We have characterized the EV-like dermatosis of acquired HIV in 4 adolescents. Multiple HPV types were isolated in skin tissue samples, including β-HPV, but also high levels of HPV 1 and 2. ARV did not improve the EV eruption.
Abstract
Background. We have previously described the presentation of epidermodysplasia verruciformis (EV)–like eruptions in almost a quarter of hospitalized adolescents with vertically-acquired human immunodeficiency virus (HIV) infection in Harare, Zimbabwe, a region with a high prevalence of HIV infection.
Methods. We performed a clinical case note review and skin biopsy from affected sites in 4 HIV-infected adolescents with EV-like lesions in Harare. Biopsies were processed for histology and for human papillomavirus (HPV) typing.
Results. All patients had long-standing skin lesions that pre-dated the diagnosis of HIV by several years. The histology of skin biopsies from all patients was consistent with EV. In each biopsy, EV-associated β-HPV type 5 was identified (additionally, type 19 was found in 1 biopsy). Cutaneous wart–associated HPV types 1 and 2 were detected in all biopsies, together with genital lesion–associated HPV types 6, 16, and 52, (as well as ≥3 other genital lesion–associated HPV types). Despite immune reconstitution with combination antiretroviral therapy (cART), there was no improvement in EV-like lesions in any patient.
Conclusions. EV is a disfiguring and potentially stigmatizing condition among this patient group and is difficult to treat; cART appears to have no impact on the progression of skin disease. Among adolescents with longstanding HIV-induced immunosuppression and with high levels of sun exposure, close dermatological surveillance for potential skin malignancy is required.
Classical epidermodysplasia verruciformis (EV) is a human papillomavirus (HPV)–related condition, manifest as widespread, persistent, banal macules or papules resembling common flat warts or pityriasis versicolor. It starts on the hands and forehead in childhood [1], spreading to involve other sun-exposed sites. With time, lesions may become more prominent and verrucous, and may progress to invasive squamous cell carcinoma.
The disease results from a genetic susceptibility to chronic disseminated infection with HPV of the ß papillomavirus group. Evidence suggests a genetic heterogeneity of the disease rather than X-linked or autosomal-dominant inheritance. Homozygous mutations in either the EVER1 and EVER2 gene located on chromosome 17 have been frequently identified [2, 3]. Although classically an autosomal-recessive condition, acquired EV has been reported in immunodeficiency states, including sporadic reports in adults with human immunodeficiency virus (HIV) infection [4–10].
We have previously described the presentation of EV-like eruptions, commonly termed planar warts, in up to a quarter of hospitalized adolescents with vertically acquired HIV infection in Harare, Zimbabwe [11]. Here, we report the histological findings and results of HPV typing performed on skin biopsies taken from affected sites in 4 HIV-infected adolescents in Harare.
CASE REPORTS
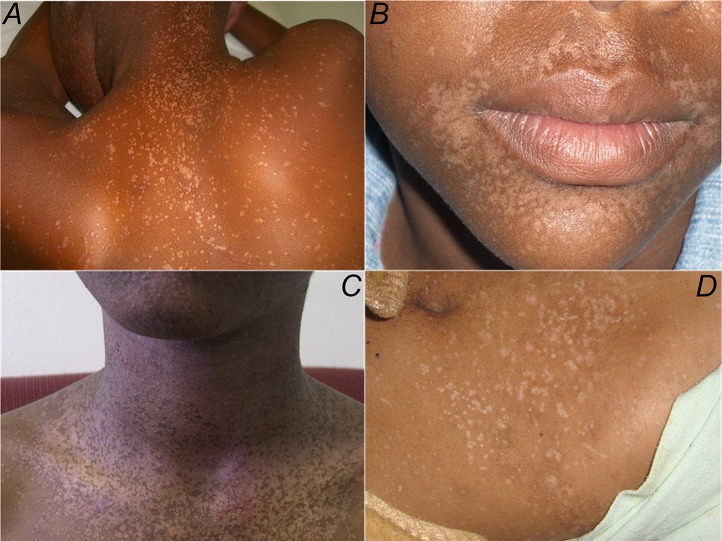
Patient 1 was diagnosed with HIV infection in 2005 at 13 years of age. Since the age of 8, he had complained of a persistent, nonitchy skin rash covering his face and arms. There was no family history of skin disease and no other past medical history of note. A physical examination revealed widespread flat, nonscaly, hypopigmented macules from 1 to 15 mm. The lesions were distributed on the sun-exposed areas of his upper torso, face, neck, and forearms. The genital area, other mucous membranes, scalp, and nails were unaffected. Koebnerization was frequent. A clinical diagnosis of planar warts was made. No specific treatment was available. In July 2005, he commenced combination antiretroviral therapy (cART) with nevirapine, lamivudine, and stavudine. His nadir CD4 T-cell count was 193 cells/μL (8% of the total lymphocyte count). After starting cART, his CD4 T-cell count improved to 964 cells/μL (25% of the total lymphocyte count) in 2008 and he remained clinically well. However, the cutaneous eruption had not improved (Figure 1A ).
Figure 1.
A–D, Physical examination of all 4 cases revealed multiple hypopigmented papules distributed on the trunk, neck, face, and upper limbs.
Patient 2 was found to be infected with HIV in 2005, at the age of 12. Since 2001 at age 8, he complained of a persistent nonitchy hypopigmented rash covering sun-exposed areas, including the legs, but with no genital lesions. There was no family history of skin disease. He commenced cART in March of 2007. The nadir CD4 T-cell count at that time was 186 cells/μL (11% of the total lymphocyte count). Sixteen months later, he was clinically well and his CD4 T-cell count had improved to 479 cells/μL (23%). The skin eruption remained unchanged despite the introduction of cART (Figure 1B ).
Patient 3 was diagnosed with HIV infection in 2007 at the age of 15. Since the age of 7, he had complained of a persistent maculopapular eruption similar in distribution and morphology to patients 1 and 2. He commenced cART in December of 2007 with a nadir CD4 T-cell count of 180 cells/μL. In 2011, his CD4 T-cell count was 414 cells/μL and his skin manifestations remained unchanged (Figure 1C).
Patient 4 was found to be infected with HIV in 2002 when he was 13 years old, following a diagnosis of pulmonary tuberculosis. At that time, disseminated flat warts were noted involving the face and limbs with scattered truncal lesions; these had been present for several years. He commenced cART in 2006, but the nadir CD4 T-cell count was not recorded. In 2010, his CD4 T-cell count was 97 cells/μL and the skin rash remained unchanged (Figure 1D ).
All 4 patients were vertically infected with HIV: all were maternal orphans, denied previous sexual intercourse, and reported no blood transfusions or intravenous drug use.
MATERIALS AND METHODS
Tissue Samples
Diagnostic punch skin biopsies were taken from each patient with informed consent (Table 1). The samples were split: one half was processed for light microscopy and the other half was fixed in formalin for transport, and then rinsed in phosphate buffered saline at 4°C before homogenization. Tissue was digested with a 200 μg/mL solution of proteinase K overnight at 37°C, and DNA was extracted using phenol/chloroform and ethanol precipitation.
Table 1.
Human Papillomavirus Types Isolated
| Patient | Site of Skin Biopsy | HPV Types | HPV Type Summary | ||
| β/EV Types | Cutaneous Wart–Associated Types | Genital Types | HPV Types With Highest Signal Intensities (Quantitative and Semiquantitative Data) | ||
| 1 | Neck | 5 | 1, 2 | 6, 16, 18, 45, 52, 53, 54 | HPV 1 +++ |
| HPV 2 ++ | |||||
| HPV 6+ | |||||
| 2 | Shoulder | 5,19 | 1, 2 | 6, 11, 16, 18, 31, 33, 52 | HPV 1+++ |
| HPV 2++ | |||||
| 3 | Arm | 5 | 1, 2 | 6, 11, 16, 31, 51, 52, 66 | HPV 1+++ |
| HPV 2++ | |||||
| 4 | Neck | 5 | 1, 2 | 6, 11, 16, 18, 31, 52, 53, 66 | HPV 1+++ |
| HPV 2++ | |||||
| HPV 6+ | |||||
Abbreviations: EV, epidermodysplasia verruciformis; HPV, human papillomavirus.
HPV Typing
Two comprehensive HPV typing systems were utilized in 2 separate specialist HPV research laboratories that could identify cutaneous wart–associated HPV types, β (EV-associated) types, and genital HPV types. First, polymerase chain reaction (PCR) was performed, followed by sequencing using primers that amplify a wide spectrum of HPV types; PGMY, GP5+/GP6+, CP65/CP70, CP66/CP70, and CH1F/CN1R [12, 13]. Next, confirmatory HPV typing was done using a Luminex-based system (cutaneous HPV) and 2 reverse hybridization line probe assays (LiPA) (genital and β-HPV) [14–16]. The final HPV typing outcome was obtained by combining the results of all the methods used. HPV viral load assays were available for common β and genital HPV types (HPV 5 and HPV 6, 11, 16, and 18, respectively) [17].
RESULTS
Histopathology
The histological findings in each patient’s biopsy included basket-weave orthokeratosis overlying a prominent granular cell layer. Acanthosis, mild spongiosis, focal lymphocyte exocytosis, koilocytes, and occasional prickle layer keratinocytes with enlarged nuclei were also observed. These histological appearances are those of a plane wart as is typically seen in epidermodysplasia verruciformis (Figure 2).
Figure 2.
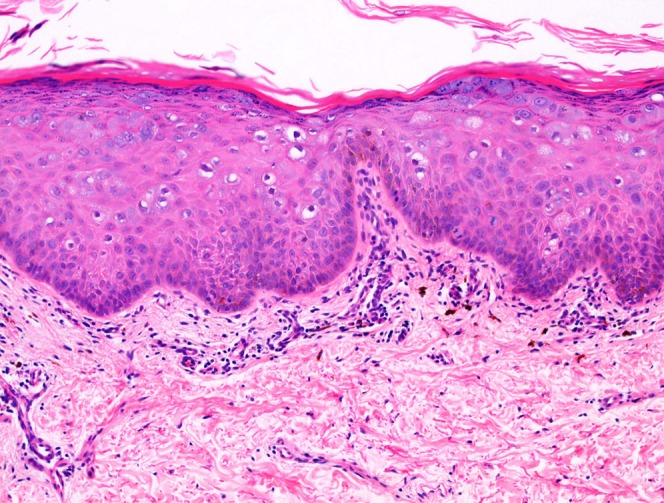
Punch biopsy of skin showing an acanthotic epidermis containing numerous koilocytes. The features are those of a plane wart, which is a classic histological feature of epidermodysplasia verruciformis. Hematoxylin and eosin stain, 100x original magnification.
HPV Typing
HPV 5, a β-type (EV-associated) HPV, was detected in all biopsy samples by LiPA, but very low copy numbers were detected by real-time quantitative PCR. HPV 19, another β-HPV, was isolated in sample 2. Multiple cutaneous wart–associated and genital HPV types were also isolated from each sample (Table 1). HPV types 1 and 2, usually found in plantar warts, were isolated in all samples with both PCR/sequencing and Luminex techniques; all samples had high HPV 1 signals. A large number of genital HPV types were identified (7 or 8 in each sample). HPV viral load testing was available for HPV 6, 11, 16, and 18. However, only HPV 6 was found in levels above the detectable limit in samples from patients 1 and 3; the copy number was too low to permit accurate quantification.
DISCUSSION
We describe acquired EV in 4 adolescents with longstanding, untreated vertically acquired HIV infection. Histology and HPV typing are consistent with EV. In addition to identifying “expected” EV-associated β-HPV types in skin biopsies from affected areas, HPV types 1 and 2 were detected in each biopsy, as well as 7 or more HPV types normally associated with genital lesions. Despite immune reconstitution with cART, there was no improvement in the EV lesions, from which multiple HPV strains were isolated after several years of otherwise successful treatment. HPV 1 and 2, classically associated with plantar warts, were also isolated in skin biopsies from our patients, together with HPV 5, commonly found in patients with congenital EV [18]. HPV 3, a cutaneous wart–associated HPV type, has previously been identified in EV lesions from 1 HIV-infected individual; by contrast, HPV 1 or 2 have not been described [9].
HPV infection is frequent in immunocompromised patients, but there are few reports of EV occurring in acquired immunodeficiency states [4–10]. Prior to this study, only 18 cases of HIV-associated EV have been reported, and only 2 described congenital HIV and EV [10, 19]. One of these 2 patients was found to be homozygous for the C912A T polymorphism in the TMC8/EVER2 gene [19].
More than 100 HPV types are recognised and classified into 3 clinical categories: anogenital or mucosal, cutaneous wart–associated, and β-HPV types. β-HPV viruses are ubiquitous and nonpathogenic in the normal population [20].
Classical EV results from a cell-mediated immune deficiency and an accompanying underlying genetic susceptibility to EV-HPV. This results in an inhibition of natural cytotoxic mechanisms against HPV-infected keratinocytes, leading to the development of skin lesions. Thus, is it possible that these 4 long-term survivors had an undefined, underlying genetic susceptibility, but it is more likely that they had a long-standing, acquired cell-mediated immune defect secondary to a chronic, untreated HIV infection.
These vertically HIV-infected adolescents probably first experienced HPV infection in early childhood when their cell-mediated immune systems were already impaired by HIV, whereas adults who are infected with HIV later in life have robust immune mechanisms against HPV infection that were developed in childhood when their immune systems were still intact. This might explain why EV is not as common in HIV-infected adults as it is in older HIV-infected children. Close contact between these immunosuppressed children and other HPV-infected children in an HIV clinic or with HIV-infected adults with multiple HPV types is a possible explanation for the unusually high number of genital and nongenital HPV types isolated in each skin biopsy. Laboratory contamination is an unlikely explanation for the detection of HPV types 19, 45, 51, 52, 53, 54, and 66, as these types were not stored in the 2 research laboratories.
There is no definitive treatment for EV. Lee et al [10] reviewed the treatment of twenty patients with congenital EV and concluded that oral retinoids (with or without interferon) were effective, as was low-dose oral retinoid maintenance. Relapses were common upon treatment cessation and caution is advised when prescribing retinoids to adolescents with child-bearing potential due to the recognized teratogenicity [10]. Topical imiquimod, intralesional interferon, 5-fluorouracil, and cimetidine have all been used as treatment, but with variable success [1, 6, 8, 10]. Previous reports suggest that treatment of HIV-associated EV is less successful than that for classical EV. Haas et al [7] report the resolution of lesions upon starting cART in 1 adult patient. A recent treatment trial of glycolic acid in HIV-positive children in Botswana showed a trend toward flattening and color normalization in flat warts, although complete resolution was observed in only 10% of patients. Response was greatest in those developing warts after starting ART and those with fewer HPV types [21]. In this resource-limited setting, alternative treatments were not available and immune reconstitution with cART had no impact on the appearance of the lesions. This group of adolescents was left with a disfiguring and potentially stigmatizing condition in high-prevalence communities where the skin condition is frequently identified as being associated with HIV infection.
Classical EV is associated with a high risk of lesional transformation to squamous cell carcinoma, occurring in up to 70% of cases [1, 22]. Malignant lesions usually occur in the third and fourth decades of life, but there are case reports of carcinoma appearing as early as 15 and 17 years of age [1]. The oncogenic potential of HPV 5 and 8 is well established but other EV-HPV, including HPV 10, 14, 20, and 47, have also been implicated [18, 22]. Both ultraviolet B and diagnostic X-ray radiation have been identified as oncogenic cofactors in HPV 5–related malignancies [1, 22]. Earlier malignant transformation in skin lesions exposed to sunlight and radiotherapy has been reported [1]. No malignancies were encountered in our series or other reported cases of HIV-associated EV, but as cART becomes more widely available, adolescents surviving to adulthood will need close dermatological surveillance. This is particularly important among this group of children with longstanding immune deficiency and high levels of sun exposure.
In summary, we describe 4 HIV-infected adolescents with EV-like lesions. Preliminary results suggest that among long-term survivors of vertically-acquired HIV, this might be a common clinical HPV phenotype. It is a disfiguring condition and difficult to treat because no impact on the progression of skin disease has been observed after the administration of cART. EV is relatively rare in HIV-infected adults but appears to be frequent in vertically-infected children, and close dermatological surveillance for potential skin malignancy is required.
Notes
Financial support. R. A. F. was funded by the Wellcome Trust.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Gul U, Kilic A, Gonul M, Cakmak SK, Bayis SS. Clinical aspects of epidermodysplasia verruciformis and review of the literature. Int J Dermatol. 2007;46:1069–72. doi: 10.1111/j.1365-4632.2006.03014.x. [DOI] [PubMed] [Google Scholar]
- 2.Jablonska S, Orth G, Jarzabek-Chorzelska M, et al. Epidermodysplasia verruciformis versus disseminated verrucae planae: is epidermodysplasia verruciformis a generalized infection with wart virus? J Invest Dermatol. 1979;72:114–19. doi: 10.1111/1523-1747.ep12530383. [DOI] [PubMed] [Google Scholar]
- 3.Ramoz N, Taieb A, Rueda LA, et al. Evidence for a nonallelic heterogeneity of epidermodysplasia verruciformis with two susceptibility loci mapped to chromosome regions 2p21-p24 and 17q25. J Invest Dermatol. 2000;114:1148–53. doi: 10.1046/j.1523-1747.2000.00996.x. [DOI] [PubMed] [Google Scholar]
- 4.Bonamigo R, Maldonado G, Londero RM, Cartell A. Epidermodysplasia verruciformis–like disorder in a teenager with HIV and HCV infections. Pediatr Dermatol. 2007;24:456–7. doi: 10.1111/j.1525-1470.2007.00487.x. [DOI] [PubMed] [Google Scholar]
- 5.Barzegar C, Paul C, Saiag P, et al. Epidermodysplasia verruciformis–like eruption complicating human immunodeficiency virus infection. Br J Dermatol. 1998;139:122–7. doi: 10.1046/j.1365-2133.1998.02328.x. [DOI] [PubMed] [Google Scholar]
- 6.Davison SC, Francis N, McLean K, Bunker CB. Epidermodysplasia verruciformis–like eruption associated with HIV infection. Clin Exp Dermatol. 2004;29:311–12. doi: 10.1111/j.1365-2230.2004.01503.x. [DOI] [PubMed] [Google Scholar]
- 7.Haas N, Fuchs PG, Hermes B, Henz BM. Remission of epidermodysplasia verruciformis–like skin eruption after highly active antiretroviral therapy in a human immunodeficiency virus–positive patient. Br J Dermatol. 2001;145:669–70. doi: 10.1046/j.1365-2133.2001.04426.x. [DOI] [PubMed] [Google Scholar]
- 8.Carre D, Dompmartin A, Verdon R, et al. Epidermodysplasia verruciformis in a patient with HIV infection: no response to highly active antiretroviral therapy. Int J Dermatol. 2003;42:296–300. doi: 10.1046/j.1365-4362.2003.01707_2.x. [DOI] [PubMed] [Google Scholar]
- 9.Jacobelli S, Laude H, Carlotti A, et al. Epidermodysplasia verruciformis in human immunodeficiency virus–infected patients: a marker of human papillomavirus-related disorders not affected by antiretroviral therapy. Arch Dermatol. 2011;147:590–6. doi: 10.1001/archdermatol.2010.399. [DOI] [PubMed] [Google Scholar]
- 10.Lee KC, Risser J, Bercovitch L. What is the evidence for effective treatments of acquired epidermodysplasia verruciformis in HIV-infected patients? Arch Dermatol. 2010;146:903–5. doi: 10.1001/archdermatol.2010.194. [DOI] [PubMed] [Google Scholar]
- 11.Lowe S, Ferrand RA, Morris-Jones R, et al. Skin disease among human immunodeficiency virus–infected adolescents in Zimbabwe: a strong indicator of underlying HIV infection. Pediatr Infect Dis J. 2010;29:346–51. doi: 10.1097/INF.0b013e3181c15da4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berkhout RJ, Tieben LM, Smits HL, Bavinck JN, Vermeer BJ, ter Schegget J. Nested PCR approach for detection and typing of epidermodysplasia verruciformis–associated human papillomavirus types in cutaneous cancers from renal transplant recipients. J Clin Microbiol. 1995;33:690–5. doi: 10.1128/jcm.33.3.690-695.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harwood CA, Spink PJ, Surentheran T, et al. Degenerate and nested PCR: a highly sensitive and specific method for detection of human papillomavirus infection in cutaneous warts. J Clin Microbiol. 1999;37:3545–55. doi: 10.1128/jcm.37.11.3545-3555.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Koning M, Quint W, Struijk L, et al. Evaluation of a novel highly sensitive, broad-spectrum PCR-reverse hybridization assay for detection and identification of beta-papillomavirus DNA. J Clin Microbiol. 2006;44:1792–800. doi: 10.1128/JCM.44.5.1792-1800.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Koning MN, ter Schegget J, Eekhof JA, et al. Evaluation of a novel broad-spectrum PCR-multiplex genotyping assay for identification of cutaneous wart–associated human papillomavirus types. J Clin Microbiol. 2010;48:1706–11. doi: 10.1128/JCM.02122-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levi JE, Kleter B, Quint WG, et al. High prevalence of human papillomavirus (HPV) infections and high frequency of multiple HPV genotypes in human immunodeficiency virus–infected women in Brazil. J Clin Microbiol. 2002;40:3341–5. doi: 10.1128/JCM.40.9.3341-3345.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weissenborn SJ, Wieland U, Junk M, Pfister H. Quantification of beta-human papillomavirus DNA by real-time PCR. Nat Protoc. 2010;5:1–13. doi: 10.1038/nprot.2009.153. [DOI] [PubMed] [Google Scholar]
- 18.Orth G. Host defenses against human papillomaviruses: lessons from epidermodysplasia verruciformis. Curr Top Microbiol Immunol. 2008;321:59–83. doi: 10.1007/978-3-540-75203-5_3. [DOI] [PubMed] [Google Scholar]
- 19.Hohenstein E, Rady PL, Hergersberg M, et al. Epidermodysplasia verruciformis in a HIV-positive patient homozygous for the c917A-->T polymorphism in the TMC8/EVER2 gene. Dermatology. 2009;218:114–18. doi: 10.1159/000174084. [DOI] [PubMed] [Google Scholar]
- 20.Astori G, Lavergne D, Benton C, et al. Human papillomaviruses are commonly found in normal skin of immunocompetent hosts. J Invest Dermatol. 1998;110:752–5. doi: 10.1046/j.1523-1747.1998.00191.x. [DOI] [PubMed] [Google Scholar]
- 21.Moore RL, de Schaetzen V, Joseph M, et al. Acquired epidermodysplasia verruciformis syndrome in HIV-infected pediatric patients: prospective treatment trial with topical glycolic acid and human papillomavirus genotype characterization. Arch Dermatol. 2012;148:128–30. doi: 10.1001/archdermatol.2011.268. [DOI] [PubMed] [Google Scholar]
- 22.Sehgal VN, Luthra A, Bajaj P. Epidermodysplasia verruciformis: 14 members of a pedigree with an intriguing squamous cell carcinoma transformation. Int J Dermatol. 2002;41:500–3. doi: 10.1046/j.1365-4362.2002.01539.x. [DOI] [PubMed] [Google Scholar]