Abstract
Rickettsia conorii, an obligate intracellular bacterium and the causative agent of Mediterranean spotted fever, preferentially infects microvascular endothelial cells of the mammalian hosts leading to onset of innate immune responses, characterized by the activation of intracellular signaling mechanisms, release of pro-inflammatory cytokines and chemokines, and killing of intracellular rickettsiae. Our recent studies have shown that interferon (IFN)-β, a cytokine traditionally considered to be involved in antiviral immunity, plays an important role in the autocrine/paracrine regulation of host defense mechanism and control of R. conorii growth in the host endothelial cells. Here, we show that R. conorii infection induces the expression of ISG15 (an interferon-stimulated gene coding a protein of 17 kD) and UBP43 (an ISG15-specific protease) at the levels of mRNA and protein and report the evidence of ISGylation of as yet unidentified target proteins in cultured human microvascular endothelium. Infection-induced expression of ISG15 and UBP43 requires intracellular replication of rickettsiae and production of IFN-β, because treatment with tetracycline and presence of an antibody capable of neutralizing IFN-β activity resulted in near complete attenuation of both responses. Inhibition of R. conorii-induced ISG15 by RNA interference results in significant increase in the extent of rickettsial replication, whereas UBP43 knockdown yields a reciprocal inhibitory effect. In tandem, these results demonstrate the stimulation of interferon-β-mediated innate immune mechanisms capable of perturbing the growth and replication of pathogenic rickettsiae and provide first evidence for ISG!5-mediated post-translational modification of host cellular proteins during infection with an intracellular bacterium.
Keywords: Endothelial cells, Interferon, ISG15, Rickettsia conorii, UBP43
1. Introduction
Rickettsia conorii is an obligate intracellular gram-negative bacterium, which causes Mediterranean spotted fever (MSF), a serious and occasionally fatal exanthematic disease typically characterized by fever, maculopapular rash, and ‘tache-noire’ at the site of tick bite [1]. The past few years have witnessed increased recognition and spread of pathogenic Rickettsia species, including R. conorii, throughout the world and identification of new and previously unsuspected vectors capable of transmitting the disease to humans [2,3]. Additionally, more recent descriptions of MSF patients have also attributed previously underappreciated complications such as acute myocarditis [4], acute pancreatitis [5], and persistent encephalitis [6] as compounding factors of the progression and outcome of human R. conorii infections.
Because microvascular endothelium lining of blood vessels is the primary target of a majority of pathogenic rickettsiae, ‘vasculitis’ as a result of inflammation, dysfunction, and damage to the vasculature represents a hallmark feature of rickettsial pathogenesis in their mammalian hosts. As expected, endothelial cells infected with spotted fever group rickettsiae launch a combination of responses, which are governed by intracellular signaling pathways leading to the activation of transcriptional control and pathogen clearance mechanisms in the host [7,8]. In this regard, our recent findings demonstrate that R. conorii infection of human microvascular endothelial cells in vitro induces the expression and secretion of interferon-β (IFN-β: a type I IFN), leading to the autocrine/paracrine stimulation of Janus Kinase - Signal Transducer and Activator of Transcription (JAK-STAT) signaling [9].
Known to be induced by type I IFNs, the human ISG15 gene encodes for the Interferon-stimulated protein of 17 kDa, a ubiquitin-like modifier protein, which can also function as a cytokine [10]. Interestingly, ISG15 within the cells can be detected as both free, unconjugated protein as well as in covalent complexes with other cellular proteins via a process termed ‘ISGylation’. Since ISG15 expression and activity is under tight control of specific signaling mechanisms governing innate immunity and ISGylation is known to modulate JAK-STAT signal transduction pathway, we hypothesized an important role for ISG15 in host cell responses to pathogenic rickettsiae. In the present study, we report on the expression of ISG15 and UBP43 (the only known ISG15-specific deconjugating protease that removes ISG15 from its conjugates by deISGylation) in microvascular endothelial cells infected with R. conorii. Our data clearly suggest increased expression of ISG15 and UBP43 and implicate a role for both of these proteins in host defense via pronounced effects on intracellular replication of R. conorii.
2. Materials and Methods
2.1. Cell culture, R. conorii infection, and tetracycline treatment
Human dermal microvascular endothelial cells (HMECs) were grown in MCDB 131 medium (Invitrogen) containing 10% FBS (Aleken Biologicals), 10 ng/ml Epidermal growth factor (Becton-Dickinson), 1 μg/ml Hydrocortisone (Sigma), and 10 mM L-glutamine (Invitrogen). Rickettsia conorii (Malish 7 strain) was propagated in Vero cells, purified by density gradient centrifugation, and titered by plaque formation assay as described previously [11]. HMECs were infected with R. conorii for 3 h, at which time initial inoculum of medium supplemented with the bacteria was removed and replaced with culture medium only. Endothelial cells infected with approximately 6×103 pfu/cm2 of R. conorii were subjected to the isolation of total RNA and preparation of protein lysates following established laboratory protocols [9, 12]. For the analysis of intracellular R. conorii replication by quantitative RT-PCR, HMECS were infected with approximately 6×102 pfu for every cm2 of culture surface area. To inhibit intracellular growth of R. conorii, tetracycline (Sigma) at a final concentration of 20 μg/ml was introduced into the culture medium at 3 h post-infection [9,11].
2.2. IFN-β treatment of HMECs and neutralization of IFN-β activity
To investigate ISG15 and UBP43 expression, HMECs were treated with 10 ng/ml of recombinant human IFN-β (PBL Interferon Source). For neutralization of IFN-β, an antibody against human IFN-β (R&D Systems) was added to the culture medium after R. conorii infection at a final concentration of 10 μg/ml.
2.3. Quantitation of R. conorii by real-time PCR
Either uninfected or R. conorii-infected HMECs from the wells of 6-well culture plates were scraped into the medium and collected by centrifugation at 10,000 g for 30 min. Total DNA (host and rickettsial) was extracted using DNeasy Blood and Tissue Kit (Qiagen) according to the manufacturer's instructions and quantified by a Nanodrop spectrophotometer (ND-1000, Thermo Scientific). The PCR for rickettsial outer membrane protein A (ompA) was performed using primer pair RR190.547F and RR190.701R with established specificity for spotted fever group rickettsiae [9,13]. The copy number of R. conorii DNA in each well was normalized to the total DNA.
2.4. siRNA for ISG15 and UBP43
ON-TARGETplus smart pools of siRNA for ISG15 and UBP43 along with control non-interfering siRNA were purchased from Thermo Scientific. HMECs at or near 80% confluence were transfected with 100 nM siRNA using Lipofectamine 2000™ (Invitrogen) following the manufacturer's recommendations. After 6 h, cells were placed in fresh culture medium and allowed to recover at 37°C for 12 h prior to R. conorii infection.
2.5 Western blotting
Cells were washed with PBS and suspended in a protein lysis buffer containing 2% w/v sodium dodecyl sulfate (SDS). The samples were separated on a 10% polyacrylamide-SDS gel under denaturing conditions, followed by wet gel transfer to a nitrocellulose membrane in a blotting apparatus at 100V for 90 min. Primary antibodies against ISG15 and UBP43 were purchased from Cell Signaling Technology. An antibody against human α-tubulin from Accurate Chemical & Scientific Corporation was used to control for any variations in loading of samples in different lanes. For detection, IgG-HRP secondary antibodies compatible with the primary antibodies and Western Lightning enhanced chemiluminescence reagent (PerkinElmer) were used.
2.6.Gene expression analysis by quantitative Real-time PCR
Total RNA isolated using TRI™ Reagent (Molecular Research Center, Inc) was purified further using a qPCR-grade RNA purification kit (Qiagen). Conversion of RNA to corresponding cDNA was carried out using a RT2 First Strand Kit (Qiagen). Primers for human ISG15, UBP43 and GAPDH, and SYBR Green Master mix were obtained from Qiagen. Quantitative PCR reactions were performed according to the manufacturer's instructions in a MyiQ cycler (BioRad). Gene expression was normalized to GAPDH and relative expression was calculated by ΔΔCt method.
2.7. Densitometric and Statistical analysis
Blots were scanned in the grayscale mode at a resolution setting of 600 dpi and band intensities were calculated with an image analysis program namely ImageJ, version 1.42. These values were then normalized to the housekeeping protein α-tubulin and the protein expression levels were determined relative to the controls. Statistical significance between the test and control groups was evaluated by Student t-test's and differences were considered to be statistically significant at a p value of equal to or less than 0.05.
3. Results
3.1. R. conorii infection of HMECs induces the expression of mRNAs encoding ISG15 and UBP43
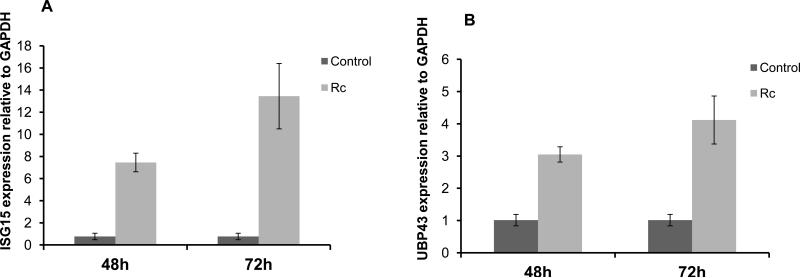
Microvascular endothelium lining of small and medium-sized blood vessels represents the primary target of infection and an important player in host defense against pathogenic rickettsiae. Therefore, we initially determined the expression of ISG15 and ISG15-specific isopeptidase UBP43 during R. conorii infection of HMECs. Compared to the relatively low basal expression in uninfected cells, the mRNA expression of both ISG15 (Figure 1A) and UBP43 (Figure 1B) was significantly higher in R. conorii-infected HMECs at 48 and 72 h post-infection. The intensity of changes in ISG15 mRNA expression was, however, more pronounced than that of UBP43.
Figure 1.
Activation of ISG15 and UBP43 mRNA expression during R. conorii infection of microvascular endothelium in vitro. Total RNA collected from HMECs infected with R. conorii (Rc) for 48 and 72 h was subjected to quantitative real-time PCR to determine the steady-state levels of transcripts for ISG15 and UBP43 using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a housekeeping gene. Changes in the contents of ISG15 (A) and UBP43 (B) are shown as the mean ± standard error of the mean from a minimum of three independent experiments. For the ease of comparison, the basal mRNA levels of each gene in uninfected cells (Control) were given a value of 1.
3.2. R. conorii infection and IFN-β treatment of HMECs stimulates the expression of free ISG15 and both isoforms of UBP43: evidence for ISGylation of target proteins
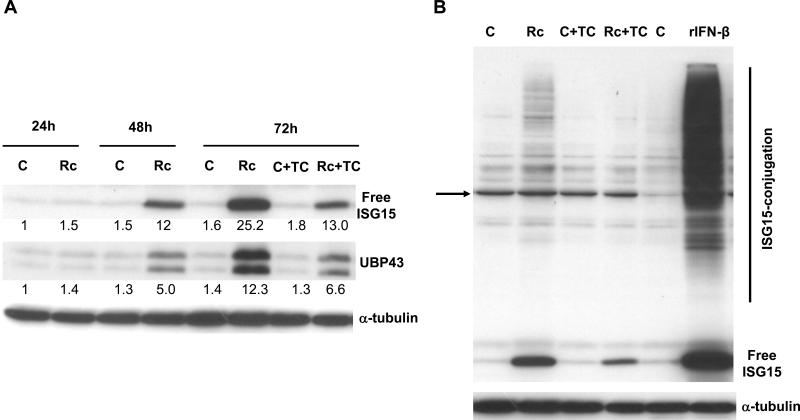
Our follow up experiments to evaluate the steady-state levels of protein products by Western blot analysis further revealed that infection-induced changes in the transcript levels were paralleled by robust increases in the cellular levels of both proteins under investigation (Figure 2A). Clearly, there was significantly higher intracellular accumulation of free, unconjugated ISG15 and both intact as well as truncated version (splice variant) of UBP43 in HMECs infected with R. conorii for 48 and 72 h. Further, treatment of HMECs with recombinant human IFN-β (rh IFN-β) resulted in robust induction in the expression of ISG15 leading to significantly higher steady-state levels of free intracellular ISG15 as well as increased conjugation of ISG15 to other cellular proteins (Figure 2B). Interestingly, the results also yield evidence for ISGylation of target cellular proteins during R. conorii infection of HMECs, the intensity of which was considerably lower in comparison to that triggered by rh IFN-β. Inhibition of intracellular replication of rickettsiae by treatment with tetracycline resulted in partial attenuation of ISG15 and UBP43 expression as well as ISGylation at 72 h after infection (Figure 2A and 2B), indicating that metabolically active, viable rickettsiae are required to induce ISG15 and UBP43 expression.
Figure 2.
R. conorii infection of HMECs up-regulates ISG15 and UBP43 protein expression and ISGylation. A. Western blot anaIysis of ISG15 and UBP43 in HMECs infected with R. conorii for different durations of time (24, 48, and 72 h). The antibody for UBP43 was capable of detecting both full length (39kDa) and amino-terminal truncated (34kDa) isoforms of UBP43. Also shown in the effect of tetracycline (TC) treatment on the levels of ISG15 and UBP43 expression during R. conorii infection. The results of a typical experiment (n=3) are presented. The relative intensity of each band in comparison to the level of baseline expression in uninfected controls is also shown. B. Evidence for protein ISGylation during R. conorii infection and IFN-β treatment of HMECs. Protein extracts were prepared from HMECs infected with R. conorii, treated with recombinant human IFN-β, or those left uninfected and untreated for controls. Equal amounts of protein were separated on an SDS-PAGE gel and electro-transferred to a nitrocellulose membrane. The membrane was then probed with an anti-ISG15 antibody. The arrow indicates a non-specific, cross-reactive protein band present in all experimental conditions, the intensity of which is variable even among simultaneously processed controls. The levels of free ISG15 in response IFN-β treatment and infection in the presence and absence of tetracycline and the effect of inhibition of rickettsial replication on ISGylation are also presented.
3.3. Autocrine and/or paracrine effects of IFN-β contribute to R. conorii-induced ISG15 and UBP43 expression in vascular endothelial cells
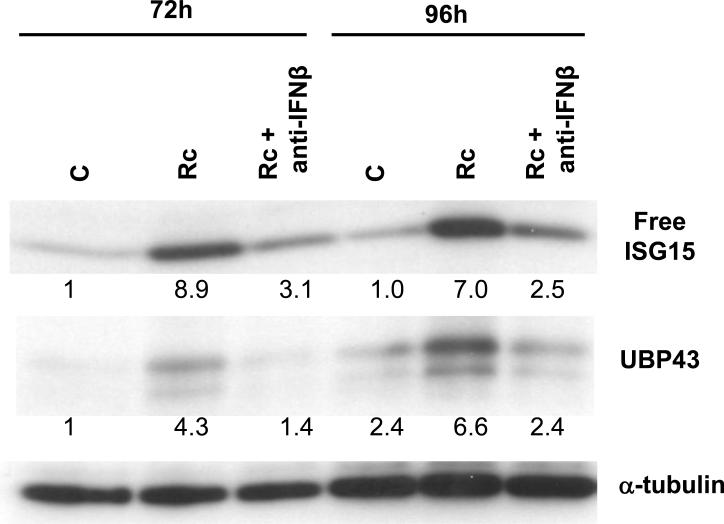
Recent evidence from our laboratory has suggested a prominent role for IFN-β in STAT1 activation response of R. conorii-infected HMECs [9]. Because ISG15 is an interferon-responsive gene, we predicted a potential role for IFN-β in stimulating the ISG15/UBP43 expression. Infection in the presence of an antibody capable of neutralizing the activity of IFN-β resulted in nearly complete abrogation of accumulation of both free intracellular ISG15 as well as UBP43 in infected HMECs (Figure 3).
Figure 3.
IFN-β secreted by R. conorii-infected endothelial cells induces ISG15 and UBP43 expression. An antibody capable of neutralizing the activity of IFNβ was added to the culture medium during the infection. Total protein lysates were prepared at different times post-infection and processed for the detection of ISG15 and UBP43 by western blotting. The relative intensity of each band in comparison to the level of baseline expression in uninfected controls at 72 h, which was assigned a value of 1, was determined by densitometry and normalization to the housekeeping protein α-tubulin.
3.4. siRNA-mediated knock-down of ISG15 and UBP43 has opposite effects on intracellular R. conorii replication
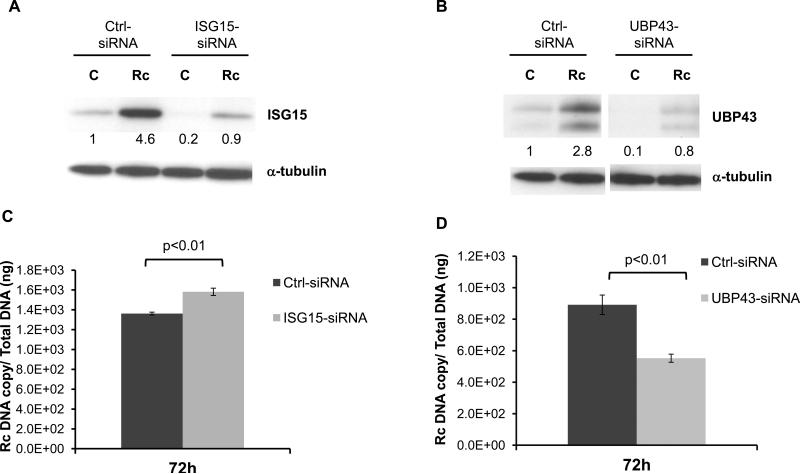
Finally, we utilized gene-specific siRNAs to investigate the potential involvement of ISG15 and UBP43 in host defense mechanisms during rickettsial infection. Introduction of siRNAs against ISG15 and UBP43 by transfection into HMECs prior to R. conorii infection effectively inhibited the expression of corresponding proteins whereas a scrambled non-specific siRNA sequence had no effect on infection-induced expression of ISG15 and UBP43 (Figure 4A and 4B). Replication of R. conorii in HMECs transfected with siRNA for ISG15 was significantly enhanced in comparison to the level of replication in cells receiving scrambled non-interfering siRNA (Figure 4C). In contrast, rickettsial replication in host cells with lower levels of UBP43 due to siRNA-mediated interference was significantly lower than corresponding controls (Figure 4D).
Figure 4.
Effect of ISG15 and UBP43 knockdown on R. conorii replication in HMECs. HMECs were transfected with ISG15 and UBP43-specific siRNA or non-target control siRNA prior to infection with R. conorii. To ensure the efficacy of knockdown, the levels of ISG15 (A) and UBP43 (B) were first investigated by Western blot analysis. Next, the levels of intracellular replication of R. conorii in HMECs transfected with siRNAs against ISG15 and UBP43 were determined by rickettsial outer membrane protein A (rOMPA)-based quantitative PCR. Rickettsial replication was significantly increased in ISG15-knockdown cells (C), but was significantly lower than the corresponding controls in UBP43-siRNA transfected cells (D).
4. Discussion
Although secretion of type I interferons (IFN-α and IFN-β) from virus-infected cells is considered to be a hallmark of antiviral immunity and potential physiological functions of ISG15 in antiviral defense mechanisms have been the subjects of intense investigation [10,14,15], their roles in the realm of bacterial interactions with the host cells and ensuing pathogenesis or host defense pathways still remain poorly appreciated. The possible protective effects of type I IFNs in rickettsial infections were initially realized in L929 cells (a murine aneuploid fibrosarcoma cell line) infected with an avirulent strain of R. prowazekii, a prototypical typhus group species [16]. In these experiments, the anti-rickettsial and anti-viral activities against R. prowazekii and vesicular stomatitis virus were present in the culture supernatants from R. prowazekii-infected fibroblast-like L929 cells and could only be neutralized by antibodies against murine IFNα/β, but not IFNγ. More recently, we have demonstrated that a similar IFN-β-mediated response during infection of human microvascular endothelial cells with R. conorii adversely affects the intracellular replication of the pathogen through the activation of STAT1 [9].
The results of this study yield first evidence for the transcriptional regulation and expression of ISG15 (a type-I interferon-responsive gene) and UBP43 (an ISG15-specific protease), and concomitantly demonstrate increased levels of intracellular ISG15 conjugates in microvascular endothelial cells during R. conorii infection. In addition, our data not only suggest that IFN-β is a potent inducer of the expression of free ISG15 and its conjugation to as yet unidentified target proteins in human microvascular endothelium, but also attribute infection-induced changes in the host cell gene expression predominantly to the autocrine and/or paracrine effects of IFN-β. Finally, our findings implicate both ISG15 and UBP43 in the regulation of intracellular rickettsial replication in microvascular endothelial cells, the primary targets of infection in humans and animal models [7-9]. ISG15 and UBP43, thus, represent important components of IFN-β-stimulated transcriptional responses in host endothelial cells.
The robust induction of ISG15 mRNA and protein in response to R. conorii infection and significant increase in the extent of intracellular rickettsial replication in host cells unable to express ISG15 due to siRNA interference implies a potentially important role for ISG15 in anti-bacterial defense mechanisms. In the context of antiviral defense, ISG15 over-expression in cell culture model systems suppresses the budding of Ebola VP40-like virus particles and interferes with the assembly and release of HIV [17,18]. Strong evidence further supports a role for ISG15 in protecting the mammalian hosts from several viruses including Sindbis, influenza A and B, and herpes simplex virus (HSV-1) [19]. Intriguingly, ISG15 not only binds to the cellular proteins within the host cells, but can also target and ISGylate specific viral proteins, for example NS1 protein of influenza A virus, to interfere with their replication [20]. To counteract these strategies aimed at limiting the spread of infection, a number of viruses encode specific proteins to antagonize the ISGylation machinery of the host [21]. Further studies are, therefore, warranted to identify the host cell and/or bacterial proteins that may undergo ISGylation during intracellular growth and replication of pathogenic rickettsiae.
UBP43 belongs to the family of ubiquitin isopeptidases and specifically cleaves ISG15-target protein complexes. Consequently, UBP43 plays a vital role in maintaining the intricate balance of ISG15-conjugated proteins in the cells. UBP43 has been reported to be induced by viral infection and plays an important role in innate immunity against viruses [22,23]. Bacterial lipopolysaccharide (LPS) strongly activates UBP43 expression in macrophages in an interferon regulatory factor-3 (IRF-3)-dependent manner [24]. Also, macrophages lacking UBP43 display increased expression of IFN-stimulated cytokines and chemokine genes in response to LPS and UBP43 knock-out mice are able to control the growth of Salmonella typhimurium more effectively than the wild-types [25]. Although rickettsial LPS has significantly lower endotoxic activity than that from E. coli [26, Sahni et al. unpublished observations], the possibility of its contribution to R. conorii-induced UBP43 expression in endothelium is currently under investigation. Nevertheless, a decrease in the rate of rickettsial replication as a result of UBP43 knock-down is in agreement with controlled replication of S. typhimurium in UBP43-deficient host and lends support to a beneficial effect against pathogenic microbes. UBP43 can bind to interferon alpha receptor 2 (IFNAR2) subunit to inhibit the interactions between JAK and IFNAR2 proteins, thereby negatively regulating IFN-β signaling in mouse fibroblasts [27]. Alternatively, UBP43 silencing may also increase ISG15 conjugation to its cellular binding partners, thereby enhancing host innate immune responses against rickettsial replication. Therefore, an intriguing possibility that remains to be investigated further is that UBP43 may perform a dual role in R. conorii-infected endothelium by regulating both IFN-β-mediated STAT1 activation and cellular protein conjugation to ISG15.
In conclusion, the induction of ISG15 and UBP43 expression and increased ISG15 conjugation upon infection of the target host cells with R. conorii suggests that protein modifications resulting from ISGylation may play a role in innate defense mechanisms against this group of intracellular pathogenic bacteria. Further, reduced rate of replication in UBP43-deficient host cells, which are likely to have elevated levels of ISGylated proteins, indicates that the balance of ISG15 association and dissociation with its targets serves as an important determinant of the overall intensity of antibacterial responses. Although molecular mechanisms underlying newly identified antibacterial functions of ISG15 have yet to be determined, development of novel strategies aimed at modulating the ISGylation system to enhance the cellular immune response may lead to accelerated elimination of bacterial infections.
Highlights.
 Increased ISG15/UBP 43 expression in Rickettsia conorii-infected host endothelium.
Increased ISG15/UBP 43 expression in Rickettsia conorii-infected host endothelium. Involvement of interferon-β-based regulatory mechanism in ISG15/UBP43 expression.
Involvement of interferon-β-based regulatory mechanism in ISG15/UBP43 expression. First evidence of ISGylation of target host proteins in response to a bacterium.
First evidence of ISGylation of target host proteins in response to a bacterium. Antibacterial activity of ISG15 against intracellular rickettsiae.
Antibacterial activity of ISG15 against intracellular rickettsiae. Implication of antiviral host defense strategies against a pathogenic bacterium.
Implication of antiviral host defense strategies against a pathogenic bacterium.
Acknowledgments
We thank Dr. Marina Eremeeva for advice on the PCR-based quantification of rickettsiae and for providing the plasmid used in these experiments. We thankfully acknowledge the financial support provided by USPHS research grants AI040689, AI067613, and AI076697 from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health, Bethesda, MD, through the course of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leroy Q, Raoult D D. Mediterranean spotted fever. Infect. Dis. Clin. North Am. 2008;22:515–530. doi: 10.1016/j.idc.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Sousa R, França A, Dória Nòbrega S, Belo A, Amaro M, Abreu T, Poças J, Proença P, Vaz J, Torgal J, Bacellar F, Ismail N, Walker DH. Host- and microbe-related risk factors for and pathophysiology of fatal Rickettsia conorii infection in Portuguese patients. J. Infect. Dis. 2008;198:576–585. doi: 10.1086/590211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicholson WL, Allen KE, McQuiston JH, Breitschwerdt EB, Little SE SE. The increasing recognition of rickettsial pathogens in dogs and people. Trends Parasitol. 2010;26:205–212. doi: 10.1016/j.pt.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Shah SS, McGowan JP. Rickettsial, ehrlichial and Bartonella infections of the myocardium and pericardium. Front. Biosci. 2003;8:e197–201. doi: 10.2741/995. [DOI] [PubMed] [Google Scholar]
- 5.Rombola F. Mediterranean spotted fever presenting as an acute pancreatitis. Acta Gastroenterol. Belg. 2011;74:91–92. [PubMed] [Google Scholar]
- 6.Aliaga L, Sánchez-Blázquez P, Rodríguez-Granger J, Sampedro A, Orozco M, Pastor J. Mediterranean spotted fever with encephalitis. J. Med. Microbiol. 2009;58:521–525. doi: 10.1099/jmm.0.004465-0. [DOI] [PubMed] [Google Scholar]
- 7.Sahni SK. Endothelial cell infection and hemostasis. Thromb. Res. 2007;119:531–549. doi: 10.1016/j.thromres.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Valbuena G, Walker DH. Infection of the endothelium by members of the order Rickettsiales. Thromb. Haemost. 2009;102:1071–1079. doi: 10.1160/TH09-03-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colonne PM, Eremeeva ME, Sahni SK. Beta interferon-mediated activation of Signal Transducer and Activator of Transcription protein 1 interferes with Rickettsia conorii replication in human endothelial cells. Infect. Immun. 2011;79:3733–3743. doi: 10.1128/IAI.05008-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Cunha J, Knight E, Jr., Haas AL, Truitt RL, Borden EC. Immunoregulatory properties of ISG15, an interferon-induced cytokine. Proc. Natl. Acad. Sci. USA. 1996;93:211–215. doi: 10.1073/pnas.93.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rydkina E, Sahni SK, Santucci LA, Turpin LC, Baggs RB, Silverman DJ. Selective modulation of antioxidant enzyme activities in host tissues during Rickettsia conorii infection. Microb. Pathog. 2004;36:293–301. doi: 10.1016/j.micpath.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Sahni SK, Kiriakidi S, Colonne PM, Sahni A, Silverman DJ. Selective activation of signal transducer and activator of transcription (STAT) proteins STAT1 and STAT3 in human endothelial cells infected with Rickettsia rickettsii. Clin. Microbiol. Infect. 2009;15:303–304. doi: 10.1111/j.1469-0691.2008.02248.x. [DOI] [PubMed] [Google Scholar]
- 13.Eremeeva ME, Dasch GA, Silverman DJ. Evaluation of a PCR assay for quantitation of Rickettsia rickettsii and closely related spotted fever group rickettsiae. J. Clin. Microbiol. 2003;41:5466–5472. doi: 10.1128/JCM.41.12.5466-5472.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuniga EI, Hahm B, Oldstone MB. Type I interferon during viral infections: multiple triggers for a multifunctional mediator. Curr. Top. Microbiol. Immunol. 316:337–357. doi: 10.1007/978-3-540-71329-6_16. 92007. [DOI] [PubMed] [Google Scholar]
- 15.Kim KI, Zhang DE. ISG15, not just another ubiquitin-like protein. Biochem. Biophys. Res. Commun. 2003;307:431–434. doi: 10.1016/s0006-291x(03)01216-6. [DOI] [PubMed] [Google Scholar]
- 16.Turco J, Winkler HH. Interferon-alpha/beta and Rickettsia prowazekii: induction and sensitivity. Ann. NY Acad. Sci. 1990;590:168–186. doi: 10.1111/j.1749-6632.1990.tb42219.x. [DOI] [PubMed] [Google Scholar]
- 17.Okumura A, Lu G, Pitha-Rowe I, Pitha PM. Innate antiviral response targets HIV-1 release by the induction of ubiquitin-like protein ISG15. Proc. Natl. Acad. Sci. USA. 2006;103:1440–1445. doi: 10.1073/pnas.0510518103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okumura A, Pitha PM, Harty RN RN. ISG15 inhibits Ebola VP40 VLP budding in an L-domain-dependent manner by blocking Nedd4 ligase activity. Proc. Natl. Acad. Sci USA. 2008;105:3974–3979. doi: 10.1073/pnas.0710629105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skaug B, Chen ZJ. Emerging role of ISG15 in antiviral immunity. Cell. 2010;143:187–190. doi: 10.1016/j.cell.2010.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao C, Hsiang TY, Kuo RL, Krug RM. ISG15 conjugation system targets the viral NS1 protein in influenza A virus-infected cells. Proc. Natl. Acad. Sci. USA. 2010;107:2253–2258. doi: 10.1073/pnas.0909144107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeon YJ, Yoo HM, Chung CH. ISG15 and immune diseases. Biochim. Biophys. Acta. 2010;1802:485–496. doi: 10.1016/j.bbadis.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ritchie KJ, Hahn CS, Kim KI, Yan M, Rosario D, Li L, de la Torre JC, Zhang DE. Role of ISG15 protease UBP43 (USP18) in innate immunity to viral infection. Nat. Med. 2004;10:1374–1378. doi: 10.1038/nm1133. [DOI] [PubMed] [Google Scholar]
- 23.Kim JH, Luo JK, Zhang DE. The level of hepatitis B virus replication is not affected by protein ISG15 modification but is reduced by inhibition of UBP43 (USP18) expression. J. Immunol. 2008;181:6467–6472. doi: 10.4049/jimmunol.181.9.6467. [DOI] [PubMed] [Google Scholar]
- 24.Malakhova O, Malakhov M, Hetherington C, Zhang DE. Lipopolysaccharide activates the expression of ISG15-specific protease UBP43 via interferon regulatory factor 3. J. Biol. Chem. 2002;277:14703–14711. doi: 10.1074/jbc.M111527200. [DOI] [PubMed] [Google Scholar]
- 25.Kim KI, Malakhova OA, Hoebe K, Yan M, Beutler B, Zhang DE. Enhanced antibacterial potential in UBP43-deficient mice against Salmonella typhimurium infection by up-regulating type I IFN signaling. J. Immunol. 2005;175:847–854. doi: 10.4049/jimmunol.175.2.847. [DOI] [PubMed] [Google Scholar]
- 26.Hackstadt T. The biology of rickettsiae. Infect. Agents Dis. 1996;5:127–143. [PubMed] [Google Scholar]
- 27.Malakhova O, Kim K, Luo J, Zou W, Kumar KS, Fuchs SY, Shuai K, Zhang DE. UBP43 is a novel regulator of interferon signaling independent of its ISG15 isopeptidase activity. EMBO J. 2006;25:2358–2367. doi: 10.1038/sj.emboj.7601149. [DOI] [PMC free article] [PubMed] [Google Scholar]