Abstract
Background
Congress has authorized the U.S. Food and Drug Administration (FDA) to provide industry sponsors with a 6-month extension of drug marketing rights under the Pediatric Exclusivity Provision if FDA-requested pediatric drug trials are conducted. The cost and economic return of pediatric exclusivity to industry sponsors has been shown to be highly variable. We sought to determine the cost of performing pediatric exclusivity trials within a single therapeutic area and the subsequent economic return to industry sponsors.
Methods
We evaluated 9 orally administered anti-hypertensive drugs submitted to the FDA under the Pediatric Exclusivity Provision from 1997–2004 and obtained key elements of the clinical trial designs and operations. Estimates of the costs of performing the studies were generated and converted into after-tax cash outflow. Market sales were obtained and converted into after-tax inflows based on 6 months of additional patent protection. Net economic return and net return-to-cost ratios were determined for each drug.
Results
Of the 9 anti-hypertensive agents studied, an average of 2 studies per drug was performed, including at least 1 pharmacokinetic study and a safety and efficacy study. The median cost of completing a pharmacokinetic trial was $862,000 (range: $556,000–1.8 million). The median cost of performing safety and efficacy trials for these agents was $4.3 million (range: $2.1 million–12.9 million). The ratio of net economic return to cost was 17 (range: 4–64.7).
Conclusion
We found that, within a cohort of anti-hypertensive drugs, the Pediatric Exclusivity Provision has generated highly variable, yet lucrative returns to industry sponsors.
Keywords: clinical trials, hypertension, pediatrics, drugs, cost-benefit analysis
In 1997, Congress passed the United States Food and Drug Administration (FDA) Modernization Act. Section 505a of this act, known as the Pediatric Exclusivity Provision, grants 6 months of marketing exclusivity to pharmaceutical companies in exchange for testing the safety, efficacy, and pharmacokinetics of drugs currently used in the pediatric population.1 Since its inception, sponsors have complied with 173 of the 297 written requests for pediatric studies of on-patent drugs currently prescribed to children.2 This has resulted in over 130 pediatric labeling changes to date.3 Between 1990 and 1997, only 11 agents had undergone sufficient testing of dosing, safety, and efficacy to qualify for and subsequently undergo pediatric labeling changes.4
Despite the success of this provision (and its renewal under the 2002 Best Pharmaceuticals for Children Act and most recently in October 2007), critics contend that the program yields highly lucrative returns to the innovator drug industry. The benefits of 6 months of marketing protection to the entire moiety are thought to greatly exceed the costs of performing the pediatric studies. We recently evaluated the economic return of performing pediatric trials across a broad range of therapeutic indications,5, 6 demonstrating that the cost of performing pediatric clinical trials across a broad range of clinical areas was highly variable. This prior study, however, examined the economic return associated with a single drug per clinical indication. The generalizability of these findings within a single clinical indication was not determined. In the present study, we examine the economic return and the net return-to-investment ratio of clinical trials for multiple agents within a single clinical indication. Furthermore, we provide a breakdown of the estimated costs of performing trials in children based upon our cost modeling approach and identify potential predictors of cost in pediatric hypertension studies.
Methods
Anti-hypertensive drug cohort
Between 1997 and 2004, the FDA received completed clinical study reports for 12 antihypertensive agents. The clinical study reports were submitted to the FDA under the Pediatric Exclusivity Provision. Two of the authors (JL and DB) evaluated the clinical study reports for the 12 anti-hypertensive agents, including 10 orally administered and 2 intravenously administered agents. Due to significant variations in trial design (i.e., assessments in the intensive care unit versus clinic, severity of illness, length of study), intravenously administered agents were excluded from our analysis. The clinical study reports for 1 of the orally administered anti-hypertensive agents were incomplete at the time of our evaluation, and therefore this agent could not be used in our analysis. Of the remaining 9 anti-hypertensive agents, clinical reports were separated by drug and type of clinical study (safety and efficacy versus pharmacokinetic). Each anti-hypertensive drug was studied in children with a safety and efficacy trial, as well as at least 1 pharmacokinetic study. Pharmacokinetic studies were either single-dose or multi-dose. Bioequivalence or bioavailability studies were often required if a new pediatric formulation was provided in the trials; these studies were performed in adults. The bioavailability studies compared the pharmacokinetics of suspension versus tablet formulation, while the bioequivalence studies tested the pharmacokinetics of coated tablet versus small tablet formulation.
Cash outflows
For an individual anti-hypertensive agent, the net economic return as well as the net return-to-investment ratio was determined by first calculating estimates of cash outflow as previously described.5 Cash outflows are approximations of the cost of performing a clinical trial. These were generated by using commercially available trial cost estimator software (Fast Track Systems, Inc., Conshohocken, PA). Total costs were generated as the sum of contract research organization (CRO) costs, net pharmaceutical costs, per patient site costs, and central laboratory costs. Central laboratory costs were estimated using an internal pricing tool supplied by Covance Central Laboratory Services (Indianapolis, IN). The Covance Central Laboratory cost estimation resource provides cost in an 8-service template, including costs for database construction, transportation, laboratory services, and specimen management, but excludes costs for drug manufacturing and drug packaging. Laboratory testing that was not performed centrally—as specified by the clinical study reports—was estimated as local laboratory testing costs and was included in the average cost per patient visit. Total values were generated and included low, medium, and high cost estimates for each trial. Reimbursements for participants were calculated using the trial cost estimator software and included in total cost estimates. All costs for participation in PK studies were assumed to be independent of those associated with efficacy studies.
High cost estimates were used to approximate cash outflow and were adjusted for inflation (adjusted total cost) and totaled across trials for a single anti-hypertensive agent. Total adjusted costs per anti-hypertensive agent were further adjusted for a tax rate of 30% to obtain an after-tax cash outflow value. This value was used to determine the net economic return and the net economic return-to-investment ratio per anti-hypertensive agent.
Cash outflow estimates were confirmed by a 2-person review. One author acquired values for cost determinants from the study reports, and another reviewed the data and ensured that the data were obtained correctly. All cash outflow estimates were evaluated for accuracy.
Cash inflows
Yearly sales data, derived from sales audit data (IMS Health, IMS National Sales Perspective™, 2002–2004, extracted February 2005, Retail & Non-Retail, 2005–2006), were obtained from IMS Health, Inc. (Fairfield, CT).7 All sales figures for the last 3 years prior to patent expiration, the last 3 years for which data were available, or for years 2002 to 2004 were obtained. To avoid bias, sales figures were adjusted to a reference year of 2005 using pharmaceutical consumer price indices. The average of these values was obtained. These values were discounted by 10% to reflect discounts to managed care. Sales estimates were additionally discounted 8% per year to reflect the expected returns per year for an individual agent. We also assumed a contribution margin of 50% (sales revenue minus variable costs; i.e., for every $1 worth of product, the company pays an additional 50 cents in variable costs) and a tax rate of 30% (industry range: 25–35%).8 Resultant adjusted values were approximations of the after-tax cash inflow per each anti-hypertensive agent studied.
Net return calculations and procedures for minimizing error
Estimates of net economic return per drug were calculated based upon cash outflows and cash inflows adjusted to their 2005 values. Net return-to-investment ratios were calculated to reflect 6 months of marketing exclusivity by averaging cash inflows over 3 years to obtain an estimate of average annual cash inflow and dividing this value by 2 to obtain a 6-month estimate. The estimated cash outflow per drug was then subtracted from the 6-month cash inflow estimate to calculate net return. Net return-to-investment per anti-hypertensive agent was quantified by dividing net return by estimated cash outflow.
Statistical analysis
All cash inflow and outflow data were imported into SAS version 9.1.3 (Cary, NC). Summary statistics were provided for demographic and cost-related data, and a nonparametric approach was employed when appropriate for small samples (Wilcoxon rank-sum test).
Results
Study characteristics
Among the 9 orally administered anti-hypertensive agents, there were 24 clinical study reports, including 9 safety and efficacy studies, 9 single-dose or multi-dose pharmacokinetic studies, and 6 bioequivalence or bioavailability studies. Twenty-five percent of the studies were bioequivalence or bioavailability studies and were performed in adults, while the remaining studies (75%) were performed in children. Of the 9 anti-hypertensive agents studied, 7 underwent successful labeling changes as a result of the studies.3, 9 Two drugs did not undergo labeling changes and were found to have insufficient data to support safe and/or effective use in children. Further studies were recommended for these 2 agents (Table 1). These results may have been due to ineffective dosing.
Table I.
Anti-hypertensive studies and labeling changes following response to written request
| Drug | Year Submitted |
Class | Summary of Label Change | Trial | Study Type | Lower Age | Upper Age |
|---|---|---|---|---|---|---|---|
| CCB1 | 2001 | Calcium channel blocker |
Dose, PK information for ages 6–17 yrs ADVE profile in pediatric patients similar to that seen in adults |
A B |
Efficacy PK |
6 yrs 6 mos |
17 yrs 17 yrs |
| ACE1 | 2003 | ACE inhibitor |
Dose, PK information for ages 6–16 yrs Clearance higher and t-1/2 life less Not indicated for children < 6 yrs or GFR < 30 ml/min/1.73m^2 Suspension information provided |
A B B C |
PK, Eff PK PK PK (ts) |
6 yrs 1 mo 1 mo 18 yrs |
16 yrs 16 yrs 16 yrs 45 yrs |
| ACE2 | 2000 1999 |
ACE inhibitor |
Labeling changes for age 1 mo to 16 yrs; Information on dose, efficacy and suspension preparation provided |
A B C |
Efficacy PK PK (ts) |
6 yrs 1 mo 18 yrs |
16 yrs 16 yrs 45 yrs |
| CCB2 | 2001 | Calcium channel blocker |
<Additional studies recommended> | A | Efficacy | 6 yrs | 12 yrs |
| ACE3 | 2002 | ACE inhibitor |
New dosing/pharmacokinetic data; New dosing for children > 50 kg |
A B |
Efficacy PK |
6 yrs 1 mo |
16 yrs 16 yrs |
| ARB1 | 1999 2004 |
Angiotensin II receptor blocker |
Up to 4.5 mg/kg once daily not effective in lowering blood pressure in patients 6–16 yrs |
A B C C |
Efficacy PK PK (tt) PK (tt) |
6 yrs 1 yrs 18 yrs 18 yrs |
17 yrs 16 yrs 55 yrs 55 yrs |
| ACE4 | 2000 | ACE inhibitor |
Labeling change for children 6–16 yrs; Not recommended for pediatric patients < 6 yrs of GFR <30 ml/min/1.73m^2 Suspension information provided |
A B C |
Efficacy PK PK (ts) |
6 yrs 1 mo 18 yrs |
16 yrs 16 yrs 45 yrs |
| ARB2 | 2001 | Angiotensin II receptor blocker |
Labeling change for children 6–16 yrs; Not recommended for pediatric patients < 6 yrs of GFR <30 ml/min/1.73m^2 Suspension information provided |
A B C |
Efficacy PK PK (ts) |
6 yrs 1 mo 18 yrs |
16 yrs 16 yrs 45 yrs |
| ACE5 | 2001 2002 |
ACE inhibitor |
<Additional studies recommended> | A B |
Efficacy PK |
5 yrs 1 mo |
16 yrs 6 yrs |
A: Safety, efficacy or tolerability studies, B: Pharmacokinetic studies, single or multiple doses, C: Bioequivalence or bioavailability studies.
PK, pharmacokinetic; ts, tablet versus suspension formulation; tt, coated versus small tablet formulation.
Study reports summary
The 24 trials enrolled 2506 children. Twenty-one hundred children (84%) completed the studies. Efficacy studies enrolled approximately 4 times as many children as pharmacokinetic studies (efficacy = 1962, pharmacokinetic = 544). Approximately 20% (399/1962) of children enrolled did not complete the efficacy studies, but nearly all enrollees of the pharmacokinetic trials completed the studies (537/544; 99%). The median number of enrollees per antihypertensive agent for all agents reviewed was 51 (range: 16–441), with a median of 143 subjects (range: 110–441) enrolled in efficacy and safety studies and 30 subjects (range: 24–74) enrolled in pharmacokinetic studies (Table 2). The median duration for all of the studies was 17.5 months (range: 5–50 months). The time to complete efficacy studies was approximately 50% longer in duration than pharmacokinetic studies (median: 24 months vs. 16 months). The median duration of bioequivalence studies was 6 months (range: 5–7 months).
Table II.
Trial descriptions
| Drug | Protocol | Duration (mos) |
# Sites | North America |
# Visits per site |
# Pts enrolled |
# Pts completed |
CRF | Unique CRF |
Tables, figures, listings |
|---|---|---|---|---|---|---|---|---|---|---|
| CCB1 | A | 19 | 51 | 46 | 6 | 344 | 268 | 13 | 12 | 107 |
| B | 16 | 6 | 6 | 5 | 74 | 73 | 12 | 9 | 41 | |
| ACE1 | A | 50 | 33 | 33 | 6 | 143 | 107 | 70 | 21 | 105 |
| B | 11 | 20 | 20 | 4 | 61 | 57 | 42 | 20 | 86 | |
| B | 7 | 1 | 1 | 8 | 30 | 30 | 33 | 18 | 62 | |
| C | 5 | 1 | 1 | 10 | 30 | 30 | 49 | 32 | 108 | |
| ACE2 | A | 14 | 19 | 16 | 7 | 110 | 110 | 56 | 28 | 50 |
| B | 11 | 7 | 6 | 7 | 40 | 40 | 50 | 38 | 29 | |
| C | 6 | 1 | 1 | 7 | 16 | 16 | 48 | 21 | 20 | |
| CCB2 | A | 24 | 27 | 27 | 6 | 133 | 128 | 47 | 18 | 166 |
| ACE3 | A | 30 | 78 | 62 | 11 | 376 | 253 | 72 | 29 | 153 |
| B | 28 | 6 | 5 | 6 | 43 | 43 | 36 | 20 | 25 | |
| ARB1 | A | 25 | 53 | 32 | 10 | 441 | 318 | 51 | 25 | 111 |
| B | 26 | 4 | 4 | 5 | 23 | 22 | 25 | 19 | 16 | |
| C | 7 | 1 | 1 | 12 | 30 | 30 | 50 | 25 | 21 | |
| C | 6 | 1 | 1 | 12 | 30 | 30 | 50 | 25 | 22 | |
| ACE4 | A | 20 | 22 | 13 | 7 | 115 | 103 | 61 | 29 | 52 |
| B | 19 | 9 | 7 | 8 | 52 | 52 | 63 | 41 | 37 | |
| C | 7 | 1 | 1 | 12 | 25 | 24 | 70 | 33 | 16 | |
| ARB2 | A | 20 | 54 | 23 | 5 | 177 | 164 | 42 | 26 | 55 |
| B | 19 | 10 | 5 | 6 | 50 | 50 | 54 | 31 | 56 | |
| C | 6 | 1 | 1 | 10 | 16 | 16 | 63 | 34 | 27 | |
| ACE5 | A | 28 | 27 | 20 | 5 | 123 | 112 | 37 | 18 | 154 |
| B | 11 | 4 | 4 | 4 | 24 | 24 | 18 | 16 | 9 | |
Protocol: A = safety and efficacy study, B = single-dose or multi-dose pharmacokinetic study, C = bioavailability or bioequivalence study
Duration, number of months from start of study to end of study; # Sites, number of clinical sites; North America, number of North American sites; CRF, number of case report forms per subject; Tables, figures, listings, number of tables, figures, and listings included in clinical study report.
The median number of case report forms completed per child was 50, and the median number of tables, figures, and listings per study was 51 for all studies. A subject visited a study site a median of 7 times throughout a trial. Efficacy studies included a greater number of tables, figures, and listings (107) than pharmacokinetic studies (27) (P = 0.0014). However, there was no statistically significant difference in the number of case report forms (P = 0.46) or in the number of visits completed by subjects (P = 0.48).
Study costs, sales, and cash flows
The median cost per written request to evaluate a particular anti-hypertensive drug in this cohort was $6 million (range: $3.8 million–14.2 million). These costs included estimated coordinating center, sponsor management, site payment, and central laboratory costs (when applicable) adjusted to 2005 dollars. The median value to conduct efficacy and safety clinicaltrials per drug was $4.3 million (range: $2.1 million–12.9 million), while a similar estimate for pharmacokinetic studies was $862,000 (range: $556,000–1.8 million). The median single- or multi-dose pharmacokinetic study cost was $1,117,092 (range: $634,396–1.8 million), while the median cost for a bioequivalence or bioavailability study was $731,856 (range: $555,663–862,457). The difference in study cost for single-dose or multi-dose pharmacokinetic studies versus study costs for bioequivalence and bioavailability studies was statistically significant (P = 0.026). The median cost per enrollee for efficacy studies was $31,546 (range: $18,941–$51,179), while the median cost per enrollee for pharmacokinetic studies or bioequivalence/bioavailability studies was $28,982 (range: $13,356–$47,311) and $30,071 (range: $23,851–$35,977), respectively. After-tax cash outflow, study costs adjusted for inflation and an industry average tax rate of 30% were $4.8 million (range: $2.7 million–10.4 million).
Drug sales also varied extensively. The median annual sales value per drug was $687 million (range: $218 million–$2.4 billion). There were 2 blockbuster anti-hypertensive agents with sales exceeding $1 billion (Table 3). The median 6-month cash inflow per drug was $60 million (range: $17.5 million–$320 million).
Table III.
Coordinating center, site monitoring, and central laboratory financial costs
| Drug | Trial | Coordinating | Coordinating | Sponsor | Site | Site | Central | Total | Total | Annual Sales |
|---|---|---|---|---|---|---|---|---|---|---|
| Center Costs– Low |
Center Costs– High |
Management Costs |
Payments– Low |
Payments– High |
Laboratory Payments |
Costs– Low |
Costs– High |
Estimate in Thousands |
||
| CCB1 | A | 1,335,830 | 3,701,125 | 868,656 | 290,731 | 410,201 | 120,878 | 2,616,095 | 5,100,859 | $2,572,824 |
| B | 250,628 | 583,357 | 288,686 | 61,050 | 84,360 | 18,605 | 618,969 | 975,009 | ||
| ACE1 | A | 1,567,634 | 3,931,301 | 989,482 | 306,876 | 355,026 | 56,903 | 2,920,895 | 5,332,713 | $414,940 |
| B | 525,926 | 1,205,477 | 391,201 | 145,665 | 164,806 | 21,849 | 1,084,641 | 1,783,333 | ||
| B | 170,372 | 363,870 | 157,726 | 72,230 | 94,533 | 18,267 | 418,596 | 634,396 | ||
| C | 186,287 | 392,333 | 184,947 | 85,283 | 104,100 | 34,164 | 490,680 | 715,544 | ||
| ACE2 | A | 616,557 | 1,372,005 | 436,371 | 169,941 | 223,762 | 51,378 | 1,274,248 | 2,083,516 | $861,356 |
| B | 284,222 | 625,181 | 275,698 | 146,995 | 197,768 | 18,446 | 725,360 | 1,117,092 | ||
| C | 142,354 | 311,714 | 145,193 | 81,319 | 86,392 | 12,364 | 381,230 | 555,663 | ||
| CCB2 | A | 1,102,811 | 2,621,783 | 743,969 | 590,153 | 872,818 | 58,517 | 2,495,450 | 4,297,087 | $180,380 |
| ACE3 | A | 3,455,444 | 8,520,504 | 1,759,668 | 1,454,497 | 2,294,963 | 373,190 | 7,042,799 | 12,948,325 | $277,485 |
| B | 283,226 | 659,036 | 444,827 | 110,166 | 127,323 | 15,045 | 853,264 | 1,246,231 | ||
| ARB1 | A | 1,957,254 | 4,521,229 | 1,234,825 | 2,951,279 | 4,227,413 | 324,750 | 6,468,107 | 10,308,216 | $686,725 |
| B | 237,254 | 562,956 | 388,655 | 52,302 | 59,731 | 29,503 | 707,713 | 1,040,844 | ||
| C | 176,357 | 392,159 | 207,689 | 114,651 | 118,658 | 29,663 | 528,360 | 748,168 | ||
| C | 175,647 | 390,059 | 207,689 | 117,692 | 120,634 | 44,027 | 545,055 | 762,409 | ||
| ACE4 | A | 709,453 | 1,648,164 | 553,956 | 188,843 | 256,731 | 45,657 | 1,497,910 | 2,504,508 | $664,669 |
| B | 383,812 | 870,716 | 393,105 | 134,612 | 259,496 | 0 | 911,530 | 1,523,317 | ||
| C | 163,026 | 349,821 | 164,464 | 168,720 | 334,224 | 13,949 | 510,159 | 862,457 | ||
| ARB2 | A | 1,191,285 | 3,125,146 | 785,957 | 257,021 | 368,800 | 0 | 2,234,264 | 4,279,903 | $1,324,444 |
| B | 353,881 | 822,583 | 388,949 | 120,546 | 205,051 | 0 | 863,376 | 1,416,582 | ||
| C | 153,895 | 332,456 | 152,361 | 75,149 | 84,933 | 5,928 | 387,333 | 575,677 | ||
| ACE5 | A | 999,098 | 2,437,998 | 760,168 | 172,432 | 246,240 | 88,798 | 2,020,496 | 3,533,203 | $532,765 |
| B | 170,908 | 392,422 | 243,954 | 60,504 | 62,016 | 7,673 | 483,040 | 706,065 | ||
Annual sales estimates are in (000$) and are reflective of total annual sales during the last year prior to patent expiration.
Average sales by drug class were also variable, but formal significance testing of this difference was not performed due to small sample sizes.
Net economic return and net economic return-to-investment
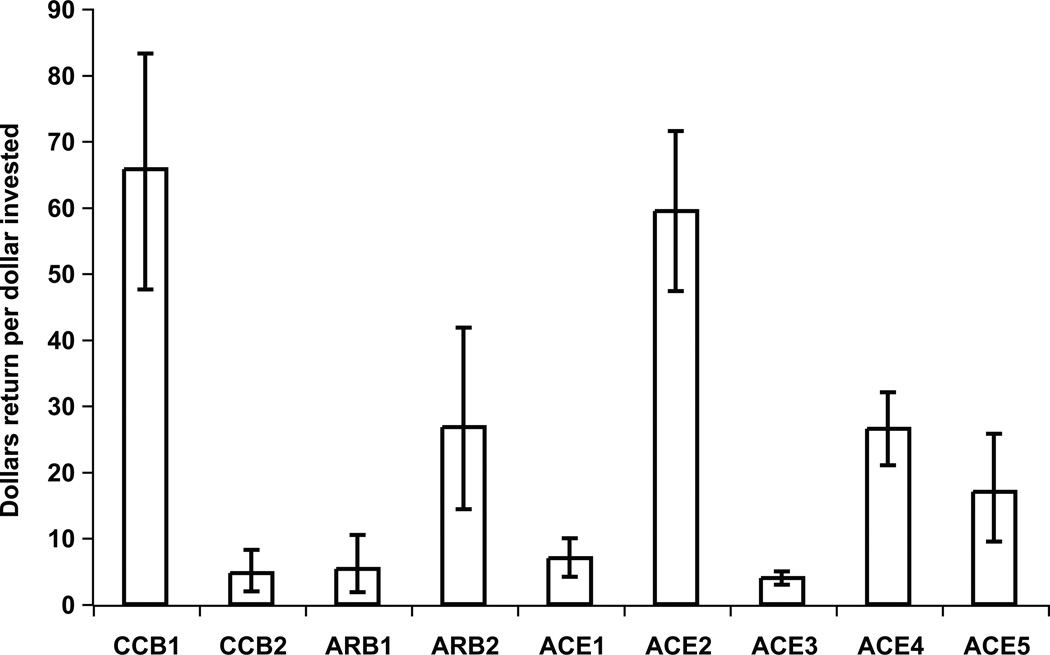
Net economic return for 6 additional months of marketing exclusivity varied by antihypertensive drug but remained positive for all drugs for the 6 months of additional exclusivity (Figure 1). Variation in net sales return greatly exceeded the variability in the cost of performing clinical trials: the standard deviation for cash inflow was $93 million, while the standard deviation in cash outflow per written request was $2.8 million. All studies resulted in a positive net return according to our analysis during the 6-month period of additional marketing exclusivity. The range in net return-to-investment after adjusting for discounts, taxes, and inflation far exceeded the cost of studying the drug and ranged from $14.5 million to $316 million (median: $53 million). For every $1 invested, 4 of the 9 agents studied had a return of less than $10, and 5 out of 9 had a return of greater than $20 for every $1 invested. The overall range was $4 to nearly $70 per $1 invested. Sensitivity analyses that adjusted discount rates for values between 5% and 20%, as well as adjustments in the contribution margin for values between 40% and 60%, also revealed positive net returns for all anti-hypertensive agents reviewed (Figure 1, error bars).
Figure 1. Dollars in net return for each dollar invested.
Economics of 6-month period of marketing exclusivity per study request: net return-to-investment ratio by anti-hypertensive agent. Error bars represent ranges in net return-to-investment due to variations in discount rate (range: 5–20%) and contribution margin (range: 40–60%) secondary to conducted sensitivity analyses.
Study cost predictors
Coordinating center, site management, and pharmaceutical costs generated using Fast Track Systems revealed that, among these cost variables, coordinating research center (CRO) costs comprised the greatest proportion of the total. Forty-one to 73% percent of the total cost for studying an anti-hypertensive agent was related to coordinating center incurred costs (Table 3). A review of coordinating center costs revealed that, for the majority of studies, site management and project management contributed the greatest percentage to the total cost estimate (Table 4).
Table IV.
Breakdown of study costs (efficacy studies only)
| Contract research organization | 70.5% |
|---|---|
| Site management | 25.1 |
| Project management | 21.1 |
| Travel | 6.2 |
| Data management | 4.9 |
| Manuscript preparation | 4.9 |
| Pre-study preparation | 4.2 |
| Randomization | 2.4 |
| Other | 1.7 |
| Sponsor | 19.0% |
| Investigator site | 10.9% |
| Central laboratory | 1.7% |
Sponsor costs are net pharmaceutical costs and represent the net costs of outsourcing clinical studies (external pharmaceutical costs) versus performing clinical studies internally (internal pharmaceutical costs).
Discussion
Our study suggests that net economic return-to-investment within the single pediatric indication of hypertension is strongly positive but highly variable. We found that of the 9 antihypertensive agents reviewed in this study, not one of the sponsors lost money by participating in the Pediatric Exclusivity Program. Furthermore, we found that the net return-to-investment as previously reported by Li et al. for a single hypertensive agent was relatively low when compared with the estimated net returns within a larger cohort of anti-hypertensive agents. Our most recent findings, coupled with our previous work, suggest that variability in the net return-to-investment following participation in the Pediatric Exclusivity Provision exists across and within clinical indications.
The benefits of examining a larger cohort of agents within a single clinical indication are not limited to sample size, but include the ability to assess for variations in cost that may be temporally associated with the introduction of an agent into the market and also with the popularity of its use among adults. One potential source of variation in the net return is related to the degree to which an agent penetrates the adult market. There are an estimated 65 million hypertensive adults in the United States, in addition to the estimated 5 million adults with clinical conditions such as heart failure who are also prescribed anti-hypertensive agents.10, 11 Thus, economic returns on anti-hypertensive drugs are expected to be high due to large sales volumes of agents while on patent. Agents such as CCB1 and ACE2 experienced the greatest net returns-to-investment, with net returns in excess of $316 million and $160 million, respectively. Sales data suggest that either the agents were broadly prescribed or that the inflow cost of such agents greatly exceeded that of other agents, resulting in higher sales values. Another potential source of variation is the order in which a particular agent is introduced into the market within a specific class of anti-hypertensive drugs. It is probable that agents with the earliest patents are also the agents with the greatest net return following exclusivity extension. ACE2 and ARB2 had earlier submission dates (Table 1; patent extension given to the latest submission date). These 2 agents also had the greatest net return-to-investment within their respective classes. Finally, variation in return could also be due to lower costs associated with studying the agents with the greatest net return and higher costs associated with studying drugs with the lowest net return. Overall, there appeared to be no trend to suggest this finding, and furthermore, sales values greatly exceeded cost values, and variations in sales greatly exceeded variations in cost.
Limitations
Limitations of our study include the number of agents available for study and the limits of our market analysis. We obtained the study reports of all anti-hypertensive drugs that had successfully undergone labeling changes (and 2 additional agents) prior to 2005. We generated cost estimates using software that is commonly used to design budgets for performing clinical trials. Attempts were made to limit measurement error by using a 2-person review of the data. Sales data were derived from audit information, and all inflow and outflow estimates were approximated in 2005 dollars in order to avoid bias. However, our model is a market-adjusted account of the difference in inflow versus outflow costs as a consequence of the exclusivity provision. It does not account for the returns required by pharmaceutical companies in order to support the development of new drugs, costs of formulation changes, marketing costs, distribution costs, or costs to society secondary to delayed entry of generics. It also does not account for costs incurred by pharmaceutical companies in choosing to develop 1 agent over another agent. Our model was limited by additional assumptions. We assumed: (1) fixed costs associated with the introduction of these pharmaceutical agents (i.e., warehouse construction, marketing research) occurred early in the product life-cycle and therefore were assumed to be minimal during the exclusivity period; (2) differences in incremental cash flow were more accurate predictors of return from investment than net profit; and (3) estimates of net return could be obtained by discounting tax-adjusted cost and sales values to the date of clinical study report submission to the FDA for analysis. However, our estimates of incremental cash outflows and inflows were consistent with previous estimates.4 Finally, we could not measure the potential gain to patients and society by conducting these trials.
Perspectives
Despite the high economic return to industry, these studies have been performed for the benefit of pediatric health and have provided necessary information for a significant pediatric health problem. Historically, only 25% of drugs used in children have sufficient labeling information to support their use in children.12 Insufficient labeling information has contributed to off-label use of agents in children, subjecting them to potential adverse events and to exposure to agents with unestablished efficacy. It is therefore important to provide appropriate labeling information to ensure benefits for this vulnerable population.4 Prior to the passage of the Best Pharmaceuticals for Children Act and the Pediatric Exclusivity Provision, legislation to encourage the testing of drugs in children was largely ineffective, and few studies were completed.13 With the rising epidemic of childhood obesity, conditions such as hypertension are increasing in prevalence, and now more than 10% of obese children and 1.3–5.3% of non-obese children in the United States are hypertensive.14 When one considers the potential impact of untreated hypertension, it is clear that proper testing of the safety, dosing, and efficacy of antihypertensive agents in children is essential.15–18 Furthermore, uncontrolled hypertension in the pediatric population is associated with increased morbidity in childhood.19
As a consequence of the 2002 Best Pharmaceuticals for Children Act, several antihypertensive agents have now been approved for use in the pediatric population. Of the 138 labeling changes that have occurred, just over 5% are now approved for use as anti-hypertensive therapies. Ideally all studies will result in labeling changes. However, of the 9 agents reviewed in this study, 2 did not. This is problematic because the likelihood that these agents will be retested is minimal and the prospect of having the necessary safety, efficacy, and dosing data to properly administer these agents to children, unlikely. However, in cases where drugs do not undergo labeling changes, there should be more transparency about the findings of the study. Greater transparency is necessary with regard to safety and/or lack of efficacy. Currently, labeling information is publicly available on the FDA Web site.20
Passage of the Best Pharmaceuticals for Children Act and the Pediatric Exclusivity Provision have resulted in the most significant increase in the number of label changes for drugs commonly administered to children to date. Without this provision, few drugs would undergo testing for safety, efficacy, and pharmacokinetics in the pediatric population. The rationale for granting pediatric exclusivity independent of label changes is the value associated with collecting information for use of agents in children in well-controlled and designed trials.21 Previously failed efforts to encourage label changes and the tremendous success of the Pediatric Exclusivity Provision suggest that society may need to bear the cost of testing drugs in children under this provision in order to protect future generations.
Acknowledgments
Sources of funding
Dr. Baker-Smith is funded by NIH T32 grant (HL069749-04). Drs. Li and Benjamin received support from the National Institute of Child Health & Human Development (NICHD) 1U10-HD45962-04 and the U.S. Food and Drug Administration. Drs. Benjamin, Li, and Califf received support from 1UL1RR024128-01.
The views expressed are those of the authors. No official endorsement by the U.S. Food and Drug Administration is provided or should be inferred.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The Duke Clinical Research Institute was the coordinating center for 2 of the trials of drugs mentioned in this study.
References
- 1.Department of Health and Human Services, U.S. Food and Drug Administration. [Accessed November 27, 2007];The Pediatric Exclusivity Provision: January 2001 Status Report to Congress. Available at: http://www.fda.gov/cder/pediatric/reportcong01.pdf.
- 2. [Accessed November 27, 2007];Approved active moieties to which FDA has issued a written request for pediatric studies under Section 505A of the Federal Food, Drug, and Cosmetic Act. U.S. Food and Drug Administration Web site. Available at: http://www.fda.gov/cder/Pediatric/wrlist.htm.
- 3. [Accessed November 27, 2007];Pediatric exclusivity labeling changes. U.S. Food and Drug Administration Web site. Available at: http://www.fda.gov/cder/pediatric/labelchange.htm.
- 4.Breslow LH. The Best Pharmaceuticals for Children Act of 2002: the rise of the voluntary incentive structure and congressional refusal to require pediatric testing. Harvard J Legis. 2003;40:133–193. [PubMed] [Google Scholar]
- 5.Li JS, Eisenstein EL, Grabowski HG, et al. Economic return of clinical trials performed under the pediatric exclusivity program. JAMA. 2007;297:480–488. doi: 10.1001/jama.297.5.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.US General Accounting Office. [Accessed November 27, 2007];Testimony before the Committee on Health, Education, Labor, and Pensions, US Senate. Pediatric drug research: substantial increase in studies of drugs for some children but challenges remains. GAO-01-705 T. Available at: http://www.gao.gov/new.items/d01705t.pdf.
- 7.IMS Health home page. [Accessed January 5, 2007]. Available at: http://www.imshealth.com. [Google Scholar]
- 8.Grabowski HG, Vernon JM, Dimasi JA. Returns on research and development for 1990s new drug introductions. Pharmacoeconomics. 1994;20(suppl 3):11–29. doi: 10.2165/00019053-200220003-00002. [DOI] [PubMed] [Google Scholar]
- 9.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 suppl 4th report):555–576. [PubMed] [Google Scholar]
- 10.Hypertension in America: a national reading. Am J Manag Care. 2005;11(13 suppl):S383–S385. [PubMed] [Google Scholar]
- 11.Hunt SA American College of Cardiology. American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2005;46:e1–e82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 12.Wilson JT. An update on the therapeutic orphan. Pediatrics. 1999;104:585–590. [PubMed] [Google Scholar]
- 13.Roberts R. Pediatric drug labeling: improving the safety and efficacy of pediatric therapies. JAMA. 2003;290:905–911. doi: 10.1001/jama.290.7.905. [DOI] [PubMed] [Google Scholar]
- 14.Ogden CL, Flegal KM, Carroll MD, et al. Prevalence and trends in overweight among U.S. children and adolescents, 1999–2000. JAMA. 2002;288:1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 15.Meyer AA, Joharchi MS, Kundt G, et al. Predicting the risk of early atherosclerotic disease development in children after repair of aortic coarctation. Eur Heart J. 2005;26:617–622. doi: 10.1093/eurheartj/ehi037. [DOI] [PubMed] [Google Scholar]
- 16.Belay B, Belamarich P, Racine AD. Pediatric precursors of adult atherosclerosis. Pediatr Rev. 2004;25:4–16. doi: 10.1542/pir.25-1-4. [DOI] [PubMed] [Google Scholar]
- 17.Daniels SR. Hypertension-induced cardiac damage in children and adolescents. Blood Press Monit. 1999;4:165–170. [PubMed] [Google Scholar]
- 18.Strong JP. The natural history of atherosclerosis in childhood. Ann N Y Acad Sci. 1991;623:9–15. doi: 10.1111/j.1749-6632.1991.tb43714.x. [DOI] [PubMed] [Google Scholar]
- 19.Lauer RM, Clarke WR. Childhood risk factors for high adult blood pressure: the Muscatine Study. Pediatrics. 1989;84:633–641. [PubMed] [Google Scholar]
- 20. [Accessed April 10, 2008];Pediatric exclusivity labeling changes as of April 10, 2008. U.S. Food and Drug Administration Web site. Available at: http://www.fda.gov/cder/pediatric/labelchange.htm.
- 21.Benjamin DK, Smith PB, Murphy MD, et al. Peer-reviewed publication of clinical trials completed for pediatric exclusivity. JAMA. 2006;296:1266–1273. doi: 10.1001/jama.296.10.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]