SUMMARY
Monocytes serve as a central defense system against infection and injury but can also promote pathological inflammatory responses. Considering the evidence that monocytes exist in at least two subsets committed to divergent functions, we investigated whether distinct factors regulate the balance between monocyte subset responses in vivo. We identified a microRNA (miRNA), miR-146a, which is differentially regulated both in mouse (Ly-6Chi/Ly-6Clo) and human (CD14hi/CD14loCD16+) monocyte subsets. The single miRNA controlled the amplitude of the Ly-6Chi monocyte response during inflammatory challenge whereas it did not affect Ly-6Clo cells. miR-146a–mediated regulation was cell-intrinsic and depended on Relb, a member of the non-canonical NF-κB/Rel family, which we identified as a direct miR-146a target. These observations not only provide novel mechanistic insights into the molecular events that regulate responses mediated by committed monocyte precursor populations but also identify novel targets to manipulate Ly-6Chi monocyte responses while sparing Ly-6Clo monocyte activity.
INTRODUCTION
Monocytes are the circulating precursors of several types of macrophages and dendritic cells (Geissmann et al., 2010). They confer protection of injured or infected tissue but also propagate chronic diseases (Auffray et al., 2009; Qian and Pollard, 2010; Shi and Pamer, 2011). At least two CD11b+ CD115+ monocyte populations exist in mice: (i) Ly-6Chi (Gr-1+ CCR2+ CX3CR1lo) cells respond to pro-inflammatory cues such as CCL2 (or MCP-1), migrate to inflamed sites and draining lymph nodes and can differentiate into antigen-presenting dendritic cells (Cheong et al., 2010) and orchestrate inflammatory functions (Swirski et al., 2007; Tacke et al., 2007); and (ii) Ly-6Clo (Gr-1− CCR2−CX3CR1hi) cells patrol the resting endothelium (Auffray et al., 2007), can be recruited to tissue after the onset of inflammation, and participate in granulation tissue formation (Nahrendorf et al., 2007). Ly-6Chi monocytes recirculate into the bone marrow where they can convert into Ly-6Clo monocytes (Varol et al., 2007). Monocyte heterogeneity is conserved at least in part in mice and humans: mouse Ly-6Chi monocytes share phenotypic and functional features with human CD14hi cells, whereas mouse Ly-6Clo monocytes resemble human CD14lo CD16+ cells (Cros et al., 2010).
Infection (Shi and Pamer, 2011), injury (Nahrendorf et al., 2007), atherosclerosis (Swirski et al., 2007; Tacke et al., 2007); cancer (Movahedi et al., 2010) and other pathophysiological conditions alter monocyte subset ratios. Changes of ratios can occur rapidly (e.g., hours after pathogenic infection), be long lasting (e.g. in chronic inflammatory disorders), and typically result in the selective amplification of pro-inflammatory Ly-6Chi cells. Human studies have underscored the relevance of studying monocyte subsets because an imbalance in their relative proportion is linked to several diseases (Ziegler-Heitbrock, 2007). The factors that regulate the balance between monocyte subset responses are largely unknown. The identification of such factors is potentially useful as it may offer new vantage points for tailoring immune responses to a desired phenotype.
RESULTS
Mir-146a Is A Candidate Regulator Of Monocyte Functional Heterogeneity
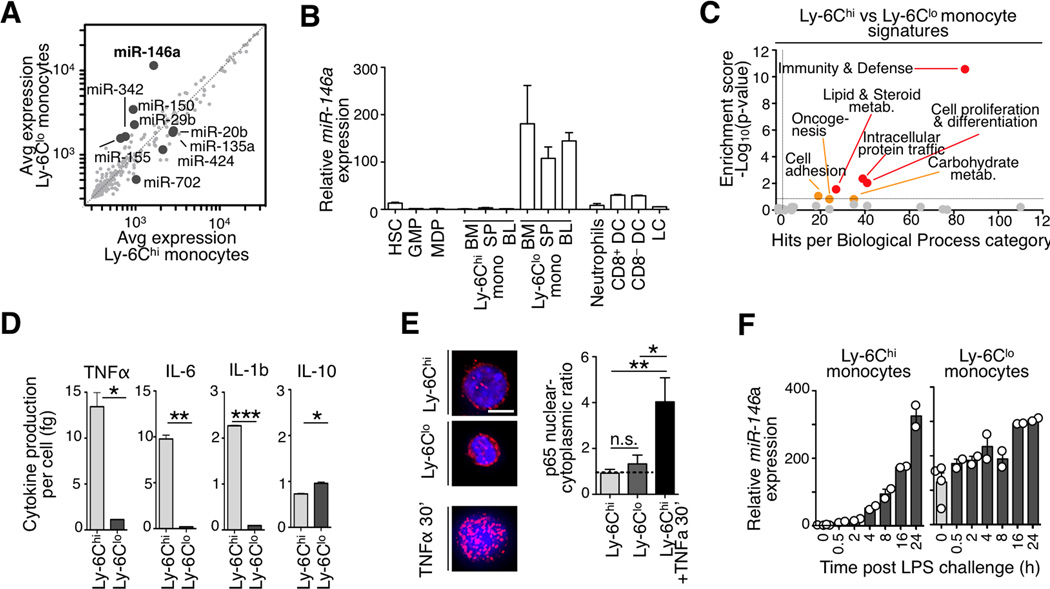
MicroRNAs (miRNAs) regulate target genes at the post-transcriptional level and can control distinct functional properties in cell types that are closely related ontogenically. miRNAs are known to regulate the development and function of various immune cell types (O'connell et al., 2010) but have to date not been investigated in the context of monocyte heterogeneity. Here we compared the expression levels of 380 miRNAs in sorted monocyte subsets (Fig S1a) and defined significant genes as those with at least 2-fold differential expression and a p<0.05 (Student’s t-test). The approach identified 9 miRNAs, which were highly expressed either in Ly-6Chi (miR-20b,-135a,-424,-702) or in Ly-6Clo monocytes (miR-146a, -150, -155, -342, -29b) (Fig 1a).
Figure 1. Mouse monocyte subsets show distinct miR-146a and inflammatory profiles.
a) Microarray analysis of miRNA expression in Ly-6Chi vs. Ly-6Clo splenic monocytes. Genes with >2-fold change among subsets and p<0.05 are highlighted (n=4 biological replicates).
b) Relative miR-146a expression in various hematopoietic cell types. Expression is relative to splenic Ly-6Chi monocytes (n=3 animals for all cell populations except for spleen monocytes, n=7).
c) Analysis of differentially expressed genes in Ly-6Chi vs. Ly-6Clo blood monocytes using the Panther database of biological functional categories.
d) Quantification of TNFα, IL-6, IL-1b and IL-10 production by splenic monocytes 8 h after LPS challenge (n=3–4).
e) p65 immunofluoresence staining in sorted monocyte subsets. Ly-6Chi monocytes stimulated for 30’ with TNFα prior to fixation served as a positive control for effective nuclear translocation. Scale bar: 10 µm
f) Time-course analysis of miR-146a levels after LPS challenge. Expression is relative to Ly-6Chi monocytes at time 0 h (n=2–4).
Data are presented as mean±SEM. (* p<0.05,** p<0.01, *** p<0.001, Student’s t-test).
Independent assays indicated ~2 orders of magnitude higher expression of miR-146a in Ly-6Clo monocytes when compared to hematopoietic stem cells (HSC), granulocyte/macrophage progenitors (GMP), macrophage/dendritic cell progenitors (MDP) and Ly-6Chi monocytes in steady-state (Fig 1b). Thus, monocytes express miR-146a only at a late maturation stage and selectively in the Ly-6Clo subset. Steady-state dendritic cell populations expressed miR-146a at intermediate levels (Fig 1b).
miRNAs and their respective target genes are often mutually exclusively expressed in a given tissue (Farh, 2005). In keeping with previous observations that miR-146a suppresses NF-κB-dependent inflammatory pathways (Taganov et al., 2006), we confirmed with two independent genome-wide profiling methods that splenic miR-146alo Ly-6Chi monocytes showed increased inflammatory signatures (Swirski et al., 2009) and expressed components of the NF-κB signaling cascade at higher levels than their miR-146ahi Ly-6Clo counterparts (Fig 1c, S1b). Also, splenic and blood miR-146alo Ly-6Chi monocytes stimulated with lipopolysaccharide (LPS) produced more TNFα, IL-6 and IL-1b inflammatory cytokines than miR-146ahi Ly-6Clo cells (Fig 1d and S1c).
The elevated miR-146a expression in Ly-6Clo cells and the inflammatory profile of Ly-6Chi cells reported above were likely not due to a premature activation artifact induced by the isolation procedure because IκBα protein levels were similar in both monocyte subsets ex vivo (Fig S1d) and NF-κB subunit p65 only became detectable in the nucleus of Ly-6Chi cells upon in vitro challenge (Fig 1e). The cause for constitutive (NF-κB-independent) miR-146a expression in Ly-6Clo cells will require additional investigation.
Differential Mir-146a Expression In Monocytes In Steady-State And Inflammation
We addressed the regulation of miR-146a expression in monocyte subsets upon ex vivo challenge with either LPS, heat killed Listeria monocytogenes (HKLM) or TNFα. miR-146a was induced only in Ly-6Chi monocytes, in response to all stimuli, and reached levels matching those in Ly-6Clo cells (Fig S1e). In vivo LPS challenge studies confirmed the in vitro findings (Fig S1f). miR-146a expression in Ly-6Chi cells increased within 4 h after LPS challenge and reached levels equivalent to those found in Ly-6Clo cells after 16 h (Fig 1f). Thus, miR-146a expression is constitutive in Ly-6Clo monocytes and inducible in Ly-6Chi monocytes. LPS-stimulated Ly-6Chi monocytes were CD11c+ MHC IIhigh (Ly-6Chi) and thus distinct from Ly-6Clo monocytes (Fig S1g).
Mir-146a Controls Monocyte Subset Ratios During Inflammatory Reactions
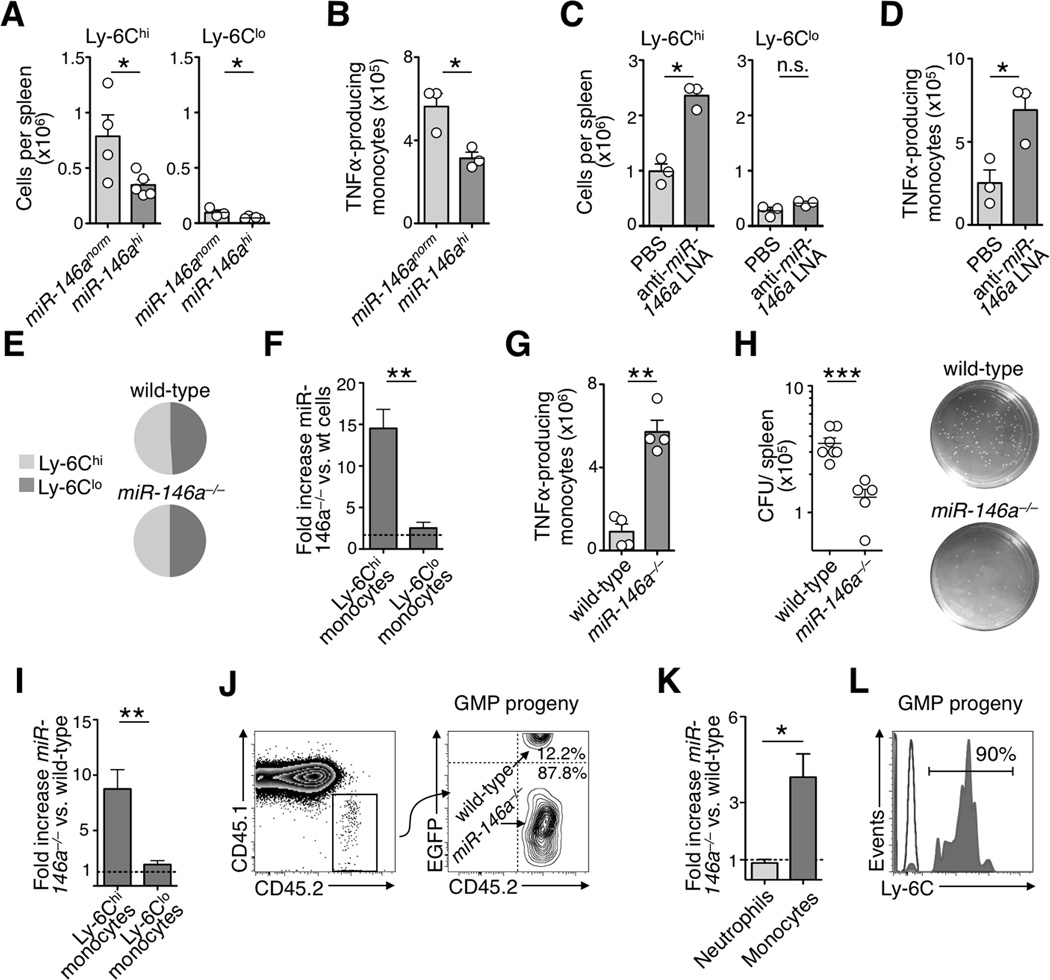
To investigate the role of miR-146a in monocytes in vivo, we generated mice in which miR-146a expression was either up- or down-regulated experimentally. To constitutively over-express miR-146a we reconstituted mice with HSC transduced to co-express EGFP and miR-146a (Fig S2a–b). miR-146a overexpression did not alter monocyte numbers or subset ratios in steady-state (Fig S2c); however, upon Listeria monocytogenes (Lm) infection (Shi and Pamer, 2011), it prevented the unfolding of a full-fledged TNFα–producing Ly-6Chi monocyte response (Fig 2a,b).
Figure 2. Effects of ectopic miR-146a expression or miR-146a silencing on the Ly-6Chi monocyte response.
a) Monocyte counts in spleen of mice reconstituted with miR-146a–expressing (miR-146ahi) or control (miR-146anorm) vector and challenged with Lm (n=3–5 from 2 independent experiments).
b) Number of TNFα-producing monocytes after ex vivo re-stimulation (same mice as in a).
c) Monocyte counts in spleen of mice that received either anti-miR-146a LNA or PBS and were challenged with live Lm for 24 h (n=3).
d) Number of TNFα-producing monocytes after LNA treatment (same mice as in c).
e) Ly-6Chi/Ly-6Clo monocyte ratios in wild-type (1.03±0.03) and miR-146a−/− (1.05±0.04) mice in steady-state (mean±SEM).
f) Fold increase of Ly-6Chi and Ly-6Clo miR-146a−/− monocytes in peritoneal cavity compared to their wild-type counterparts in bone marrow chimeras 4 d after peritoneal LPS injection (n=4 from 2 independent experiments).
g) Number of TNFα-producing wild-type or miR-146a−/− monocytes (same mice as in f).
h) Colony forming unit (CFU) assay to quantify viable Lm from the spleen of wild-type (n=6) and miR-146a−/− (n=5) mice 24 h after infection.
i) Fold increase of Ly-6Chi and Ly-6Clo miR-146a−/− monocytes compared to their wild-type counterparts in bone marrow chimeras 24 h after peritoneal thioglycollate injection (n=3).
j) Tracking of EGFP+ wild-type and EGFP− miR-146a−/− CD45.2 GMP progeny. Right dot plot shows CD45.2 Lin− CD11b+ CD115+ donor GMP-derived monocyte (representative of 4 independent experiments).
k) Fold increase of miR-146a−/− neutrophils and monocytes (GMP donor-derived) compared to their wild-type counterparts in the peritoneal cavity 4 d after LPS challenge (n=4 from 2 independent experiments).
l) Ly-6C expression by donor GMP-derived monocytes (same mice as in k).
Data are presented as mean±SEM. (* p<0.05, ** p<0.01, *** p<0.001, Student’s t-test).
To suppress miR-146a expression in vivo we used two independent approaches. The first one involved systemic delivery of anti-miRNA locked nucleic acid (LNA) formulations (Fig S2d,e). LNA treatment did not alter monocyte subset ratios in steady-state (Fig S2f) but it increased the number of TNFα–producing Ly-6Chi monocytes at Lm infected sites (Fig 2c,d).
The second approach to suppress miR-146a expression used recently described mice with targeted deletion of the miR-146a gene (Boldin et al., 2011) (Fig S2g). miR-146a−/− mice contained both monocyte subsets thus Ly-6Chi→Ly-6Clo monocyte conversion should not require miR-146a. Also, miR-146a knockdown did neither alter the ratio (Fig 2e) nor the phenotype (Fig S2h) of monocyte subsets in 8 wk old mice. To compare miR-146a−/− and wild-type monocyte responses as they developed in the same environments, we reconstituted wild-type (CD45.1) mice with equal numbers of miR-146a−/− (CD45.2) and wild-type (EGFP+ CD45.2) cells (Fig S2i). The absence of miR-146a strongly amplified TNFα–producing Ly-6Chi peritoneal monocytes in response to LPS challenge (Fig 2f, g). Ly-6Chi monocytes mediate immune defense in early phase of Lm infection (Shi and Pamer, 2011). Accordingly, Lm-infected miR-146a−/− mice contained reduced numbers of viable Lm 24 h post infection when compared to Lm-infected wild-type mice (Fig 2h). Amplification of the Ly-6Chi monocyte response in absence of miR-146a was confirmed in a model of sterile peritonitis induced by thioglycollate (Fig 2i).
Cell-Intrinsic Mir-146a-Mediated Regulation Of The Ly-6Chi Monocyte Response
The experiments above involved indiscriminate alteration of miR-146a expression in all hematopoietic cells. We reasoned that injection of miR-146a−/− GMP into wild-type mice would permit to track miR-146a−/− monocytes in a wild-type environment because miR-146a is only upregulated upon progenitor cell maturation. Specifically, we co-administered equal numbers of miR-146a−/− (CD45.2 EGFP−) and wild-type (CD45.2 EGFP+) GMP into non-irradiated wild-type (CD45.1) mice, which were subsequently challenged with LPS i.p. (Fig S2j). Wild-type and miR-146a−/− hematopoietic progenitor cells show comparable clonogenic potential (Boldin et al., 2011; Fig S2k) and the transferred cells’ progeny contained monocytes and neutrophils, as expected. miR-146a−/− monocytes recruited to the peritoneal cavity outnumbered their wild-type counterparts (Fig 2j, k) and were Ly-6Chi (Fig 2l); in marked contrast, miR-146a−/− neutrophils—which do not upregulate miR-146a in vivo—mounted a response that was similar to their wild-type counterparts (Fig 2k). Thus miR-146a should regulate Ly-6Chi monocytes at least in part in a cell-intrinsic manner.
Mir-146a Controls Ly-6Chi Monocyte Proliferation And Trafficking In Inflammatory Conditions
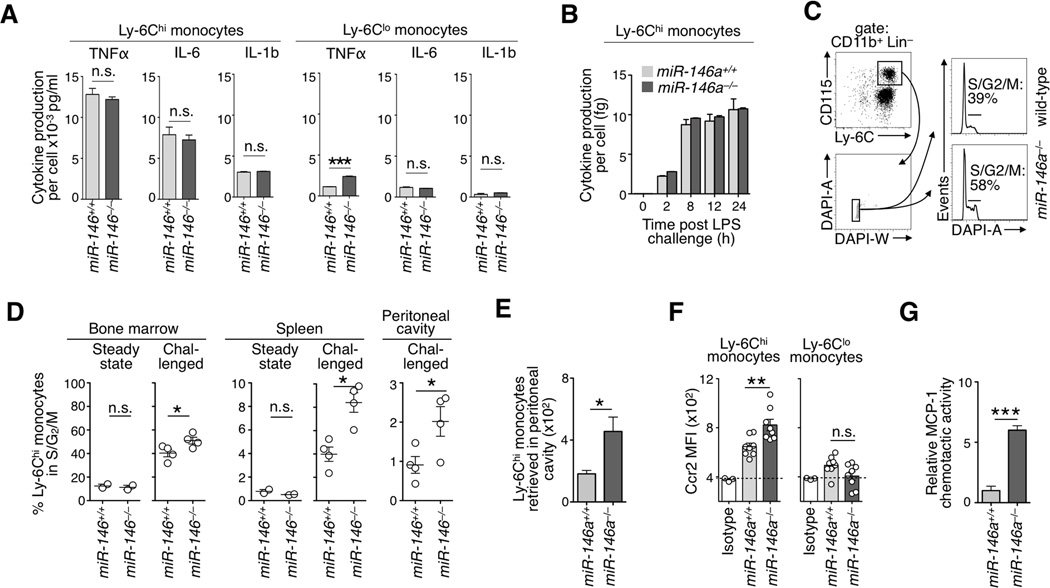
In contrast to previous descriptions for other cell types (Nahid et al., 2009; Boldin et al., 2011), including macrophages (Fig S3a), the absence of miR-146a did not detectably alter inflammatory cytokine production by Ly-6Chi and Ly-6Clo monocytes on a per-cell basis (Fig 3a,b). However, LPS challenge increased the percentage of miR-146a−/− Ly-6Chi monocytes undergoing cell division in bone marrow (Fig 3c, d) and to a lower extent in the spleen and peritoneal cavity (Fig 3d). The absence of miR-146a did not affect proliferation of Ly-6Clo monocytes; Fig S3b. Co-cultures of miR-146a−/− and wild-type cells also indicated a proliferative advantage for bone marrow miR-146a−/− Ly-6Chi monocytes (Fig S3c, d).
Figure 3. Altered proliferation and trafficking of miR-146a−/− Ly-6Chi monocytes during inflammation.
a) Cytokine production of sorted wild-type and miR-146a−/− monocyte subsets after in vitro LPS stimulation. Cytokine production is expressed per cell (n=2–3).
b) Time course TNFα production by Ly-6Chi monocytes upon LPS challenge in vitro (n=2).
c) Gating strategy for DAPI staining of bone marrow Ly-6Chi monocytes. Histograms show data for LPS stimulated wild-type or miR-146a−/− animals.
d) Quantification of cell cycle status in wild-type and miR-146a−/− animals in steady state (n=2) or after 4 consecutive days of LPS injection i.p. (n=4) in bone marrow, spleen and peritoneal cavity.
e) Number of donor wild-type and miR-146a−/− EGFP+ CD45.2 Ly-6Chi monocytes retrieved in the peritoneal cavity 6 h after transfer into LPS-treated CD45.1 recipient mice (n=4).
f) Flow cytometry-based cell surface CCR2 mean-fluorescence intensity (MFI) in wild-type and miR-146a−/− blood monocytes (n=8).
g) In vitro chemotactic activity of wild-type (EGFP+) and miR-146a−/− (EGFP−) Ly-6Chi monocytes toward MCP-1 (n=4).
Data are presented as mean±SEM. (* p<0.05, ** p<0.01, *** p<0.001, Student’s t-test).
In addition, co-injection of bone marrow miR-146a−/− (EGFP− CD45.2) and control (EGFP+ CD45.2) Ly-6Chi monocytes into LPS-treated wild-type (CD45.1) mice showed higher accumulation of miR-146a−/− cells at the site of inflammation within only 6 h (Fig 3e). The chemokine CCL2 controls Ly-6Chi monocyte migration to inflamed sites (Shi and Pamer, 2011). Interestingly, miR-146a−/− blood Ly-6Chi—but not Ly-6Clo—monocytes expressed the cognate receptor CCR2 at higher levels than their wild-type counterparts (Fig 3f, S3e) and migrated more efficiently toward a CCL2 gradient in vitro (Fig 3g).
These observations indicate that miR-146a controls the expansion of Ly-6Chi monocytes during acute inflammatory conditions in part through elevated proliferation of Ly-6Chi monocytes—predominantly in the bone marrow—and increased trafficking to inflamed sites.
Relb Is A Mir-146a Target
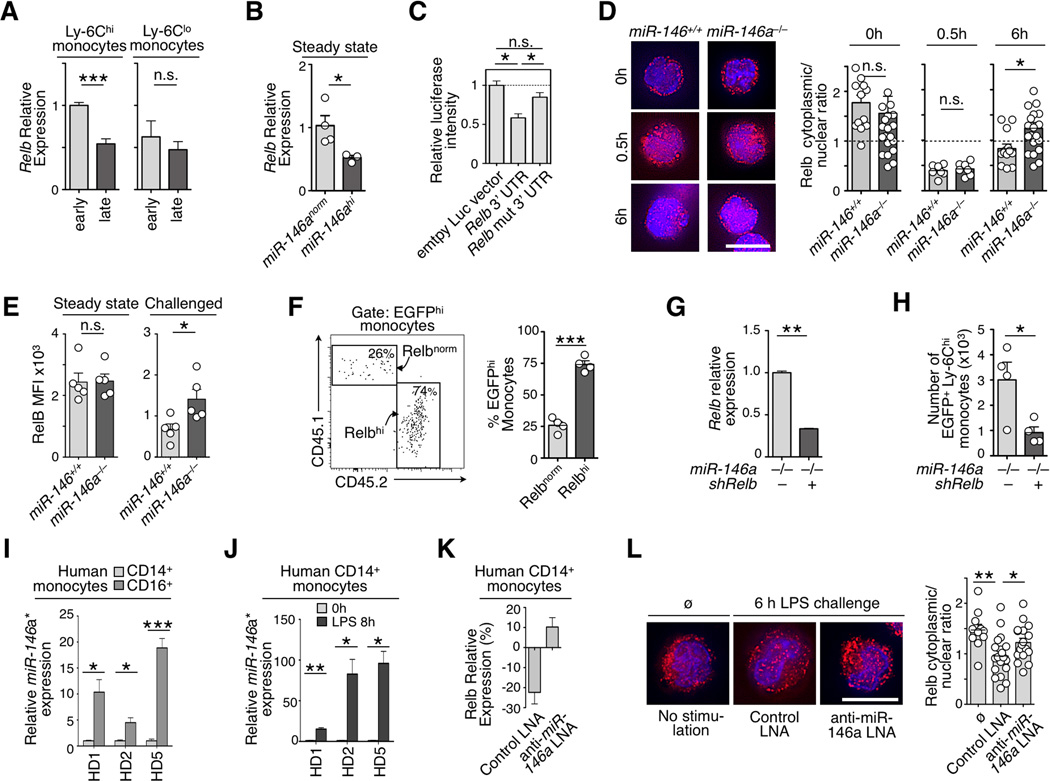
We aimed to find endogenous miR-146a target genes that contribute to altering the monocyte response. The screening approach, which compared the expression profiles of miR-146a–predicted target genes in Ly-6Chi and Ly-6Clo monocytes either at 2 h or 8 h after Lm challenge (Fig S4a and supplementary information), identified the transcription factor Relb (Fig 4a). Experimental evidence also indicates that Relb is a miR-146a target. First, ectopic miR-146a expression in resting Ly-6Chi monocytes in vivo reduced Relb transcript levels (Fig 4b). Second, NIH-3T3 cells transfected with a luciferase reporter plasmid expressing Relb 3’ UTR (ENSMUST00000049912) containing a potential miR-146a binding sequence showed reduced luciferase activity upon miR-146a overexpression. The phenotype was rescued by mutating the seed sequence (Fig 4c). Third, immunofluorescence microscopy with a validated anti-Relb Ab (Fig S4b) showed efficient nuclear translocation of Relb protein at 30 min after LPS challenge in both wild-type and miR-146a−/− Ly-6Chi monocytes; however at 6 h cytoplasmic Relb levels were recovered more prominently in the miR-146a−/− cells (Fig 4d). Fourth, flow cytometry analysis confirmed that Relb protein levels remained higher in miR-146a−/− Ly-6Chi monocytes upon LPS challenge (Fig 4e).
Figure 4. Relb is a miR-146a target in monocytes.
a) Relative Relb mRNA expression in monocytes subsets recruited to the peritoneal cavity at 2 h (early) and 8 h (late) post inflammatory challenge. Data are normalized to Ly-6Chi monocytes at 2 h (n=3).
b) Relative Relb mRNA expression in steady-state Ly-6Chi monocytes that over-express miR-146a (miR-146ahi) or not (miR-146anorm) (n=3).
c) Luciferase reporter assay for miR-146a–dependent regulation of Relb 3’ UTR. Luciferase activity was measured in NIH3T3 cells transfected with control empty vector, Relb 3’ UTR or a mutated version of the Relb 3’ UTR.
d) Immunofluorescence staining of Relb protein in wild-type or miR-146a−/− Ly-6Chi monocytes at 0, 0.5 and 6 h after LPS challenge. (Images are representative of n=7–21 cells analyzed per condition). Scale bar 10µm. Quantification shows cytoplasmic vs. nuclear fluorescence signal ratios.
e) Flow cytometry evaluation of intracellular Relb protein expression levels in wild-type or miR-146a−/− Ly-6Chi blood monocytes in steady-state or 6 h after LPS challenge (n=5).
f) Tracking of EGFP+ monocytes reconstituted with a Relb–overexpressing (Relbhi) or control (Relbnorm) vector in the peritoneal cavity 7 d after LPS challenge. Gating shows a representative result of the two competing monocyte populations (n=4 animals per group).
g) shRNA–mediated knockdown in EGFP+ cells measured by real-time PCR in miR-146a−/− Ly-6Chi monocytes (n=3).
h) Accumulation in the peritoneal cavity of EGFP+ miR-146a−/− monocytes transfected either with a shRelb or control construct 7 d after transfer into LPS challenged recipients.
i) Differential miR-146a* expression in CD14+ (CD16−) and CD16+(CD14−) monocytes from 3 healthy donors (HD) ex vivo.
j) Induction of miR-146a* in CD14+(CD16−) monocytes 6 h post LPS challenge (same donors as in i; n=3 technical replicates per group).
k) Percent change of Relb mRNA expression in CD14+(CD16−) monocytes of HD5 6 h post LPS challenge in presence of a scrambled or anti-miR-146a LNA (n=3).
l) Immunofluorescence staining of Relb protein in CD14+(CD16−) monocytes analyzed ex vivo (ø) or treated as in k. (Images are representative of n=11–19 cells analyzed per condition). Scale bar 10µm.
Quantification shows cytoplasmic vs. nuclear fluorescence signal ratios.
Data are presented as mean±SEM. (* p<0.05, ** p<0.001, *** p<0.0001, Student’s t-test).
Modulation Of Relb Expression Affects The Ly-6Chi Monocyte Response
To investigate whether modulation of Relb affects the monocyte response, we generated both Relbhi EGFPhi CD45.1 HSC (which expressed Relb from a cDNA sequence that could not be regulated by miR-146a) and control Relbnorm EGFPhi CD45.2 HSC, which were adoptively transferred at a 1:1 ratio into LPS-treated CD45.1/2 recipient animals (Fig S4c). Relb overexpression did not alter HSC expansion (Fig S4d) but amplified the monocyte response in vivo (Fig 4f) and thus recapitulated the phenotype observed for miR-146a−/− Ly-6Chi monocytes.
We also injected LPS-treated CD45.1 mice either with miR-146a−/− shRelb EGFPhi HSC (which expressed a miR30-hairpin based shRNA to silence Relb to the levels found in challenged wild-type monocytes) or with miR-146a−/− EGFPhi HSC (which expressed a control EGFP vector) (Fig 4g, S4e). Relb silencing did not alter HSC expansion (Fig S4f) but decreased miR-146a−/− Ly-6Chi monocyte responses in vivo (Fig 4h). These data indicate that miR-146a can control Ly-6Chi monocyte fate in response to acute inflammatory challenge via Relb targeting.
miR-146a And Relb Expression In Human Monocytes
The human Relb 3’UTR contains a binding site for the alternative processing isoform miR-146a-3p (miR-146a*) instead of the “canonical” miR-146a-5p isoform (miR-146a) (Transcript ENST00000221452, Fig S4g). miR-146a, and most notably miR-146a*, were detected at higher levels in human CD16+(CD14lo) monocytes than in their CD14+(CD16−) counterparts ex vivo (Fig 4i, S4h,i), and were selectively induced in CD14+ (CD16−) monocytes 6 h post LPS challenge (Fig 4j and S4j,k). miR-146a* was also detected in mouse monocytes (Fig S4l). These data are in line with previous findings that CD14loCD16+ monocytes resemble Ly6Clo cells and respond less well to LPS in comparison to CD14+CD16+ and CD14+CD16− monocytes, which resemble mouse Ly-6Chi monocytes (Cros et al., 2010). Furthermore, human CD14+ monocytes challenged with LPS decreased Relb mRNA levels (Fig 4k), although treatment with a LNA to suppress miR-146a* induction (Fig S4m) was sufficient to prevent Relb downregulation (Fig 4k, l).
DISCUSSION
This study provides functional evidence that miR-146a and Relb differentially regulate monocyte subsets. Following inflammatory challenge, modulation of miR-146a expression tunes the amplitude of the Ly-6Chi–but not the Ly-6Clo—monocyte response: premature miR-146a induction aborts Ly-6Chi cell amplification whereas lack of miR-146a induction leads to expansion and increased recruitment of these cells. miR-146a in monocytes targets Relb, which expression levels tune the amplitude of Ly-6Chi monocyte responses.
Recent work has identified miR-146a as a negative regulator of the canonical NF-κB inflammatory cascade by targeting Traf6 and Irak1/2 (O'connell et al., 2010) and as a tumor suppressor gene by decreasing transcription of NF-κB–targeted genes (Boldin et al., 2011; Zhao et al., 2011). The present study extends the role of miR-146a to the control of Relb, which is mostly implicated in the non-canonical NF-κB pathway (Vallabhapurapu and Karin, 2009). Relb has sizable effects on mononuclear phagocytes as it controls dendritic cell development in humans (Platzer et al., 2004) and mice (Burkly et al., 1995; Cejas et al., 2005; Wu et al., 1998), and the generation of monocyte-derived osteoclasts (Vaira et al., 2008). In accordance with the present study, the non-canonical NF-κB pathway activator CD40L also controls Ly-6Chi monocyte expansion (Lutgens et al., 2010). Of note, miR-146a can regulate proinflammatory gene expression by controlling RelB-dependent reversible chromatin remodeling (El Gazzar et al., 2011).
Ly-6Clo monocytes constitutively express miR-146a in accordance with their non-inflammatory properties (Nahrendorf et al., 2007; Auffray et al., 2009). Nevertheless, miR-146a−/− Ly-6Clo cells did not mount an inflammatory response that was notably higher than their wild-type counterparts. It is possible that miR-146a does not play a significant role in Ly-6Clo cells; yet, other regulatory mechanisms may keep Ly-6Clo cells in check in absence of miR-146a. The study of Ly-6Clo cells that bear defects in several candidate factors (e.g., miR-146a and other miRNAs) may serve to address this question. Either way, the present findings indicate that selective targeting of the miR-146a pathway should control Ly-6Chi monocyte responses while preserving Ly-6Clo cells.
Previous work has identified that miR-146a−/− macrophages produce higher levels of inflammatory cytokines than their wild-type counterparts (Boldin et al., 2011); however, we could not recapitulate these findings in miR-146a−/− monocytes. Challenged miR-146a−/− and wild-type Ly-6Chi monocytes may produce the same amount of cytokines on a per-cell basis because miR-146a up-regulation is induced after the initial burst of inflammatory cytokine production (4–24 h vs 0–8 h, respectively). Yet, miR-146a−/− Ly-6Chi monocytes will contribute more cytokine production at target sites not only because more of these cells are recruited but also because they can give rise locally to miR-146a−/−macrophages, which exhibit heightened inflammatory functions.
The findings presented here place miR-146a and Relb as key regulators of monocyte subset population dynamics. miR-146a and Relb preferentially control Ly-6Chi monocytes, which are cells that selectively expand in many chronic inflammatory conditions. Targeting of miR-146a or Relb may serve to suppress adverse inflammatory Ly-6Chi monocyte responses while sparing Ly-6Clo monocyte activity.
EXPERIMENTAL PROCEDURES
Mouse and human samples
The studies used 6–12 wk old mice. The institutional subcommittee on research animal care at Massachusetts General Hospital approved the animal studies. Human blood was obtained from healthy volunteers and collected in heparinized collection tubes in accordance to a protocol approved by the Committee on microbiological safety at Harvard Medical School.
Monoclonal Antibodies (Mabs), Flow Cytometry And Cell Sorting
Cell staining and cell sorting was performed as described in supplemental methods.
Gene expression arrays and analysis
Gene expression studies were performed in accordance to MIAME guidelines and are described in supplemental methods.
In Vivo Challenges
LPS from Escherichia coli (serotype O55:B5, Sigma) was given at 0.4 mg/kg in PBS daily i.p. for 4 d (or 7 d when indicated). Lm bacteria (strain EGDe, ATCC) were expanded in Brain Heart Broth (Fluka) and given i.v. at 3×103 CFU. Thioglycollate was given i.p. as a 4% solution in 1 ml RMPI.
In Vitro Challenges
5–6×104 isolated cells were plated in complete medium (RPMI, Cellgro Mediatech Inc.), 10% FCS (Stem Cell Technologies), 100 U/ml Pen/strep, 2 mM L-Glu (both Cellgro Mediatech Inc.) in round bottom 96-well plates. Stimulations included LPS (100 ng/ml, Sigma), rmTNFα (50 ng/ml, Peprotech) and HKLM (5×108/ml, heat killed Lm, Invivo Gen). Luminex cytokine assays (R&D Biosciences) were analyzed on a Luminex FlexMap 3D (Agilent) instrument.
Statistical Analysis
Results were analyzed with Prism 4.0 (GraphPad). P-values were determined using Student’s t tests. A p-value <0.05 was taken as statistically significant and higher significance is indicated in the figure legends. All graphs show mean ± SEM.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Mike Waring, Andrew Cosgrove and Adam Chicoine (Ragon Institute of MGH, MIT and Harvard) for cell sorting; Borja Saez (Harvard Medical School), Patrick Stern and David Feldser (MIT) for help with retroviral gene transfer. Charles Vanderburg and Anna Krichevsky (Harvard Medical School) for help with analytical RNA techniques; and Yoshiko Iwamoto and Joshua Dunham (MGH Center for Systems Biology) for help with immunofluorescence staining and imaging. Martin Etzrodt is part of the International PhD program ‘Cancer and Immunology’ at the University of Lausanne, Switzerland. This work was supported in part by National Institutes of Health grants NIH-R01 AI084880 and P30 DK043351 (to M.J. Pittet).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accession Numbers
The microarray data newly generated in this study are available on the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/gds) under the accession number GSE32364.
Supplemental Information
Supplemental Information includes Supplemental Experimental Procedures and Figures.
REFERENCES
- Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- Boldin M, Taganov K, Rao D, Yang L, Zhao J, Kalwani M, Garcia-Flores Y, Luong M, Devrekanli A, Xu J, Sun G, Tay J, Linsley P, Baltimore D. miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. Journal of Experimental Medicine. 2011;208:1189–1201. doi: 10.1084/jem.20101823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkly L, Hession C, Ogata L, Reilly C, Marconi LA, Olson D, Tizard R, Cate R, Lo D. Expression of relB is required for the development of thymic medulla and dendritic cells. Nature. 1995;373:531–536. doi: 10.1038/373531a0. [DOI] [PubMed] [Google Scholar]
- Cejas PJ, Carlson LM, Kolonias D, Zhang J, Lindner I, Billadeau DD, Boise LH, Lee KP. Regulation of RelB expression during the initiation of dendritic cell differentiation. Molecular and cellular biology. 2005;25:7900–7916. doi: 10.1128/MCB.25.17.7900-7916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong C, Matos I, Choi J, Dandamudi DB, Shrestha E, Longhi MP, Jeffrey KL, Anthony RM, Kluger C, Nchinda G, Koh H, Rodriguez A, Idoyaga J, Pack M, Velinzon K, Park CG, Steinman RM. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell. 2010;143:416–429. doi: 10.1016/j.cell.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cros J, Cagnard N, Woollard K, Patey N, Zhang S, Senechal B, Puel A, Biswas SK, Moshous D, Picard C, Jais J, D'cruz D, Casanova J, Trouillet C, Geissmann F. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33:375–386. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Gazzar M, Church A, Liu T, McCall CE. MicroRNA-146a regulates both transcription silencing and translation disruption of TNF-{alpha} during TLR4-induced gene reprogramming. J Leukoc Biol. 2011;90:509–519. doi: 10.1189/jlb.0211074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farh K. The Widespread Impact of Mammalian MicroRNAs on mRNA Repression and Evolution. Science. 2005;310:1817–1821. doi: 10.1126/science.1121158. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science (New York, NY) 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkin J, Schichl YM, Koeffel R, Bauer T, Richter S, Konradi S, Gesslbauer B, Strobl H. miR-146a is differentially expressed by myeloid dendritic cell subsets and desensitizes cells to TLR2-dependent activation. Journal of immunology. 2010;184:4955–4965. doi: 10.4049/jimmunol.0903021. [DOI] [PubMed] [Google Scholar]
- Lutgens E, Lievens D, Beckers L, Wijnands E, Soehnlein O, Zernecke A, Seijkens T, Engel D, Cleutjens J, Keller AM, Naik SH, Boon L, Oufella HA, Mallat Z, Ahonen CL, Noelle RJ, de Winther MP, Daemen MJ, Biessen EA, Weber C. Deficient CD40-TRAF6 signaling in leukocytes prevents atherosclerosis by skewing the immune response toward an antiinflammatory profile. The Journal of experimental medicine. 2010;207:391–404. doi: 10.1084/jem.20091293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J, Mack M, Pipeleers D, In't Veld P, De Baetselier P, Van Ginderachter JA. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–5739. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- Nahid MA, Pauley KM, Satoh M, Chan EK. miR-146a is critical for endotoxin-induced tolerance: implication in innate immunity. The Journal of biological chemistry. 2009;284:34590–34599. doi: 10.1074/jbc.M109.056317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahrendorf M, Swirski F, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. Journal of Experimental Medicine. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'connell R, Rao D, Chaudhuri AA, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nature Reviews Immunology. 2010;10:111–122. doi: 10.1038/nri2708. [DOI] [PubMed] [Google Scholar]
- Platzer B, Jörgl A, Taschner S, Höcher B, Strobl H. RelB regulates human dendritic cell subset development by promoting monocyte intermediates. Blood. 2004;104:3655–3663. doi: 10.1182/blood-2004-02-0412. [DOI] [PubMed] [Google Scholar]
- Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swirski F, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taganov K, Boldin M, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira S, Johnson T, Hirbe AC, Alhawagri M, Anwisye I, Sammut B, O'Neal J, Zou W, Weilbaecher KN, Faccio R, Novack DV. RelB is the NF-kappaB subunit downstream of NIK responsible for osteoclast differentiation. Proc Natl Acad Sci USA. 2008;105:3897–3902. doi: 10.1073/pnas.0708576105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- Varol C, Landsman L, Fogg DK, Greenshtein L, Gildor B, Margalit R, Kalchenko V, Geissmann F, Jung S. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med. 2007;204:171–180. doi: 10.1084/jem.20061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, D'Amico A, Winkel KD, Suter M, Lo D, Shortman K. RelB is essential for the development of myeloid-related CD8alpha-dendritic cells but not of lymphoid-related CD8alpha+ dendritic cells. Immunity. 1998;9:839–847. doi: 10.1016/s1074-7613(00)80649-4. [DOI] [PubMed] [Google Scholar]
- Zhao JL, Rao DS, Boldin MP, Taganov KD, O'Connell RM, Baltimore D. NF-kappaB dysregulation in microRNA-146a-deficient mice drives the development of myeloid malignancies. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:9184–9189. doi: 10.1073/pnas.1105398108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. 2007;81:584–592. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.