Abstract
This study evaluated individual differences in levels of C-reactive protein (CRP) measured in saliva, cross-sectionally and prospectively, in relation to systemic inflammation and risk for cardiovascular disease (CVD). Plasma and saliva samples, later assayed for CRP, were collected multiple times from an ethnically diverse group of women seeking help from domestic violence crisis shelters-agencies (N = 107; mean age at study start = 34 years). Plasma and saliva CRP levels were moderately associated cross-sectionally and across two years. There were indications that saliva CRP levels were, on average, higher in the morning than evening. Higher levels of saliva and plasma CRP were associated with a higher body mass index, but did not differ between women who did and did not smoke. Salivary CRP reliably discriminated between high and low levels of plasma CRP, using a clinically relevant cutoff point of 3 mg/L, recommended by the American Heart Association. Results build upon an emerging literature suggesting that under specific conditions levels of CRP in saliva may reflect low-grade inflammation and have the potential to serve as a screen for CVD risk status.
Keywords: saliva, C-reactive protein, inflammation, intimate partner violence, validation, cardiovascular disease
Childhood maltreatment and intimate partner violence (IPV) are associated with persistent activation of inflammatory processes (e.g., Danese et al., 2007; Fernandez-Botran et al., 2011). Studies indeed suggest that inflammation is a key mechanism through which harsh life events affect the development of physical health problems (Danese et al., 2007; Felitti et al., 1998). Circulating levels of C-reactive protein (CRP) have been employed clinically as an indicator of general inflammation and as a marker of cardiovascular disease risk status (CVD; Pearson et al., 2003). A handful of studies focused on the assessment of CRP in saliva, and the findings raise the intriguing possibility that saliva may afford a minimally invasive alternative to blood sampling for monitoring CRP (e.g., Quellet-Morin et al., 2011). Several significant gaps in knowledge remain, and in this study we attempt to fill that gap by exploring the correlates and concomitants of salivary CRP in a high-risk sample of women exposed to IPV.
Recent technical advances have made the assessment of a wide range of analytes in saliva possible, creating new opportunities to study how biological and social processes interact and influence health outcomes and human behavior. Compared to other biospecimens such as urine or blood, the assessment of biomarkers in saliva has several advantages (Granger et al., 2007; Granger et al., in press; Pfaffe et al., 2011): collecting oral fluids does not require skilled professionals and special laboratory equipment, it is generally stress- and pain-free and therefore less burdensome for participants/patients, and it allows for self- and home-based collection. Several immune markers can be detected and reliably assessed in oral fluids, including secretory immunoglobulin A (SIgA), cytokines (e.g., IL-6, IL-8, IL-1β, TNF-α), and acute phase proteins such as CRP (Granger et al., in press; Pfaffe et al., 2011).
The degree to which levels of immune and inflammatory markers in oral fluids represent systemic immune activity is a key issue. Levels of many immune-related analytes are different in oral fluids than in serum/plasma, and serum-saliva correlations for cytokines and other surrogate markers of inflammation are typically modest (Granger et al., 2006). The assumption is that the immune system is highly compartmentalized and that local-mucosal secretory and regulatory mechanisms (infiltrating neutrophils, macrophages), the status of oral hygiene, injury to the mouth, and periodontal disease are major contributors to the degree of inflammation in the mouth and levels of these analytes in oral fluids (Fernandez-Botran et al., 2011; Minetto et al., 2005; Sjögren et al 2005).
By contrast, the literature suggests that CRP levels in oral fluids are strongly and positively associated with levels in the circulation (e.g., Megson et al., 2010; Quellet-Morin et al., 2011). CRP is primarily produced by the liver, and levels in circulation increase dramatically in response to local and systemic inflammation (Black et al., 2004). Mildly elevated CRP in the circulation is associated with chronic inflammation and a heightened risk for CVD through its involvement in atherosclerosis (e.g., Melander et al., 2009; Ridker et al., 1998; Wilson et al., 2008; for reviews see Bisoendial et al., 2010; Pearson et al., 2003), whereas markedly high concentrations of CRP (> 10 mg/L) generally indicates acute infections or inflammatory diseases (Dhingra et al., 2007). Although the molecule is too large to pass from the circulation into the salivary glands by diffusion or ultrafiltration (see Granger et al, in press), it is hypothesized to enter saliva, as do many larger serum constituents, as a component of gingival crevicular fluid (GCF; Megson et al., 2010).
The few studies that have investigated the association between circulating and salivary CRP levels have included healthy adults and have reported varying results. Kopanczyk and colleagues (2010) and Dillon and colleagues (2010) found no significant association between CRP in saliva and blood. In contrast, Quellet-Morin and colleagues (2011) reported a moderate-to-strong association between saliva and serum CRP levels for a sample of 61 healthy adults, who were carefully screened for infectious, immune or salivary gland disorders. In addition, salivary CRP was associated with other correlates of inflammation and synthesis of acute phase proteins (i.e., smoking, Body Mass Index (BMI), serum levels of IL-6).
Two studies have been conducted focusing on saliva CRP in clinical samples. Floriano and colleagues (2009, see also Miller et al., 2010) determined levels of salivary CRP in a sample of 48 patients with existing acute myocardial infarction (AMI) and healthy controls. A significant association between serum and saliva levels of CRP was observed. From more than 20 salivary biomarkers, CRP displayed the best discriminating ability for distinguishing between AMI patients and controls. Similarly, Punyadeera and colleagues (2011) reported a strong association (r = .92) between serum and saliva levels of CRP in patients with pre-existing cardiac conditions. The importance of systematic variation in participants’ health in new efforts to validate the measurement of salivary CRP as an inflammatory marker was also reflected in the study by Dillon and colleagues (2010). Although no significant correlation was detected between serum and saliva CRP levels for the entire sample, for individuals with very high blood CRP levels (> 10 mg/L) the correlation with serum CRP increased dramatically.
We extend the literature investigating saliva CRP by employing a high risk sample including women exposed to IPV, which is often viewed as an extreme and chronic stressor, associated with many acute and chronic medical conditions (e.g., Campbell et al., 2002; Ellsberg et al., 2008; Woods et al., 2008), including a heightened risk for CVD (e.g., Scott-Storey et al., 2009). Previous studies on adverse life experiences have demonstrated that a history of IPV is associated with persistent elevation in inflammation levels, even after the women left the abusive relationship (Fernandez-Botran et al., 2011; Newton et al., 2011). The enduring activation of inflammatory processes can be explained by (a combination of) factors, including the frequent and multiple injuries resulting from physical violence; the post-traumatic stress, anxiety and depressive symptoms which develop in at least two thirds of battered women (Golding, 1999; Jones et al., 2001); and the negative health behaviors associated with IPV (Breiding et al., 2008). Furthermore, dysregulation of the endocrine and autonomic nervous systems resulting from chronic stress can have substantial adverse effects on the immune system (Chrousos, 1995; Glaser & Kiecolt-Glaser, 2005; Sorrels & Sapolsky, 2007). For example, Danese and colleagues (2007) suggested that persistent elevation of CRP levels in survivors of child abuse is due to the reduced ability of glucocorticoid signaling to control the hypothalamic-pituitary-adrenal (HPA) axis response to stress.
In the present study, we examine associations between salivary and plasma CRP, as well as the capacity of salivary CRP to predict high versus low levels of plasma CRP using a clinically relevant cutpoint used to assess CVD risk status (3 mg/L; Pearson et al., 2003). We investigate whether saliva and plasma CRP were comparable in their associations with other correlates of inflammation (BMI and smoking) and with physical and oral health. In addition, we explore whether salivary CRP discriminates between the risk categories for developing CVD, as stated by the American Heart Association (AHA) for serum levels of CRP. Based on the Framingham Study (Dhingra et al., 2007), women with CRP concentrations of 10 mg/L and higher were included as a separate category in these analyses to explore the ability of salivary CRP to screen for acute infections and inflammatory diseases. The current study employs a longitudinal design (three data collection waves separated by a year) and the diurnal collection of saliva samples at each data collection time. The multiple assessments of CRP in plasma and saliva provide a unique opportunity to investigate diurnal variation in salivary CRP, concurrent and prospective associations between saliva and plasma CRP, as well as their associations with BMI, smoking, physical and oral health.
Method
Sample
Data for the current study come from a convenience sample of women seeking help from three domestic violence crisis shelters and community agencies in a Midwestern State. These women were recruited for participation in a larger 2-year longitudinal study involving seven waves of data collection. Women 18 years of age or older, currently in an intimate abusive relationship, and residing in either a shelter or help-seeking in the community were eligible for the study. Women were excluded from participating if they had a known autoimmune disease or cancer at study entry. One hundred, fifty-seven women met eligibility criteria and agreed to participate. Signed informed consent was obtained.
The mean age of the women in this sample at study start was almost 34 years (SD = 9.5). The baseline sample had a high percentage of both African-American (47%) and Caucasian (46%) participants, and this ratio of ethnicity was consistent across waves. Seventy-seven percent of the women in the sample had a high school education or higher, but the majority had not completed college. The average annual income was between $10,000 and $15,000. At study start, the women had been in an intimate abusive situation an average of 5.35 years (SD = 6.69), with a range of one month to 34 years. The percentage of women who smoked at each data collection wave varied between 63 and 71%.
The current study used data from three of the seven waves of data collection, namely, baseline (Wave 1), one year (Wave 4) and two years (Wave 7). Retention rates were 132 women (84%) at one year after study entry and 128 (82%) at two years. Women who did not participate in the study at wave 4 and 7 were significantly younger (p’s < .03) and spent a shorter period of time in an abusive relationship compared to those women who stayed in the study (p’s < .02). Participants received a monetary acknowledgement in appreciation for their participation. The study was approved by the Institutional Review Board for the Protection of Human Research Participants at a major Midwestern university.
Procedure
At baseline, one-year and two-year data collection sessions, women completed questionnaires and had blood drawn by an RN or licensed phlebotomist using universal precautions. Wave 1 blood samples were drawn between the hours of 7:30 and 9:30 pm; waves 4 and 7 blood samples were drawn between 6 and 7 pm. Each sterile blood tube was immediately taken to the laboratory for processing. In some cases, tubes were not completely filled due to technical or venous access issues, thus had insufficient volume for tests.
After completing the questionnaire and having blood drawn, women were given specific instructions on the passive drool method for saliva collection. Participants received extensive verbal and written instructions that addressed times for sample collection, rinsing the mouth out prior to sample collection, avoiding coughing or clearing the throat into the collection tube, abstaining from eating, brushing teeth, using mouthwash, chewing gum or smoking for the hour prior to saliva collection. Instructions also directed participants to refrigerate the samples immediately after collection. Posters reminding women of collection times and outlining the instructions for the sample collection, including refrigeration of the samples, were posted throughout the shelter. At follow-up data collection times the protocol related to saliva collection, including refrigeration of samples, was re-emphasized. For each data collection wave, participants were asked to collect saliva at bedtime and upon awakening the next morning. Samples were taken to the hospital laboratory later that day by a member of the study team and frozen at −80°C.
Measures
Demographic and general health descriptors
Women self-reported their age, race/ethnicity, levels of education, annual income, and current smoking. Body mass index (BMI) was calculated using self-report height; weight was obtained using the study scale.
Physical and oral health symptoms
Physical symptoms were assessed using a modified version of the Pennebaker Inventory of Limbic Languidness (PILL) (Pennebaker, 1982; Pennebaker et al., 1977). The modified scale M-PILL assesses the frequency of 60 common physical symptoms and sensations, including physical health problems experienced by abused women and gynecologic-related items (Woods et al., 2008). Participants rated the frequency within the past 9 months with which they had experienced each symptom, on a scale of 0 (not at all) to 5 (more than once a day). Two M-PILL items addressed oral health, namely jaw pain and toothaches/dental pain, and the symptom severity for each of these items was included in the analyses. For the remaining items, the total number of physical symptoms women reported experiencing at least once a month for the past nine months was calculated and used in the analyses as a general health symptom variable.
High sensitivity plasma CRP
Blood for C-reactive protein was collected, centrifuged, and aliquoted into four separate tubes per sample at each data collection wave. The aliquot samples were stored in a minus 80° freezer until transported to Johns Hopkins University (JHU) School of Nursing for analysis. All samples at each wave were run in duplicate in a single batch. Quantification of CRP was determined using the steps outlined in the commercial enzyme immunoassay kit by R & D Systems, Minneapolis, MN. The plasma CRP quantification had a ceiling value of 12.55 mg/L.
High sensitivity salivary CRP
All samples were assayed for CRP using a commercially available immunoassay without modification to the manufacturer's recommended protocol. The test volume was 15 µL, with a range of standards from 93.75 to 3000 pg/mL, and the assay had a lower limit of sensitivity of 10 pg/mL. Samples were thawed to room temperature, centrifuged at 3000 rpm for 15 mins to remove mucins, and diluted 1:10 prior to assay. All samples were assayed in duplicate, and the average of the duplicates was used in the statistical analyses. Intra- and inter-assay coefficients of variation were less than 10% and 15% respectively.
Analytic approach
Differences between morning and evening salivary CRP values were examined separately for each wave. Next, we investigated the associations of salivary and plasma CRP with measures of general physical health symptoms and oral health symptoms, as well as with BMI and smoking. We also examined the stability of CRP levels over a period of two years.
Then, the concurrent and across-wave associations between plasma and salivary CRP were investigated in a number of different ways. Correlations between continuous measures of CRP were calculated. For each wave, analyses of covariance (ANCOVA) were conducted to test whether salivary CRP levels differed significantly between the three risk categories for CVD, based on serum levels of CRP as developed by the AHA and including a fourth category based on the Framingham Study (Dhingra et al., 2007).
Furthermore, we conducted sequential logistic regression analyses to predict high versus low levels of plasma CRP based on a clinically relevant cutoff of 3 mg/L (Pearson et al., 2003). Physical health, BMI and smoking were entered into the model as predictors in the first step. To prevent overfitting the logistic regression model, which occurs when too many predictors are included given the size of the groups, we included the two items on oral health in the general health variable, describing the total number of symptoms women reported experiencing at least once a month. Thus, each logistic regression includes four predictors. Salivary CRP was entered in the second step to test whether it significantly improved the prediction of high plasma CRP over and above the health-related variables. The significance of each model (step) was evaluated by calculating the difference in −2 log likelihood between the models, which is distributed as Χ2 with the difference in the number of parameters between the models as the degrees of freedom. The contribution of each individual predictor to the model was tested by the Wald test, and by calculating odds ratios.
Finally, the capacity of salivary CRP to predict high versus low plasma CRP was further evaluated using Receiver Operating Characteristic (ROC) curve analysis. The ROC curve is generated by plotting the probability of detecting true positives (sensitivity) and false positives (1 – specificity) for an entire range of cutoff points. The area under the ROC curve is a measure of the model’s ability to discriminate between individuals with high and low plasma CRP; acceptable discrimination is indicated when the area under the curve is at least .70 (Hosmer & Lemeshow, 2000). The cutoff point that optimizes the sensitivity/specificity ratio was selected using Youden’s J statistic (Youden, 1950). Apart from calculating the probability that individuals with high and low plasma CRP levels were classified correctly (sensitivity and specificity), we also examined classification accuracy from the perspective of low versus high salivary CRP (i.e., the probability that individuals with salivary CRP levels below or above the cutoff point were correctly classified as having low or high plasma CRP). The ROC curve analyses were conducted using salivary CRP as a predictor of plasma CRP, and then were repeated using the probabilities for high levels of plasma CRP calculated by the full logistic regression model, in order to test whether the inclusion of health variables in the prediction improved classification accuracy.
Results
Diurnal variation in salivary CRP
Descriptive statistics for plasma and salivary levels of CRP are displayed in Table 1. Morning levels of salivary CRP could not be assessed for the majority of the sample at wave 7 due to insufficient specimen volume (samples were first tested for two other analytes). A log transformation was applied to correct for the skewed distribution, and outlying values were winsorized (i.e., replaced with the next highest value of the remaining distribution; Tabachnik & Fidell, 2001). Differences in morning and evening levels of salivary CRP were tested for each wave separately. For wave 1, morning levels of CRP were significantly higher than evening CRP levels, t (95) = 5.42, p < .01. For waves 4 and 7, however, there were no significant differences between morning and evening levels, t (118) = −1.59, p = .12 and t (13) = 1.14, p = .28, respectively (note however that for wave 7, only 14 individuals had both morning and evening CRP values). For all waves, morning and evening levels of saliva CRP were strongly and positively correlated: (a) wave 1, r (94) = .74, p < .01; (b) wave 4, r (117) = .84, p < .01; and (c) wave 7, r (12) = .94, p < .01. Therefore, (untransformed) morning and evening levels of saliva CRP were averaged for each wave separately, after which a log transformation was applied and outlying values were winsorized. When one of the two measures for a wave was missing, the remaining assessment of salivary CRP was used to represent the average level at the respective wave. Median levels of CRP in plasma were between 1257 (wave 1) to 2097 (wave 4) times higher than were levels of CRP in saliva.
Table 1.
Descriptive statistics for health symptoms, BMI, plasma and saliva CRP at each wave
| N | Mean | Median | SD | |
|---|---|---|---|---|
| Wave 1 | ||||
| Physical health symptoms | 157 | 18.86 | 18.00 | 12.87 |
| Jaw pain | 157 | 0.79 | 0.00 | 1.55 |
| Toothache/dental pain | 157 | 1.38 | 0.00 | 1.85 |
| Body Mass Index | 152 | 29.37 | 28.26 | 7.31 |
| Saliva CRP morning (pg/ml) | 104 | 7791.43 | 2419.07 | 10539.52 |
| Saliva CRP evening (pg/ml) | 101 | 4920.06 | 1093.12 | 8287.94 |
| Mean saliva CRP (pg/ml) | 109 | 6311.88 | 1893.70 | 8847.14 |
| Plasma CRP (mg/L) | 114 | 3.96 | 2.38 | 4.08 |
| Wave 4 | ||||
| Physical health symptoms | 132 | 11.92 | 9.00 | 10.81 |
| Jaw pain | 132 | 0.41 | 0.00 | 1.09 |
| Toothache/dental pain | 132 | 0.83 | 0.00 | 1.50 |
| Body Mass Index | 127 | 29.85 | 29.01 | 7.38 |
| Saliva CRP morning (pg/ml) | 128 | 5664.24 | 1599.66 | 9356.68 |
| Saliva CRP evening (pg/ml) | 123 | 4627.82 | 1228.09 | 8027.18 |
| Mean saliva CRP (pg/ml) | 132 | 5096.35 | 1378.02 | 8190.34 |
| Plasma CRP (mg/L) | 123 | 4.35 | 2.89 | 4.01 |
| Wave 7 | ||||
| Physical health symptoms | 128 | 11.54 | 9.00 | 10.58 |
| Jaw pain | 128 | 0.40 | 0.00 | 1.09 |
| Toothache/dental pain | 128 | 0.75 | 0.00 | 1.47 |
| Body Mass Index | 126 | 29.97 | 28.71 | 7.67 |
| Saliva CRP morning (pg/ml) | 14 | 4277.60 | 3213.08 | 5177.95 |
| Saliva CRP evening (pg/ml) | 119 | 2663.95 | 1047.86 | 4009.67 |
| Mean saliva CRP (pg/ml) | 119 | 2661.83 | 1044.38 | 3989.85 |
| Plasma CRP (mg/L) | 103 | 3.54 | 1.61 | 3.81 |
Associations of salivary and plasma CRP levels with health, BMI, and smoking
No saliva or plasma CRP measure was significantly associated with general physical health symptoms at any of the measurement waves (Table 2). For wave 7, higher levels of salivary CRP were significantly associated with greater jaw pain (r [117] = .19, p = .04) and toothache/dental pain (r [117] = .21, p = .03). Furthermore, a higher BMI was associated with higher levels of saliva CRP (r’s between .17 and .26, p’s < .06) and also with higher levels of plasma CRP (r’s between .35 and .46, p’s < .01) at the same time of assessment. In addition, some of the salivary and all of the plasma CRP measures were associated with BMI values from different measurement waves. Finally, for each wave, there was no significant difference in salivary levels of CRP between individuals who did or did not smoke (p’s > .14). This was also the case for plasma CRP: there were no significant differences between smokers and non-smokers at each data collection wave (p’s > .61).
Table 2.
Pearson’s correlations of saliva and plasma CRP (log) with physical and oral health
| Saliva 1 | Saliva 4 | Saliva 7 | Plasma 1 | Plasma 4 | Plasma 7 | |
|---|---|---|---|---|---|---|
| Physical health – 1 | −.08 | .03 | .10 | −.09 | .16 | .08 |
| Physical health – 4 | −.07 | .00 | .09 | −.02 | .14 | .02 |
| Physical health – 7 | −.05 | .01 | .04 | −.05 | .09 | .00 |
| Jaw pain – 1 | −.17 | −.07 | −.01 | −.03 | −.02 | .05 |
| Jaw pain – 4 | −.21 | −.15 | .07 | −.15 | −.14 | −.14 |
| Jaw pain – 7 | .11 | .05 | .19* | .09 | −.08 | .00 |
| Toothache/dental pain – 1 | −.07 | .08 | .09 | −.16 | .12 | .03 |
| Toothache/dental pain – 4 | .13 | .10 | .23* | .21* | .08 | .00 |
| Toothache/dental pain – 7 | .11 | .13 | .21* | .10 | .06 | .05 |
| BMI – 1 | .20* | .17 | .14 | .37** | .32** | .44** |
| BMI – 4 | .03 | .17 | .15 | .30** | .35** | .42** |
| BMI – 7 | .22* | .27** | .26** | .27** | .35** | .46** |
p < .05,
p < .01, N = 84–132.
Stability of salivary CRP and associations with plasma CRP
As displayed in Table 3, salivary CRP levels were moderately stable across time, as demonstrated by r’s ranging between .46 and .61 (p’s < .01). A repeated measures ANOVA with saliva CRP as the within subjects factor revealed a significant main effect of time (F [2, 156] = 6.41, p < .01). Contrasts indicated that mean levels of salivary CRP were significantly lower at wave 7 compared to wave 4 (F [1, 78] = 7.12, p = .01) and wave 1 (F [1,78] = 10.86, p < .01]. There were no significant differences in salivary CRP between wave 1 and 4 (F [1,78] = 1.08, p = .30). Note however, that the majority of wave 7 respondents’ means were based on an evening sample only, compared to wave 4 and 1.
Table 3.
Pearson’s correlations between diurnal mean saliva CRP and plasma CRP measures (log)
| Saliva 1 | Saliva 4 | Saliva 7 | Plasma 1 | Plasma 4 | Plasma 7 | |
|---|---|---|---|---|---|---|
| Saliva CRP – 1 | - | |||||
| Saliva CRP – 4 | .61** | - | ||||
| Saliva CRP – 7 | .46** | .57** | - | |||
| Plasma CRP – 1 | .53** | .32** | .32** | - | ||
| Plasma CRP – 4 | .33** | .38** | .20* | .53** | - | |
| Plasma CRP – 7 | .39** | .38** | .49** | .58** | .70** | - |
p < .05,
p < .01, N = 73 – 122.
Similarly, moderate to high stability was observed for plasma CRP (r’s between .53 and .70, p’s < .01, see Table 3). A repeated measures ANOVA with the three assessments of plasma CRP as the within subjects factor indicated a significant main effect of time (F [1.84, 128.71] = 3.38, p = .04). Plasma CRP at wave 4 was significantly higher compared to wave 1 (F [1, 70] = 4.46, p = .04) and wave 7 (F [1, 70] = 6.32, p = .01). No significant differences were observed in plasma CRP between wave 1 and 7 (F [1, 70] = 0.04, p = .84).
At each wave, the associations between salivary and plasma CRP levels were significant, ranging from r (120) = .38, p < .01 for wave 4 to r (107) = .53, p < .01 for wave 1 (Table 3). The remaining cross-wave associations between saliva and plasma CRP levels were all significant, with correlation coefficients ranging between r (107) = .20, p = .04 and r (71) = .39, p < .01.1
Predicting American Heart Association (AHA) risk categories for CRP
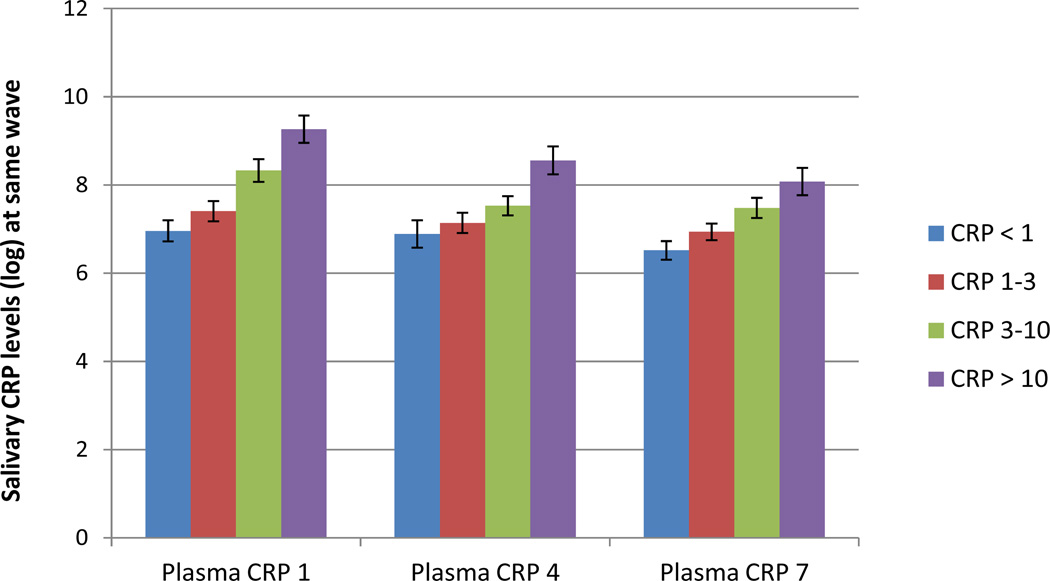
Analyses of Covariance (ANCOVA) were conducted to examine whether mean salivary CRP significantly differed between the four risk categories for developing CVD, controlling for smoking, BMI, physical health, and jaw and dental pain (see Figure 1 and Table 4). For each wave, the full ANCOVA model was statistically significant for wave 1, F (8,96) = 5.93, p < .01; for wave 4, F (8, 108) = 3.27, p < .01; and for wave 7, F (8, 84) = 4.69, p < .01. After controlling for smoking, physical and oral health, adjusted mean levels of salivary CRP differed significantly across the four categories (p’s < .01).
Figure 1.
Salivary CRP levels (M, SE) for American Heart Association (AHA) risk categories for developing cardiovascular diseases, based on serum CRP.
Note. CRP < 1 mg/L = low risk; CRP 1–3 mg/L = average risk; CRP 3–10 mg/L = high risk (Pearson et al., 2003). The fourth category, CRP > 10 mg/L, may indicate acute inflammation and was based on the Framingham Study (Dhingra et al., 2007).
Table 4.
ANCOVA Tests of differences in salivary CRP for AHA risk categories based on plasma CRP
| n (%) | M (SE)a | F | Contrasts | |
|---|---|---|---|---|
| Wave 1 | 5.93** | 1 = 2, 1 < 3, 1 < 4 | ||
| 1. CRP < 1 mg/L | 31 (30) | 6.96 (0.24) | 2 < 3, 2 < 4, 3 < 4 | |
| 2. CRP 1 – 3 mg/L | 31 (30) | 7.41 (0.23) | ||
| 3. CRP 3 – 10 mg/ | 26 (25) | 8.33 (0.26) | ||
| 4. CRP > 10 mg/L | 17 (16) | 9.27 (0.31) | ||
| Wave 4 | 3.27** | 1 = 2, 1 = 3, 1 < 4 | ||
| 1. CRP < 1 mg/L | 21 (18) | 6.89 (0.31) | 2 = 3, 2 < 4, 3 < 4 | |
| 2. CRP 1 – 3 mg/L | 38 (32) | 7.14 (0.23) | ||
| 3. CRP 3 – 10 mg/L | 39 (33) | 7.53 (0.22) | ||
| 4. CRP > 10 mg/L | 19 (16) | 8.56 (0.32) | ||
| Wave 7 | 4.69** | 1 = 2, 1 < 3, 1 < 4 | ||
| 1. CRP < 1 mg/L | 29 (31) | 6.52 (0.21) | 2 = 3, 2 < 4, 3 = 4 | |
| 2. CRP 1 – 3 mg/L | 30 (32) | 6.94 (0.19) | ||
| 3. CRP 3 – 10 mg/L | 22 (24) | 7.48 (0.23) | ||
| 4. CRP > 10 mg/L | 12 (13) | 8.08 (0.31) | ||
Note. AHA category 1 = low risk; category 2 = average risk; category 3 = high risk (Pearson et al., 2003). The fourth category was based on the Framingham Study (Dhingra et al., 2007), and may indicate acute inflammation. Salivary CRP values were log-transformed.
Adjusted mean levels of salivary CRP.
p < .01
Contrasts indicated some parallels between salivary CRP levels and plasma CRP as categorized according to the risk categories. There were no significant differences between low (< 1 mg/L) and average (1–3 mg/L) risk categories (p’s > .15). For wave 1 and 7, salivary CRP was significantly higher for women in the high risk (3–10 mg/L) category compared to women in the low risk category (p’s < .01). For all waves, levels of salivary CRP were higher for women with plasma levels of 10 mg/L or higher compared to women in the low risk category (p’s < .01). Significant differences in salivary CRP between the average and high risk categories were found only for wave 1 (p < .01). Finally, women with the highest levels of plasma CRP had also higher salivary CRP compared to women in the average risk category (for all waves, p’s < .01) and compared to women in the high risk category (for wave 1 and 4, p’s < .03, for wave 7, p = .12).
Predicting high levels of plasma CRP by salivary CRP: logistic regressions
For each wave, a sequential logistic regression analysis was performed to predict high versus low levels of plasma CRP based on the threshold of 3 mg/L. Table 5 shows the regression coefficients, Wald statistics and the odds ratios for each of the predictors. Results from the ROC analyses, including sensitivity and specificity, can be found in Table 6.
Table 5.
Logistic regression analyses predicting high (> 3 mg/L) versus low levels of plasma CRP
| B | Wald Χ2 | p | Odds ratio (95% CI) | Χ2 | |
|---|---|---|---|---|---|
| Wave 1 | |||||
| Step 1 | 14.65** | ||||
| Physical health symptoms | −0.01 | 0.60 | .44 | 0.99 (0.96 – 1.02) | |
| Smoking | −0.72 | 2.15 | .14 | 0.49 (0.19 – 1.27) | |
| Body mass index | 0.11 | 10.63 | <.01 | 1.11 (1.04 – 1.18) | |
| Step 2 | 25.17** | ||||
| Salivary CRP | 0.86 | 19.12 | <.01 | 2.37 (1.61 – 3.49) | |
| Wave 4 | |||||
| Step 1 | 15.53** | ||||
| Physical health symptoms | 0.05 | 6.15 | .01 | 1.05 (1.01 – 1.09) | |
| Smoking | 0.12 | 0.07 | .79 | 1.12 (0.48 – 2.65) | |
| Body mass index | 0.09 | 9.37 | <.01 | 1.10 (1.03 – 1.16) | |
| Step 2 | 10.81** | ||||
| Salivary CRP | 0.48 | 9.67 | <.01 | 1.62 (1.20 – 2.20) | |
| Wave 7 | |||||
| Step 1 | 12.09** | ||||
| Physical health symptoms | 0.02 | 1.01 | .31 | 1.02 (0.98 – 1.06) | |
| Smoking | −0.32 | 0.43 | .51 | 0.72 (0.28 – 1.90) | |
| Body mass index | 0.10 | 10.04 | <.01 | 1.11 (1.04 – 1.18) | |
| Step 2 | 12.07** | ||||
| Salivary CRP | 0.74 | 10.37 | <.01 | 2.10 (1.34 – 3.29) | |
p < .05,
p < .01, N = 93 – 107.
Note. Jaw pain and dental pain/toothaches were included in the general health symptoms variable. Salivary CRP values were log-transformed.
Table 6.
ROC curve analysis for high versus low plasma CRP
| Area (95% CI) |
SE | p | Specificity | Specificity | % Low salivary CRP correctly classified as low plasma CRP |
% High salivary CRP correctly classified as high plasma CRP |
|
|---|---|---|---|---|---|---|---|
| Wave 1 | |||||||
| Salivary CRP | .80 (.71–.88) | .04 | < .01 | 80% | 77% | 85% | 70% |
| Health variables + salivary CRP | .85 (.78–.93) | .04 | < .01 | 93% | 68% | 93% | 67% |
| Wave 4 | |||||||
| Salivary CRP | .71 (.62–.81) | .05 | < .01 | 68% | 75% | 71% | 71% |
| Health variables + salivary CRP | .77 (.68–.86) | .04 | < .01 | 86% | 59% | 81% | 68% |
| Wave 7 | |||||||
| Salivary CRP | .75 (.65–.86) | .05 | < .01 | 71% | 79% | 83% | 66% |
| Health variables + salivary CRP | .80 (.70–.89) | .05 | < .01 | 71% | 76% | 82% | 63% |
Note. Cut-off value based on Youden’s J statistic.
For wave 1, data from 105 women were available for analysis, and for 43 women of this group, plasma CRP levels were higher than 3 mg/L. Compared to a constant-only model, the model with physical health, BMI and smoking (step 1) was statistically significant, Χ2 (3) = 14.65, p < .01. According to the Wald criterion, only BMI and none of the other health variables significantly predicted high plasma CRP, Χ2 (1) = 10.63, p < .01. The addition of salivary CRP in the second step significantly improved the prediction of plasma CRP, Χ2 (1) = 25.17, p < .01, indicating that salivary CRP reliably distinguished between high and low levels of plasma CRP over and above the health variables, Wald’s Χ2 (1) = 19.12, p < .01, odds ratio 2.37.
The logistic regression analysis for wave 4 was based on 117 participants, including 58 women with high levels of plasma CRP. More physical health symptoms and a higher BMI (p’s < .02) were significantly associated with high levels of plasma CRP. After controlling for health, higher levels of salivary CRP significantly predicted high plasma CRP (p < .01). For wave 7, the logistic regression analysis included 93 participants, and of this group 34 individuals had high levels of plasma CRP. BMI predicted high plasma CRP (p < .01) and salivary CRP significantly contributed to the prediction of high plasma CRP after controlling for health (p < .01).
Predicting high levels of plasma CRP by salivary CRP: ROC curve analyses
Table 6 presents the results of the ROC curve analyses for each wave. Based on salivary CRP alone, the area under the curve (AUC) ranged between .71 (wave 4) and .80 (wave 1) (p’s < .01), which is considered acceptable to excellent discrimination between low and high plasma CRP. Between 68 and 80% of the women with high plasma CRP were correctly classified (sensitivity), whereas 75–79% of the participants with low plasma CRP were correctly classified (specificity). Of the women with salivary CRP levels below the cutoff point, 71–85% were correctly identified as having low plasma CRP, whereas 66–71% of the participants with higher levels of salivary CRP were correctly placed in the high plasma CRP group.
When ROC analyses were conducted with the predicted probabilities based on the full logistic regression model (including health variables), the AUC ranged between .77 and .85 (p’s < .01) with a sensitivity of 71–93% and specificity of 59–76%. In addition, 81–93% of the women with low salivary CRP were correctly identified as having low plasma CRP, and 63–68% of the women with high salivary CRP levels were correctly classified as having high plasma CRP.
Discussion
In a prospective longitudinal study of high risk women, we observed moderate associations between CRP measured in saliva and plasma and significant associations of both saliva and plasma CRP with BMI (but not smoking) within each wave of assessment. Salivary CRP reliably distinguished between individuals with high and low plasma levels of CRP, even after controlling for general and oral health. ROC analyses showed that the classification accuracy was adequate, especially for lower levels of salivary CRP. In addition, saliva CRP discriminated significantly between the risk categories for CVD based on plasma CRP. Finally, results indicate that plasma and saliva CRP were moderately stable across a period of two years, and there was some evidence for diurnal variation in saliva CRP, with a consistent, although not always significant, pattern of higher morning values than evening values.
The high-risk nature of the sample was reflected in the high plasma CRP levels at each assessment, with 37 to 49% of these relatively young women having concentrations higher than the clinically relevant cutpoint of 3 mg/L. This is consistent with previous studies demonstrating that chronic stress such as IPV or childhood maltreatment is associated with immune function alterations, including elevation of serum CRP levels (Danese et al., 2007; Fernandez-Botran et al., 2011) and higher leukocytes and lymphocyte cell counts (Woods et al., 2005).
The longitudinal design of the current study with saliva collected in the morning and evening for each data collection wave allowed us to investigate more basic characteristics of salivary CRP. There was statistically significant diurnal variation in salivary CRP levels for one of the three data collection waves, with higher morning values than evening values (note however that the significance test for wave 7 had low statistical power). This result suggesting a possible diurnal difference in salivary CRP concentrations is consistent with two previous studies on serum CRP (Koc et al., 2010; Rudnicka et al., 2007; but see also Meier-Ewert et al., 2001), and calls for more research with multiple samples collected within and across days in populations with expected high and low CRP levels.
To the best of our knowledge this is the first study showing that individual differences in saliva CRP are moderately stable over a long period of time, with correlation coefficients similar to plasma CRP. Although the stability in CRP as assessed in the two biospecimens may reflect a stable trait component, the chronic nature of IPV, with approximately half of the women remaining in an abusive relationship over a long period of time, may also account for the stability in CRP concentrations. An additional explanation has been suggested by Fernandez-Botran and colleagues (2011), who stated that elevated CRP levels are more likely to be the result of a chronic, cumulative effect, in contrast to plasma IL-6 which is more susceptible to change in response to physiological and psychosocial stimuli.
Several results from the current study support the potential use of salivary CRP as an indicator for systemic levels of inflammation. For each data collection wave, we observed a moderate association between CRP as assessed in plasma and saliva, and it is noteworthy that the saliva-plasma CRP concentrations were significantly correlated even when the measures were taken two years apart. Further, the associations with smoking and BMI were similar for saliva and plasma CRP: women who smoked did not have higher CRP concentrations in either saliva or plasma than women who did smoke, but higher BMI values were consistently associated with higher salivary and plasma CRP. Finally, salivary CRP significantly discriminated between low and high plasma CRP after controlling for physical and oral health symptoms, with sensitivity ranging between 71 and 93%, and specificity between 59 and 76%.
Overall, results indicated that discrimination between low and high plasma CRP was especially accurate for low levels of salivary CRP. For example, for wave 1, 93% of the women with low salivary CRP were correctly classified as having low plasma CRP, compared to 67% of the women with high salivary CRP who were correctly identified as having high plasma CRP. Thus, the noninvasive assessment of salivary CRP as an alternative marker for inflammation may prove to be especially valuable in terms of screening: whereas low salivary CRP levels are likely to reflect low plasma CRP concentrations, elevated CRP levels in saliva warrants further risk assessment including a venipuncture to measure serum CRP. The less accurate prediction of plasma CRP in the case of high salivary CRP concentrations may be due to insufficient control for local inflammatory processes, oral hygiene and diseases. Megson and colleagues (2010) have demonstrated that CRP in the GCF is likely to be of systemic origin, and as such could be indicative of systemic inflammation (see also Pradeep et al., 2010). Since whole saliva contains secretions from the salivary glands as well as from GCF, this would account for the significant associations between plasma and saliva CRP levels. However, as for some of the cytokines found in oral fluids, the question of local production of CRP in the oral cavity must be considered. Indeed, although CRP is primarily synthesized in the liver, two studies showed increased CRP mRNA expression in the submandibular glands of rats with inflammation, which was preceded by a transient increase in the level of mRNAs for the cytokines IL-1β, IL-6 and TNF-α (Wei et al., 2001; Yao et al., 2005).
Thus, although higher levels of CRP in whole saliva appear to have a systemic origin, poor oral hygiene and local inflammation processes may also be an important contributor, explaining the lower classification accuracy in the case of high salivary CRP. Future studies on salivary CRP as an inflammatory marker should include a more detailed oral health assessment. In the current study, the measurement of physical and oral health focused on the frequency of occurrence of specific symptoms over the previous nine months. Two items were included regarding jaw and dental pain, which were taken from a larger questionnaire on physical health, and which are not a complete and validated measure of oral health status. Future studies should incorporate more questions directly related to periodontal disease (associated with increased levels of CRP in GCF and serum, e.g., Megson et al., 2010; Noack et al., 2001), as well as more objective measures of oral health. Detailed and validated health measures at the time of sampling would provide more information on the association between saliva and plasma CRP in individuals with varying health-related problems, and may improve the classification accuracy for individuals with high salivary CRP.
Concluding, our study focusing on a high-risk sample of women exposed to IPV provides further support for the validity of salivary CRP as an alternative marker of inflammation and a potential risk index for CVD. When combined with a comprehensive evaluation of oral health, salivary CRP has the potential to be used within a broad spectrum of applications where it may assist in screening programs, the diagnosis of CVD and in monitoring overall health, disease status and treatment response (Pfaffe et al., 2011). Indeed, results from the current study suggest that salivary CRP discriminates between the risk categories for CVD developed by the AHA (Pearson et al., 2003). Although not all of the contrasts were significant, results are consistent with those reported by Floriano and colleagues (2009) who demonstrated that salivary CRP was among the salivary biomarkers that reliably distinguished between patients with existing acute myocardial infarction and controls (see also Punyadeera et al., 2011). Because saliva sampling is minimally invasive, does not require specialized laboratory equipment and trained personnel, and can be performed in community- and home-based settings, measurement of salivary CRP can also be included in large-scale epidemiological studies. Further replication of the results of the current study in samples with systematic variation in physical and oral health is needed, as well as more basic research on the origin of CRP in whole saliva and on possible diurnal variation.
Research highlight.
This study provides support for the validity of salivary CRP as a minimally invasive marker of systemic inflammation and points to its potential use as a screening tool.
Acknowledgements
this research was supported by NINR/NICHHD (R01 009286) and an Ohio Board of Regents Grant to SW. DO was supported by a Rubicon award (446-10-026) from the Netherlands Organization for Scientific Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: in the interest of full disclosure, DAG is founder and Chief Strategy and Scientific Advisor at Salimetrics LLC (State College, PA). DAG’s relationship with Salimetrics LLC is managed by the policies of the Conflict of Interest Committee at the Johns Hopkins University School of Medicine. Salimetrics donated a portion of the salivary CRP kits used in this study.
Follow-up partial correlation analyses indicated that all of their values remained similar to the zero-order correlations reported in Table 3, after controlling for smoking, physical symptoms, jaw and toothaches/dental pain and BMI. Specifically, the mean difference in correlations was .05, range .00–.12; only the association between saliva CRP at wave 7 and plasma CRP at wave 4 was no longer significant.
References
- Bisoendial RJ, Boekholdt SM, Vergeer M, Stroes ESG, Kastelein JJP. C-reactive protein is a mediator of cardiovascular disease. Eur. Heart J. 2010;31:2087–2095. doi: 10.1093/eurheartj/ehq238. [DOI] [PubMed] [Google Scholar]
- Black S, Kushner I, Samols D. C-reactive protein. J. Biol. Chem. 2004;279:48487–48490. doi: 10.1074/jbc.R400025200. [DOI] [PubMed] [Google Scholar]
- Breiding MJ, Black MC, Ryan GW. Chronic disease and health risk behaviors associated with partner violence. 18 US states/territories 2005. Ann. Epidemiol. 2008;18:538–544. doi: 10.1016/j.annepidem.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Campbell J, Jones AS, Dienemann J, Kub J, Schollenberger J, O’Campo P, Gielen AC, Wynne C. Intimate partner violence and physical health consequences. Arch. Intern. Med. 2002;162:1157–1163. doi: 10.1001/archinte.162.10.1157. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N. Engl. J. Med. 1995;332:1351–1362. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc. Natl. Acad. Sci. USA. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra R, Gona P, Nam B, D’Agostino RB, Wilson PWF, Benjamin EJ, O’Donnell CJ. C-reactive protein, inflammatory conditions, and cardiovascular disease risk. The Am. J. Med. 2007;120:1054–1062. doi: 10.1016/j.amjmed.2007.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon MC, Opris DC, Kopanczyk R, Lickliter J, Cornwell HN, Bridges EG, Nazar AM, Grove Brdiges K. Detection of homocysteine and C-reactive protein in the saliva of healthy adults. Comparison with blood levels. Biomarker Insights. 2010;5:57–61. doi: 10.4137/bmi.s5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsberg M, Jansen HAFM, Heise L, Watts CH, Garcia-Moreno C WHO Multi-country Study on Women’s Health and Domestic Violence against Women Study team. Intimate partner violence and women’s physical and mental health in the WHO multi-country study on women’s health and domestic violence. An observational study. Lancet. 371:1165–1172. doi: 10.1016/S0140-6736(08)60522-X. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am. J. Prev. Med. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Fernandez-Botran R, Miller JJ, Burns VE, Newton TL. Correlations among inflammatory markers in plasma, saliva, and oral mucosal transudate in post-menopausal women with past intimate partner violence. Brain Beh. Immun. 2011;25:314–321. doi: 10.1016/j.bbi.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floriano PN, Christodoulides N, Miller CS, Ebersole JL, Spertus J, Rose BG, Kinane DF, Novak MJ, Steinhubl S, Acosta S, Mohanty S, Dharshan P, Yeh C, Redding S, Furmaga W, McDevitt JT. Use of saliva-based nano-biochip tests for acute myocardial infarction at the point of care. A feasibility study. Clin. Chem. 2009;55:1530–1538. doi: 10.1373/clinchem.2008.117713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction. Implications for health. Nat. Rev. Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Golding JM. Intimate partner violence as a risk factor for mental disorders. A meta-analysis. J. Fam. Violence. 1999;14:99–132. [Google Scholar]
- Granger DA, Granger GA, Granger SW. Immunology and developmental psychopathology. In: Cicchetti D, Cohen D, editors. Developmental Psychopathology, Vol 2. Developmental Neuroscience. New York: John Wiley & Sons; 2006. pp. 677–709. [Google Scholar]
- Granger DA, Kivlighan KT, Fortunato C, Harmon AG, Hibel LC, Schwartz E, Whembolua G. Integration of salivary biomarkers into developmental and behaviorally-oriented research. Problems and solutions for collecting specimens. Physiol. Behav. 2007;92:583–590. doi: 10.1016/j.physbeh.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Granger DA, Johnson SB, Szanton SL, Out D, Lau Schumann L. Incorporating salivary biomarkers into nursing research. An overview and review of best practices. Biol. Res. Nurs. doi: 10.1177/1099800412443892. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosmer DW, Lemeshow S. Applied logistic regression. 2nd edition. New York: John Wiley & Sons; 2000. [Google Scholar]
- Jones L, Hughes M, Understaller U. Post-traumatic stress disorder (PTSD) in victims of domestic violence. A review of the research. Trauma Violence Abuse. 2001;2:99–119. [Google Scholar]
- Koc M, Karaarslan O, Abali G, Batur MK. Variation in high-sensitivity C-reactive protein levels over 24 hours in patients with stable coronary artery disease. Tex. Heart Inst. J. 2010;37:42–48. [PMC free article] [PubMed] [Google Scholar]
- Kopanczyk R, Opris DC, Lickliter J, Bridges EG, Nazar AM, Grove Bridges K. C-reactive protein levels in blood and saliva show no correlation in young, healthy adults. FASEB J. 2010;24:lb409. doi: 10.4137/bmi.s5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megson E, Fitzsimmons T, Dharmapatni K, Barthold PM. C-reactive protein in gingival crevicular fluid may be indicative of systemic inflammation. J. Clin. Periodontol. 2010;37:797–804. doi: 10.1111/j.1600-051X.2010.01603.x. [DOI] [PubMed] [Google Scholar]
- Melander O, Newton-Cheh C, Almgren P, Hedblad B, Berglund G, Engström G, Persson M, Smith JG, Magnusson M, Christensson A, Struck J, Morgenthaler NG, Bergmann A, Pencina MJ, Wang TJ. Novel and conventional biomarkers for prediction of incident cardiovascular events in the community. JAMA. 2009;302:49–57. doi: 10.1001/jama.2009.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier-Ewert HK, Ridker PM, Rifal N, Price N, Dinges DF, Mullington JM. Absence of diurnal variation of C-reactive protein concentrations in healthy human subjects. Clin. Chem. 2001;47:426–430. [PubMed] [Google Scholar]
- Miller CS, Foley JD, Bailey AL, Campell CL, Humphries RL, Christodoulides N, Floriano PN, Simmons G, Bhagwandin B, Jacobson JW, Redding SW, Ebersole JL, McDevitt JT. Current developments in salivary diagnostics. Biomark. Med. 2010;4:171–189. doi: 10.2217/bmm.09.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minetto M, Rainoldi A, Gazzoni M, Terzolo M, Borrione P, Termine A, Saba L, Dovio A, Angeli A, Paccotti P. Differential responses of serum and salivary interleukin-6 to acute strenuous exercise. Eur. J. Appl. Physiol. 2005;93:679–686. doi: 10.1007/s00421-004-1241-z. [DOI] [PubMed] [Google Scholar]
- Newton TL, Fernandez-Botran R, Miller JJ, Lorenz DJ, Ellison Burns V, Fleming KN. Markers of inflammation in midlife women with intimate partner violence histories. J. Womens Health. doi: 10.1089/jwh.2011.2788. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noack B, Genco RJ, Trevisan M, Grossi S, Zambon JJ, De Nardin E. Periodontal infections contribute to elevated systemic C-reactive protein level. J. Periodontol. 2001;72:1221–1227. doi: 10.1902/jop.2000.72.9.1221. [DOI] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, III, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease. Application to clinical and public health practice. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- Pennebaker JW. The psychology of physical symptoms. New York: Springer-Verlag; 1982. [Google Scholar]
- Pennebaker JW, Burnam MA, Schaeffer MA, Harper MA. Lack of control as a determinant of perceived physical symptoms. J. Pers. Social Psychol. 1977;35:167–174. doi: 10.1037//0022-3514.35.3.167. [DOI] [PubMed] [Google Scholar]
- Pfaffe T, Cooper-White J, Beyerlein P, Kostner K, Punyadeera C. Diagnostic potential of saliva. Current state and future applications. Clin. Chem. 2011;57:675–687. doi: 10.1373/clinchem.2010.153767. [DOI] [PubMed] [Google Scholar]
- Pradeep AR, Manjunath S, Kathariya R. Progressive periodontal disease has a simultaneous incremental elevation of gingival crevicular fluid and serum CRP levels. J Invest. Clin. Dentistry. 2010;78:133–138. doi: 10.1111/j.2041-1626.2010.00022.x. [DOI] [PubMed] [Google Scholar]
- Punyadeera C, Dimeski G, Kostner K, Beyerlein P, Cooper-White J. One-step homogeneous C-reactive protein assay for saliva. J. Immunol. Methods. 2011;373:19–25. doi: 10.1016/j.jim.2011.07.013. [DOI] [PubMed] [Google Scholar]
- Quellet-Morin I, Danese A, Williams B, Arseneault L. Validation of a high-sensitivity assay for C-reactive protein in human saliva. Brain Beh. Immun. 2011;25:640–646. doi: 10.1016/j.bbi.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98:731–733. doi: 10.1161/01.cir.98.8.731. [DOI] [PubMed] [Google Scholar]
- Rudnicka AR, Rumley A, Lowe GDO, Strachan DP. Diurnal, seasonal, and blood-processing patterns in levels of circulating fibrinogen, fibrin D-dimer, C-reactive protein, tissue plasminogen activator, and von Willebrand factor in a 45-year-old population. Circulation. 2007;115:996–1003. doi: 10.1161/CIRCULATIONAHA.106.635169. [DOI] [PubMed] [Google Scholar]
- Scott-Storey K, Wuest J, Ford-Gilboe M. Intimate partner violence and cardiovascular risk. Is there a link? J. Adv. Nurs. 2009;10:2186–2197. doi: 10.1111/j.1365-2648.2009.05086.x. [DOI] [PubMed] [Google Scholar]
- Sjögren E, Leanderson P, Kristenson M, Ernerudh J. Interleukin-6 levels in relation to psychosocial factors. Studies on serum, saliva and in vitro production by blood mononuclear cells. Brain Beh. Immun. 2005;20:270–278. doi: 10.1016/j.bbi.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Sorrels SF, Sapolsky RM. An inflammatory review of glucocorticoid actions in the CNS. Brain Beh. Immun. 2007;21:259–272. doi: 10.1016/j.bbi.2006.11.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. Boston: Allyn and Bacon; 2001. [Google Scholar]
- Wei W, Parvin MN, Tsumura K, Akamatsu T, Tada J, Kanamori N, Hosoi K. Induction of C-reactive protein, serum amyloid P component, and kininogens in the submandibular and lacrimal glands of rats with experimentally induced inflammation. Life Sci. 2001;69:359–368. doi: 10.1016/s0024-3205(01)01129-8. [DOI] [PubMed] [Google Scholar]
- Wilson PWF, Pencina M, Jacques P, Selhub J, D’Agostino R, O’Donnell CJ. C-reactive protein and reclassification of cardiovascular risk in the Framingham Heart Study. Circ. Cardiovasc. Qual. Outcomes. 2008;1:92–97. doi: 10.1161/CIRCOUTCOMES.108.831198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SJ, Hall RJ, Campbell JC, Angott DM. Physical health and posttraumatic stress disorder symptoms in women experiencing intimate partner violence. J Midwifery Womens Health. 2008;53:538–546. doi: 10.1016/j.jmwh.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SJ, Wineman M, Page GG, Hall RJ, Alexander TS, Campbell JC. Predicting immune status in women from PTSD and childhood and adult violence. ANS Adv. Nurs. Sci. 2005;4:306–319. doi: 10.1097/00012272-200510000-00003. [DOI] [PubMed] [Google Scholar]
- Yao C, Wei W, Li X, Hosoi K. Acute phase protein induction by experimental inflammation in the salivary gland. J Oral Pathol Med. 2005;34:364–367. doi: 10.1111/j.1600-0714.2005.00321.x. [DOI] [PubMed] [Google Scholar]
- Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]