As a long-term strategy to decrease HIV acquisition in South African women, pre-exposure prophylaxis is both effective, reducing lifetime risk of infection to 27%, and cost-effective, with an incremental cost-effectiveness ratio of $2700 per years of life saved.
Abstract
Background. Recent trials report the short-term efficacy of tenofovir-based pre-exposure prophylaxis (PrEP) for prevention of human immunodeficiency virus (HIV) infection. PrEP’s long-term impact on patient outcomes, population-level transmission, and cost-effectiveness remains unknown.
Methods. We linked data from recent trials to a computer model of HIV acquisition, screening, and care to project lifetime HIV risk, life expectancy (LE), costs, and cost-effectiveness, using 2 PrEP-related strategies among heterosexual South African women: (1) women receiving no PrEP and (2) women not receiving PrEP (a tenofovir-based vaginal microbicide). We used a South African clinical cohort and published data to estimate population demographic characteristics, age-adjusted incidence of HIV infection, and HIV natural history and treatment parameters. Baseline PrEP efficacy (percentage reduction in HIV transmission) was 39% at a monthly cost of $5 per woman. Alternative parameter values were examined in sensitivity analyses.
Results. Among South African women, PrEP reduced mean lifetime HIV risk from 40% to 27% and increased population discounted (undiscounted) LE from 22.51 (41.66) to 23.48 (44.48) years. Lifetime costs of care increased from $7280 to $9890 per woman, resulting in an incremental cost-effectiveness ratio of $2700/year of life saved, and may, under optimistic assumptions, achieve cost savings. Under baseline HIV infection incidence assumptions, PrEP was not cost saving, even assuming an efficacy >60% and a cost <$1. At an HIV infection incidence of 9.1%/year, PrEP achieved cost savings at efficacies ≥50%.
Conclusions. PrEP in South African women is very cost-effective by South African standards, conferring excellent value under virtually all plausible data scenarios. Although optimistic assumptions would be required to achieve cost savings, these represent important benchmarks for future PrEP study design.
Studies suggest that pre-exposure prophylaxis (PrEP) for human immunodeficiency (HIV) infection could make an important contribution to changing the trajectory of the HIV epidemic. Over the past 2 years, 4 trials—CAPRISA 004, Pre-Exposure Prophylaxis Initiative (iPrEx), CDC Botswana (TDF2), and Partners PrEP—demonstrated the efficacy of tenofovir-based PrEP for HIV infection, using alternative methods of PrEP delivery in different populations [1–4]. Reported HIV protective efficacy ranged from 39% (CAPRISA 004, vaginal gel) [1] to 44% (iPrEx, oral), [2] 63% (TDF2, oral) [3], and 73% (Partners PrEP, oral) [4] with effectiveness estimates ranging as high as 72%–97% among the most adherent patients. Populations studied include heterosexual women, men who have sex with men, and heterosexual serodiscordant couples. More recently, PrEP studies have led to cause for more-measured enthusiasm. Both the phase-III FEM-PrEP trial of oral chemoprophylaxis in women and the oral tenofovir-only arm of the VOICE trial failed to demonstrate protective effects and were terminated early [5–7]. One possible explanation for differences in oral versus vaginal efficacy is that vaginal concentration of orally administered drug may be very sensitive to levels of adherence [8]. However, optimism was again tempered with the termination of the vaginal gel arm of the VOICE trial when interim results demonstrated no efficacy, compared with placebo [9]. Though the gel-related VOICE results conflict with those seen in CAPRISA, differences in dosing regimens may be a contributing factor, and data from the South African FACTS 001 trial, duplicating the CAPRISA dosing, are anxiously anticipated [10].
Because of the complexity of the evidence base, with different trials demonstrating different degrees of efficacy in different populations using different dosing regimens, we sought to provide threshold benchmarks to practitioners and funders in resource-limited settings when considering PrEP (either vaginal or oral) as a potential intervention in a population of South African women at high risk of infection. To do so, we also considered many critical implementation parameters not evaluable in the trials, such as the long-term impact of PrEP on transmission and costs, and how these parameters are influenced by target population risk, behavioral changes, acquired viral resistance, and toxicity.
METHODS
Analytic Overview
We used the Cost-Effectiveness of Preventing AIDS Complications (CEPAC)–International model [11–14], a mathematical simulation of HIV screening and disease, to project clinical, epidemiologic, and economic outcomes associated with tenofovir-based PrEP gel in heterosexual women in South Africa. We simulated a cohort of HIV-uninfected South African women under 2 strategies: (1) No PrEP, which included HIV screening every 5 years, and (2) PrEP, which included tenofovir-based, pericoitally delivered, vaginal gel, with monthly HIV screening. Under both strategies, patients identified as HIV infected received the current South African HIV treatment standard of care [15, 16]. Although CAPRISA 004 provided estimates for base case PrEP efficacy and program costs, sensitivity analyses were intentionally conducted to encompass the spectrum of costs and efficacies from all the reported successful orally and vaginally dosed PrEP trials [1–4]. CAPRISA 004 trial–based estimates were first applied to baseline South African incidence values; we then used sensitivity analyses to widely vary incidence, HIV screening frequency, PrEP efficacy, acquired nucleoside reverse-transcriptase resistance, and costs. Model-based outcomes included projected lifetime HIV infection risk, life expectancy, costs, and incremental cost-effectiveness.
We measured cost-effectiveness in 2010 US dollars per year of life saved (YLS), reporting economic outcomes from a modified societal perspective (excluding patient time and travel costs), using a 3% annual discount rate [17]. We applied the recommendations of the World Health Organization (WHO) Commission on Macroeconomics and Health to denote strategies with incremental cost-effectiveness ratios less than the per capita gross domestic product (GDP; $7200 for South Africa) as very cost-effective and ratios <3 times the per capita GDP as cost-effective ($21 600) [18–20].
Model Overview
The CEPAC-International Model is a state-transition, Monte Carlo simulation of HIV acquisition, detection, and clinical care [11–14]. Model users define cohort attributes (eg, mean age, sex, CD4 cell count, HIV RNA distributions, and other demographic and/or clinical characteristics) and therapeutic alternatives (eg, number, sequencing, and efficacy of antiretroviral [ART] regimens). Two components of the model were used: the PrEP Module, which incorporates the mechanics of PrEP administration and monitoring, the incidence of HIV infection in the initially uninfected cohort, and the components of HIV screening; and the Disease Model, which includes details of those who become HIV infected, including the natural history, clinical management, and costs of HIV disease.
PrEP Module
The PrEP Module captures the attributes of a PrEP program, including target population characteristics (eg, age and sex distributions and HIV infection incidence) and intervention characteristics (eg, efficacy, behavioral changes, HIV screening frequency, and toxicity) [11–14]. In the No PrEP strategy, population- and age-based incidence rates determined the risk of HIV infection. In the PrEP strategy, the background HIV infection incidence was attenuated by the efficacy of the PrEP intervention (39% in the base case), defined as a reduction in primary HIV infection incidence. To remain conservative, we assumed that after initiation, PrEP was continued until HIV infection or death and examined this assumption in sensitivity analyses. For persons receiving PrEP, a defined proportion of the cohort experienced PrEP-related adverse events (eg, tenofovir-related nephrotoxicity) [21–23].
Infection was detected using an HIV test, either through current testing availability (No PrEP: mean, every 5 years) [14] or through the increased screening frequency accompanying the PrEP program. HIV screening (and other laboratory monitoring) in the PrEP program were in concordance with the study protocols of CAPRISA 004 and other trials; HIV-uninfected women receiving PrEP were followed up with monthly HIV tests and had biannual chemistry panels performed [1]. Although we adhered strictly to the trial protocols for purposes of modeling the study, we understand that monthly testing is neither practical nor feasible in a country-wide PrEP program. We considered HIV testing at alternative frequencies, as seldom as biannually, to examine this assumption in sensitivity analyses. We assumed a point-of-care rapid HIV test, with 99.6% sensitivity and 98.0% specificity, without the ability to detect primary HIV infection during the window period [24]. After HIV detection, PrEP was discontinued and treatment was provided according to details in the Disease Model. To produce conservative cost-effectiveness estimates, we assumed that PrEP continued lifelong; we relaxed this assumption in sensitivity analyses and examined the impact of stopping PrEP at different ages.
Disease Model
Details of the CEPAC-International model have been reported previously [11–14]. In brief, when women in the simulation acquired HIV infection, they transitioned to the Disease Model and progressed with the natural history of HIV infection. They were only eligible for HIV-related monitoring, care, and therapy after HIV detection. Their clinical course was represented by monthly transitions between health states, defined by CD4 cell count, HIV RNA level, and history of opportunistic infection. In the model, HIV RNA level determined the rate of monthly CD4 cell count decrease, whereas absolute CD4 cell count determined the probability of opportunistic infection and chronic HIV-related mortality [25, 26]. In accordance with current standards of care, patients who received a diagnosis of HIV infection attended biannual clinic visits for CD4 cell count assessment [15, 16, 27].
When laboratory and clinical monitoring revealed that criteria for ART initiation were met (CD4 cell count, <200 cells/μL; WHO stage IV disease; or CD4 cell count <350 cells/μL with tuberculosis), women received up to 2 sequential ART regimens (Table 1). During the first year of ART, patients were monitored biannually for CD4 cell count and HIV RNA level and yearly thereafter [15, 16, 27]. When ART resulted in HIV RNA suppression, CD4 cell count increased with a concomitant reduction in the risk of opportunistic infection and death [25, 30–33]. The Disease Model captures the impact of PrEP-associated resistance by allowing a user-defined proportion of the population who received PrEP (but became infected regardless) to have a decreased probability of virologic suppression during first-line ART. Patients with virologic suppression were also at monthly risk for treatment failure, resulting in virologic rebound and CD4 cell count decrease [30–33]. ART failure was defined as a 1-log increase in HIV RNA level, as observed by standard laboratory monitoring [15, 16]. Detection of failure of a first-line regimen resulted in an immediate switch to the second-line regimen, which the patient received until death [15, 16, 27].
Table 1.
Model Inputs for Select Parameters
| Variable | Base Case Value | Range in Sensitivity Analysis | Reference |
| Baseline cohort characteristics | |||
| Age, mean years (SD) | 23.9 (3) | 17.9–29.9 | [1] |
| Sex, % female | 100 | … | Assumption |
| Annual HIV infection incidence by age, % | |||
| ≤25 years | 2.2 | 1.1–44 | [28] |
| ≥26 years | 1.0 | 0.5–20 | [28] |
| PrEP characteristics | |||
| PrEP efficacy,a % | 39 | 10–90 | [1] |
| PrEP toxicity,b %/month | 0.02 | 0.01–2 | |
| Probability of PrEP resistance among those infected on PrEP | 0.05 | 0.05–1.0 | Assumption |
| HIV test characteristics | |||
| Average background HIV test frequency | Every 5 years | Once biannually | [14] |
| Sensitivity, % | 99.6 | … | [24] |
| Specificity, % | 98.0 | 98–100 | [24] |
| Initial CD4 cell count, mean cells/μL (SD) | 664 (294) | … | [25] |
| HIV RNA distribution after acute infection, % | |||
| >100 000 copies/mL | 42 | … | [29] |
| 30 001–100 000 copies/mL | 28 | … | [29] |
| 10 001–30 000 copies/mL | 18 | … | [29] |
| 3001–10 000 copies/mL | 8 | … | [29] |
| 501–3000 copies/mL | 2 | … | [29] |
| 21–500 copies/mL | 1 | … | [29] |
| Efficacy of antiretroviral therapy, first- and second-line regimens | |||
| HIV RNA suppressed at 6 months, % | 74.7 | 10% decrease for 0--100% of cohort | [30] |
| ART failure rate after 6 months, %/month | 1.59 | … | [31, 32] |
| CD4 increase at 6 months, mean cells/μL | 148 | … | [33] |
| Discount rate, %/year | 3 | 1–5 | [34] |
| Costs, 2010 US$ | |||
| PrEP program costs | |||
| Tenofovir-based PrEP, monthly (annual) | 5 (55) | 2.31–23.05 | [35] |
| HIV test,c per test (annual) | 6 (70) | 2.33–4.67 | [35] |
| Chemistry panel,d per test (annual) | 32 (63) | … | |
| Antiretroviral therapy, annual | |||
| TDF/3TC/EFV | 169 | … | [35] |
| d4T/3TC/EFV | 105 | 120–200 | [35] |
| AZT/3TC/LPV/r | 504 | … | [35] |
| Minor drug toxicity | 14 | … | [36] |
| Major drug toxicity | 1948 | … | [36] |
| Cotrimoxazole prophylaxis, monthly | 1 | … | [35] |
| Minor drug toxicity | 14 | … | [25, 37, 38] |
| Major drug toxicity | 1948 | … | [25, 37, 38] |
| CD4 test, per test | 12 | … | [38] |
| HIV RNA test, per test | 62 | … | [38] |
| Routine care, monthly (ranges by CD4 cell count) | 55–782 | … | [25, 37, 39] |
| Inpatient hospital care, per day | 278 | … | [37] |
| Outpatient hospital care, per visit | 14 | … | [37] |
Abbreviations: AZT, zidovudine; d4T, stavudine; EFV, efavirenz; HIV, human immunodeficiency virus; LPV/r, ritonavir-boosted lopinavir; PrEP, pre-exposure prophylaxis; SD, standard deviation; TDF, tenofovir; 3TC, lamivudine.
PrEP efficacy is defined as a percentage reduction in the monthly incidence of HIV infection.
Derived from the estimated upper confidence limit in the CAPRISA 004 trial.
The cost of the HIV test assumes a rapid test with pretest counseling. Reactive tests are confirmed with a second rapid test, with a cost of $2.
The cost of the chemistry panel assumes the costs of urea, creatinine, bilirubin, aspartate aminotransferase, and alanine aminotransferase testing, as well as the costs of reagents, staff salary, equipment, overhead, and facilities.
The clinical trajectory of each person’s condition was tracked from entry into the simulation until death, regardless of HIV status. Multiple individual simulations were then aggregated to achieve stable estimates of survival and costs.
Model Input Data
Demographic Parameters
Data on the demographic and clinical characteristics of the simulated cohort were derived from South African studies [1, 28, 40–42]. The target population was South African women (mean age, 23.9 years) [1]. The incidence of HIV infection was age adjusted for the female South African population. In the base case, for women aged <25 years, annual incidence was 2.2% (Table 1) [28].
PrEP Input Parameters
In the base case, we used the trial-based PrEP efficacy estimate of 39% [1]. Although trials have not demonstrated significant PrEP-related toxicity, we conservatively assumed that PrEP recipients have toxicity at a frequency of 0.02% per month (derived from the CAPRISA trial upper confidence limit) [1], with a resultant risk of death of 1 in 10 000. Similarly, the trials provided little evidence of later ART resistance in those who experienced PrEP failure [1, 2, 43]. We remain >1000-fold in excess of the upper confidence limit and assumed in the base case that 5% of the PrEP-receiving cohort became infected with ART-resistant virus; we further assumed that those infected with resistant virus have a resultant 10% absolute decrease in the rate of virologic suppression of the first-line ART regimen.
Costs
Costs of vaginal gel were estimated by multiplying the reported $0.32/dose (applicator and gel) by the recommended 2 doses per sex act (once before and once after sex) by 7.2 acts (the mean number of acts per woman per month). Thus, the mean PrEP gel and applicator cost was $5 per woman per month [35]. Because this cost estimate accounts for all reported sex acts, it is higher than the gel costs from CAPRISA 004 ($0.32/dose [44] × 6 applicators returned per month [1] = $1.92/month) and is likely to be a higher estimate than costs that may materialize for a widely available vaginal gel (approximately $0.32/dose) [45]. In the context of a PrEP program, monthly HIV tests and biannual chemistry panels added annual costs of $70 and $63 [46, 47]. Total yearly PrEP program-related costs (the cost of PrEP, HIV tests, and chemistry panels) were approximately $188. Annual ART costs were $105–$504 [35]. Direct medical care costs for HIV care (clinic visits, inpatient days, and monitoring tests) were derived using health care utilization and unit costs from the Cape Town AIDS Cohort (Table 1) [37, 39].
Sensitivity Analyses
We examined the impact of assuming a wide range of values for many individual parameters, including but not limited to annual incidence of HIV infection, PrEP efficacy, frequency of HIV testing, age, age-based discontinuation of PrEP, and costs. We simulated the important interplay between behavioral risk, adherence, and costs in the model with use of multiway sensitivity analyses on the annual incidence (reflecting risk), PrEP efficacy (reflecting potency and adherence), and costs (reflecting adherence, programmatic differences, and drug pricing). We also varied the probability of fatal toxicity, the proportion of the cohort with PrEP-related resistance, and its associated decrement in virologic suppression. The most influential parameters were then simultaneously varied.
RESULTS
Base Case
In a South African female population, with mean age of 23.9 years and an annual incidence of HIV infection of 2.2% for persons ≤25 years (annual incidence of 1% for persons ≥26 years), lifetime projected HIV infection risk was 40%. Discounted (undiscounted) population life expectancy was 22.51 (41.66) years, and the per-person lifetime cost was $7280 (Table 2). PrEP, with 39% efficacy, decreased the lifetime risk of HIV infection to 27% and increased discounted (undiscounted) life expectancy to 23.48 (44.48) years. Mean discounted lifetime costs also increased to $9890 per person, resulting in an incremental cost-effectiveness ratio of $2700/YLS, compared with the No PrEP strategy.
Table 2.
Base Casea Results and Select Scenario Analyses
| Undiscounted Results | Discounted Results | ||||
| Strategy | Lifetime HIV Infection Risk,b % | Per-person Life Expectancy, years | Per-person Lifetime Costs,b US$ | Per-person Life Expectancy, years | ICER, US$/YLS |
| No PrEP | 40 | 41.66 | 7280 | 22.51 | … |
| PrEP | 27 | 44.48 | 9890 | 23.48 | 2700 |
Abbreviations: HIV, human immunodeficiency virus; ICER, incremental cost-effectiveness ratio; PrEP, pre-exposure prophylaxis; YLS, year of life saved.
Incidence 2.2%.
Lifetime infection risk is projected from the cohort starting age of 23.9 years.
One-way Sensitivity Analysis
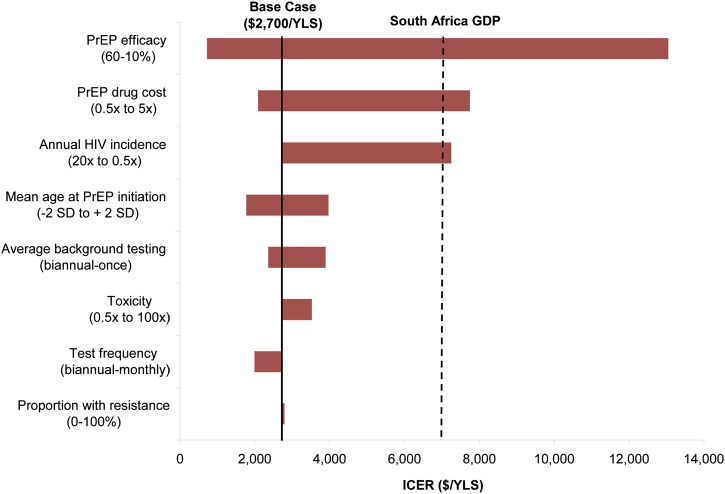
Three parameters had the largest impact on cost-effectiveness: PrEP efficacy, PrEP drug costs, and annual incidence of HIV infection in the target population (Figure 1). PrEP remained very cost-effective (<$7200/YLS) even with varying tenofovir resistance (proportion of population ranging from 0% to 100%) or increasing frequency of fatal PrEP-associated toxicity (0.5 times to 100 times). The Centers for Disease Control and Prevention suggests that HIV testing in a PrEP program be conducted every 3 months [48]; this testing frequency (compared with monthly testing in the base case) produced a similar life expectancy but at reduced cost, resulting in a more attractive incremental cost-effectiveness ratio of $1600/YLS. Results also remained very cost-effective when PrEP was discontinued at ages 35–45 years. No single input parameter change, within the ranges considered, rendered PrEP cost saving.
Figure 1.
One-way sensitivity analyses: incremental cost-effectiveness of pre-exposure prophylaxis (PrEP). This tornado diagram summarizes the results of multiple 1-way sensitivity analyses on the incremental cost-effectiveness of PrEP. Each horizontal bar represents the range of incremental cost-effectiveness ratios (ICERs) resulting from variations of a given model parameter across its plausible range, as indicated at opposite ends of each bar. The range for each bar is reported in the direction of the ICER for that bar. For example, higher human immunodeficiency virus (HIV) infection incidences and higher PrEP efficacies result in lower PrEP ICERs and are therefore reported from high to low as the bar moves from left to right. The bold vertical line indicates the base case ICER ($2700 per year of life saved). A bar reaching zero on the left would indicate cost saving (none of the bars reach that benchmark). The dashed vertical line represents the per capita gross domestic product (GDP) for South Africa, a threshold denoting a very cost-effective use of resources, by international standards [18–20].
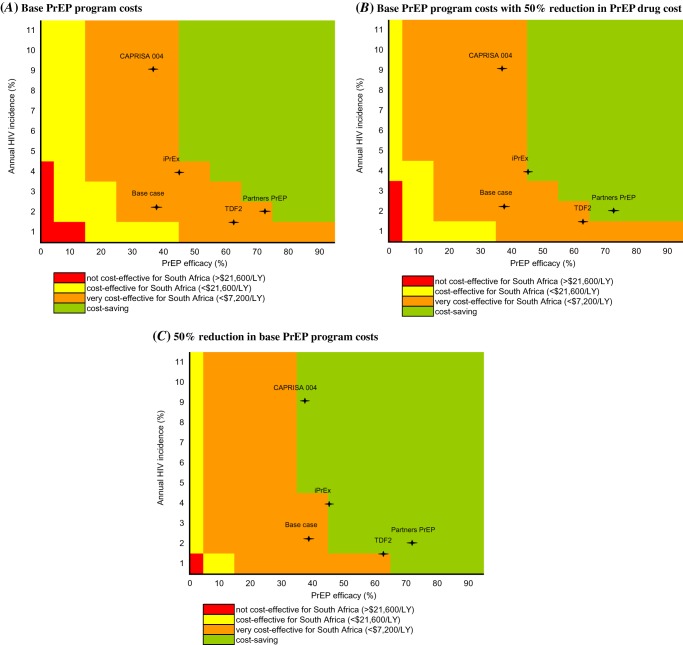
Multiway Sensitivity Analysis: The Impact of Incidence, Efficacy, and Cost
Simultaneously varying the aforementioned most influential parameters produced results ranging from not cost-effective to cost saving (Figure 2). However, at values represented by all of the reported trials, results were robust; PrEP was very cost-effective and bordered on cost saving (Figure 2). Decreasing PrEP drug costs alone by 50% produced moderately more favorable results, and reducing total PrEP program costs by 50% resulted in cost savings at 3 of the incidence and efficacy combinations represented by the trials.
Figure 2.
Multiway sensitivity analyses: incremental cost-effectiveness of pre-exposure prophylaxis (PrEP). This figure reports the ranges of incremental cost-effectiveness ratios for PrEP as a function of the 3 most influential parameters identified in Figure 1: human immunodeficiency virus (HIV) infection incidence of 1%–11% annually (vertical axis), PrEP efficacy of 0%–95% (horizontal axis), and PrEP program cost (the 3 vertical panels). The color indicates the incremental cost-effectiveness ratio achieved by each combination of parameters, ranging from not cost-effective in red (ratio >$21 600 per year of life saved [YLS]), to cost-effective in yellow ($7200–21,600/YLS), to very cost-effective in orange (<$7200/YLS), to cost saving in green. A, Base PrEP program costs. B, Base PrEP program costs with 50% reduction in PrEP drug cost (from $55/year to $28/year). C, 50% reduction in base PrEP program costs (from $188/year to $94/year). The base case and CAPRISA 004, iPrEX, TDF2 and Partners PrEP trial point estimates are indicated in each panel by the (+).
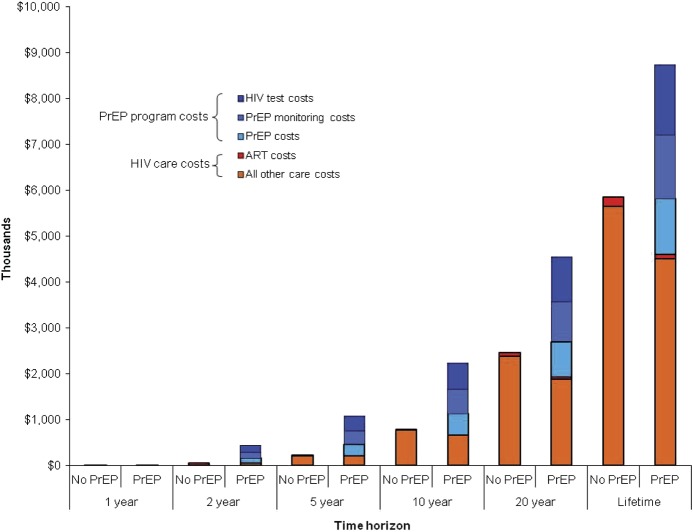
Budgetary Impact of PrEP for HIV-Uninfected Women in South Africa
Under base case assumptions, the savings from reduced HIV care costs with PrEP programs (compared with the HIV care costs without PrEP programs) will not offset the additional costs associated with the provision of PrEP (Figure 3). At 5 years, cumulative discounted costs will be $1075 per person in the PrEP program. Of these costs, 24% will be attributable to PrEP medications, 27% to PrEP laboratory monitoring costs, 31% to HIV testing costs, and 19% to HIV care costs for persons who become newly infected. By 20 years, HIV care costs for persons who become newly infected will account for 41% of total costs. To achieve coverage for half the 11.1 million women in South Africa aged 15–44 years who are HIV uninfected and potentially PrEP eligible, the discounted PrEP budget over the next 5 years would be $6.0 billion [49].
Figure 3.
Projected costs per woman over time, without and with pre-exposure prophylaxis (PrEP). This figure presents the components of the cumulative costs at 1, 2, 5, 10, and 20 years and over a woman’s lifetime, with No PrEP and PrEP availability. PrEP costs (shaded blue) are divided by their components, including drug costs only, PrEP monitoring costs (chemistry panels), and human immunodeficiency virus (HIV) testing costs. HIV care costs for those who became infected (shaded orange) include the costs for HIV-related care and antiretroviral therapy (ART; shaded red). Costs are scaled per 1000 people and reported in thousands (2010 US dollars).
DISCUSSION
Among South African women, vaginal PrEP would be a highly cost-effective intervention. At $2700/YLS, PrEP remained below the WHO-suggested very cost-effective threshold in South Africa, under a range of model parameter values chosen to capture the performance of both vaginal and oral alternatives. Cost savings could only be achieved under optimistic assumptions, in which PrEP was targeted to populations in which the incidence of HIV infection is high (≥5%/year), with high efficacy (≥50%) and at lower PrEP drug costs (<$40/year). The cost-effectiveness results compare very favorably with those that have been published for ART and CD4 cell count laboratory monitoring for HIV in South Africa [12, 50, 51].
Improvements in adherence—whether through easier regimens, such as a vaginal ring, targeting populations likely to be most receptive, or training providers to encourage compliance—are crucial to maximizing the effectiveness of a PrEP program. The results from FEM-PrEP and the early termination of the TDF-only arm in VOICE might suggest that efficacy of oral chemoprophylaxis is exquisitely sensitive to suboptimal adherence, because vaginal levels are marginal for protection even with the best adherence [5–8]. The more recent termination of the vaginal gel arm of VOICE, with no statistical benefit over placebo, results in more confusion in light of the earlier CAPRISA findings [9]. It is still not known whether daily gel administration, as in VOICE, may provide inferior protection than the before and after pericoital regimen studied in CAPRISA 004 [1].
Although they have not emerged as significant issues in clinical trials, PrEP-induced toxicity, resistance, and risk compensation remain an implementation concern. We found that the clinical benefits of PrEP at the population level overwhelmingly offset any plausible level of adverse reactions, resistant breakthrough infections, or behavioral disinhibition [11, 52]. Of note, risk-reduction effects have been demonstrated in microbicide, pre- and postexposure prophylaxis, and vaccine studies [1, 2, 53–59]. The challenge will be to sustain these effects if PrEP becomes a public health program with less frequent clinical monitoring.
Despite its excellent value, PrEP implementation will not pay for itself. This analysis suggests that the per-woman investment would be $1075/person, or $6.0 billion, for 50% coverage of all eligible women in South Africa over the next 5 years. This investment will pay substantial dividends both in HIV cases averted and in the associated care costs prevented. However, if a one-size-fits-all prevention approach proves to be infeasible, targeting PrEP to women at highest risk and/or those most likely to adhere will pay the highest returns on investment and may even prove to be cost saving.
Although our findings offer very strong evidence of the cost-effectiveness of PrEP, they are more tempered than those that have been previously reported [60]. We believe that the following modeling choices explain and justify our more conservative conclusions: first, we accounted explicitly for the indirect costs of providing PrEP (including regular HIV testing and chemistry panels); second, we captured the possibility (and the expected costs) of adverse outcomes, including PrEP-related toxicity and the potential for antiretroviral resistance attributable to prophylaxis failure; third, we recognized the time value of cost-effectiveness outcomes and that the costs of PrEP are incurred long before its prevention benefits are realized; fourth, in the absence of data demonstrating capacity to accurately target PrEP to those at the highest risk, we model it as a lifelong (or until infected) intervention.
This analysis has several limitations. We applied efficacies from short-term trials conducted in groups at high risk of HIV infection (heterosexual women, men, and men who have sex with men) at high risk to different populations and projected these results over longer periods [5, 6]. Until trials can confirm the durable efficacy of a tenofovir vaginal gel, the efficacy and optimal dosing of PrEP remain uncertain. Recognizing this uncertainty, we considered a wide range of efficacies and dosing strategies. Second, we considered only the first generation of HIV infections prevented, conservatively ignoring the additional benefit of later HIV infections averted. Last, we estimated and considered a wide range of PrEP program costs, because costs for a vaginal gel and for PrEP implementation are not yet available.
This analysis suggests that PrEP, when applied to South African women, will substantially reduce the lifetime risk of HIV infection and be very cost-effective. Given the long-awaited proof-of-concept of PrEP as an effective female-initiated prevention option, its implementation holds substantial potential for reducing the incidence of HIV infection in South Africa, where PrEP will provide excellent value for money.
Notes
Acknowledgments.
We thank Will Grogan, Sarah Lorenzana, Bethany Morris, and Yoriko Nakamura for their technical support.
Financial support.
This work was funded by the National Institute of Allergy and Infectious Diseases (R01 AI058736, P30 AI060354) and the National Institute of Mental Health (R01 MH065869 and R01 MH087328). The funding sources had no role in the design, analysis, or interpretation of the study or in the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases, the National Institute of Mental Health, or the National Institutes of Health. R. P. W. had access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Potential conflicts of interest.
K. H. M. has unrestricted educational and research grants from Gilead Sciences and Merck Pharmaceuticals. All other authors report no conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thigpen MC, Kebaabetswe PM, Smith DK, et al. 6th IAS Conference on HIV Pathogenesis, Treatment, and Prevention.: Daily oral antiretroviral use for the prevention of HIV infection in heterosexually active young adults in Botswana: results from the TDF2 study [oral abstract WELBC01] Rome, Italy. 2011. [Google Scholar]
- 4.Baeten J. Antiretroviral pre-exposure prophylaxis for HIV-1 prevention among heterosexual African men and women: the Partners PrEP study [oral abstract MOAX0106] 6th IAS Conference on HIV Pathogenesis, Treatment, and Prevention. Rome, Italy. 2011. [Google Scholar]
- 5. Family Health International. FHI to initiate orderly closure of FEM-PrEP. Available at: http://www.fhi.org/en/Research/Projects/FEM-PrEP.htm. Accessed 24 May 2011.
- 6.Stephenson J. Study halted: no benefit seen from antiretroviral pill in preventing HIV in women. JAMA. 2011;305:1952. doi: 10.1001/jama.2011.649. [DOI] [PubMed] [Google Scholar]
- 7. NIH News. NIH modifies ‘VOICE' HIV prevention study in women. Available at: http://www.nih.gov/news/health/sep2011/niaid-28.htm. Accessed 13 October 2011.
- 8.Karim SS, Kashuba AD, Werner L, Karim QA. Drug concentrations after topical and oral antiretroviral pre-exposure prophylaxis: implications for HIV prevention in women. Lancet. 2011;378:279–81. doi: 10.1016/S0140-6736(11)60878-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. MTN statement on decision to discontinue use of tenofovir gel in VOICE, a major HIV prevention study in women. Available at: http://www.mtnstopshiv.org/node/3909. Accessed January 3, 2012.
- 10. FACTS 001-Tenofovir gel study. Available at: http://www.rhru.co.za/Documents/Materials/FACTS_001_Overview_23Nov2010.pdf. Accessed 3 January 2012.
- 11.Paltiel AD, Freedberg KA, Scott CA, et al. HIV preexposure prophylaxis in the United States: impact on lifetime infection risk, clinical outcomes, and cost-effectiveness. Clin Infect Dis. 2009;48:806–15. doi: 10.1086/597095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walensky RP, Wolf LL, Wood R, et al. When to start antiretroviral therapy in resource-limited settings. Ann Intern Med. 2009;151:157–66. doi: 10.7326/0003-4819-151-3-200908040-00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walensky RP, Wood R, Ciaranello AL, et al. Scaling up the 2010 World Health Organization HIV Treatment Guidelines in resource-limited settings: a model-based analysis. PLoS Med. 2010;7:e1000382. doi: 10.1371/journal.pmed.1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walensky RP, Wood R, Fofana MO, et al. The clinical impact and cost-effectiveness of routine, voluntary HIV screening in South Africa. J Acquir Immune Defic Syndr. 2011;56:26–35. doi: 10.1097/QAI.0b013e3181fb8f24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. South Africa National Department of Health. The South African antiretroviral treatment guidelines. Available at: http://www.doh.gov.za/docs/factsheets/guidelines/art.pdf. Accessed 19 July 2010.
- 16. South Africa National Department of Health. Clinical guidelines for the management of HIV & AIDS in adults and adolescents. Available at: http://www.doh.gov.za/docs/factsheets/guidelines/adult_art.pdf. Accessed 19 July 2010.
- 17.Goldie SJ, Yazdanpanah Y, Losina E, et al. Cost-effectiveness of HIV treatment in resource-poor settings–the case of Cote d'Ivoire. N Engl J Med. 2006;355:1141–53. doi: 10.1056/NEJMsa060247. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Choosing interventions that are cost-effective (WHO-CHOICE): cost-effectiveness thresholds. Geneva: World Health Organization; 2009. Available at: http://www.who.int/choice/costs/CER_thresholds/en/index.html. Accessed 31 July 2010. [Google Scholar]
- 19. World Health Organization. Macroeconomics and health: investing in health for economic development. Report of the Commission on Macroeconomics and Health. Available at: http://whqlibdoc.who.int/publications/2001/924154550x.pdf. Accessed 25 March 2011.
- 20.International Monetary Fund. World economic outlook database. World economic and financial surveys. 2009. Available at: http://imf.org/external/pubs/ft/weo/2009/02/weodata/index.aspx. Accessed 13 October 2009. [Google Scholar]
- 21.Bygrave H, Kranzer K, Hilderbrand K, et al. Renal safety of a tenofovir-containing first line regimen: experience from an antiretroviral cohort in rural Lesotho. PLoS One. 2011;6:e17609. doi: 10.1371/journal.pone.0017609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaisiri K, Bowonwatanuwong C, Kasettratat N, Kiertiburanakul S. Incidence and risk factors for tenofovir-associated renal function decline among Thai HIV-infected patients with low-body weight. Curr HIV Res. 2010;8:504–9. doi: 10.2174/157016210793499259. [DOI] [PubMed] [Google Scholar]
- 23.Cooper RD, Wiebe N, Smith N, Keiser P, Naicker S, Tonelli M. Systematic review and meta-analysis: renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin Infect Dis. 2010;51:496–505. doi: 10.1086/655681. [DOI] [PubMed] [Google Scholar]
- 24.Sauer G, Brand T, Bester R, Beggs M, Arai HX, Janse van Rensburg E. Program and abstracts of the 13th International AIDS Conference. Durban, South Africa: 2000. Evaluation of the Abbott Determine HIV-1/2 rapid assay using samples from the Western Cape region, South Africa [abstract MoPeA2091] [Google Scholar]
- 25.Holmes CB, Wood R, Badri M, et al. CD4 decline and incidence of opportunistic infections in Cape Town, South Africa: implications for prophylaxis and treatment. J Acquir Immune Defic Syndr. 2006;42:464–9. doi: 10.1097/01.qai.0000225729.79610.b7. [DOI] [PubMed] [Google Scholar]
- 26.Mellors JW, Munoz A, Giorgi JV, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946–54. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 27. World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. Available at: http://whqlibdoc.who.int/publications/2010/9789241599764_eng.pdf. Accessed 19 July 2010.
- 28.Rehle TM, Hallett TB, Shisana O, et al. A decline in new HIV infections in South Africa: estimating HIV incidence from three national HIV surveys in 2002, 2005 and 2008. PLoS One. 2010;5:e11094. doi: 10.1371/journal.pone.0011094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawn SD, Badri M, Wood R. Tuberculosis among HIV-infected patients receiving HAART: long term incidence and risk factors in a South African cohort. AIDS. 2005;19:2109–16. doi: 10.1097/01.aids.0000194808.20035.c1. [DOI] [PubMed] [Google Scholar]
- 30.Hammond R, Harry TC. Efficacy of antiretroviral therapy in Africa: effect on immunological and virological outcome measures—a meta-analysis. Int J STD AIDS. 2008;19:291–6. doi: 10.1258/ijsa.2007.007248. [DOI] [PubMed] [Google Scholar]
- 31.Gallant JE, DeJesus E, Arribas JR, et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med. 2006;354:251–60. doi: 10.1056/NEJMoa051871. [DOI] [PubMed] [Google Scholar]
- 32.Johnson M, Grinsztejn B, Rodriguez C, et al. Atazanavir plus ritonavir or saquinavir, and lopinavir/ritonavir in patients experiencing multiple virological failures. AIDS. 2005;19:685–94. doi: 10.1097/01.aids.0000166091.39317.99. [DOI] [PubMed] [Google Scholar]
- 33.Tuboi SH, Brinkhof MW, Egger M, et al. Discordant responses to potent antiretroviral treatment in previously naive HIV-1-infected adults initiating treatment in resource-constrained countries: the antiretroviral therapy in low-income countries (ART-LINC) collaboration. J Acquir Immune Defic Syndr. 2007;45:52–9. doi: 10.1097/QAI.0b013e318042e1c3. [DOI] [PubMed] [Google Scholar]
- 34.Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost effectiveness in health and medicine. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 35. The Clinton Foundation HIV/AIDS Initiative. Antiretroviral (ARV) price list. November 2010. Available at: http://www.clintonfoundation.org/files/chai_arv_priceList_201011_english.pdf. Accessed 8 March 2011.
- 36.Kumarasamy N, Venkatesh KK, Cecelia AJ, et al. Spectrum of adverse events after generic HAART in southern Indian HIV-infected patients. AIDS Patient Care STDS. 2008;22:337–44. doi: 10.1089/apc.2007.0093. [DOI] [PubMed] [Google Scholar]
- 37.Cleary S, Boulle A, McIntyre D, et al. Cost-effectiveness of antiretroviral treatment for HIV-positive adults in a South African township. Médecins Sans Frontières, Health Systems Trust; 2004. Available at: http://www.hst.org.za/uploads/files/arv_cost.pdf. Accessed 31 July 2010. [Google Scholar]
- 38.Gauteng Department of Health. Gauteng Hospitals Numeric. Gauteng Province, South Africa: 2004. [Google Scholar]
- 39.Badri M, Cleary S, Maartens G, et al. When to initiate highly active antiretroviral therapy in sub-Saharan Africa? A South African cost-effectiveness study. Antivir Ther. 2006;11:63–72. [PubMed] [Google Scholar]
- 40.Middelkoop K, Myer L, Mark D, et al. Adolescent and adult participation in an HIV vaccine trial preparedness cohort in South Africa. J Adolesc Health. 2008;43:8–14. doi: 10.1016/j.jadohealth.2007.11.144. [DOI] [PubMed] [Google Scholar]
- 41.Shisana O RT, Simbayi LC, Parker W, et al. SABSSM III Implementation Team. South African national HIV prevalence, incidence, behaviour and communication survey 2008: a turning tide among teenagers? Cape Town: HSRC Press; 2009. [Google Scholar]
- 42.Joint United Nations Programme on HIV/AIDS (UNAIDS) Global report: UNAIDS report on the global AIDS epidemic 2010. Geneva: UNAIDS; 2010. Available at: http://www.unaids.org/globalreport/documents/20101123_GlobalReport_full_en.pdf. Accessed 23 May 2011. [Google Scholar]
- 43.Liegler T, Abdel-Mohsen M, Atchison R, et al. Program and abstracts of the 18th Conference on Retroviruses and Opportunistic Infections. Boston, MA, Feb 27–March 2, 2011: Drug resistance and minor drug resistant variants in iPrEx [abstract 97LB] [Google Scholar]
- 44.Dugger C. African studies give women hope in HIV fight. Vulindela, South Africa: New York Times; A1. 2010. Available at: http://www.nytimes.com/2010/07/20/world/africa/20safrica.html?pagewanted=all. Accessed 21 February 2012. [Google Scholar]
- 45.Schoofs M, Wonacott P. New gel cuts risk of HIV infection. The Wall Street Journal. A1 2010. Available at: http://online.wsj.com/article/SB10001424052748704720004575377140651050822.html. Accessed 21 February 2012. [Google Scholar]
- 46.Bassett IV, Giddy J, Nkera J, et al. Routine voluntary HIV testing in Durban, South Africa: the experience from an outpatient department. J Acquir Immune Defic Syndr. 2007;46:181–6. doi: 10.1097/QAI.0b013e31814277c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gilks CF. Program and abstracts of the 5th IAS Conference on HIV Pathogenesis, Treatment and Prevention. Cape Town, South Africa: 2009. Cost effectiveness analysis of routine laboratory or clinically driven strategies for monitoring anti-retroviral therapy in Uganda and Zimbabwe [oral presentation] [Google Scholar]
- 48. Centers for Disease Control and Prevention. Pre-exposure prophylaxis (PrEP) for HIV prevention: Promoting safe and effective use in the US. Available at: http://www.cdc.gov/hiv/prep/pdf/PrEPfactsheet.pdf. Accessed 13 October 2011.
- 49.The World Bank. World development indicators. World Data Bank; 2011. Available at: http://databank.worldbank.org/ddp/home.do. Accessed 21 February 2012. [Google Scholar]
- 50.Bendavid E, Young SD, Katzenstein DA, Bayoumi AM, Sanders GD, Owens DK. Cost-effectiveness of HIV monitoring strategies in resource-limited settings: a southern African analysis. Arch Intern Med. 2008;168:1910–8. doi: 10.1001/archinternmed.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walensky RP, Ciaranello AL, Park JE, Freedberg KA. Cost-effectiveness of laboratory monitoring in sub-Saharan Africa: a review of the current literature. Clin Infect Dis. 2010;51:85–92. doi: 10.1086/653119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abbas U, Glaubius R, Mubayi A, Hood G, Mellors J. Program and abstracts of the 18th Conference on Retroviruses and Opportunistic Infections. Boston, MA, Feb 27–March 2, 2011: Predicting the impact of ART and PrEP with overlapping regimens on HIV transmission and drug resistance in South Africa [abstract 98LB] [Google Scholar]
- 53.Amico R, Liu A, McMahan V, et al. Adherence indicators and PrEP drug levels in the iPrEx study [abstract 95LB] Program and abstracts of the 18th Conference on Retroviruses and Opportunistic Infections. Boston, MA, Feb 27–March 2, 2011.
- 54.Bartholow BN, Buchbinder S, Celum C, et al. HIV sexual risk behavior over 36 months of follow-up in the world's first HIV vaccine efficacy trial. J Acquir Immune Defic Syndr. 2005;39:90–101. doi: 10.1097/01.qai.0000143600.41363.78. [DOI] [PubMed] [Google Scholar]
- 55.Cohen MS, Gay C, Kashuba AD, Blower S, Paxton L. Narrative review: antiretroviral therapy to prevent the sexual transmission of HIV-1. Ann Intern Med. 2007;146:591–601. doi: 10.7326/0003-4819-146-8-200704170-00010. [DOI] [PubMed] [Google Scholar]
- 56.Matthews LT, Baeten JM, Celum C, Bangsberg DR. Periconception pre-exposure prophylaxis to prevent HIV transmission: benefits, risks, and challenges to implementation. AIDS. 2010;24:1975–82. doi: 10.1097/QAD.0b013e32833bedeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peterson L, Taylor D, Roddy R, et al. Tenofovir disoproxil fumarate for prevention of HIV infection in women: a phase 2, double-blind, randomized, placebo-controlled trial. PLoS Clin Trials. 2007;2:e27. doi: 10.1371/journal.pctr.0020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Skoler-Karpoff S, Ramjee G, Ahmed K, et al. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1977–87. doi: 10.1016/S0140-6736(08)61842-5. [DOI] [PubMed] [Google Scholar]
- 59.van Griensvan F, Keawkungwal J, Tappero JW, et al. Lack of increased HIV risk behavior among injection drug users participating in the AIDSVAX B/E HIV vaccine trial in Bangkok, Thailand. AIDS. 2004;18:295–301. doi: 10.1097/00002030-200401230-00020. [DOI] [PubMed] [Google Scholar]
- 60.Williams BG, Abdool Karim SS, Karim QA, Gouws E. Epidemiological impact of tenofovir gel on the HIV epidemic in South Africa. J Acquir Immune Defic Syndr. 2011;58:207–10. doi: 10.1097/QAI.0b013e3182253c19. [DOI] [PMC free article] [PubMed] [Google Scholar]