Abstract
HIV-1 associated nephropathy is a rapidly progressive form of focal segmental glomerulosclerosis typically seen in patients of African ancestry. HIV-transgenic mice can develop an HIVAN-like renal disease. Zhong et al. show that the oral administration of a cyclic nucleotide phosphodiesterase 4 inhibitor and a retinoic acid receptor-alpha agonist can prevent the development of HIVAN in transgenic mice, acting through a cAMP dependent mechanism that is independent of HIV-1 genes. These findings suggest that endogenous host factors play a critical role in HIVAN.
The late physician-scientist Dr. Judah Folkman, considered by many to be the father of anti-angiogenic cancer therapies, showed that certain types of experimental cancers in mice could be cured by disrupting their blood supply without directly attacking the tumor cells. His revolutionary treatment is currently being tested in patients with angiogenic tumors, including AIDS-Kaposi’s Sarcoma (KS). Although the pathogenesis of HIV-associated nephropathy (HIVAN) is clearly different from AIDS-KS, HIVAN is characterized by the excessive proliferation of renal glomerular and tubular epithelial cells, leading to the microcystic transformation of renal tubules and rapid development of focal segmental glomerulosclerosis (FSGS). Therefore, the new study by Zhong et al.1 (this issue), challenges us to ask a similar type of question: Can we cure HIVAN in transgenic (Tg) mice without attacking the HIV-1 genes?
Briefly, the study by Zhong et al. show that the oral administration of two drugs that increase the intracellular levels of cAMP, Roflumilast and Am580, are capable of curing HIVAN in Tg mice without affecting the expression of HIV-1 genes1. Roflumilast is a cyclic nucleotide phospodiesterase 4 (PDE4) inhibitor that increases the intracellular concentration of cAMP by blocking its degradation. Am580 is a specific retinoic acid receptor-alpha (RARα) agonist that has several systemic and renal effects, including increasing the intracellular levels of cAMP. Both drugs, acting in a synergistic manner, prevented the progression of the renal disease in HIV-Tg mice by blocking the de-differentiation and proliferation of podocytes through a cAMP/PKA/CREB dependent pathway1 (Fig. 1, red color). This pathway requires the participation of the RAR-α in the nucleus of podocytes, in addition to the direct activation of cAMP and PKA in the cytoplasm of these cells. The mechanism by which retinoic acid receptor agonists induce a direct activation of cAMP in the cytoplasm is not well understood, but similar findings have been documented in acute myeloblastic leukemia cells 1. However, Am580 did not prevent the progression of HIVAN in dual HIV-Tg / RARα knock- out mice 2, suggesting that the nuclear RARα plays a key role in this process. Thus, more studies are needed to determine how the RARα may induce the rapid activation of cAMP in the cytoplasm, and to validate this pathway in normal human podocytes. Finally, Zhong et al. concluded that the beneficial effects of Roflumilast and Am580 were independent of HIV-viral genes. Overall, these provocative findings raise several interesting issues that need to be discussed.
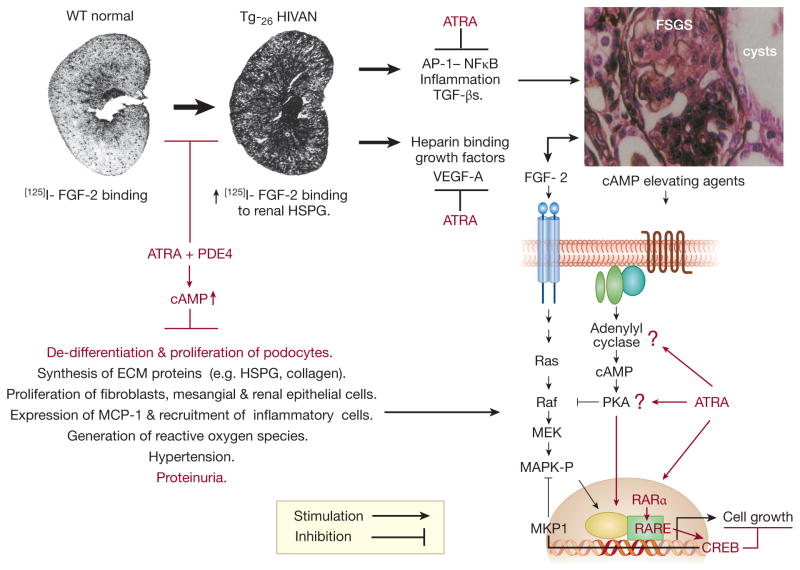
Figure 1. A summary of the possible roles that all-trans retinoic acid (ATRA) and Roflumilast (PDE4) may have to prevent the progression of HIVAN in HIV-Tg26 mice.
Zhong et al.1 showed that ATRA + PDE4 prevented the progression of HIVAN in Tg mice by blocking the de-differentiation and proliferation of podocytes through a cAMP/PKA/CREB dependent mechanisms that is independent of HIV-1 genes (highlighted in red color). Based on the pathogenesis of the renal disease in Tg26 mice3–5, and the known beneficial effects of these drugs in other experimental models of renal diseases 6–8, we have highlight additional pathological pathways (black color) that might be affected as well. Abbreviations: ATRA = all-trans retinoic acid analogs; PDE4 = cyclic nucleotide phosphodiesterase-4 inhibitor; cAMP = cyclic adenosine monophosphate; FGF-2 = Fibroblast Growth Factor-2; HSGP = heparan sulfate proteoglycans; ECM = extracelllular matrix proteins; MCP-1 = Monocyte Chemoattractant Protein 1; AP-1= Activator Protein-1; NFkB = Nuclear Factor Kappa B; TGF-β = Transforming Growth Factor Beta; VEGF-A = Vascular Endothelial Cell Growth Factor-A; FSGS= Focal Segmental Glomerulosclerosis; Ras = rat sarcoma G protein; Raf = rapidly accelerated fibrosarcoma protein kinase; MEK = mitogen activated protein kinase / Erk kinase; MAPK-P = mitogen-activated protein kinase - phosphorylated / ERK-P; MKP-1 = mitogen activated protein kinase phosphatase-1; PKA = protein kinase A; RAR α = retinoic acid receptor alpha; RARE = retinoid acid responsive element; CREB = cAMP responsive element binding protein.
First, we should mention that Zhong et al. used a new colony of HIV-Tg mice that was re-derived from the parent HIV-Tg26 mouse line. Therefore, to understand how HIVAN can be cured in these mice, it is necessary to review the natural history of the renal disease in Tg26 mice. Briefly, these mice carry a replication-deficient Δ gag/pol PNL4-3 HIV proviral genomic DNA driven by the endogenous HIV-1 promoter 3–4. The mRNA expression of HIV-1 is detected in a wide range of cells, including glomerular and tubular epithelial cells 3–5. Viral proteins cannot however, be detected in the circulation or peripheral blood mononuclear cells. Heterozygous mice, are born with normal kidneys, but develop progressive renal disease during their first months of life 3–4. Usually, male mice develop a more rapid and progressive renal disease when compared to female mice 3–5. Severe edema and nephrotic syndrome occur approximately between 60 and 250 days of age 3–5. At this time point, Tg26 mice usually showed BUN levels exceeding 200 mg/dl and elevated serum creatinine (> 1 mg/dl) and die of uremia and ascitis 3–4. However, not all mice develop renal disease, and others do it in an asynchronous and unpredictable manner 3–5. Thus, a remarkable finding of the study of Zhong et al. is that all untreated control HIV-Tg mice, both males and females, developed chronic renal failure in a synchronized manner during the first nine weeks of life. Moreover, the prevalence of the renal disease in the control group was increased relative to the parent Tg26 mouse line3–4, and to the expected prevalence (~ 80%) in the re-derived mouse colony. We should considered that Zhong et al. pre-selected mice at four weeks of age based on urine dipsticks protein values of 1+ / 2+ (30 –100 mg/dl). However, similar levels of proteinuria can be detected in Tg26 mice without renal disease3, due to the normal excretion of low molecular weight tubular proteins and pheromones. In summary, one limitation of the study by Zhong et al. is that we do not know how many mice in each group had pre-existing renal disease at the beginning of the study.
Another limitation of this study is the definition of chronic renal failure (BUN levels > 30 mg/dl). Previous publications in Tg26 mice reported similar BUN levels in mice without renal disease, and used cut-off BUN values above 45 mg/dl 3–4. These BUN values, revealed a much better correlation with the renal histological findings associated with chronic renal failure, showing all glomeruli with segmental or global glomerulosclerosis in combination with microcystic tubular dilatation 3–4. In contrast, 30% to 40 % of the mice treated with Roflumilast or Am580 alone, were reported as having chronic renal failure, but showed only “mild” glomerular sclerotic changes, very few collapsed glomeruli, and mild tubulo-interstitial injury scores. The data presented by Zhong et al. also appear to exclude the possibility that these mice suffered weight loss, or pre-renal failure secondary to drug-induced diarrhea, dehydration, severe edema, nephrotic syndrome, or ascitis. Thus, it is unclear why these mice developed chronic renal failure with such renal histological and clinical findings. Moreover, a previous study done by the same group, showed that a more prolonged treatment with Am580 alone was capable of preventing the progression of HIVAN in Tg26 mice 2. Finally, for the purpose of comparing the outcome of the renal disease in mice belonging to the original Tg26 line 3–4 vs. those used in this study, both the renal histological and clinical findings at nine weeks of age appear to be more severe in Tg26 mice 3–5.
Nevertheless, when the findings of Zhong et al. are considered in the context of previous studies 6–8, it is clear that Roflumilast and Am580 have the potential to improve the outcome of HIVAN acting through different mechanisms and renal cell types (Fig.1). For example, Zhong et al. began their treatment when mice were four weeks of age. Renal electron microscopy studies done in Tg26 mice with renal disease at this early time point, show effacement of foot processes, expansion of the mesangial matrix narrowing the lumen of glomerular capillaries, simplification and atrophy of tubular epithelial cells, and thickening of glomerular and tubular basement membranes 3, 5. No renal epithelial proliferative changes however, are detected at this early stage 3, 5. Thus, in addition to the pro-differentiation changes in podocytes described by Zhong et al., these drugs have the potential to improve the outcome of HIVAN by blocking the synthesis and accumulation of renal extracellular matrix proteins. Previous studies suggest that these changes can be mediated by cAMP and/or TGF-β dependent mechanisms 8–9. In addition, blocking the synthesis of heparan sulfate proteoglycans (Fig. 1), may prevent the renal accumulation of heparin binding growth factors, including VEGF-A and FGF-2, which can induce renal proliferative changes during the late stages of HIVAN in Tg26 mice5. Moreover, previous studies showed that drugs that increase the intracellular levels of cAMP, can also decrease the mitogenic activity of FGF-2 in cultured human primary renal epithelial cells by inhibiting the MAPK signaling pathway10 (Fig 1). In summary, it is tempting to speculate that retinoids, in combination with PDE4 inhibitors, may ameliorate the progression of HIVAN independently of HIV-genes, by blocking the de-differentiation of podocytes and the synthesis of extracellular matrix proteins during the early stages of the renal disease, and by preventing the mitogenic activity of heparin binding growth factors during the late stages of HIVAN.
Finally, Roflumilast and Am580 have powerful systemic effects, which could also modulate the outcome of HIVAN in humans and transgenic mice. For example, retinoids can decrease the blood pressure of mice with renal disease 8, and may improve their renal outcome by modulating the activity of NO synthase, endothelin-1, and/or angiotensin II type 1 receptors 6. Most notably, these drugs have powerful anti-inflammatory activity, and can affect the recruitment of inflammatory cells, as well as the transcription of inflammatory genes acting through AP-1 or NFκβ related mechanisms 6. (Fig.1). Roflumilast also inhibits the expression of the cytokine monocyte chemoattractant protein 1 (MCP-1), and the generation of reactive oxygen species 7. Based on its anti-inflammatory activity, Roflumilast was approved by the FDA to treat flares of chronic obstructive pulmonary disease. Unfortunately, this drug cannot be used in patients with liver disease, and has known adverse interactions with several HIV-medications. Alternatively, retinoids are being used in clinical trials to treat skin lesions and tumors, including AIDS-KS, and in patients with minimal change disease or FSGS. Overall, we hope that these studies will provide valuable information to assess the clinical value of Roflumilast and Am580 in patients with HIVAN.
Acknowledgments
This work was supported in part by the National Institutes of Health grants: R01-HL- 55605, R01-DK049419, and R01 HL-102497.
Footnotes
Disclosures.
None
References
- 1.Zhong Y, Wu Y-W, Liu R, et al. Roflumilast enhances the renal protective effects of retinoids in a HIV-1 transgenic mouse model with rapidly progressive renal failure. Kidney Int. doi: 10.1038/ki.2011.467. in press ( this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ratnam KK, Feng X, Chuan PY, et al. Role of the retinoic acid receptor – alpha in HIV-associated nephropathy. Kidney Int. 2011;9:624–634. doi: 10.1038/ki.2010.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dickie P, Felser J, Eckhaus M, et al. HIV-associated nephropathy in transgenic mice expressing HIV-1 genes. Virology. 1991;185:109–119. doi: 10.1016/0042-6822(91)90759-5. [DOI] [PubMed] [Google Scholar]
- 4.Kopp JB, Klotman ME, Adler SH, et al. Progressive glomerulosclerosis and enhanced renal accumulation of basement membranes components in mice transgenic for human immunodeficiency virus type 1 genes. Proc Nat Acad Sci USA. 1992;89:1577–1581. doi: 10.1073/pnas.89.5.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ray PE, Bruggeman LA, Weeks BS, et al. bFGF and its low affinity- receptors in the pathogenesis of HIV-associated nephropathy in transgenic mice. Kidney Int. 1994;46:759–772. doi: 10.1038/ki.1994.331. [DOI] [PubMed] [Google Scholar]
- 6.Wagner J. Potential role of retinoids in the therapy of renal disease. Nephrol Dial Transplant. 2001;16:441–444. doi: 10.1093/ndt/16.3.441. [DOI] [PubMed] [Google Scholar]
- 7.Cheng J, Grande JP. Cyclic nucleotide phosphodiesterase (PDE) inhibitors: Novel therapeutics agents for progressive renal disease. Exp Biol Med. 2007;232:38–51. [PubMed] [Google Scholar]
- 8.Morath C, Dechow C, Lehrke I, et al. Effects of retinoids on the TGF-β system and extracellular matrix in experimental glomerulonephritis. J Am Soc Nephrol. 2001;12:2300–2309. doi: 10.1681/ASN.V12112300. [DOI] [PubMed] [Google Scholar]
- 9.Duncan MR, Frazier SK, Abramson S, et al. Connective tissue growth factor mediates transforming factor β-induced collagen synthesis: down-regulation by cAMP. FASEB J. 1999;13:1774–1786. [PubMed] [Google Scholar]
- 10.Izevbgie EB, Gutkind JS, Ray PE. Isoproterenol inhibits Fibroblast Growth factor-2 induced growth of renal epithelia cells. Pediatr Nephrol. 2000;14:726–734. doi: 10.1007/pl00013426. [DOI] [PubMed] [Google Scholar]