Abstract
Human induced pluripotent stem (iPS) cells potentially provide a unique resource for generating patient-specific cardiomyocytes to study cardiac disease mechanisms and treatments. However, existing approaches to cardiomyocyte production from human iPS cells are inefficient, limiting the application of iPS cells in basic and translational cardiac research. Furthermore, strategies to accurately record changes in iPS cell-derived cardiomyocyte action potential duration (APD) are needed to monitor APD-related cardiac disease and for rapid drug screening. We examined whether modulation of the bone morphogenetic protein 4 (BMP-4) and Wnt/β-catenin signaling pathways could induce efficient cardiac differentiation of human iPS cells. We found that early treatment of human iPS cells with BMP-4 followed by late treatment with small molecule Wnt inhibitors led to a marked increase in production of cardiomyocytes compared to existing differentiation strategies. Using immunocytochemical staining and real-time intracellular calcium imaging, we showed that these induced cardiomyocytes expressed typical sarcomeric markers, exhibited normal rhythmic Ca2+ transients, and responded to both β-adrenergic and electric stimulation. Furthermore, human iPS cell-derived cardiomyocytes demonstrated characteristic changes in action potential duration in response to cardioactive drugs procainamide and verapamil using voltage-sensitive dye-based optical recording. Thus, modulation of the BMP-4 and Wnt signaling pathways in human iPS cells leads to highly efficient production of cardiomyocytes with typical electrophysiological function and pharmacologic responsiveness. The use of human iPS cell-derived cardiomyocytes and the application of calcium- and voltage-sensitive dyes for the direct, rapid measurement of iPS cell-derived cardiomyocyte activity promise to offer attractive platforms for studying cardiac disease mechanisms and therapeutics.
Keywords: cardiomyocyte, embryonic stem cell, induced pluripotent stem cell, cardiac differentiation, optical recording
Introduction
The recent innovation of induced pluripotent stem (iPS) cells made from human somatic cells by forced gene expression circumvents many of the ethical issues encountered in human embryonic stem (ES) cell research and opens an exciting avenue to the generation of patient-specific cardiomyocytes for cardiac disease-modeling and therapeutics[1–8]. Cardiomyocytes derived from human iPS cells have been shown to be the functional equivalent of their human ES cell-derived counterparts [9]. However, the procurement of cardiomyocytes from human iPS cells using current approaches is inefficient [1, 6, 9], significantly limiting the application of iPS cells in basic and translational cardiac research.
Cardiomyocytes can be derived from human ES cells using both spontaneous and growth factor-directed approaches [10–19]. Treatment of human ES cells with multiple growth factors such as bone morphogenetic protein 4 (BMP-4), basic fibroblast growth factor (bFGF), activin A, vascular endothelial growth factor (VEGF) and the peptide Wnt inhibitor dickkopf homolog 1 (DKK-1) is able to improve the generation of cardiomyocytes [10, 16]. However, these approaches vary in their efficiency in human ES cell lines since cardiac differentiation efficacy is often cell-line dependent. Additionally, the use of multiple growth factors makes these approaches costly. Thus, it is of interest to develop novel applicable, efficient strategies to induce cardiac differentiation of human ES cells as well as iPS cells.
Small molecules have been used widely to modulate signaling pathways critical in the self-renewal and lineage differentiation of stem cells due to their potency, affordability, and ability to enter cells [15, 20–22]. Chen et al. recently discovered two small molecule Wnt inhibitors: IWR-1 (inhibitor of Wnt response 1) and IWP-1 (inhibitor of Wnt production 1), which are potentially novel anti-tumor agents [23]. In this study, we investigated whether these small molecules modulate cardiac differentiation of human ES and iPS cells. We further hypothesized that by enhancing both early mesoderm and subsequent cardiac mesoderm formation through the early treatment with BMP-4 coupled with late inhibition of the Wnt/β-catenin activity by small molecules, respectively, cardiomyocyte differentiation of human ES and iPS cells will be enhanced.
The generation of cardiomyocytes from human iPS cells would potentially facilitate both the study of mechanisms of hereditary cardiac arrhythmic disorders [2, 4] and screening for drug-induced pro-arrhythmia related to the prolongation of action potential duration (APD) [24, 25]. Variations in disease severity, incomplete disease penetrance and the high-throughput requirement for drug discovery point to the desirability of a highly efficient platform to accurately assess changes in human cardiomyocyte APD. The voltage-sensitive dye-based optical recording is a highly efficient system to measure APD and has been performed in guinea-pig cardiomyocytes [26] and in intact animal hearts [27, 28]. However, whether this approach is feasible to monitor changes in human iPS cell-derived cardiomyocytes’ APDs in response to cardioactive drugs remains to be investigated.
In the present study, we discovered that early treatment of human ES and iPS cells with BMP-4 coupled with late Wnt inhibition by small molecules markedly increases the production of cardiomyocytes with typical electrophysiological function and pharmacologic responsiveness. Using human iPS cell-derived cardiomyocytes, we then successfully applied calcium- and voltage-sensitive dyes for the direct, rapid monitoring of iPS cell-derived cardiomyocyte activity. Our study provides attractive platforms for studying cardiac disease mechanisms and therapeutics.
Materials and Methods
Human ES and iPS Cells Culture and differentiation
The human ES and iPS cells were cultured on irradiated mouse embryonic fibroblasts (MEF) and were maintained in undifferentiated ES culture medium for 5–6 days. Cells were detached with collagenase type IV (Invitrogen) to make embryoid bodies (EBs) of about 100–200 cells. Upon aggregation, ES cells begin to differentiate in a manner mimicking embryonic development. EBs were cultured in suspension in differentiation medium for 4 days. EBs were attached to plates coated with 0.1% gelatin on day 4 at 1:1 ratio, and either small molecules or DMSO were added on day 5. The medium was changed every other day. After 10 days, the medium’s FBS content (20%) was reduced to 2.5%. EBs were examined daily for beating starting on day 12. Detailed information of human ES and iPS cell culture can be found in Supplemental Methods.
Quantitative RT-PCR
See Supplemental Methods for details.
Immunostaining and Confocal Microscopy
See Supplemental Methods for details.
Fluorescence-activated cell sorting (FACS) analysis
See Supplemental Methods for details.
Western Blots
See Supplemental Methods for details.
Calcium Transient Measurements
For calcium imaging, beating EBs were manually dissected and digested with Collagenase A and Collagenase B (1:1, Roche, 6.67 mg/mL) and plated onto gelatin-coated cover slips. After 2–3 days in culture the beating cardiomyocyte clusters were loaded with 25 μM cell permeant calcium indicator dye fluo-4 AM (Invitrogen) together with 0.1% Pluronic F-127 (Invitrogen) in Tyrode solution. Before imaging, the cells were allowed to de-esterify the fluo-4 AM for 30 minutes in Tyrode solution at 37°C. Fluorescence was measured by manually defining each region of interest and quantified in relation to baseline fluorescence (F/F0). The samples were electrically stimulated by field pacing (10 V, 10 ms bipolar pulses at 0.2–4 Hz). The samples were stimulated with the β-adrenoceptor agonist isoproterenol (Sigma) at 500 nM. See Supplemental Methods for details.
Action Potential Recordings
Beating clusters were derived as described above and were stained with 7.5μM di-4-ANEPPS (Invitrogen), washed and imaged during perfusion with Tyrode’s solution at 1 to 2 ml/min at 37°C. To test drugs, 25 μM procainamide (Sigma, P9391) or 375 nM verapamil (Sigma, V4629) were added to the perfusion solution. These concentrations were chosen to simulate the tissue concentration resulting from clinical administration of these drugs. Action potentials were induced by field stimulation and recordings were captured before and during drug perfusion and after a washout by Tyrode’s solution. Action potential duration (APD) was calculated from the time of maximal rise in membrane potential to the time at 90% of repolarization for each beating cluster from three consecutive beats. See Supplemental Methods for details.
Statistical Analysis
Data were presented as mean ± standard error of the mean (SEM) from three independent experiments. Sigma Plot was used to administer a one-tailed Student’s t-test to action potential measurements in experiments using arrhythmogenic drugs because it was known that those drugs have particular lengthening or shortening effects on APD. All other experiments were analyzed with two-tailed Student’s t-tests. A probability value <0.05 was considered statistically significant.
Results
BMP-4 Promotes Cardiac Differentiation of Human ES Cells
We determined the effects of BMP-4 on the expression of BrachyuryT and mesoderm posterior 1 (MESP1), two transcriptional factors that are transiently expressed in early mesoderm in human ES cells upon differentiation [10, 13]. As shown in Fig. 1A-1D, BMP-4 treatment of human embryoid bodies (EBs: aggregates of cells derived from ES cells) in suspension culture for 4 days resulted in a marked increase in expression of both BrachyuryT and MESP1. To determine if up-regulation of mesodermal gene expression leads to enhanced cardiac differentiation, we re-plated these EBs in differentiation media without BMP-4 for another 8 days. We observed a significant increase in beating EBs in samples treated with BMP-4 vs. control, with an optimal cardiogenic effect at 25 ng/ml (Fig. 1E). Thus, BMP-4 treatment of human EBs in suspension for 4 days markedly promotes mesoderm formation and subsequent cardiomyocyte production from human ES cells.
Figure 1. Effect of BMP-4 on cardiac differentiation in human ES cells.
A, Schematic diagram of protocol used for cardiac differentiation of human ES and iPS cells. B, Undifferentiated human ES cells (hESCs), human iPS cells (hiPSCs) and EBs derived from hESCs and hiPSCs. Scale bar, 500 μm. C and D, qPCR quantification of the expression of two transcriptional factors, Brachyury T (C) and mesoderm posterior 1 (MESP1, D), that are transiently expressed in early mesoderm. The EBs were cultured in differentiation medium with BMP-4 (0–50 ng/ml) for 4 days in suspension. E, EBs were attached to gelatin-coated plates for further differentiation after 4 day suspension culture with BMP-4 (0–50 ng/ml). Beating clusters were quantified on day 15 of differentiation. Mean ± SEM (n=3 independent experiments). *p<0.05 vs. 0 ng/ml BMP-4 treatment; #p<0.05 vs. 12.5 ng/ml BMP-4 treatment.
Effects of IWR-1 on β-Catenin Expression Level and Genes Involved in Early Cardiogenesis of Human ES Cells
We next investigated whether inhibition of Wnt/β-catenin signaling can enhance BMP-4-directed cardiac differentiation of human ES cells. We utilized a recently discovered small molecule Wnt inhibitor IWR-1 [23], which abolishes destruction of Axin protein, a molecule involved in the degradation of β-catenin [29]. We harvested human EBs treated with BMP-4 in suspension for 4 days followed by the presence or absence of IWR-1 for an additional 2 days. Treatment with IWR-1 significantly decreased β-catenin protein levels in differentiating human ES cells (Fig. 2A). To investigate whether the IWR-1-mediated decrease of β-catenin in differentiating human ES cells leads to induction of genes involved in early cardiogenesis, we performed qRT-PCR analyses for NKX2.5, ISL1, GATA4 and MEF2C, genes commonly expressed in cardiac mesoderm or cardiac progenitor cells [10, 17, 21]. The mRNA levels for NKX2.5, ISL1, GATA4 and MEF2C were all found to increase significantly in EBs treated with BMP-4 followed by IWR-1 compared to controls treated with BMP-4 alone (Fig. 2B-2E).
Figure 2. Effects of Wnt inhibitors on the expression of β-catenin protein and cardiac mesodermal/progenitor marker genes.
A, EBs made from H7 human ES cells were cultured in the presence of BMP-4 (25 ng/ml) for 4 days in suspension, followed by a 2-day treatment with IWR-1 (10 μM) or DMSO. EBs were then harvested for detection of β-catenin protein by Western blot analysis (Left panel). Right panel: Quantification of β-catenin protein. Mean ± SEM (n=3), *p<0.05. (B-E) EBs were treated with BMP-4 (25 ng/ml) in suspension for 4 days and then treated with IWR-1 (10 μM) or vehicle for another 2 days. EBs were harvested and subjected to qRT-PCR analysis for cardiac mesodermal/progenitor genes: NKX2.5 (B), ISL1 (C), GATA4 (D) and MEF2C (E). Mean ± SEM (n=3), *p<0.05.
Addition of Small Molecule Wnt Inhibitors after Mesoderm Induction with BMP-4 Further Enhances Cardiac Differentiation of Human ES Cells
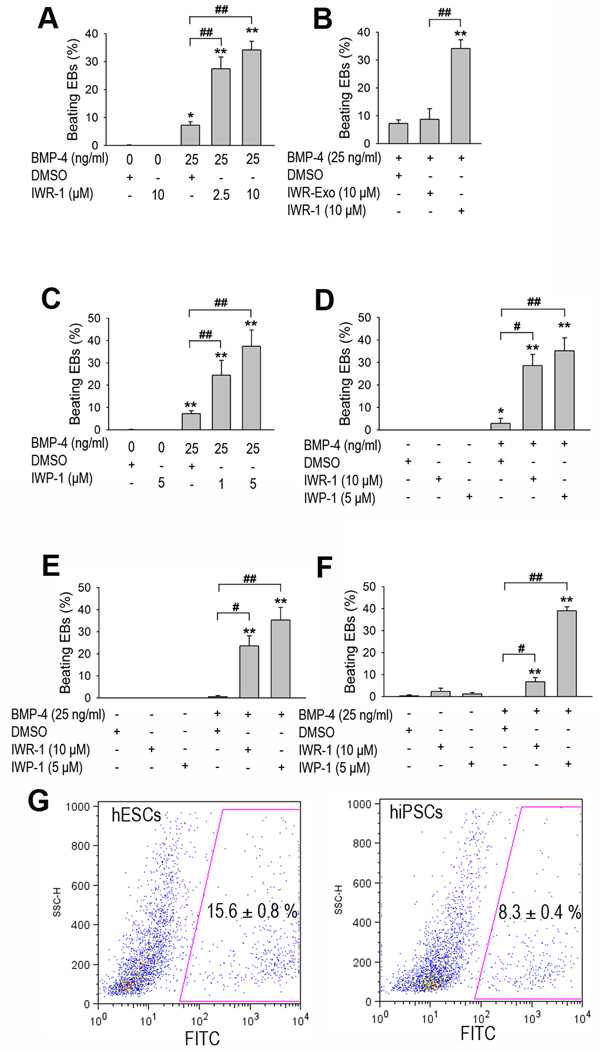
To investigate whether IWR-1 induction of cardiac differentiation depends on BMP-4 induction, EBs were cultured in either the presence or the absence of BMP-4 for 4 days and then treated with IWR-1 (Fig. 1A). A marked increase in beating cardiomyocyte clusters was observed after 12–14 days of differentiation (27.5% and 34.1% for 25 ng/ml BMP-4 with 2.5 µM and 10 µM IWR-1, respectively) compared to 7.2% for BMP-4 alone and 0 beating clusters for IWR-1 treatment alone (Fig. 3A). IWR-1-exo, a diastereomeric form of IWR-1 that exhibits a decreased ability to inhibit Wnt signaling compared to IWR-1[23], failed to promote cardiomyocyte differentiation of human EBs pretreated with BMP-4 (Fig. 3B), suggesting a specific effect of IWR-1 in promoting cardiac differentiation. Moreover, IWP-1, a small molecule that inhibits the activity of the membrane-bound acyltransferase essential to the lipid modification and signaling ability of Wnt proteins [23], was also able to promote cardiomyocyte cluster formation in human EBs pre-treated with BMP-4 (24.4% and 37.7% beating EBs for 25 ng/ml BMP-4 with 1 µM and 5 µM IWP-1, respectively; Fig. 3C). When we tested the H1 human ES cell line, we observed a comparable effect of BMP-4 and Wnt inhibitors in promoting cardiac differentiation (28.6% and 35.2% beating EBs for 25 ng/ml BMP-4 with 10 µM IWR-1 and 5 µM IWP-1, respectively, compared to 2.9% for 25 ng/ml BMP-4 alone; Fig. 3D). Taken together, these results show that small molecule Wnt inhibitors can synergistically interact with BMP-4 in promoting cardiac differentiation of human ES cells.
Figure 3. Effects of Wnt inhibitors on cardiac differentiation of human ES and iPS cells.
EBs were cultured in the presence or absence of BMP-4 (25 ng/ml) in suspension culture for 4 days, followed by a treatment of DMSO (vehicle control) or Wnt small molecule inhibitors for 2 additional days, and the number of beating EBs or cardiomyocytes was quantified on day 15. (A) Effect of BMP-4 and IWR-1 on formation of beating EBs made from human H7 ES cells. (B) Effect of BMP-4 and IWR-1 or IWR-Exo (a diastereomeric form of IWR-1 used as a negative control) on formation of beating EBs made from human H7 ES cells. (C) Effect of BMP-4 and another small molecule Wnt inhibitor, IWP-1, on formation of beating EBs made from human H7 ES cells. (D-F) Effects of BMP-4 and Wnt inhibitors on cardiac differentiation of human ES cell line H1 (D) and human iPS cell lines: fetal lung fibroblasts IMR90 C1 iPS (E) and neonatal foreskin C1 iPS cells (F). (G) Day 15 EBs of H7 human ES cells (hESCs; left panel) and neonatal foreskin C1 iPS cells (hiPSCs; right panel) induced by BMP-4 and IWP-1 were dissociated into single cells, fixed, immunostained with cardiac troponin T (cTnT), and quantified by FACS. Mean ± SEM (n=3 independent experiments). *p<0.05 vs. DMSO; **p<0.01 vs. DMSO, #p<0.05 vs. BMP-4 + DMSO; ##p<0.01 vs. BMP-4 + DMSO.
BMP-4 and Small Molecule Wnt Inhibitors Synergistically Promote Cardiac Differentiation of Human iPS Cells
Because signaling pathways modulating cardiac differentiation of human iPS cells are largely unknown, we investigated whether a combination of BMP-4 treatment and Wnt inhibition was also able to improve cardiac differentiation in human iPS cells. We generated EBs using the fetal lung fibroblasts IMR90 C1 iPS cell line [7]. Early BMP-4 treatment followed by Wnt inhibition using either IWR-1 or IWP-1 increased cardiomyocyte cluster formation significantly in iPS cells compared to treatment with BMP-4 alone or a small molecule Wnt inhibitor alone (23.6% and 35.3% for 25 ng/ml BMP-4 with 10 µM IWR-1 and 5 µM IWP-1, respectively, vs 0.53% for 25 ng/ml BMP-4 alone and 0 beating clusters for IWR-1 or IWP-1 alone; Fig. 3E).
To test whether this strategy also would promote cardiac differentiation of other human iPS cell lines, we generated EBs using the neonatal foreskin C1 iPS cells [7]. The combination of early BMP-4 treatment and late Wnt inhibition resulted in a marked increase in cardiomyocyte differentiation compared to control treatment (6.8% and 39.0% for 25 ng/ml BMP-4 with 10 µM IWR-1 and 5 µM IWP-1, respectively, vs 0 beating clusters for 25 ng/ml BMP-4 alone; Fig. 3F), suggesting a cardiogenic effect of BMP-4 and Wnt inhibition independent of the iPS cells’ origin. To determine the percentage of cardiomyocytes within the cultures, we performed flow cytometry analysis using cardiac troponin T (cTnT) as a marker. Early treatment of H7 human ES and neonatal foreskin C1 iPS cells with BMP-4 coupled with late Wnt inhibition by IWP-1 led to 15.6% and 8.3% cardiomyocytes production in culture, respectively (Fig. 3G). These observations indicate that BMP-4 and small molecular Wnt inhibitors synergistically promote cardiac differentiation of human iPS cells as they do in human ES cells.
Small Molecule-Induced Cardiomyocytes Derived from Human ES and iPS Cells Express Typical Sarcomeric Markers
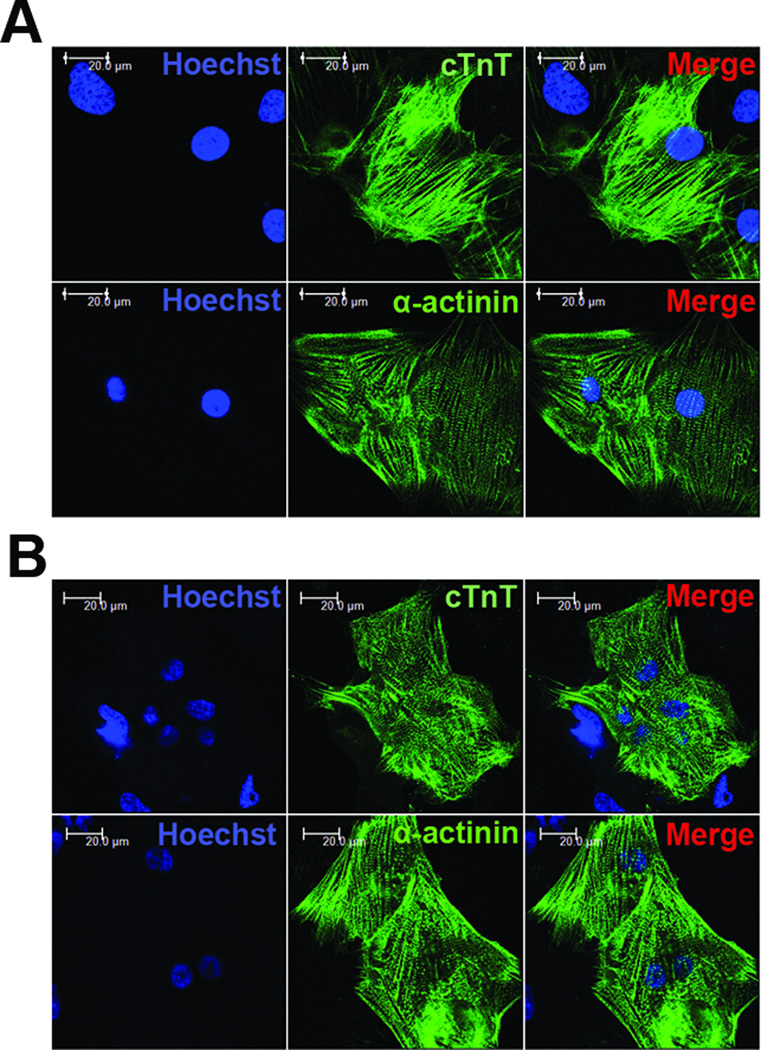
To evaluate the expression of myofilament proteins in BMP-4/IWR-1-induced human ES and iPS cell-derived cardiomyocytes (hES-CMs; hiPS-CMs), we performed immunostaining for cTnT. As shown in (Fig. 4A and 4B; top panel), hES-CMs and hiPS-CMs exhibited comparable striated, sarcomeric labeling. We also performed immunolabeling for α-sarcomeric actinin, another highly specific myofilament protein, using cariomyocytes enzymatically dissociated from beating EBs. Immunofluorescent imaging showed a clear striated pattern for α-sarcomeric actinin in both hESCMs and hiPS-CMs (Fig. 4A and 4B; bottom panel). These results show that BMP-4/IWR-1-induced hES-CMs and hiPS-CMs develop well-organized sarcomeric structures.
Figure 4. Immunolabeling of cTnT and α-actinin in hES-CMs and hiPS-CMs.
Beating EBs around day 20 of H7 human ES cells (A) or fetal lung fibroblasts IMR90 C1 iPS cells (B) were manually dissected and enzymatically dissociated into small cardiomyocyte clusters, which were then replated onto 0.1% gelatin-coated coverslips. Cells were fixed and immunostained with antibodies for cTnT and α-actinin antibodies. Nuclei were counterstained with Hoechst 33258. Scale bars, 20 µm.
Real-time Calcium [Ca2+]i Transient Analysis of Small Molecule-Induced hES-CMs and hips-CMs
To characterize whether small molecule-induced hES-CMs and hiPS-CMs adopt a fully differentiated cardiac phenotype, we enzymatically dissociated beating EBs into small beating cardiomyocyte clusters, loaded them with the calcium indicator dye fluo-4 AM, and performed real-time intracellular calcium [Ca2+]i imaging (Fig. 5A and 5B). Both hES-CMs (Fig. 5C) and hips-CMs (Fig. 5D) in the beating clusters displayed [Ca2+]i oscillations. The duration of the calcium transients between hES-CMs and hiPS-CMs showed modest differences possibly due to variation in differentiation state of cardiomyocytes in each cluster. More importantly, however, isoproterenol stimulation induced a marked increase in the frequency of [Ca2+]i transients in both hES-CMs (Fig. 5C) and hiPS-CMs (Fig. 5D), suggesting the existence of functional β-adrenergic signaling pathways. In addition, increasing frequencies of external field stimulation resulted in faster [Ca2+]i oscillations in both hES-CMs (Fig. 5E) and hiPS-CMs (Fig. 5F).
Figure 5. Calcium transients in hES-CMs and hiPS-CMs.
The beating cardiomyocyte clusters derived from beating EBs were loaded with the cell permeant calcium indicator dye fluo-4 AM. Fluorescence was measured by manually defining a region of interest centered on spontaneously beating clusters and comparing that measurement to baseline fluorescence (F/F0). A and B, pseudo-color images show minimal (Ca2+ min) and maximal (Ca2+ max) fluo-4 fluorescence intensity of H7 human ES (A) and fetal lung fibroblasts IMR90 C1 iPS cell-derived cardiomyocyte clusters (B). C and D, the frequency of [Ca2+] transients was increased during isoproterenol stimulation in human ES (C) and human iPS cell-derived cardiomyocyte clusters (D). E and F, [Ca2+] transients of cardiomyocyte clusters differentiated from human ES (E) and human iPS cells (F) responded to electric pacing at multiple frequencies (0.5, 1 and 1.5 Hz).
Optical Measurement of Action Potential Duration of Small Molecule-Induced hES-CMs and hiPS-CMs
To determine whether voltage-sensitive dye-based optical recording might be an effective approach to measure APD in hiPS-CMs and to further validate the cardiomyocyte phenotype, we assessed membrane potential changes in both hES-CMs and hiPS-CMs within the beating clusters. Compared to the characteristics of embryonic atrial-, ventricular- and nodal-like action potentials shown in the previous studies [9, 14, 18], the characteristics of the action potentials of the cardiomyocytes we derived showed predominant embryonic ventricular-like action potential waveforms that displayed rapid upstrokes and distinct plateau phases (Fig. 6A-D insets, solid line). Variations in baseline APDs between experiments (Fig. 6A-D insets, solid line) were most likely due to compositional heterogeneity in the cluster. Importantly, however, when treated with procainamide, a sodium and potassium dual channel blocker [30, 31], both hES-CMs and hips-CMs exhibited significantly lengthened APDs (Fig. 6A and 6C, respectively). This effect was reversible as APDs returned to normal following drug washout with vehicle solution. In contrast, the L-type calcium channel blocker verapamil [25, 30, 31] reversibly shortened APDs in both hES-CMs and hiPS-CMs (Fig. 6B and 6D, respectively). These results suggest that the ion channels on hES-CMs and hiPS-CMs are functional and can be pharmacologically modulated.
Figure 6. Effect of channel modulating drugs on the action potential durations of hES-CMs and hiPS-CMs.
Beating cardiomyocyte clusters derived from H7 human ES and fetal lung fibroblasts IMR90 C1 iPS cells were stained with the voltage sensitive dye di-4-ANEPPS and electrically paced at 0.5 Hz. Insets in each panel show the representative evoked action potentials before (solid line: A: 201 ms; B: 209 ms; C: 211 ms; D: 161 ms) and 15 minutes after application (dashed line: A: 251 ms; B: 180 ms; C: 246 ms; D: 134 ms) of procainamide (25 μM) or verapamil (375 nM). Note that action potentials in the insets are shown in normalized units. APD90 (the time of maximal rise in membrane potential to the time at 90% of repolarization) was normalized to baseline. Graphs show the time-dependence of responses after (A) procainamide and (B) verapamil of human ES cell-derived cardiomyocyte clusters and (C) procainamide and (D) verapamil of human iPS cell-derived cardiomyocyte clusters as well as washout recovery for all samples. Mean ± SEM (n=3). *p < 0.05 vs. base line; # p< 0.05 vs. 15-min of drug treatment.
Discussion
Human iPS cells potentially provide an attractive platform for generating patient-specific cardiomyocytes for cardiac disease-modeling, drug screening and regenerative therapy [1, 2, 4]. Through sequential BMP-4 treatment and Wnt inhibition by small molecules, we used human iPS cells and efficiently generated functional cardiomyocytes. These hiPS-CMs expressed typical sarcomeric markers and exhibited typical electrophysiological characteristics, such as responsiveness to β-adrenergic and electric stimulation and to drugs that modulate ion channels contributing to action potentials. Thus, our study lays a foundation for the application of human iPS cells to basic and clinical cardiac research. Furthermore, we established a highly efficient platform to monitor changes in APDs of hiPS-CMs using voltage-sensitive dye-based optical recording. Thus, our hiPS-CMs provide an attractive platform for APD-related disease modeling, pro-arrhythmia screening for novel therapeutics and drug discovery.
Differentiation of human iPS cells into cardiomyocytes in the past has been inefficient [1, 6, 9]. Our cardiac differentiation efficiency (35.3% beating EB for fetal lung IMR C1 iPS and 39.0% for neonatal foreskin C1 iPS cells induced by BMP-4 and IWP-1) is significantly higher (about 8-fold) than that reported by Zhang et al. (about 4.5% beating EB for either iPS line) [9]. We were able to derive approximately 60 and 40 beating EBs per well of a 6-well plate from fetal lung IMR C1 and neonatal foreskin C1 human iPS cells, respectively (Fig. S2). Furthermore, cTnT FACS analysis revealed that 8.3% of the cells derived from Foreskin C1 iPS cells induced by BMP-4 and IWP-1 were cardiomyocytes (Fig. 3G).
We found that BMP-4 treatment resulted in a marked increase in early mesoderm lineage commitment (as evidenced by increased Brachyury T and Mesp1 expression) and beating efficiency in ES cell-derived cardiomyocytes (Fig. 1E), consistent with a role of BMP-4 in promoting cardiogenesis [10, 13, 16]. We next investigated whether inhibition of the Wnt pathway using the small molecule IWR-1 can enhance BMP-4 directed cardiac differentiation. IWR-1 not only increased expression of genes commonly expressed in cardiac mesoderm/progenitor cell (Fig. 2), it also significantly improved cardiac differentiation when introduced after the application of BMP-4 (Fig. 3), suggesting a critical, stage-specific role of BMP stimulation and Wnt inhibition during cardiac differentiation of human ES and iPS cells. Previous studies have suggested that inhibition of the Wnt/β-catenin signaling pathway is required for efficient cardiac mesoderm/progenitor specification from early mesoderm [10, 21, 32, 33]. Our results are consistent with a model in which our small molecule Wnt antagonists inhibit Wnt activity in early mesoderm in a cell-autonomous manner. To our knowledge, it has not yet been reported that Wnt inhibition in ectodermal or endodermal lineages could enhance cardiogenesis. This possibility warrants further investigation in murine models in which Wnt activity could be conditionally deleted in ectodermal or endodermal lineages and then cardiogenesis quantified.
Combinatorial approaches with BMP-4, bFGF, activin A, VEGF and DKK-1 leads to a highly efficient cardiac differentiation of human ES cell lines [10, 16]. However, such strategies have not been tested in multiple human ES or iPS cell lines. In addition, the application of multiple costly growth factors compromises the general use of these strategies. By contrast, the current study achieved a highly efficient cardiac differentiation of H7 (71 beating EBs/well, Fig. S1 or 37.7% beating EB, Fig. 3C) and H1 (60 beating EBs/well, Fig. S1 or 35.2% beating EB; Fig. 3D) human ES cells by using a single growth factor BMP-4 coupled with a small molecule Wnt inhibitor IWP-1. Based on cTnT FACS data, 15.6% of the cells derived from H7 ES cell culture induced by BMP-4 and IWP-1 were cardiomyocytes (Fig. 3G). RT-PCR results suggested that our differentiation protocol could generate various subtypes of cardiomyocytes: atrial (MLC2A: atrial myosin light chain), ventricular (MLC2V: ventricular myosin light chain) and nodal-like (HCN4: hyperpolarization-activated cyclic nucleotide-gated channel 4) cardiomyocytes (Fig. S3). Notably, these small molecules can be produced in large quantities with relatively straight forward synthetic schemes [23]. Because cardiac differentiation efficacy may depend on which cell line is used, our novel BMP-4/small molecule Wnt inhibitor strategy provides an easily applicable means to optimize cardiomyocyte production from human ES and iPS cell lines utilized in individual research laboratories.
The hiPS-CMs and hES-CMs expressed typical sarcomeric markers (Fig. 4), exhibited normal Ca2+ transient rhythm, and responded to both β-adrenergic and electric stimulation (Fig. 5), validating the cells’ phenotypic and functional identities. Furthermore, the hES-CMs and hiPS-CMs were affected by the L-type calcium channel blocker, verapamil, and the sodium and potassium channel blocker, procainamide, in a reversible manner (Fig. 6). Therefore, our findings suggest that hES-CMs and hiPS-CMs created from this study express functional ion channels contributing to action potentials that can be modulated by procainamide and verapamil. Our results are consistent with those described by Zhang et al.[9], supporting the notion that hiPS-CMs are functionally similar to hES-CMs. Notably, the calcium transient duration (Fig. 5C-D) and baseline APD (Fig. 6A-D) between hES-CMs and hiPS-CMs cluster showed modest differences possibly due to the existence of non-cardiomyocytes within each beating cluster and/or the difference in differentiation state of cardiomyocytes in the cluster. Future efforts will be needed to develop strategies to derive highly purified, phenotypically mature hES-CMs and hiPS-CMs.
Another important feature of this study is that we were able to demonstrate the differentiation pathway in a number of cell lines. The initial findings in H7 and H1 ES cells were then extended to fetal lung and neonatal foreskin iPS lines created by Dr. James Thompson [7]. These results establish yet another tie between these two cell types. The fact that our protocol appears to be highly effective in multiple lines of ES and iPS cells is important in suggesting that we have identified a strategy that might be broadly applicable for cardiac differentiation.
Hereditary and drug-induced cardiac arrhythmias are devastating diseases for both the family and the medical community. Abnormal action potential due to irregular cardiac depolarization and repolarization is one of the integral mechanistic features of cardiac arrhythmias. The generation of functional hiPS-CMs provides an attractive platform for disease-modeling and drug development. Currently, patch-clamp technology is the gold standard for studying APD-related cardiac diseases [2, 4]. However, it is technically demanding and unable to detect the propagation and spatial heterogeneity of action potentials in multicellular models. Extracellular field potentials detected by electrodes integrated into the cultureware represent another promising model for studying cardiac electrophysiology and drug screening [24, 25]. However, they only report the electrical activities of cardiomyocytes proximal to the electrodes. Furthermore, integration of electrodes into the cultureware is an expensive proposition as it is unlikely that cultureware used for this purpose will be reusable.
Patch clamping is a traditional method for analysis of single cell action potentials. In this study, using voltage-sensitive dye-based optical recording, we successfully recorded changes in the APD of hiPS-CMs in response to two cardioactive drugs: procainamide and verapamil (Fig. 6). Optical recording of action potentials in conjunction with the generation of patient-specific iPS cells can be used to identify and study abnormal cardiac electrophysiology caused by genetic diseases. It is also scalable for high-throughput functional screening for unintended or off-target effects of novel cardiac and non-cardiac medicine. Scalability also allows for safe testing of pharmaceutical intervention on a patient-by-patient basis. Therefore, optical recordings of hiPS-CMs using affordable voltage- and calcium-sensitive dyes were established and used in this study. These dye based techniques have been validated in a variety of systems [26–28] and in this study we show that they represent an attractive option for screening and functional characterization of cardiac arrhythmia defects and for drug toxicity screening and optimization for treatment.
Despite the advances presented in this work, several challenges remain. First, techniques are needed to develop phenotypically mature cardiomyocytes as opposed to the embryonic phenotype observed both in this study and with other current techniques [9, 14, 18]. For example, these pluripotent stem cells-derived cardiomyocytes tend to have slower upstroke velocity compared to adult cardiomyocytes. In addition, it has been shown that murine and human ES cells-derived cardiomycytes lack transverse tubules that exist in adult myocardium and are essential for highly efficient excitation-contraction coupling [34]. Physiological stimuli such as electrical pacing, mechanical stretch and shear stress may help to promote cardiomyocyte maturation. Second, hips-CM clusters represent a mixed population of cardiomyocytes containing atrial, ventricular and nodal cardiomyocytes [9, 14, 18]. Two groups have reported methods for the purification of ventricular cardiomyocytes from human ES cells [35, 36], but it is important to extend this methodology to iPS cells. Third, the iPS cells were generated using viral transformation; to ensure the safety of cardiac regeneration, it will be critically important to derive functional cardiomyocytes from virus- and transgene-free human iPS cells generated by direct delivery of reprogramming proteins and mRNAs [37–39].
Conclusion
In summary, we have exploited the BMP-4 and Wnt signaling pathways to induce highly efficient cardiac differentiation of human ES and iPS cells. Our study shows that small molecule-induced hES-CMs and hiPS-CMs express cardiac-specific myofilament proteins and respond to β-adrenergic stimulation and electric pacing. By showing that hES-CMs and hiPS-CMs respond to treatment with ion channel-modulating drugs, this work helps to lay a foundation for the use of hiPS-CMs to predict drug-induced cardiac arrhythmia or to screen for and study inherited electrical defects. We believe this study provide a crucial foundation for future studies that will identify additional steps in the cardiac differentiation pathway of pluripotent cells and will improve cardiac differentiation efficiency.
Supplementary Material
Acknowledgements
We thank Dr. James Thompson for providing human iPS cell lines and Dr. Caihong Qiu for technical support for culturing human iPS cells. We acknowledge Dr. Frederick Sigworth for providing advice on measuring APs. We thank Drs. Lawrence Young, Jeffrey Bender, Raymond Russell, Kerry Russell, Diane Krause, Ivana Kuo and Esra Cagavi Bozkulak for their critical reviews of the manuscript. We appreciate Drs. Rong Ju and Yong Deng’s assistance on our experiments. Thanks to Dr. Michael Simons for sharing his lab equipment.
Source of Funding
This work was supported by the Yale startup fund, the Connecticut Stem Cell 09SCAYALE10, NIH 1K02HL101990-01 and UL1 RR024139 (YQ), NIH 5T32 HL007950 (PJ), the Finnish Foundation for Cardiovascular Research (JP), Welch Foundation I-1665 and NIH 1R01GM076398-01 (LL), and NIH DK57751 and DK061747 (BEE).
Footnotes
Disclosures
Lawrence Lum is listed as an inventor on a pending patent application for the IWR-1 and IWP-1 compounds.
References
- 1.Carvajal-Vergara X, Sevilla A, D'Souza SL, Ang YS, Schaniel C, Lee DF, et al. Patient-specific induced pluripotent stem-cell-derived models of LEOPARD syndrome. Nature. Jun 10;465(7299):808–812. doi: 10.1038/nature09005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Itzhaki I, Maizels L, Huber I, Zwi-Dantsis L, Caspi O, Winterstern A, et al. Modelling the long QT syndrome with induced pluripotent stem cells. Nature. Jan 16; doi: 10.1038/nature09747. [DOI] [PubMed] [Google Scholar]
- 3.Maherali N, Sridharan R, Xie W, Utikal J, Eminli S, Arnold K, et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007 Jun 7;1(1):55–70. doi: 10.1016/j.stem.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Moretti A, Bellin M, Welling A, Jung CB, Lam JT, Bott-Flugel L, et al. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N Engl J Med. Oct 7;363(15):1397–1409. doi: 10.1056/NEJMoa0908679. [DOI] [PubMed] [Google Scholar]
- 5.Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008 Jan 10;451(7175):141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007 Nov 30;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 7.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007 Dec 21;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 8.Zwi L, Caspi O, Arbel G, Huber I, Gepstein A, Park IH, et al. Cardiomyocyte differentiation of human induced pluripotent stem cells. Circulation. 2009 Oct 13;120(15):1513–1523. doi: 10.1161/CIRCULATIONAHA.109.868885. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, et al. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009 Feb 27;104(4):e30–e41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008 May 22;453(7194):524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 11.Xu C, Police S, Rao N, Carpenter MK. Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circ Res. 2002 Sep 20;91(6):501–508. doi: 10.1161/01.res.0000035254.80718.91. [DOI] [PubMed] [Google Scholar]
- 12.Tran TH, Wang X, Browne C, Zhang Y, Schinke M, Izumo S, et al. Wnt3a-induced mesoderm formation and cardiomyogenesis in human embryonic stem cells. Stem Cells. 2009 Aug;27(8):1869–1878. doi: 10.1002/stem.95. [DOI] [PubMed] [Google Scholar]
- 13.Takei S, Ichikawa H, Johkura K, Mogi A, No H, Yoshie S, et al. Bone morphogenetic protein-4 promotes induction of cardiomyocytes from human embryonic stem cells in serum-based embryoid body development. Am J Physiol Heart Circ Physiol. 2009 Jun;296(6):H1793–H1803. doi: 10.1152/ajpheart.01288.2008. [DOI] [PubMed] [Google Scholar]
- 14.Mummery C, Ward-van Oostwaard D, Doevendans P, Spijker R, van den Brink S, Hassink R, et al. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation. 2003 Jun 3;107(21):2733–2740. doi: 10.1161/01.CIR.0000068356.38592.68. [DOI] [PubMed] [Google Scholar]
- 15.Leschik J, Stefanovic S, Brinon B, Puceat M. Cardiac commitment of primate embryonic stem cells. Nat Protoc. 2008;3(9):1381–1387. doi: 10.1038/nprot.2008.116. [DOI] [PubMed] [Google Scholar]
- 16.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007 Sep;25(9):1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 17.Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, et al. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001 Aug;108(3):407–414. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He JQ, Ma Y, Lee Y, Thomson JA, Kamp TJ. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ Res. 2003 Jul 11;93(1):32–39. doi: 10.1161/01.RES.0000080317.92718.99. [DOI] [PubMed] [Google Scholar]
- 19.Fukuda K, Yuasa S. Stem cells as a source of regenerative cardiomyocytes. Circ Res. 2006 Apr 28;98(8):1002–1013. doi: 10.1161/01.RES.0000218272.18669.6e. [DOI] [PubMed] [Google Scholar]
- 20.Chen S, Do JT, Zhang Q, Yao S, Yan F, Peters EC, et al. Self-renewal of embryonic stem cells by a small molecule. Proc Natl Acad Sci U S A. 2006 Nov 14;103(46):17266–17271. doi: 10.1073/pnas.0608156103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qyang Y, Martin-Puig S, Chiravuri M, Chen S, Xu H, Bu L, et al. The renewal and differentiation of Isl1+ cardiovascular progenitors are controlled by a Wnt/beta-catenin pathway. Cell Stem Cell. 2007 Aug 16;1(2):165–179. doi: 10.1016/j.stem.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 22.Ueno S, Weidinger G, Osugi T, Kohn AD, Golob JL, Pabon L, et al. Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc Natl Acad Sci U S A. 2007 Jun 5;104(23):9685–9690. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009 Feb;5(2):100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka T, Tohyama S, Murata M, Nomura F, Kaneko T, Chen H, et al. In vitro pharmacologic testing using human induced pluripotent stem cell-derived cardiomyocytes. Biochem Biophys Res Commun. 2009 Aug 7;385(4):497–502. doi: 10.1016/j.bbrc.2009.05.073. [DOI] [PubMed] [Google Scholar]
- 25.Braam SR, Tertoolen L, van de Stolpe A, Meyer T, Passier R, Mummery CL. Prediction of drug-induced cardiotoxicity using human embryonic stem cell-derived cardiomyocytes. Stem Cell Res. Mar;4(2):107–116. doi: 10.1016/j.scr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Hardy ME, Lawrence CL, Standen NB, Rodrigo GC. Can optical recordings of membrane potential be used to screen for drug-induced action potential prolongation in single cardiac myocytes? J Pharmacol Toxicol Methods. 2006 Sep-Oct;54(2):173–182. doi: 10.1016/j.vascn.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Panakova D, Werdich AA, Macrae CA. Wnt11 patterns a myocardial electrical gradient through regulation of the L-type Ca(2+) channel. Nature. Aug 12;466(7308):874–878. doi: 10.1038/nature09249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akar FG, Rosenbaum DS. Transmural electrophysiological heterogeneities underlying arrhythmogenesis in heart failure. Circ Res. 2003 Oct 3;93(7):638–645. doi: 10.1161/01.RES.0000092248.59479.AE. [DOI] [PubMed] [Google Scholar]
- 29.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004 Sep;5(9):691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 30.Ridley JM, Milnes JT, Benest AV, Masters JD, Witchel HJ, Hancox JC. Characterisation of recombinant HERG K+ channel blockade by the Class Ia antiarrhythmic drug procainamide. Biochem Biophys Res Commun. 2003 Jun 27;306(2):388–393. doi: 10.1016/s0006-291x(03)00980-x. [DOI] [PubMed] [Google Scholar]
- 31.Cheng HC, Incardona J, McCullough B. Isolated perfused and paced guinea pig heart to test for drug-induced changes of the QT interval. J Pharmacol Toxicol Methods. 2006 Nov-Dec;54(3):278–287. doi: 10.1016/j.vascn.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Marvin MJ, Di Rocco G, Gardiner A, Bush SM, Lassar AB. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev. 2001 Feb 1;15(3):316–327. doi: 10.1101/gad.855501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneider VA, Mercola M. Wnt antagonism initiates cardiogenesis in Xenopus laevis. Genes Dev. 2001 Feb 1;15(3):304–315. doi: 10.1101/gad.855601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lieu DK, Liu J, Siu CW, McNerney GP, Tse HF, Abu-Khalil A, et al. Absence of transverse tubules contributes to non-uniform Ca(2+) wavefronts in mouse and human embryonic stem cell-derived cardiomyocytes. Stem Cells Dev. 2009 Dec;18(10):1493–1500. doi: 10.1089/scd.2009.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huber I, Itzhaki I, Caspi O, Arbel G, Tzukerman M, Gepstein A, et al. Identification and selection of cardiomyocytes during human embryonic stem cell differentiation. Faseb J. 2007 Aug;21(10):2551–2563. doi: 10.1096/fj.05-5711com. [DOI] [PubMed] [Google Scholar]
- 36.Fu JD, Jiang P, Rushing S, Liu J, Chiamvimonvat N, Li RA. Na+/Ca2+ exchanger is a determinant of excitation-contraction coupling in human embryonic stem cell-derived ventricular cardiomyocytes. Stem Cells Dev. Jun;19(6):773–782. doi: 10.1089/scd.2009.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jia F, Wilson KD, Sun N, Gupta DM, Huang M, Li Z, et al. A nonviral minicircle vector for deriving human iPS cells. Nat Methods. Mar;7(3):197–199. doi: 10.1038/nmeth.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009 Jun 5;4(6):472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. Nov 5;7(5):618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.