin EV. Evolution of mosaic operons by horizontal gene transfer and gene displacement in situ. Fenome. Biol. 2003;4:R55. doi: 10.1186/gb-2003-4-9-r55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dendekar T, Snel B, Huynen M, Bork P. Conservation of gene order: a fingerprint of proteins that physically interact. Trends Biochem. Sci. 1998;23:432–328. doi: 10.1016/s0968-0004(98)01274-2. [DOI] [PubMed] [Google Scholar]
- 40.Fondi M, Emiliani G, Fani R. Origin and evolution of operons and metabolic pathways. Res. Microbiol. 2009;160:502–512. doi: 10.1016/j.resmic.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 41.Puehringer S, Metlitzky M, Schwarzenbacher R. The pyrroloquinoline quinone biosynthesis pathway revisited: as structural approach. BMC Biochemistry. 2008;9:8. doi: 10.1186/1471-2091-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Podzelinska K, He S-M, Wathioer M, Yakunin A, Proudfoot M, Hove-Jenson B, Zechel DL, Jia Z. Structure of PhnP, a phosphodiesterase of the carbon-phosphorous lyase pathway for phosphonate degradation. J. Biol. CHem. 2009;284:17216–17226. doi: 10.1074/jbc.M808392200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vetting MW, Wackett LP, Que L, Jr, Lipscomb JD, Ohlendorf DH. Crystaollographic comparison of manganese- and iron-dependent homoprotocatechuate 2,3-dioxygnases. J. Bacteriology. 2004;186:1945–1958. doi: 10.1128/JB.186.7.1945-1958.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bruijnincx PCA, van Koten G, Gebbink RJMK. Mononuclear non-heme iron enzymes with the 2-His-l-carboxylate facial triad: recent developments in enzymology and modeling studies. Chem. Soc. Rev. 2008;37:2716–2744. doi: 10.1039/b707179p. [DOI] [PubMed] [Google Scholar]
- 45.Koehntop KD, Emerson JP, Que L., Jr The 2-His-1-carboxylate facial triad: a versatile platform for dioxyygen activation by mononuclear non-heme iron(II) enzymes. J. Biol. Inorg. Chem. 2005;10:87–93. doi: 10.1007/s00775-005-0624-x. [DOI] [PubMed] [Google Scholar]
- 46.Bellamacina CR. Protein motifs. 9. The nicotinamide dinucleotide binding motif: A comparison of nucleotide binding proteins. FASEB J. 1996;10:1257–1269. doi: 10.1096/fasebj.10.11.8836039. [DOI] [PubMed] [Google Scholar]
- 47.Dym O, Eisenber D. Sequence-structure analysis of FAD-containing proteins. Protein Sce. 2001;10:1712–1728. doi: 10.1110/ps.12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Magnusson OT, Toyama H, Saeki M, Schwarzenbacher R, Klinman JP. The structure of a biosynthetic intermediate of pyrroloquinoline quinone (PQQ) and elucidation of the final step of PQQ biosynthesis. J. Am. Chem. Soc. 2004;126:5342–5343. doi: 10.1021/ja0493852. [DOI] [PubMed] [Google Scholar]
- 49.Toms AV, Haas AL, Park JH, Begley TP, Ealick SE. Structural characterization of the regulatory proteins TenA and TenI from Bacillus subtilis and identification of TenA as a thiaminase II. Biochemistry. 2005;44:2319–2329. doi: 10.1021/bi0478648. [DOI] [PubMed] [Google Scholar]
- 50.Magnusson OT, RoseFigura JM, Toyama H, Schwarzenbacher R, Klinman JP. Pyrroloquinoline quinone biogenesis: Characterization of PqqC and its H84N and H84A active site variants. Biochemistry. 2007;46:7174–7186. doi: 10.1021/bi700162n. [DOI] [PubMed] [Google Scholar]
- 51.RoseFigura JM, Puehringer S, Scharzenbacher R, Toyama H, Klinman JP. Characterization of a protein-generated O(2) binding pocket in PqqC, a cofactorless oxidase catalyzing the final step in PQQ production. Biochemistry. 2011;50:1556–1566. doi: 10.1021/bi1015474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wecksler SR, Stoll S, Tran H, Magnusson OT, Wu S-P, King D, Britt RD, Klinman JP. Pyrroloquinoline quinone biogenesis: Demonstration that PqqE from Klebsiella pneumoniae is a radical S-adenosyl-L-methionine enzyme. Biochemistry. 2009;48:10151–10161. doi: 10.1021/bi900918b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brindley AA, Zajicek R, Warren MJ, Ferguson SJ, Rigsby SEJ. NirJ, a radical SAM family member of the d(1) heme biogenesis cluster. FEBS Lett. 2010;584:2461–2466. doi: 10.1016/j.febslet.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 54.Vey JL, Drennan CL. Structural insights into radical generation by the radical SAM superfamily. Chem. Rev. 2011;111:2487–2506. doi: 10.1021/cr9002616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nicolet Y, Drennan CL. AdoMet radical proteins - from structure to evolution - alignment of divergent protein sequences reveals strong secondary structure element conservation. Nucleic Acids Res. 2004;13:4015–4025. doi: 10.1093/nar/gkh728. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.