The role of antibiotic susceptibility testing on clinical outcome is unclear. In a randomized, controlled trial of bacterial keratitis, a higher minimum inhibitory concentration to moxifloxacin was significantly associated with worse visual acuity.
Abstract
Background. For bacterial infections, the susceptibility to antibiotics in vitro has been associated with clinical outcomes in vivo, although the importance of minimum inhibitory concentration (MIC) has been debated. In this study, we analyzed the association of MIC on clinical outcomes in bacterial corneal ulcers, while controlling for organism and severity of disease at presentation.
Methods. Data were collected as part of a National Eye Institute–funded, randomized, controlled trial (the Steroids for Corneal Ulcers Trial [SCUT]). All cases enrolled in SCUT had a culture-positive bacterial corneal ulcer and received moxifloxacin. The MIC to moxifloxacin was measured by E test. Outcomes included best spectacle-corrected visual acuity, infiltrate/scar size, time to re-epithelialization, and corneal perforation.
Results. Five hundred patients with corneal ulcers were enrolled in the trial, and 480 were included in this analysis. The most commonly isolated organisms were Streptococcus pneumoniae and Pseudomonas aeruginosa. A 2-fold increase in MIC was associated with an approximately 0.02 logMAR decrease in visual acuity at 3 weeks, approximately 1 letter of vision loss on a Snellen chart (0.019 logMAR; 95% confidence interval [CI], .0040–.033; P = .01). A 2-fold increase in MIC was associated with an approximately 0.04-mm larger infiltrate/scar size at 3 weeks (0.036 mm; 95% CI, .010–.061; P = .006). After controlling for organism, a higher MIC was associated with slower time to re-epithelialization (hazards ratio, 0.92; 95% CI, .86–.97; P = .005).
Conclusions. In bacterial keratitis, a higher MIC to the treating antibiotic is significantly associated with worse clinical outcomes, with approximately 1 line of vision loss per 32-fold increase in MIC.
Clinical Trials Registration: NCT00324168.
Corneal opacity is the fourth leading cause of blindness globally [1]. Infectious keratitis is a leading cause of corneal blindness, with an estimated annual occurrence of 1.5–2 million cases worldwide; the true incidence may be much higher [2]. Bacteria such as Streptococcus pneumoniae and Pseudomonas aeruginosa are common etiologic agents of infectious keratitis and are responsible for as much as half of the corneal ulceration in South India and typically a larger proportion in the United States and Europe [3–7]. Treatment of advanced bacterial keratitis is difficult and can lead to poor visual outcomes and blindness, and antimicrobial-resistant bacteria are increasingly found [8].
In systemic bacterial infections, in vitro susceptibility is thought to predict clinical outcomes [9, 10]. In ocular infections, a high concentration of antibiotic is delivered directly to the site of infection using topical antibiotics. Thus, it is possible that in vitro susceptibility does not play as large a role in determining clinical outcome [11]. Recent studies have suggested that in vitro susceptibility may predict clinical outcome in bacterial keratitis; however, it has been difficult to separate the effect of susceptibility from that of species of organism [12–14].
As part of the National Eye Institute–funded Steroids for Corneal Ulcers Trial (SCUT), all isolates were tested for the minimum inhibitory concentration (MIC) to moxifloxacin, the antibiotic used per protocol. Specific clinical features, such as visual acuity or infiltrate/scar size, were measured, which allowed correction with baseline measurements for each clinical outcome. This afforded the opportunity to assess the MIC’s effect during the course of treatment, an analysis that can be difficult to perform in other disease settings. In this report, we analyzed the association between MIC and clinical outcome, while controlling for organism and presenting clinical measurements.
METHODS
Trial Methods
The SCUT (National Eye Institute [NEI] U10-EY015114) was an NEI-funded, randomized, placebo-controlled, double-masked multicenter clinical trial designed to assess any benefit in clinical outcome from the use of topical corticosteroids as adjunctive therapy in the treatment of bacterial keratitis. Methods for the trial have been described in depth previously [15]. In brief, patients were enrolled at the Aravind Eye Care System (Madurai, Tirunelveli, and Coimbatore) in India, Dartmouth Medical School, and the F. I. Proctor Foundation at the University of California, San Francisco. Eligible patients had a culture-positive bacterial corneal ulcer and had been on topical moxifloxacin for at least 48 hours. Patients were randomized to receive prednisolone phosphate 1% (Bausch & Lomb Pharmaceuticals, Tampa, FL) or topical placebo (0.9% NaCl and preservative; Leiter’s Pharmacy, San Jose, CA). Both arms received topical moxifloxacin (Vigamox; Alcon, Fort Worth, TX). The moxifloxacin treatment regimen consisted of 1 drop applied every hour while awake for the first 48 hours, then 1 drop applied every 2 hours until re-epithelialization, and then 4 times a day until 3 weeks after enrollment. Institutional review board approval was granted by the University of California, San Francisco, Dartmouth Medical School, and the Aravind Eye Care System. This study conformed to the tenets of the Declaration of Helsinki, and informed consent was obtained from all subjects.
The primary outcome for the trial was best spectacle-corrected visual acuity (BSCVA) at 3 months after enrollment. Refraction, using trial frames or a phoropter, was performed at each time point using a protocol adapted from the Age-Related Eye Disease Study using a tumbling “E” chart at 4 meters and logMAR visual acuity (Precision Vision Chart 2305 and 2305A, La Salle, IL) [16]. If a patient read fewer than 10 letters at 4 meters, acuity was measured at 1 meter. If fewer than 10 letters were read at 1 meter, low vision was assessed by counting fingers, hand motions, light perception, and no light perception. In all cases, we did not use the patient’s spectacles. Secondary outcomes included BSCVA at 3 weeks, infiltrate/scar size at 3 weeks, time to re-epithelialization of the epithelial defect, and proportion of corneal perforation. Visual acuity was measured by masked, certified refractionists who used standardized Early Treatment Diabetic Retinopathy Study (ETDRS) tests at each study visit. Infiltrate/scar size and epithelial defect size were measured by masked, certified examiners using slit-lamp. Assessment of adverse events, including corneal perforation, was done at each study visit.
Corneal scrapings for smear and culture were performed after the slit-lamp examination at presentation. Two scrapings were smeared for Gram stain and potassium hydroxide wet mount. Three scrapings were inoculated onto sheep’s blood agar, chocolate agar, and potato dextrose agar or Sabouraud’s agar. The criterion for a positive bacterial culture was growth of the organism on 1 solid medium at the site of inoculation. For Staphylococcus epidermis and diphtheroids, cultures were considered positive only if moderate growth was seen on at least 2 solid media or on 1 solid medium plus a Gram-stained corneal smear [17]. All patients were checked for fungal elements on smear and culture, and any evidence of fungal infection resulted in exclusion. Quality control was performed according to the National Committee for Clinical Laboratory Standards (NCCLS) performance standards, recommendations, guidelines, and reports (NCCLS M100-S10 [M2]) [18]. Antibiotic susceptibility testing was performed using the E test method (AB BIODISK, Solna, Sweden), as was done in the SCUT pilot study [12]. All microbiological testing was performed by certified microbiologists. Isolates with more than 1 organism identified (n = 6) and isolates that had a positive culture but did not have an identifiable organism (n = 6) were excluded from the analysis.
Statistical Methods
Analysis of MIC and clinical outcomes was a prespecified outcome and was 1 of the 3 specific aims of the main trial [15]. A log2-transformation of MIC was used for all statistical models. Log2-transformed MICs were analyzed as a continuous variable. Differences in MIC across groups of organism were analyzed using analysis of variance (ANOVA). For each regression model, the corresponding baseline measure was included in the model as a covariate. The relationship of MIC and BSCVA at 3 weeks and 3 months was analyzed using multiple linear regression adjusting for baseline BSCVA and treatment arm. MIC and infiltrate/scar size at 3 weeks and 3 months was analyzed using multiple linear regression adjusting for baseline infiltrate/scar size and treatment arm. MIC and time to re-epithelialization was assessed using Cox proportional hazards regression adjusting for baseline epithelial defect size and treatment arm. Re-epithelialization time was right-censored at 21 days after enrollment. MIC and proportion of corneal perforation was assessed with a multiple logistic regression model, adjusting for baseline infiltrate depth and treatment arm. A second model for each outcome controlled for organism as a fixed effect to assess the effect of etiologic organism on the results. These models contained organism as a categorical variable to account for the effect of organism on the overall results. As a sensitivity analysis, causative organism was included in a mixed model as a random effect. All analyses were performed in Stata 10.0 statistical software (StataCorp, College Station, TX).
RESULTS
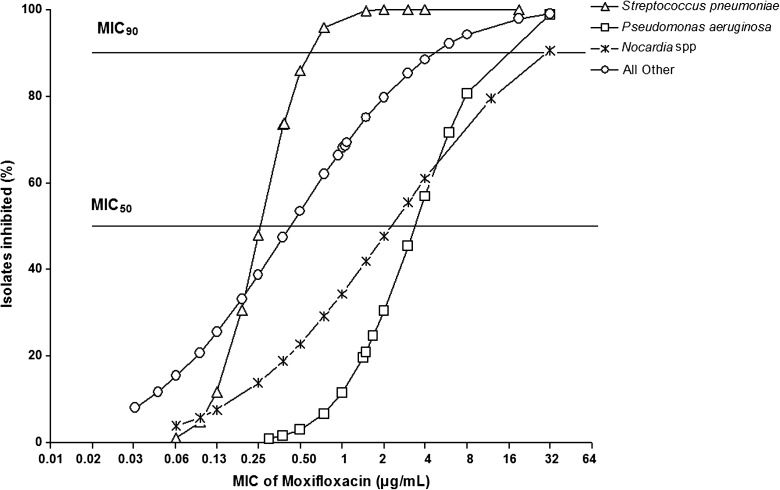
Of 500 patients enrolled in the trial, 492 had MIC results available. Of the 492 isolates with MIC results, 6 grew on culture but did not have an identifiable organism, and 6 had a mixed infection; thus, 480 isolates were included in this analysis. The most common organism isolated was S. pneumoniae (n = 248, 53%), followed by P. aeruginosa (n = 104, 22%) and Nocardia spp (n = 55, 12%) (Table 1). MICs across groups of organism were significantly different (ANOVA P < .001; Table 1), and P. aeruginosa isolates had the highest MICs for moxifloxacin. Whereas the majority of S. pneumoniae and Moraxella spp isolates were susceptible to moxifloxacin, larger proportions of P. aeruginosa, Nocardia spp, and S. aureus were resistant (Figure 1).
Table 1.
Etiologic Organism, MIC50, and MIC90 Against Moxifloxacin
| Organism | n | MIC50, μg/mL | MIC90, μg/mL |
| Streptococcus pneumoniae | 247 | 0.25 | 0.38 |
| Pseudomonas aeruginosa | 109 | 3 | 32 |
| Nocardia spp | 55 | 2 | 32 |
| Staphylococcus, coagulase-negative | 21 | 0.25 | 3 |
| Moraxella spp | 13 | 0.09 | 0.5 |
| Staphylococcus aureus | 11 | 1 | 8 |
| Streptococcus, viridans group | 10 | 0.22 | 6 |
| Corynebacterium spp | 4 | 0.75 | 32 |
| Klebsiella spp | 3 | 0.125 | 0.125 |
| Pseudomonas, non-aeruginosa | 3 | 2 | 6 |
| Enterobacter spp | 2 | 0.25 | 0.5 |
| Bacillus spp | 1 | 19 | 19 |
| Mycobacteria spp | 1 | 32 | 32 |
| Total | 480 | 0.38 | 6 |
| P < .001a | |||
Abbreviations: MIC, minimum inhibitory concentration; MIC50, MIC median; MIC90, MIC 90th percentile.
Analysis of variance.
Figure 1.
Percentage of different bacterial isolates inhibited at various concentrations of moxifloxacin. Organisms included Streptococcus pneumoniae (n = 247), Pseudomonas aeruginosa (n = 109), Nocardia spp (n = 55), and all other bacterial isolates (n = 69). Horizontal lines represent the threshold for the minimum inhibitory concentration median (MIC50) and 90th percentile (MIC90).
Median baseline BSCVA was logMAR 0.83, with approximate Snellen equivalent of 20/135, and an interquartile range (IQR) of 0.36 (20/50) to 1.7 (count fingers). Median 3-week BSCVA was logMAR 0.42, with an approximate Snellen of 20/50, and an IQR 0.16 (20/30) to 0.90 (20/160). Median 3-month BSCVA was logMAR 0.3, with an approximate Snellen of 20/40, and an IQR 0.06 (20/23) to 0.68 (20/95). Median baseline infiltrate/scar size was 2.7 mm (IQR, 1.9–4.1 mm). Median 3-week infiltrate/scar size was 2.7 mm (IQR, 1.9–3.8 mm). Median 3-month infiltrate/scar size was 2.7 mm (IQR, 1.8–3.9 mm). Median time to re-epithelialization was 7.5 days (IQR, 2.5–16 days). There were 15 perforations during the course of the trial.
A 2-fold increase in MIC was associated with 0.018 worse logMAR BSCVA at 3 weeks after enrollment (0.019 logMAR; 95% confidence interval [CI], .0040–.033; P = .013) (Table 2). When controlling for organism, this relationship remained (0.019 logMAR; 95% CI, .0042–.046; P = .019) (Table 2). Three-month BSCVA was estimated to be reduced by 0.013 logMAR per 2-fold increase in MIC (0.013 logMAR; 95% CI, −.0032–.030; P = .11). Three-week infiltrate/scar size was estimated to increase by 0.036 mm per 2-fold increase in MIC (0.036 mm; 95% CI, .010–.061; P = .006) (Table 3). When controlling for organism, this relationship remained (0.052 mm; 95% CI, .018–.088; P = .003) (Table 3). A 2-fold increase in MIC was associated with a 0.027-mm larger infiltrate/scar size at 3 months after enrollment (0.027; 95% CI, −.0016–.055; P = .064). This relationship was significant when controlling for organism (0.050 mm; 95% CI, .011–.87; P = .012). An increase in MIC was associated with a longer time to re-epithelialization; however, crude estimates were not statistically significant (hazards ratio [HR], 0.99; 95% CI, .95–1.04; P = .77) (Table 4). After controlling for organism, there was a statistically significant association between slower time to re-epithelialization and increase in MIC (HR, 0.92; 95% CI, .86–.97; P = .005) (Table 4). There was no significant association in proportion of corneal perforation in crude (odds ratio [OR], 0.95; 95% CI, .74–1.21; P = .65) or organism-adjusted (OR, 0.93; 95% CI, .73–1.19; P = .55) models. Sensitivity analyses that included organism as a random effect did not change the results.
Table 2.
Multiple Linear Regression Predicting 3-Week BSCVA (in logMAR), Correcting for Baseline BSCVA and Treatment Arm
| Coefficienta | 95% CI | P Value | |
| Covariate | |||
| log2MIC, μg/mL | 0.019 logMAR | .0040–.033 | .013 |
| Corticosteroid (vs placebo) | −0.028 logMAR | −.097–.041 | .41 |
| Baseline BSCVA | 0.75 | .70–.81 | <.001 |
| Model correcting for organisms as fixed effects | |||
| log2MIC, μg/mL | 0.025 logMAR | .0042–.046 | .019 |
| Corticosteroid (vs placebo) | −0.030 logMAR | −0.099–.039 | .40 |
| Baseline BSCVA | 0.77 | .71–.82 | <.001 |
Abbreviations: BSCVA, best spectacle-corrected visual acuity; CI, confidence interval; MIC, minimum inhibitory concentration.
Coefficient refers to line of visual acuity; a negative coefficient indicates an improvement in visual acuity.
Table 3.
Multiple Linear Regression Predicting 3-Week Infiltrate/Scar Size (in mm), Correcting for Baseline Infiltrate Size and Treatment Arm
| Coefficienta | 95% CI | P Value | |
| Covariate | |||
| log2MIC, μg/mL | 0.036 | .010–.061 | .006 |
| Corticosteroid (vs placebo) | 0.014 | −0.10–.13 | .81 |
| Baseline infiltrate size | 0.85 | .81–.88 | <.001 |
| Model correcting for organisms as fixed effects | |||
| log2MIC, μg/mL | 0.053 | .018–.088 | .003 |
| Corticosteroid (vs placebo) | −0.006 | −0.12–.11 | .92 |
| Baseline infiltrate size | 0.86 | .82–.89 | <.001 |
Abbreviations: CI, confidence interval; MIC, minimum inhibitory concentration.
Coefficient refers to infiltrate/scar diameter (geometric mean, in mm); a positive coefficient indicates an increase in infiltrate/scar size.
Table 4.
Cox Proportional Hazards Regression Predicting Time to Re-Epithelialization, Controlling for Baseline Epithelial Defect Size and Treatment Arm
| Covariate | HRa | 95% CI | P Value |
| log2MIC, μg/mL | 0.99 | .95–1.04 | .77 |
| Corticosteroid (vs placebo) | 0.90 | .73–1.09 | .28 |
| Baseline epithelial defect size | 0.65 | .60–.71 | <.001 |
| Model correcting for organisms as fixed effects | |||
| log2MIC, μg/mL | 0.92 | .86–.97 | .005 |
| Corticosteroid (vs placebo) | 0.94 | .77–1.15 | .55 |
| Baseline epithelial defect size | 0.64 | .58–.70 | <.001 |
Abbreviations: CI, confidence interval; HR, hazards ratio; MIC, minimum inhibitory concentration.
A hazards ratio below 1 indicates a slower re-epithelialization time in days.
DISCUSSION
We found a significant relationship between MIC and 3-week BSCVA when controlling for baseline BSCVA, with and without controlling for organism. These results suggest that a higher MIC results in worse visual acuity 3 weeks after enrollment in the trial, the period during which patients were treated with topical moxifloxacin. However, the effect size is small for this relationship: a loss of approximately 1 letter of visual acuity per 2-fold increase in MIC, or 1 line of visual acuity per 32-fold increase. Visual acuity is affected by many factors, including the location of the ulcer relative to the pupil and preexisting ocular diseases. This study suggests that although MIC probably has a role in determining visual acuity outcomes, the effect is not large, and other factors such as location may play a larger role. A higher MIC was associated with a larger infiltrate/scar size at 3 weeks in a crude analysis and when controlling for organism. A previous study showed an association between higher MIC and larger infiltrate/scar size at 3 months after enrollment, but it was not large enough in sample size to control for organism [12]. Here, the relationship remained significant when controlling for organism, which suggests that antibiotic susceptibility itself has an effect on outcome infiltrate/scar size. The effect size in the current study was smaller than the previous study. Previously, infiltrate/scar size was estimated to increase by 0.33 mm per 2-fold increase in MIC [12]. In the current study, at 3 weeks and 3 months, we estimate infiltrate/scar size to increase by only 0.05 mm per 2-fold increase in MIC, which suggests that our previous estimate may have been too high.
An association between in vitro susceptibility and clinical outcome suggests that tailoring antibiotic therapy according to susceptibility patterns could improve outcomes. In particular, it is important to understand this relationship during the course of treatment, which can only be assessed by controlling for presentation characteristics. In the systemic disease literature, the relationship between clinical outcome and antibiotic susceptibility is unclear [19, 20]; however, susceptible organisms are thought to respond better than resistant organisms according to the “90-60” rule, in which susceptible organisms respond approximately 90% of the time and resistant organisms respond approximately 60% of the time [9]. In ocular infections, clinical outcomes such as visual acuity or scar size have very precise measurements of severity at presentation, which allowed for isolation of the effect during the course of treatment with the antibiotic of interest. Moxifloxacin 0.5% delivers 5000 μg/mL medication directly to the corneal surface, which exceeds the MICs found in this study. Moxifloxacin is thought to have good penetration to the cornea [21], suggesting that there is an excess of drug on the ocular surface, which may overcome differences in MIC and partially explain the small effect sizes seen in this study. However, whereas the delivery dose is high compared with MICs, the drug dissipates quickly, and the therapeutic dose that is achieved may be lower than the MIC, especially in disease that reaches the anterior chamber or stroma. Previous reports suggest that levels of moxifloxacin in the aqueous humor are approximately 1.3 μg/mL [22], which is lower than the MIC50 we found in this study for some organisms, particularly P. aeruginosa. Although P. aeruginosa ulcers may have higher MICs to moxifloxacin than to other antibiotics such as ciprofloxacin, it is still frequently the drug of choice for empiric therapy for many ophthalmologists due to issues with penetration and availability [23]. In SCUT, P. aeruginosa ulcers responded successfully to moxifloxacin and, on average, had greater improvement in BSCVA during the study period than other etiologic organisms [24]. Consideration of MIC testing for P. aeruginosa and Nocardia spp cases may be prudent, given their higher MICs to moxifloxacin.
Particular organisms may be associated with different MICs, clinical outcomes, or both [21, 22]. In this study, we confirm that MICs to moxifloxacin were significantly different across the groups of organisms isolated in the study. Some studies have reported that certain classes of fluoroquinolones have relatively poor activity against Streptococcus spp, but we did not find that in this study [8, 13]. Almost all of the S. pneumoniae isolates were susceptible to moxifloxacin [25]. Although the Clinical and Laboratory Standards Institute does not publish standards for sensitivity and resistance for P. aeruginosa to moxifloxacin, in this study, P. aeruginosa had a relatively high MIC50 and MIC90, which indicates a range of susceptibilities to moxifloxacin. Previous reports have shown that moxifloxacin and other fluoroquinolones have good activity against P. aeruginosa isolated from keratitis [26]. Organisms may act as a confounding factor in the relationship between MIC and organism.
Here, we demonstrate that etiologic organism is an important factor in the association between clinical outcomes and susceptibility to the treatment antibiotic. Previous retrospective studies have shown that type of organism is predictive of outcome, and that there was an association between clinical outcome and MIC against ciprofloxacin and ofloxacin in some bacterial species but not others [13]. Because our study was prospective in nature, all patients were treated according to a standard treatment protocol, and all received monotherapy with the same antibiotic (moxifloxacin) according to a standardized treatment protocol. Because data were prospectively collected as part of a clinical trial, data collection tools and outcomes assessments were standardized. We show that for some outcomes, such as visual acuity or infiltrate/scar size, controlling for organism does not substantially change the nature of the relationship between MIC and outcome. However, there seems to be no relationship between MIC and healing time unless organism is included in the model. Previous studies in bacterial keratitis have suggested that there may be a relationship between healing time of the ulcer and susceptibility [13, 27]. These studies either corrected for severity of the ulcer [27] or used an outcome of the ratio of healing time to ulcer size [13]. By using baseline epithelial defect size as a covariate in our regression model, we are able to show specifically how healing time and other clinical outcomes are related to in vitro susceptibility during the time of treatment.
This study has several limitations. The majority of the corneal ulcers enrolled in this trial were enrolled in India [15, 28]. Geographic differences in resistance patterns have been previously reported [29], which indicate that the MICs we report in this study may not be broadly applicable to other geographic locations. However, due to the number of cases enrolled in the trial, we were able to control for organism in all of our models and could assess how the inclusion of organism as a potential confounder affected the results. Per the trial protocol, moxifloxacin was used as monotherapy in all cases enrolled in the trial. All patients received a standardized treatment regimen and were on the treatment for a standardized period of time. Susceptibility patterns across types of organism to moxifloxacin were different, but we were able to control for this in the analysis. The standardized clinical trial protocol for use of moxifloxacin also reduces the likelihood of bias due to different antibiotic protocols. Finally, all cases, regardless of their randomization to corticosteroid or placebo, were included in the analysis for this study. Overall, there was no difference in outcomes between corticosteroid and placebo-randomized patients. We included a term in each model in this study for treatment arm, to control for any possible difference in outcome that may be attributed to randomized treatment arm.
In conclusion, the results of this study show that there is a relationship between in vitro susceptibility and clinical outcomes in bacterial keratitis, but the effect is relatively small. The small effect of the MICs that predict clinical outcomes may partially explain why it is difficult to distinguish between different antibiotics in a randomized, controlled trial setting. Identification of etiologic organism as well as in vitro susceptibility is important for tailoring appropriate therapy in the treatment of bacterial keratitis. A randomized, controlled trial assessing outcomes in patients who received empiric therapy versus tailored therapy would provide conclusive evidence on the best treatment strategy for this disease.
Notes
Acknowledgments.
We thank the research staff at each of the trial sites. We are also grateful for the invaluable guidance and advice of the SCUT data and safety monitoring board: Marian Fisher, PhD (chair), Anthony Aldave, MD, Donald Everett, MA, Jacqueline Glover, PhD, K. Ananda Kannan, MD, Steven Kymes, PhD, G. V. S. Murthy, MD, and Ivan Schwab, MD.
Financial support.
This work was supported by the National Eye Institute (NEI) for the Steroids for Corneal Ulcers Trial (grant U10 EY015114). N. R. A. is supported by an NEI grant (K23EY017897) and a Research to Prevent Blindness Award. The Department of Ophthalmology at UCSF is supported by a core grant (EY02162) from the NEI; an unrestricted grant from Research to Prevent Blindness, New York, NY; and That Man May See, Inc, San Francisco, CA. Alcon provided moxifloxacin (Vigamox) for the trial. The sponsors did not have a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Resnikoff S, Pascolini D, Etya'ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–50. [PMC free article] [PubMed] [Google Scholar]
- 2.Whitcher J, Srinivasan M, Upadhyay M. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79:214–21. [PMC free article] [PubMed] [Google Scholar]
- 3.Bourcier T, Thomas F, Borderie V, Chaumeil C, Laroche L. Bacterial keratitis: predisposing factors, clinical and microbiological review of 300 cases. Br J Ophthalmol. 2003;87:834–8. doi: 10.1136/bjo.87.7.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srinivasan M, Gonzales C, George C, et al. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, South India. Br J Ophthalmol. 1997;81:965–71. doi: 10.1136/bjo.81.11.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varaprasathan G, Miller K, Lietman T, et al. Trends in the etiology of infectious corneal ulcers at the F. I. Proctor Foundation. Cornea. 2004;23:360–4. doi: 10.1097/00003226-200405000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Bharathi MJ, Ramakrishnan R, Meenakshi R, Padmavathy S, Shivakumar C, Srinivasan M. Microbial keratitis in South India: influence of risk factors, climate, and geographical variation. Ophthalmic Epidemiol. 2007;14:61–9. doi: 10.1080/09286580601001347. [DOI] [PubMed] [Google Scholar]
- 7.Leck A, Thomas P, Hagan M, et al. Aetiology of suppurative corneal ulcers in Ghana and south India, and epidemiology of fungal keratitis. Br J Ophthalmol. 2002;86:1211–15. doi: 10.1136/bjo.86.11.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexandrakis G, Alfonso E, Miller D. Shifting trends in bacterial keratitis in south Florida and emerging resistance to fluoroquinolones. Ophthalmology. 2000;107:1497–502. doi: 10.1016/s0161-6420(00)00179-2. [DOI] [PubMed] [Google Scholar]
- 9.Rex J, Pfaller M. Has antifungal susceptibility testing come of age? CID. 2002;35:982–9. doi: 10.1086/342384. [DOI] [PubMed] [Google Scholar]
- 10.Weinstein M, Reller L, Murphy J, Lichtenstein K. The clinical significance of positive blood cultures: a comprehensive analysis of 500 episodes of bacteremia and fungemia in adults. I. Laboratory and epidemiologic observations. Rev Infect Dis. 1983;5:35–53. doi: 10.1093/clinids/5.1.35. [DOI] [PubMed] [Google Scholar]
- 11.Baum J, Barza M. The evolution of antibiotic therapy for bacterial conjunctivitis and keratitis: 1970–2000. Cornea. 2000;19:659–72. doi: 10.1097/00003226-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Chen A, Prajna L, Srinivasan M, et al. Does in vitro susceptibility predict clinical outcome in bacterial keratitis? Am J Ophthalmol. 2008;145:409–15. doi: 10.1016/j.ajo.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaye S, Tuft S, Neal T, et al. Bacterial susceptibility to topical antimicrobials and clinical outcome in bacterial keratitis. Invest Ophthalmol Vis Sci. 2010;51:362–8. doi: 10.1167/iovs.09-3933. [DOI] [PubMed] [Google Scholar]
- 14.Wilhelmus K. Evaluation and prediction of fluoroquinolone pharmacodynamics in bacterial keratitis. J Ocul Pharmacol Ther. 2003;19:493–9. doi: 10.1089/108076803322473042. [DOI] [PubMed] [Google Scholar]
- 15.Srinivasan M, Mascarenhas J, Rajaraman R, et al. The Steroids for Corneal Ulcers Trial: study design and baseline characteristics. Arch Ophthalmol. 2011;130:151–7. doi: 10.1001/archophthalmol.2011.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Age-Related Eye Disease Study Research Group. Age-Related Eye Disease Study (AREDS): design implications. AREDS Report No. 1. Control Clin Trials. 1999;20:573–600. doi: 10.1016/s0197-2456(99)00031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilhelmus K, Liesegang T, Osato M, Jone D. Laboratory diagnosis of ocular infections. Washington, DC: American Society for Microbiology Press; 1994. [Google Scholar]
- 18. National Committee for Clinical Laboratory Standards. NCCLS Document M100-S10, NCCLS, Wayne, PA, January, 2000. Performance standards for antimicrobial susceptibility testing.
- 19.Yu V, Chiou C, Feldman C, et al. An international prospective study of pneumococcal bacteremia: correlation with in vitro resistance, antibiotics administered, and clinical outcome. CID. 2003;37:230–7. doi: 10.1086/377534. [DOI] [PubMed] [Google Scholar]
- 20.Smith A, Fiel S, Mayer-Hamblett N, Ramsey B, Burns J. Susceptibility testing of Pseudomonas aeruginosa isolates and clinical response to parenteral antibiotic administration: lack of association in cystic fibrosis. Chest. 2003;123:1495–502. doi: 10.1378/chest.123.5.1495. [DOI] [PubMed] [Google Scholar]
- 21.Holland E, Lane S, Kim T, Raizman M, Dunn S. Ocular penetration and pharmacokinetics of topical gatifloxacin 0.3% and moxifloxacin 0.5% ophthalmic solutions after keratoplasty. Cornea. 2008;27:314–9. doi: 10.1097/ICO.0b013e3181608561. [DOI] [PubMed] [Google Scholar]
- 22.Solomon R, Donnenfeld E, Perry H, et al. Penetration of topically applied gatifloxacin 0.3%, moxifloxacin 0.5%, and ciprofloxacin 0.3% into the aqueous humor. Ophthalmology. 2005;112:466–9. doi: 10.1016/j.ophtha.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 23.Hsu H, Nacke R, Song J, Yoo S, Alfonso E, Israel H. Community opinions in the management of corneal ulcers and ophthalmic antibiotics: a survey of 4 states. Eye Contact Lens. 2010;36:195–200. doi: 10.1097/icl.0b013e3181e3ef45. [DOI] [PubMed] [Google Scholar]
- 24.Sy A, Srinivasan M, Mascarenhas J, et al. Pseudomonas aeruginosa keratitis: outcomes and response to corticosteroid treatment. Invest Ophthalmol Vis Sci. 2011;53:267–72. doi: 10.1167/iovs.11-7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clinical and Laboratory Standards Institute. Wayne, PA: Performance standards for antimicrobial susceptibility testing; twenty-first informational supplement. 2011. [Google Scholar]
- 26.Sueke H, Kaye S, Neal T, et al. Minimum inhibitory concentrations of standard and novel antimicrobials for isolates from bacterial keratitis. Invest Ophthalmol Vis Sci. 2010;51:2519–24. doi: 10.1167/iovs.09-4638. [DOI] [PubMed] [Google Scholar]
- 27.Wilhelmus K, Abshire R, Schlech B. Influence of fluoroquinolone susceptibility on the therapeutic response of fluoroquinolone-treated bacterial keratitis. Arch Ophthalmol. 2003;121:1229–33. doi: 10.1001/archopht.121.9.1229. [DOI] [PubMed] [Google Scholar]
- 28.Srinivasan M, Mascarenhas J, Rajaraman R, et al. Corticosteroids for bacterial keratitis: the Steroids for Corneal Ulcers Trial (SCUT) Arch Ophthalmol. 2011;130:143–50. doi: 10.1001/archophthalmol.2011.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliveria A, d'Azevedo P, Francisco W, Hofling-Lima A. In vitro activity of fluoroquinolones against ocular bacterial isolates in Sao Paulo, Brazil. Cornea. 2007;26:194–8. doi: 10.1097/01.ico.0000248379.78777.f6. [DOI] [PubMed] [Google Scholar]