Abstract
Nodal is a TGF-beta related embryonic morphogen that is expressed in multiple human cancers. Detection of Nodal expression in these tissues can be challenging if issues related to Nodal transcription and protein processing are not considered. Here, we discuss certain characteristics related to Nodal expression and function, and how these can facilitate acquisition and interpretation of expression data, contributing to our understanding of the potential role of Nodal in human cancer. We also discuss how Nodal could be exploited clinically as a novel biomarker for cancer progression and therapeutic target.
Introduction
Cancer cells can exploit normally dormant embryonic pathways to promote tumorigenicity and metastasis. Understanding the impact of these embryonic signals and the regulatory programs that reactivate them holds significant potential for new cancer therapies. Studying embryonic signaling pathways in cancer, particularly aggressive cancer, has led to the discovery of the re-expression of the embryonic morphogen Nodal (1). Nodal is a member of the Transforming Growth Factor Beta (TGF-beta) super family, essential in maintaining the pluripotency of human embryonic stem cells (hESCs). Recent findings have revealed that Nodal is a critical regulator of melanoma growth, plasticity and tumorigenicity, and holds promise as a new biomarker for metastatic potential (1–3). Similar observations have been reported in gliomas and carcinomas of the breast, endometrium and prostate (4–7).
Nodal is an important regulator of early vertebrate development, including mesoderm formation, body plan establishment, and cell fate determination (8). In humans, Nodal expression is largely restricted to embryonic tissues including the trophoblast and the developing mammary gland – but is generally lost in normal adult tissues (4). Therefore, studies addressing the role of Nodal in cancer progression have focused on the mechanisms underlying its re-expression in tumor cells and the translational relevance of targeting Nodal as a novel therapy (9).
With any new discovery there are associated challenges. As investigators introduce novel findings to the literature, it is with the expectation that other scientists will confirm and extend their findings. In the case of Nodal, this has been particularly challenging and confounding due to inconsistencies and sometimes incorrect information available in public databases, in addition to lackluster reagents for human cell studies. This review is dedicated to full transparency and disclosure of some of our challenges and experiences related to the study of Nodal.
Processing and Signaling of Nodal
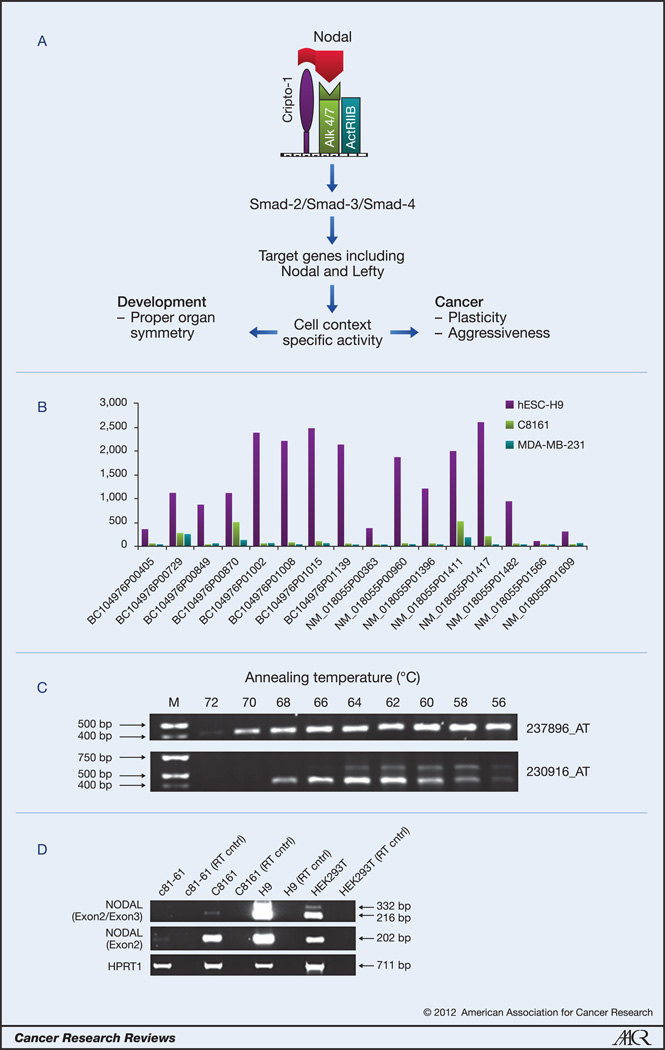
Much of our understanding of how Nodal protein is processed and propagates signaling comes from studies related to developmental biology, since Nodal is a critical factor in normal embryonic development, and regulates numerous developmental processes including gastrulation and left-right asymmetry (8,10,11). Canonical Nodal signaling is propagated via the binding of Nodal ligand to the Cripto-1 coreceptor and a complex of type I and type II activin receptors (ALK4/7 and ActRIIB, respectively), triggering phosphorylation events that activate Smad2/3 and facilitate binding to Smad4 (Figure 1 A) (11). This Smad complex associates with other transcription factors in the nucleus and propagates the transcription of target genes including Nodal itself and the Nodal antagonist, Lefty. Under normal circumstances, the positive feedback on Lefty expression as well as Nodal serves to limit signaling activity, and provides a more refined level of pathway regulation. However, in cancer cells studied, the Lefty gene is highly methylated and does not respond to Nodal signaling, allowing Nodal transcription to proceed unchecked (4, 12). Exposing tumor cells to Lefty produced by hESCs dramatically inhibits Nodal expression and reduces clonogenic potential (4).
Figure 1.
A) Schematic representation of primary Nodal signaling events. B) Microarray results (NimbleGen HG18 chip) of mRNA from human embryonic stem cells (hESC-H9), melanoma (C8161) and breast cancer (MDA-MB-231) cell lines showing wide variability in detection levels between 16 different probes all designated as recognizing Nodal. C) Results from PCR experiments show that primers corresponding to the Affymetrix U133B chip 237896_AT microarray probe produce a single PCR product that corresponds to the Nodal gene, while sequencing of the two PCR products recognized by the 230916_AT probe do not correspond to the Nodal gene. D) Semi-quantitative PCR analyses in human embryonic stem cells (H9), and human embryonic kidneys cells (HEK293T), poorly aggressive (c81-61), and highly aggressive (C8161) human melanoma cells with primers spanning the exon 2/exon 3 boundary identifies two bands which may indicate splice variants of Nodal (RT cntrl = no reverse transcriptase present).
Nodal signaling can occur in both an autocrine and paracrine fashion, and may be influenced by the processing, stability and trafficking of Nodal protein (10, 11). Nodal is translated in a precursor form consisting of a signal peptide, pro-domain and mature domain. Transfection studies with exogenous mouse Nodal suggest that the pro-form (pro- and mature domains) is cleaved to a much less stable, but highly active mature form extracellularly by the proprotein convertases Furin and Pace4 (10). Certainly, in mice, Furin and Pace4 are required for Nodal signaling (13). Transfection studies also suggest that Cripto-1 could further regulate maturation by anchoring the pro-form of mouse Nodal and one of the proprotein convertases in a complex at the plasma membrane, and may facilitate Nodal internalization as well as signaling (10). However, whether this has relevance in cancer is not yet clear. Nodal can signal independently of Cripto-1 (11), and, in melanoma cell lines, endogenous Cripto-1 is present on the surface of only a small percentage (less than 5%) of cells (4, 14), suggesting the possibility that a canonical mechanism of signaling may not necessarily occur in cancer cells. In tumor cells, it is also not clear if Nodal signals in a paracrine or autocrine fashion or both; although, by immunofluorescence microscopy, Nodal protein is observed in only a proportion of melanoma cells in culture (29–39% depending on the cell line), suggesting that the signaling range of Nodal is limited, but may also identify an important cellular subpopulation (15). Indeed, more research is needed to determine the precise mechanisms that regulate Nodal signals at the single cell level in cancer cells.
Multiple groups have now detected human Nodal protein in hESCs and various cancer cell lines with commercially available antibodies from different companies, in assays including Western blotting, immunofluorescence, immunohistochemistry, and immunoprecipitation (1, 3, 15, 7). In particular, Western blot analyses in human cancer cells indicate that the predominant species of endogenous Nodal protein are the precursor and pro- forms, though reported molecular weights vary (42–48KDa and 35–37KDa, respectively) (1, 3, 4, 15, 7). The mature form (~13–15KDa) is rarely observed, suggesting it is likely highly unstable and/or cleaved predominantly outside the cell. However, what is clear in our experience is that particular care must be taken in preparing and storing protein lysates, as Nodal protein (especially the mature form) is highly susceptible to degradation, leading to variable levels of detection over time, even between aliquots of the same sample.
Considering that Nodal is not typically expressed in adult tissues, one important question has been how Nodal is upregulated in cancer. Recent research suggests that Nodal re-expression in aggressive melanoma is regulated by a Notch signaling pathway (15). Certainly, a molecular link between these two prominent stem-cell associated pathways exists, as shown by the in vitro activity of an RBPJ-dependent Nodal enhancer element. Specifically, the expression of Notch4 and Nodal was observed to correlate in aggressive melanoma cell lines but not poorly aggressive cell lines, as well as in advanced stage melanomas. Targeting Notch4 expression or activity downregulated Nodal levels in aggressive cell lines and exogenous expression of the Notch4 intracellular domain in poorly aggressive cells upregulated Nodal levels, indicating Notch4 specifically regulates the Nodal gene in melanoma cell lines. In vitro biological assays of vascular-like network and colony formation were perturbed by Notch4 inhibition and could be partially rescued by recombinant Nodal, suggesting melanoma aggressive cell behavior is controlled at least in part by Notch4 regulation of Nodal. Whether Nodal expression is regulated by a Notch signaling pathway in other cancers is not yet known.
Expression and Detection of the Nodal Gene: Lessons Learned
Exhaustive investigations related to human Nodal expression and function in cancer have proven challenging, particularly with regard to gene expression studies. In fact, few studies have reported substantive analyses of Nodal gene expression in human cancer cells (3, 4, 15). Efforts by our group to detect a Nodal-specific amplicon in human cancer cells, and in some cases also hESCs, using primers previously published in studies with human trophoblast, breast cancer and hESC lines (16–18), were met with limited success. The reason for this discrepancy is not entirely clear, though certainly, Nodal expression in cancer cell lines is less abundant than in embryonic cells (4), which may account for some detection differences. Importantly, verification of a correlation between mRNA and protein in cancer cells has been achieved, but only when selecting semi-quantitative primers located in either exon 2 or the 3’ untranslated region of the human Nodal gene sequence or with a commercially available real-time PCR primer probe set (Applied Biosystems Hs00250630_s1) also located in exon 2 (15). As such, extensive controls to exclude detection of contaminating genomic DNA (DNAse treatment and omission of reverse transcriptase in control samples) are necessary when evaluating human Nodal mRNA levels with these tools. Notably, the only published report of human Nodal mRNA detection by in situ hybridization was achieved in human tumor xenograft tissue sections with an antisense RNA probe complementary to a portion of exon 2, and, in this instance, extensive signal amplification with a biotin-streptavidin tyramide signal amplification kit was necessary (3). Altogether, these findings indicate how important primer and sequence selection is in elucidating human Nodal gene expression, particularly in tumor cells.
Probes for the human Nodal gene utilized by manufacturers of microarray chips demonstrate considerable variability in detecting Nodal expression levels. Levels of gene expression in hESC-H9 and cancer cell lines C8161 and MDA-MB-231 were evaluated by our laboratory using the HG18 Human Gene Expression 385K Microarray Chip (NimbleGen, Roche Applied Sciences). Of the 16 probes designated as Nodal, individual detection levels were widely inconsistent, ranging from 305.4 to 2630.63 relative fluorescent units in hESC-H9s (Figure 1B). Furthermore, it is not clear that all probes designated as Nodal actually specifically detect Nodal expression. The Affymetrix chip U133B employs two probes for Nodal (237896_AT and 230916_AT), both of which contain sequences in exon 3 of the Nodal gene. PCR amplification of these two regions, for 237896_AT, gave a PCR product that was sequenced and verified as Nodal, and for 230916_AT, gave two PCR products that were both repeatedly sequenced and never corresponded to the Nodal gene sequence (Figure 1C), suggesting it is not specific for Nodal. Discordances such as these exemplify the possibility that Nodal could be incorrectly detected or disregarded as unexpressed in standard mRNA expression analyses such as microarray. This analysis highlights the importance of verification by multiple methods, especially in detecting Nodal gene expression.
Confounding the situation further is the possibility that multiple Nodal splice variants may exist. Semi-quantitative PCR analyses in hESCs with primers spanning the exon 2/exon 3 boundary identified two bands (Figure 1D). DNA sequence analysis indicated that the novel, larger amplicon included an 116bp region of intron 2 located at the exon2/3 splice junction, suggesting the possibility that it represents a novel splice variant. Consistent with this, submissions in NCBI Ace View (http://www.ncbi.nlm.nih.gov/IEB/Research/Acembly/av.cgi?db=human&ctx=ctx-28419-mweb11-22816&q=nodal&N=0) and Ensembl (http://www.ensembl.org/Homo_sapiens/Gene/Summary?g=ENSG00000156574;r=10:72192071-72207707) suggest that the human Nodal gene may generate up to 4 mRNA species. Despite this, the only validated and complete Nodal sequence (NM_018055.4) encodes a full-length protein, detectable by Western blotting and other assays. Extensive studies would be required to determine whether these putative Nodal splice variants are translated into protein or are possible regulatory RNAs, and, more importantly, whether there is tissue specificity with regard to their gene expression. Remarkably, all three putative Nodal splice variants contain a complete or large portion of exon 2, which, if expressed, may offer a plausible explanation for why primers/probes in exon 2 accurately detect Nodal gene expression, while many others do not. Nonetheless, these observations demonstrate that detection of Nodal gene expression is complex and exemplify why extreme care should be taken when evaluating Nodal expression in human cancer and other cells.
Evidence for Clinical Potential of Targeting Nodal in Cancer
Variability in response rates among patients with the same cancer type, or even in the same patient during the evolution of a specific malignancy, is often due to the high level of heterogeneity within the cancer cell population. In particular, while a subset of cancer cells may be sensitive to a certain anti-cancer drug, others may continue to proliferate unabated, because these cells have either developed resistance to that chemotherapeutic or simply do not express the target for which the drug was developed. In melanoma, for instance, several signaling molecules or pathways have been identified as potential therapeutic targets (19). One widely studied example is the mitogen-associated protein kinase signaling pathway associated with activating mutations in NRAS or BRAF, which have recently been the focus of targeted therapy in melanoma (20). However, not all melanomas present these specific mutations or activated signaling pathways, and some develop resistance to targeted therapy. Consequently, a significant proportion of melanoma patients do not benefit from these novel targeted approaches (21). The same is true in breast cancer where the hormone receptors (HR) estrogen and progesterone, useful biomarkers for predicting survival and therapeutic outcome, are either not expressed in all patients or are not expressed homogeneously within the same tumor of an individual patient. In fact, patients that were once HR positive may become HR negative or, as occasionally reported, HR negative patients can revert to HR positive, affecting the efficacy of targeted treatment (22). The ideal molecular target, therefore, would be detected in specific cancer cells responsible for disease progression and, most importantly, expressed in all affected patients. Alternative to this “magic bullet” approach would be to increase our armamentarium of inhibitors of molecular targets by identifying additional, functionally relevant targets and leading to a multi-targeted approach to eliminate more subsets of the total heterogeneous cancer cell population.
Studies from cancer stem cell (CSC) research have suggested that targeting cancer cells with stem cell-like characteristics, which appear to be responsible for the morphologic and functional heterogeneity seen in cancer, could impact survival by reducing tumor growth, metastatic spread, drug resistance and disease recurrence (23). Advances in the field of CSC research have enabled us to characterize the reemergence of specific embryonic signaling pathways, such as Nodal, thus contributing to our understanding of the molecular mechanisms that regulate cancer cell plasticity and aggressiveness (24). Since Nodal expression is not generally detected in normal tissues, but can re-emerge in a number of human cancers including melanoma, breast and prostate cancer (1, 4, 7), Nodal could represent a candidate target in aggressive cancer cells.
Our work has shown that downregulation of Nodal can be achieved, either by directly targeting Nodal with Nodal Morpholino or by exposing cells to hESC-derived Lefty, or by targeting Notch4 upstream of Nodal expression, thus reducing tumorigenicity, plasticity and aggressiveness of human melanoma and breast cancer cells (1, 4, 15). In addition, Nodal specific shRNA has been shown to reduce Nodal expression and decrease invasiveness and tumor growth of human glioma cells, both in vitro and in vivo (6). Given the complexity of Nodal detection, as previously described in this review, it must be noted that Nodal knockdown experiments must be validated by protein expression analysis, especially with RNA interference methods where PCR detection of partially degraded, non-translating transcripts could lead to potential misinterpretation of results (25). Certainly, tumors in Nude mice formed from injected Nodal-expressing aggressive human melanoma cells exhibited a decrease in tumor cell proliferation and increase in apoptosis when mice were treated intratumorally with hESC-derived Lefty (4). Similarly, aggressive melanoma cells injected retroorbitally in Nude mice and allowed to colonize to the lung formed fewer tumor cell colonies when treated with intraperitoneal injections of a function blocking anti-Nodal antibody, compared with mice injected with an isotype control immunoglobulin (26). Of note, melanoma cells observed in the lungs of antibody-treated mice showed more frequent signs of cellular distress and apoptosis compared with control mice. Collectively, these studies suggest the potential for Nodal as a target for human cancer therapy.
Implications and Future Directions
The plastic phenotype associated with aggressive tumors presents a significant challenge in the detection and targeting of cancer cells exhibiting stem cell-like characteristics. As we elucidate the embryonic signaling pathways reactivated in many cancers and their contributions to tumor cell plasticity, new strategies will emerge regarding the suppression of this elusive phenotype. One of these pathways -- the Nodal signaling pathway -- is a master regulator of tumor cell plasticity and tumorigenicity. Because Nodal is not expressed by most normal adult tissues, and is overexpressed by aggressive tumor cells, it may represent a valuable new therapeutic target. The methylation (and silencing) of Nodal’s inhibitor, Lefty, also provides an additional consideration for therapeutic application. Not only does exogenously added Lefty suppress Nodal expression in tumor cells, but Lefty can also be re-expressed in tumor cells treated with 5-azacytidine (D.A.K., personal communication). Equally noteworthy is the molecular cross-talk between Nodal and Notch, via Notch4 regulation of Nodal expression. The implications of this new finding suggest that tumor cells co-expressing Notch4 and Nodal may better define the cancer stem cell phenotype. Furthermore, suppression of this phenotype may require a combinatorial multi-target approach. Using modest patient sample numbers in immunohistochemistry studies, the diagnostic potential of Nodal in human melanoma, breast and prostate carcinoma appears promising (2, 4, 7). However, ongoing studies with ambiguous nevi will ultimately assess the prognostic and predictive power (or value) of Nodal in identifying patients at risk for melanoma disease onset and progression. Lastly, this review reveals the challenges we and others have faced with respect to accurately measuring Nodal gene and protein expression, based on the information available in the literature, public databases, and commercial reagents. Let us not forget that the role of Nodal in cancer is a relatively new observation, and as such, deserves continuous validation, thoughtful discussion and patience -- to understand its full impact on the field.
Acknowledgments
Financial support: National Cancer Institute R01CA121205 Award and Northwestern University Physical Science and Oncology Center Grant U54CA143869 to M.J.C. Hendrix.
References
- 1.Topczewska JM, Postovit LM, Margaryan NV, Sam A, Hess AR, Wheaton WW, et al. Embryonic and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. Nat Med. 2006;12:925–932. doi: 10.1038/nm1448. [DOI] [PubMed] [Google Scholar]
- 2.Yu L, Harms PW, Pouryazdanparast P, Kim DS, Ma L, Fullen DR. Expression of the embryonic morphogen Nodal in cutaneous melanocytic lesions. Mod Pathol. 2010;23:1209–1214. doi: 10.1038/modpathol.2010.101. [DOI] [PubMed] [Google Scholar]
- 3.McAllister JC, Zhan Q, Weishaupt C, Hsu MY, Murphy GF. The embryonic morphogen, Nodal, is associated with channel-like structures in human malignant melanoma xenografts. J Cutan Pathol. 2010;37(Suppl 1):19–25. doi: 10.1111/j.1600-0560.2010.01503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Postovit LM, Margaryan NV, Seftor EA, Kirschmann DA, Lipavsky A, Wheaton WW, et al. Human embryonic stem cell microenvironment suppresses the tumorigenic phenotype of aggressive cancer cells. Proc Natl Acad Sci U S A. 2008;105:4329–4334. doi: 10.1073/pnas.0800467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papageorgiou I, Nicholls PK, Wang F, Lackmann M, Makanji Y, Salamonsen LA, et al. Expression of nodal signalling components in cycling human endometrium and in endometrial cancer. Reprod Biol Endocrinol. 2009;7:122. doi: 10.1186/1477-7827-7-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee CC, Jan HJ, Lai JH, Ma HI, Hueng DY, Lee YC, et al. Nodal promotes growth and invasion in human gliomas. Oncogene. 2010;29:3110–3123. doi: 10.1038/onc.2010.55. [DOI] [PubMed] [Google Scholar]
- 7.Lawrence MG, Margaryan NV, Loessner D, Collins A, Kerr KM, Turner M, et al. Reactivation of embryonic nodal signaling is associated with tumor progression and promotes the growth of prostate cancer cells. Prostate. 2011;71:1198–1209. doi: 10.1002/pros.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schier AF, Shen MM. Nodal signalling in vertebrate development. Nature. 2000;403:385–389. doi: 10.1038/35000126. [DOI] [PubMed] [Google Scholar]
- 9.Abbott DE, Postovit LM, Seftor EA, Margaryan NV, Seftor RE, Hendrix MJ. Exploiting the convergence of embryonic and tumorigenic signaling pathways to develop new therapeutic targets. Stem Cell Rev. 2007;3:68–78. doi: 10.1007/s12015-007-0010-x. [DOI] [PubMed] [Google Scholar]
- 10.Constam DB. Running the gauntlet: an overview of the modalities of travel employed by the putative morphogen Nodal. Curr Opin Genet Dev. 2009;19:302–307. doi: 10.1016/j.gde.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Schier AF. Nodal morphogens. Cold Spring Harb Perspect Biol. 2009;1:a003459. doi: 10.1101/cshperspect.a003459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costa FF, Seftor EA, Bischof JM, Kirschmann DA, Strizzi L, Arndt K, et al. Epigenetically reprogramming metastatic tumor cells with an embryonic microenvironment. Epigenomics. 2009;1:387–398. doi: 10.2217/epi.09.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beck S, Le Good JA, Guzman M, Ben Haim N, Roy K, Beermann F, et al. Extraembryonic proteases regulate Nodal signalling during gastrulation. Nat Cell Biol. 2002;4:981–985. doi: 10.1038/ncb890. [DOI] [PubMed] [Google Scholar]
- 14.Strizzi L, Abbott DE, Salomon DS, Hendrix MJ. Potential for crypto-1 in defining stem cell-like characteristics in human malignant melanoma. Cell Cycle. 2008;7:1931–1935. doi: 10.4161/cc.7.13.6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardy KM, Kirschmann DA, Seftor EA, Margaryan NV, Postovit LM, Strizzi L, et al. Regulation of the embryonic morphogen Nodal by Notch4 facilitates manifestation of the aggressive melanoma phenotype. Cancer research. 2010;70:10340–10350. doi: 10.1158/0008-5472.CAN-10-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munir S, Xu G, Wu Y, Yang B, Lala PK, Peng C. Nodal and ALK7 inhibit proliferation and induce apoptosis in human trophoblast cells. J Biol Chem. 2004;279:31277–31286. doi: 10.1074/jbc.M400641200. [DOI] [PubMed] [Google Scholar]
- 17.Normanno N, De Luca A, Bianco C, Maiello MR, Carriero MV, Rehman A, et al. Cripto-1 overexpression leads to enhanced invasiveness and resistance to anoikis in human MCF-7 breast cancer cells. J Cell Physiol. 2004;198:31–39. doi: 10.1002/jcp.10375. [DOI] [PubMed] [Google Scholar]
- 18.Vallier L, Alexander M, Pedersen RA. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci. 2005;118:4495–4509. doi: 10.1242/jcs.02553. [DOI] [PubMed] [Google Scholar]
- 19.Palmieri G, Capone M, Ascierto ML, Gentilcore G, Stroncek DF, Casula M, et al. Main roads to melanoma. J Transl Med. 2009;7:86. doi: 10.1186/1479-5876-7-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arkenau HT, Kefford R, Long GV. Targeting BRAF for patients with melanoma. Br J Cancer. 2011;104:392–398. doi: 10.1038/sj.bjc.6606030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smalley KS, Haass NK, Brafford PA, Lioni M, Flaherty KT, Herlyn M. Multiple signaling pathways must be targeted to overcome drug resistance in cell lines derived from melanoma metastases. Mol Cancer Ther. 2006;5:1136–1144. doi: 10.1158/1535-7163.MCT-06-0084. [DOI] [PubMed] [Google Scholar]
- 22.Thompson AM, Jordan LB, Quinlan P, Anderson E, Skene A, Dewar JA, et al. Prospective comparison of switches in biomarker status between primary and recurrent breast cancer: the Breast Recurrence In Tissues Study (BRITS) Breast Cancer Res. 2010;12:R92. doi: 10.1186/bcr2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu S, Wicha MS. Targeting breast cancer stem cells. J Clin Oncol. 2010;28:4006–4012. doi: 10.1200/JCO.2009.27.5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strizzi L, Hardy KM, Seftor EA, Costa FF, Kirschmann DA, Seftor RE, et al. Development and cancer: at the crossroads of Nodal and Notch signaling. Cancer Res. 2009;69:7131–7134. doi: 10.1158/0008-5472.CAN-09-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Echeverri CJ, Beachy PA, Baum B, Boutros M, Buchholz F, Chanda SK, et al. Minimizing the risk of reporting false positives in large-scale RNAi screens. Nat Methods. 2006;3:777–779. doi: 10.1038/nmeth1006-777. [DOI] [PubMed] [Google Scholar]
- 26.Strizzi L, Postovit LM, Margaryan NV, Lipavsky A, Gadiot J, Blank C, et al. Nodal as a biomarker for melanoma progression and a new therapeutic target for clinical intervention. Expert Rev Dermatol. 2009;4:67–78. doi: 10.1586/17469872.4.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]