Abstract
Evidence suggests that patients on opiate maintenance therapy for the treatment of addiction present with opioid-induced hyperalgesia (OIH). This study compared the experimental (cold-pressor, electrical stimulation) pain responses of 82 treatment-seeking heroin-dependent adults randomized to methadone (METH, n = 11) or buprenorphine (BUP, n = 64) therapy, with matched drug free controls (n = 21). Heroin-dependent participants were evaluated at baseline (treatment entry), medication (METH or BUP) stabilization (4-8 weeks), and chronic administration (12-18 weeks), at trough (just prior to dosing) and peak (3 hours after dosing) plasma levels. Collection of the control group’s pain responses occurred twice during a single session, three hours apart. Baseline comparisons indicate that heroin-dependent individuals demonstrate significantly shorter latencies to threshold and tolerance for cold-pressor pain than the control group. Across pain stimuli and time points, little change in pain responses were found over time, the exception being cold pressor pain tolerance, for which hyperalgesia significantly increased at trough METH/BUP levels in both groups as they stabilized in treatment. We conclude that heroin-dependent individuals are hyperalgesic, and that once stabilized in treatment, are not different in pain responses regardless of treatment agent. The effects of non-pharmacologic therapy and previous heroin use may explain increased hyperalgesia found with treatment.
Perspective
To better understand the clinical phenomenon of OIH, this article describes experimental pain responses of heroin-dependent participants both prior to and over the course of maintenance therapy with methadone or buprenorphine. Hyperalgesia is present with illicit and treatment opioid use, and does not appear to appreciably improve over the course of treatment.
Keywords: opioid-induced hyperalgesia, methadone, heroin, buprenorphine, pain
Introduction
Managing reports of pain in individuals who suffer addictive disease is a well-recognized clinical challenge. Providing opioid analgesia to these patients is complicated by clinician concerns about drug-seeking behaviors, fears of legal sanctions, and a general lack of knowledge about addiction, all compounded by powerful societal prejudices against addicted individuals. These challenges are arguably greatest for those addicted to opioids, in that the class of drug abused is also a primary pharmacotherapy for the treatment of moderate to severe pain. Opioid-addicted individuals seek psychoactive effects, yet are not immune to the drug’s effects on central and peripheral opioid-relevant pain systems.
A distinguishing effect of opioid use is heightened sensitivity to pain, or opioid-induced hyperalgesia (OIH). Convergent lines of preclinical and clinical evidence indicate that opioid administration not only provides a rapid and powerful analgesia, but concurrently sets into motion certain anti-analgesic or hyperalgesic opponent processes, which can be observed both during opioid activity and withdrawal2,7,8,20, and which have been suggested to contribute to opioid tolerance 6,9. The implications of this altered pain state have become of interest to investigators and clinicians who prescribe opioid analgesics for chronic pain in all populations 7, 20.
Research during the past decade has demonstrated a robust and reliable hyperalgesia in patients on the full opioid agonist methadone or on the partial opioid agonist buprenorphine as substitution/maintenance medications for treatment of opioid addiction11,12,14. Ho and Dole19 observed over 40 years ago that methadone-maintained heroin-dependent patients were significantly more sensitive to cold pressor-induced pain than drug-free controls. Diminished tolerance to experimental (electrical stimulation [ES], cold-pressor [CP]) pain has been reliably demonstrated in methadone patients in comparison to matched drug-free heroin users10 and controls3,11,12,14,33, at both peak and trough methadone blood levels. Cross-sectional data suggest a large effect size, indicating that methadone-maintained patients (MM) are 42-76% less tolerant of CP pain than are normal controls matched on age, gender and ethnicity. These findings have implications for the management of pain in methadone patients, and support increased analgesic need in patients receiving opioid pharmacotherapy1,27,36.
In these clinical examinations of OIH, studies have focused almost exclusively on the pain responses of well-stabilized patients receiving treatment with a long-acting substitution opioid, either methadone or buprenorphine. Not described are the pain responses of opioid addicts abusing the short-acting street opioid, heroin, which due to its rapid onset and offset of action, is hypothesized to produce a robust hyperalgesic effect. Further, the way in which hyperalgesia might change as a patient moves from heroin abuse to stabilization, and ultimately, maintenance on a treatment opioid, requires description, as does its differential progression in persons on full (methadone) vs. the partial (buprenorphine) opioid agonist therapy.
The objective of the current study was to compare the pain responses of treatment-seeking heroin-dependent individuals to those of non-drug using controls on two types of experimental pain stimuli (CP and ES) to describe the degree to which heroin-dependent participants experience hyperalgesia. Further, following heroin-dependent participants over the induction and maintenance phases of opioid substitution therapy, a second goal was to assess whether these pharmacotherapies differentially affect the expression of hyperalgesia over time.
Materials and Methods
Design
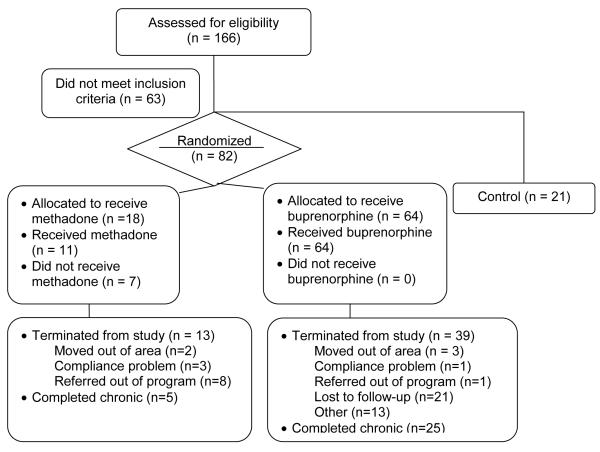
A survey design was used to describe hyperalgesia among heroin-dependent adults presenting for treatment (n = 82), and to compare the responses of these patients on standardized CP and ES assays to those of a control group with no history of substance use disorders (n = 21) (see Figure 1). Further, using a time-series design, CP and ES pain responses of heroin-dependent patients randomized to methadone (METH) or buprenorphine (BUP) therapy were compared within and between each other at; (1) baseline-prior to entering buprenorphine or methadone treatment; (2) stabilization-first instance of two consecutive opiate-free urines (between weeks 4 and 8); and (3) chronic administration- the first instance of two consecutive opiate-free urines following 12 weeks of therapy. Collection of pain measures occurred at putative trough (immediately prior to dose) and peak (three hours post dose) METH or BUP concentrations. To control for the effects of order, half of the participants were administered the CP procedure followed by the ES procedure and half administered the ES procedure followed by the CP procedure. The control group underwent experimental pain detection and tolerance measures two times in a single day, three hours apart designed to mimic peak and trough blood levels.
Figure 1.
Design and Participant disposition
Participants
A total of 82 heroin-dependent adults (18 entering METH treatment, and 64 entering BUP treatment) and 21 drug-free controls participated in this study. Studying a lower number of control subjects in comparison to heroin-dependent subjects was supported by low intergroup variation on key variables (depression, anxiety) in the former. Recruitment methods included newspaper advertisements, word of mouth, fliers posted at local treatment centers, and self-referral. Inclusion criteria included: at least 18 years of age; in good physical health; agreeable to and capable of signing an informed consent; no existing conditions that would affect sensitivity to cold (Raynaud’s disease, urticaria, etc.); no neuropathology that would affect pain responses (i.e., peripheral neuropathy, neuropathic pain); and no cardiovascular conditions that could put participants at risk for CP-induced blood pressure increases. Non-control group participants were seeking opioid maintenance therapy for the treatment of a DSM-IV diagnosed heroin dependence disorder. History of opiate use and addiction was measured by the Addiction Severity Index (ASI)26.
Heroin-dependent individuals were excluded from participation if they had: a known sensitivity to BUP or METH; dependence on alcohol, benzodiazepines or other drugs of abuse (except nicotine); any acute medical condition that would make participation medically hazardous; acute psychosis, severe depression, or in need of acute inpatient treatment/suicidal; taken Levo-Alpha Acetyl Methadol (LAAM), METH or naltrexone within 30 days of enrolling in the study; discontinued participation in an opiate-substitution (i.e., methadone, LAAM) treatment program within 30 days of enrolling in the study; or any pending legal action that could prohibit sustained participation. For the control group, a current or past history of substance abuse, current use of analgesic medication, or being a nursing or pregnant female excluded participation.
The study provided treatment with METH or BUP at no cost, and participants received brief weekly counseling from the study physician on issues of drug use and abstinence. Heroin-dependent participants were compensated with grocery or department store gift cards including $60 for each completed set (trough and peak) of pain testing (at baseline, stabilization, and maintenance). As a bonus, those who completed all six pain procedures received an additional $60. In addition, participants received $5 for each opiate-free urine test, and $10 for the fourth of four consecutive opiate-free urine tests. A total of $365 in gift cards was possible for complete study participation and compliance. Participants in the control group received $60 in cash for the completion of their one day of pain testing.
Pain Measures
Two experimental pain assays, cold-pressor (CP) and electrical stimulation (ES), were utilized to measure pain responses.
Cold Pressor Test (CP)
The CP method utilized was adapted from the procedures of Eckhardt16, which have been extensively used in the pharmaceutical development of analgesics. In this paradigm, two water containers (one containing water at room temperature water and one containing ice water), were positioned in front of the participant, and eye patches placed over the eyes to minimize distraction. Participants placed thier forearm into the room-temperature water with fingers wide apart, andwere instructed not to make contact with the sides or bottom of the container. After 1 minute and 45 seconds, a blood pressure cuff was inflated to 20 mm Hg below the obtained diastolic blood pressure to augment the pain experience with ischemia. At exactly 2 minutes, the participant removed the arm from the water and immediately placeds it in the ice bath (1.0+/−0.5C).
A water pump, placed in the cold-water container, prevented laminar warming around the immersed limb. Participants were not spoken to during the cold-water immersion in order to minimize any distraction or cues for time, which could adversely influence pain detection and tolerance levels. As detailed in standardized instructions, the participant reported when the pain was first felt (detection), and kept the arm immersed until the pain could no longer be tolerated, at which time he/she immediately removed the arm from the container (tolerance). All CP trials were truncated at 120 seconds. The dependent variables included in the analyses were the time in seconds from immersion to (a) the first detection of pain, and (b) when pain could no longer be tolerated and the arm removed from the water. All participants completed a practice run of the CP prior to actual testing to allow familiarization with the method and process involved.
Electrical Stimulation (ES)
Employed as a standardized pain induction technique for over a century, ES boasts ample empirical data supporting its validity, reliability and safety to produce a well-characterized nociceptive experience, and demonstrated to be analogous to clinical pain13,39,40. ES was delivered via cutaneous electrodes, using the commercially available SD9 Square Pulse Stimulator (Grass Products). Specifically, electro-conductive gel was applied to the earlobe and to two ear clips, which were then attached to the participant’s right ear. Eye patches covered the participant’s eyes to minimize distraction. Electrical pulses (frequency 0.7 pulses per second) of 14 milliseconds duration increased approximately every 5 seconds by 2.5-volt increments (starting at 0 volts) with a maximum voltage of 100V. Participants reported the following: (a) onset of pain (detection) and (b) point at which pain could no longer be tolerated (tolerance), and at each of these time points, the corresponding voltage was recorded. When participants indicated that tolerance had been reached, the power was immediately turned off and the ear clip and eye patches removed. An event recorder (Dash 2 EZ two-channel recorder, Grass Products) added to the SD-9 stimulator unit, provided the capability of displaying voltage as a function of time. Before testing, each participant completed a practice test of the ES pain assay.
Procedures
All study procedures were approved by the UCLA Office for the Protection of Research Subjects Institutional Review Board, and informed consent was obtained from each participant. Following a screening visit to establish study eligibility, consented participants were familiarized with the CP and ES procedures. All pain-testing sessions took place in a private setting, and one of two trained research assistants administered the ES and CP. Prior to each pain testing session, participants also underwent a brief health screening, completed the Beck Depression Inventory,4 State-Trait Anxiety Inventory38 and a measure of subjective opioid withdrawal for the opioid-dependent participants. Respiration, EKG, pulse oximetry, heart rate and blood pressure were continuously monitored prior to, during, and for at least ten minutes following each pain test to ensure return to baseline. Testing occurred at approximately the same time each morning.
For all participants, pain responses were measured on two occasions on the same day separated by three hours, so as to approximate METH/BUP trough (just prior to dosing) and peak (approximately 3 hours following dosing) blood levels. Controls completed pain testing three hours apart on a single day, whereas the heroin-dependent participants were tested on three occasions: (1) baseline; (2) stabilization; and (3) chronic administration. Following the third set of measures, participants either received a referral to a local provider for continued pharmacotherapy, or underwent an individualized dose-tapering schedule determined by the research physician.
Baseline – Day 1
Immediately prior to treatment entry, CP and ES pain detection and tolerance were assessed for the heroin-dependent participants, in a single day at two time points, three hours apart. Specific time of last heroin use was not noted, but low baseline withdrawal scores (mean = 2.55) suggest that last use was within the previous six hours.
Stabilization – Weeks 4-8
Upon entry to treatment, participants randomized to the BUP group were inducted according to the following schedule: Day One (8mg), Day Two (12mg), Day Three (16mg), and Day Four (24mg). Stabilization dose ranged between 16 and 24mg. For the participants in the METH group, the study physician followed induction procedures according to the standard clinic protocol of providing an observed split dose of 40mg methadone on Day One, after which dose increases of 10mg every other day were provided until reaching a stable daily dose (70mg - 90mg/day). As a part of treatment, participants received medication management counseling twice during the first week and once per week for the following 8 weeks. They also received relapse prevention counseling from a trained medical assistant, once a week, for an hour. Collection of urine samples for drug testing occurred weekly.
Participants who provided two consecutive opiate-free urine samples, at any point between weeks 4 and 8, were defined as having met stability criteria for treatment, and retested using the same CP and ES testing procedure as described at baseline. Participants came to the testing site approximately 22 hours following their last dose of medication, and the first set of pain tests were completed. Following testing, participants took their dose of medication. Pain testing occurred again three hours later.
Chronic Administration – Weeks 12-18
Upon completion of 12 weeks of opioid substitution therapy and provision of two consecutive clean urines, participants completed pain testing procedures in a manner identical to those used at stabilization.
Data Analysis
To evaluate differences in pain perception and tolerance between the control and heroin groups, and between the two medication groups over time, we performed mixed models analyses. For the CP data, the four analyses examining changes in response across treatment weeks were time to pain detection and time of pain tolerance for each of the two time points (peak vs. trough) by group. Similarly, for the ES data, the four analyses included the voltage of pain detection and voltage of pain tolerance. Baseline data for the control group were used for comparison to the stabilization and chronic test sessions of the heroin groups, because the control group’s responses did not significantly change with repeated (sham peak vs. trough) measurement. Transformations normalized distributions when necessary; the presented data are original values.
Results
Table 1 presents demographic characteristics of the study sample. Baseline health assessments showed that both controls and heroin-dependent participants were in general good health; overall, very few individuals reported active medical conditions, and of the 16 body systems evaluated upon physical exam, none were rated as “Clinically Significantly Abnormal.” Due to neurological and psychiatric exclusion criteria, no control or heroin-dependent participants had chronic neuropathic pain or clinically significant psychiatric illness. As anticipated, control participants reported significantly fewer health problems and symptoms of anxiety or depression than did participants in the medication groups.
Table 1.
Baseline characteristics of the Control and Heroin treatment groups.
| Control n = 21 |
Buprenorphine n = 64 |
Methadone n = 18 |
|
|---|---|---|---|
| Age (yr) | 30.14 (11) | 33.41 (9) | 34.55 (12) |
| Gender (%) | |||
| Male | 57.14 (12) | 65.63 (22) | 66.67 (12) |
| Female | 42.86 (9) | 34.38 (22) | 33.33 (6) |
| Race (%) | |||
| Anglo | 81.85 (17) | 82.81 (53) | 66.67 (12) |
| African American | 9.52 (2) | 1.56 (1) | 16.67 (3) |
| Asian American | 4.76 (1) | 0 | 5.56 (1) |
| Other | 4.76 (1) | 15.63 (10) | 11.11 (2) |
| Beck Depression Score4 | 0.67 (0.71) | 12.69 (8.47) | 13.90 (10.55) |
| State-Trait Anxiety Inventory38 | 0.73 (1.27) | 6.61 (7.62) | 6,50 (10.21) |
| Opioid withdrawal Score | -- | 2.56 (2.90) | 2.45 (3.17) |
| CP Pain threshold (sec) | |||
| Trough | 11.95 (6.10) | 12.51 (9.08) | 10.12 (6.40) |
| Peak | 11.79 (6.88) | 8.50 (4.81) | 7.11 (2.92) |
| Average | 11.87 (5.70) | 10.51 (6.41) | 8.62 (4.07) |
| CP pain tolerance(sec) | |||
| Trough | 42.80 (30.58) | 26.26 (18.64) | 18.69 (9.40) |
| Peak | 41.54 (33.00) | 18.69 (15.16) | 12.76 (4.41) |
| Average | 42.17 (30.46) | 22.48 (16.36) | 15.73 (6.33) |
| ES Pain threshold (volt) | |||
| Trough | 36.67 (10.46) | 41.58 (16.79) | 47.5 (18.17) |
| Peak | 38.00 (10.24) | 39.28 (16.82) | 44.6 (16.29) |
| Average | 37.48 (10.46) | 40.32 (13.90) | 46.8 (16.99) |
| ES pain tolerance(volt) | |||
| Trough | 62.81 (19.33) | 59.05 (20.00) | 60.1 (16.94) |
| Peak | 63.55 (20.16) | 54.29 (21.86) | 57.1 (18.28) |
| Average | 63.33 (19.90) | 56.52 (18.65) | 59.5 (17.01) |
The two treatment groups statistically differed by race
p < 0.05.
In comparison to the control group, the two medication groups were well-matched with respect to age, gender, and race. There was, however, a significant difference in racial composition between heroin users treated with BUP vs. METH (χ21, 3 = 10.78, p = 0.013), thus, race was included as a covariate in analyses comparing the two medication groups. Individuals assigned to the METH group had used opiates for significantly more years over lifetime (mean = 14.4, SD = 10.4) than those assigned to BUP (mean = 8.4, SD = 7.4), yet there was no difference between groups with respect to the number of days of opiate use in the last month (METH mean = 28 days, SD = 2; BUP mean = 30.0, SD = 0). Not unexpectedly in this population, retention decreased considerably over the course of the 18-week study, thus the evaluable sample size at the stabilization and chronic administration medication phases were notably less than those at baseline.
Cold Pressor (CP) Pain
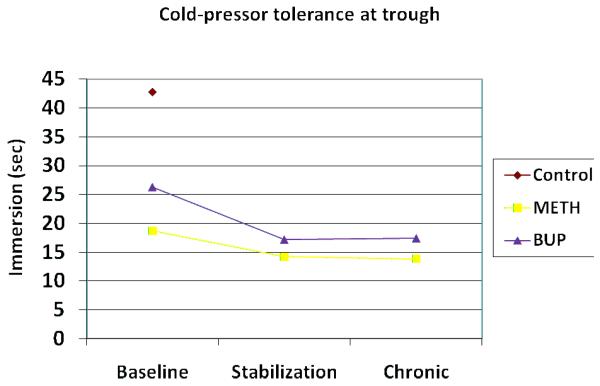
Across peak and trough time points, no group differences were found between baseline pain detection values, either between the heroin group and the control group, or between those randomized to the METH and BUP groups. Conversely, CP pain tolerance was significantly different at baseline between the heroin and control groups (t = 3.09; p=0.005), as well as between the METH and BUP groups (t = 2.67; p= 0.01) (see Fig 2 and 3).
Figure 2.
Cold-pressor pain tolerance (sec) at trough medication plasma levels
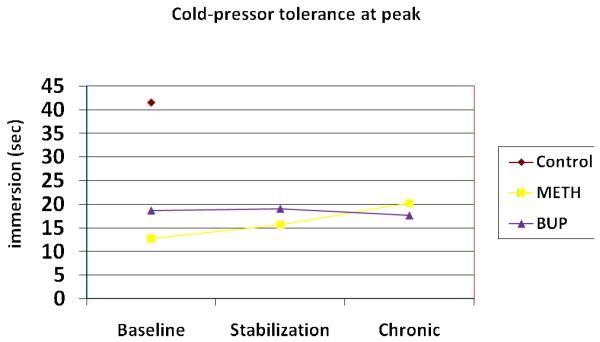
Figure 3.
Cold-pressor pain tolerance (sec) at peak medication plasma levels
Over the course of opioid substitution therapy, there were no changes in the detection of CP pain in either the METH or BUP groups. However, the METH and BUP groups each exhibited a significant decrease in CP pain tolerance between the baseline and chronic sessions (see Table 2 and Fig. 2), and were significantly different from one another (F = 6.35; p = 0.017) at 12-18 weeks. Collapsing across weeks of medication, baseline METH pain detection (mean = 8.08 sec) and baseline BUP pain detection (mean = 8.63 sec) were less than the control group (mean = 11.95 sec). Similar analyses of baseline pain tolerance, showed that the METH (mean = 14.75 sec) and BUP (mean = 18.57 sec) groups tolerated pain for significantly less time than did the control group (mean = 41.54 sec). No other statistically significant interactions or main effects were found.
Table 2.
Cold-pressor and Electrical Stimulation Pain responses of Methadone and Buprenorphine participants over Time
| Methadone | Buprenorphine | ||||||
|---|---|---|---|---|---|---|---|
|
Baseline (n=11) |
Stable (n=8) |
Chronic (n=5) |
Baseline (n=64) |
Stable (n=31) |
Chronic (n=25) |
||
|
CP detection (sec) |
trough | 10.12 (6.40) |
7.19 (2.13) |
9.26 (4.36) |
12.51 (9.08) |
8.18 (4.49) |
8.32 (4.55) |
| peak | 7.11 (2.92) |
7.61 (2.43) |
12.34 (3.86) |
8.50 (4.81) |
8.99 (4.82) |
8.55 (5.03) |
|
|
CP tolerance (sec) |
trough | 18.69 (9.40) |
14.29 (4.50) |
13.84 (5.25) |
26.26 (18.64) |
17.23 (8.81) |
17.45 (8.76) |
| peak | 12.76 (4.41) |
15.70 (5.47) |
20.44 (3.70) |
18.70 (15.16) |
19.05 (10.01) |
17.65 (9.46) |
|
|
ES detection (volt) |
trough | 47.50 (18.17) |
41.25 (7.70) |
39.60 (11.44) |
41.58 (16.79) |
38.39 (10.94) |
38.76 (13.04) |
| peak | 44.64 (16.29) |
46.00 (8.49) |
47.60 (9.53) |
39.28 (16.82) |
36.67 (11.84) |
39.83* (17.21) |
|
|
ES tolerance (volt) |
trough | 60.11 (16.94) |
54.25 (11.88) |
49.60 (13.74) |
59.05 (20.00) |
53.06 (19.27) |
53.64 (15.47) |
| peak | 57.09 (18.28) |
61.25 (12.22) |
64.80 (20.80) |
54.29 (21.86) |
53.53 (20.16) |
53.61 (17.63) |
|
Difference in ES threshold between BUP and METH (F=4.33; p=0.045)
In evaluation of CP pain responses at peak vs. trough putative plasma opioid levels, prior to dosing, significant differences in baseline tolerance were noted between the control and heroin-dependent groups (t = 2.62, p = 0.015) and between the METH and BUP groups (t = 2.35, p = 0.022). No group differences were evident on the pain detection measure at the same time point. At peak levels, group effects were more robust; significant differences were evident between the control and combined heroin groups on both CP pain detection (t=2.27 p=0.032) and tolerance (t = 3.28; p = 0.003) measures, and between the METH and BUP groups on pain tolerance as well (t = 2.75; p = 0.008). Changes within groups over time were not significant at peak or trough time points.
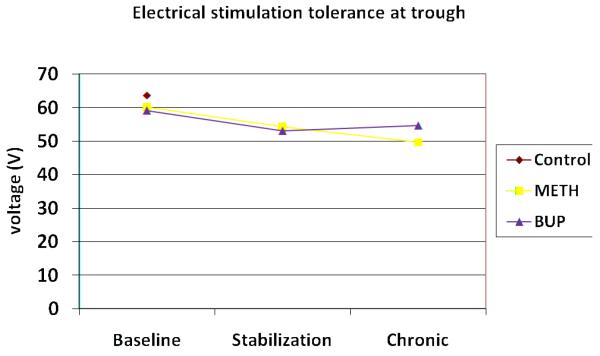
Electrical Stimulation (ES) Pain
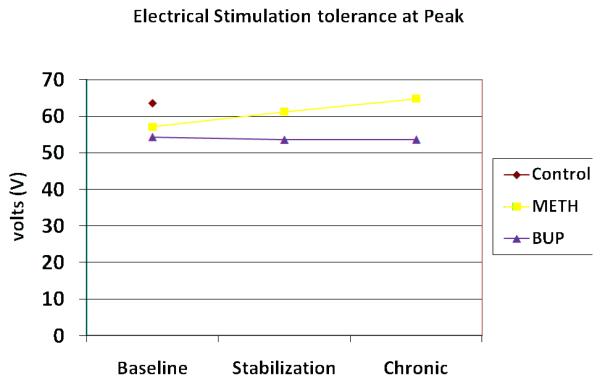
Mean baseline ES pain responses did not differ between the heroin and control groups on either detection or tolerance (see Fig 4 and 5), and at both peak and trough time points. The groups only differed on trough pain detection (t = −2.09; p = 0.041). At baseline trough levels, both ES detection and tolerance were negatively correlated with opioid withdrawal scores in the heroin group (detection r = −0.23 [p = 0.048]; tolerance r = −0.29 [p = 0.013]). Similarly, changes in ES pain responses over the course of the study were not noted in either the METH or BUP groups at peak vs. trough time points, however across time points, the groups differed from one another on ES detection at stabilization (F= 5.72; p = 0.022), and ES tolerance at the chronic session (F = 6.77, p = 0.015). Group differences for ES detection were also evident following dosing at the chronic time point (f = 4.33, p = 0.045).
Figure 4.
Electrical stimulation pain tolerance (volt) at trough medication plasma levels
Figure 5.
Electrical stimulation pain tolerance (volt) at peak medication plasma levels
Discussion
Managing pain in opioid-dependent individuals is challenging in that clinicians have little empirically derived information to guide their treatment approach. These data provide evidence that heroin-dependent individuals appear to present to treatment in a hyperalgesic state, and that maintenance therapy with either buprenorphine or methadone does not appreciatively change or worsen their pain responses.
Not surprisingly, significant differences between controls and heroin-dependent participants on the CP responses were found at baseline. Control participants tolerated the ice bath approximately twice as long as heroin-dependent participants, at both peak and trough methadone/buprenorphine levels. This finding is quite consistent across multiple studies3,11,14,15,18,33, providing further evidence that opioid-dependent participants are more sensitive to painful stimuli than are others. Although inconsistently reported in the literature14,15,19, these analyses also found increased sensitivity to CP pain threshold at putative peak methadone/buprenorphine levels, suggesting that on both CP pain responses, hyperalgesia was evident. Group differences on ES-induced pain were less apparent. Only at the trough time point were differences in pain perception noted, with heroin-dependent participants having lower voltage thresholds than control participants. In the case of ES pain, the severity of opiate withdrawal symptoms better predicted the threshold and tolerance responses of the heroin-dependent patients than did group assignment. Replicating the findings of investigators at the University of Adelaide3,14,18, hyperalgesia is not evidenced with the induction of ES pain.
That hyperalgesic responses in opioid-dependent patients vary with the type of pain stimulus used has been noted in recent reviews of OIHl17,21. Rather than disputing the presence of OIH in opiate-abusing patients, these findings suggest that CP pain may be a modality particularly sensitive to opioid-induced changes. Recent data from Ruscheweyh and colleagues35 show that variance in CP pain perception is more unique than perception of pain from other sources (heat, pinprick), supporting the position that responses sensitive to the CP procedure need not be reflected in other modalities. In fact, the genetic factors which underlie CP pain responses appear to be independent of those influencing phasic heat pain responses28. In a family-based investigation of CP pain tolerance, Birklein and colleagues4 showed that CP pain reponses were highly correlated within families, although not predicted by the opioid-relevant COMT (catechol-O-methyltransferase) gene polymorphism.
In addition to varying by modality, experimental pain responses in opiate-abusing participants appear to differ if they have undergone opiate-detoxification or are experiencing pain. For example, while Pud and colleagues33 report that CP hyperalgesia persists during detoxification, Liebmann and co-investigators23 showed that prolonged abstinence in ex-opioid addicts increases CP pain threshold in comparison to healthy controls. With his investigative team, Prosser32 reported similar findings (increased latency in detoxified participants vs normal controls) using quantitative sensory testing methodology to measure heat and pain thresholds. Recent work suggests that chronic pain (which presents in up to 55% of methadone-maintained patients29,31,34) has a similar effect on experimental pain thresholds and tolerance, such that having concurrent chronic pain increases thermal and mechanical threshold latencies30 and CP tolerance in methadone patients18. These findings suggest that the development of hyperalgesia is not only dependent on opiate use, but is also mediated by pain. The context-dependent nature of OIH may help account for mixed findings in the literature17,21.
Comparing the responses of participants assigned to METH or BUP for treatment, changes in voltage latency to the ES were minimal at both peak and trough time points for both medication groups. Across stabilization and chronic treatment, BUP participants tended to have significantly lower pain threshold and tolerance for ES pain than those treated with METH. Conversely, both prior to and following dosing, participants assigned to BUP were more tolerant of CP pain at baseline than those assigned to METH, an effect that persisted at trough plasma levels through chronic dosing. Although an apparent failure in random assignment, less hyperalgesic CP responses that occurred in the BUP-treated participants might be explained by a less severe opiate use history, as the subsample reported significantly fewer lifetime years of heroin use at baseline.
At peak time points, and similar to ES pain responses, no change in threshold or tolerance for CP pain was noted for either METH or BUP participants over time. However, at trough medication blood levels, CP pain tolerance (not threshold) decreased significantly for both groups as participants transitioned from heroin to METH/BUP maintenance. Similar to previous work14, hyperalgesic changes were most evident when the analgesic effects of the treatment opiates were likely at their lowest. Group differences in CP pain tolerance over time were not significant, although as demonstrated by Compton and colleagues12, patients maintained on the partial agonist BUP demonstrated slightly less hyperalgesia than those on METH.
Finding that OIH appeared to increase over time is in contrast to that hypothesized, as it was anticipated that participants transitioning from a short-acting (heroin) to a longer-acting (METH and BUP) opioid would be less subject to the large fluctuations in opiate blood levels associated with the development of OIH22. Also, stable dosing suggests that withdrawal hyperalgesia did not complicate responses. In that decreases for both groups occurred early in treatment (between baseline and stabilization) and not with continued METH/BUP drug exposure, it is likely that the changes are related to entering treatment as opposed to the treatment medication. The heroin-dependent participants arrived at treatment with minimal opiate withdrawal, thus it may be that heroin analgesic effects persisted at baseline (i.e., not a true “trough”). Not uncommonly, patients entering treatment report using larger than usual amounts of their drug-of-choice immediately prior to entry with the knowledge that they soon will be unable to do so. Better pain tolerance at baseline may reflect this dosing behavior.
Clearly, there are limitations to the interpretation of these data. Firstly, peak and trough blood levels of opiate were inferred from time of testing, but not established. Such inference is reasonable for patients who are receiving treatment with opiates at regular intervals, but less so for heroin-dependent patients participants, when the amount and time of last heroin use are based on self report. The degree to which their baseline pain responses reflect opiate activity is unknown, thus it will be important in future investigations to better characterize the opiate use of heroin-dependent participants. Since hyperalgesic changes were noted early in treatment (i.e., between baseline and stabilization), it will also be important to consider non-medication related effects of treatment that might affect pain responses. Stabilization in opiate treatment provides multiple health and social benefits24,25,37, yet improved pain tolerance does not appear to be one of them, which is a finding supported by the cross-sectional data as well3,11,14,15,18,33. Finally, although not uncommon in longitudinal studies of opiate addicts in treatment, participant attrition was notable, thus decreasing the power of statistical analyses over study time points.
The current findings indicate that, like methadone- and buprenorphine-maintained patients, heroin-dependent patients are significantly hyperalgesic to cold-pressor pain in comparisons to non-drug users, and once stabilized in treatment, there is no difference in the pain responses of those treated with buprenorphine versus those treated with methadone. A single, but reliable indicator in the literature (CP pain tolerance at trough plasma levels), suggests that transition to treatment with opiates worsens hyperalgesia, but the effects of non-pharmacologic treatment and previous heroin use cannot be ruled out. Based upon these data, pain clinicians are encouraged to consider the presence of OIH in the treatment plan of patients who use opiates (prescribed or illicit) secondary to an opiate addiction disorder.
Acknowledgments
The authors would like to acknowledge Ms. Geetha Doraimani for her outstanding assistance with statistical analysis of the data.
Funding for study expenses and subject payment was provided by NIDA grant DA13706.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
No conflicts of interest exist.
References
- 1.Alford DP, Compton P, Samet JH. Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Ann Intern Med. 2006;17:127–134. doi: 10.7326/0003-4819-144-2-200601170-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angst MS, Clark JD. Opioid-induced hyperalgesia; a qualitative systematic review. Anesthesiology. 2006;104:570–587. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- 3.Athanasos P, Smith CS, White JM, Somogyi AA, Bochner F, Ling W. Methadone maintenance patients are cross tolerant to the antinociceptive effects of very high plasma morphine concentrations. Pain. 2006;120:267–275. doi: 10.1016/j.pain.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. Journal of personality assessment. 1996;67:588–97. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 5.Birklein F, Depmeier C, Rolke R, Hansen C, Rautenstrauss B, Prawitt D, Magerl W. A family-based investigation of cold pain tolerance. Pain. 2008;138:111–8. doi: 10.1016/j.pain.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Célèrier E, Rivat C, Jun Y, Laulin JP, Larcher A, Reynier P, Simonnet G. Long-lasting hyperalgesia induced by fentanyl in rats: preventive effect of ketamine. Anesthesiology. 2000;92:465–72. doi: 10.1097/00000542-200002000-00029. [DOI] [PubMed] [Google Scholar]
- 7.Chang G, Chen L, Mao J. Opioid tolerance and hyperalgesia. Med Clin North Am. 2007;91:199–211. doi: 10.1016/j.mcna.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Chu LF, Angst MS, Clark D. Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clin J Pain. 2008;24:79–96. doi: 10.1097/AJP.0b013e31816b2f43. [DOI] [PubMed] [Google Scholar]
- 9.Colpaert FC. Mechanisms of opioid-induced pain and antinociceptive tolerance: signal transduction. Pain. 2002;95:287–8. doi: 10.1016/S0304-3959(01)00445-6. [DOI] [PubMed] [Google Scholar]
- 10.Compton MA. Cold-pressor pain tolerance in opiate and cocaine abusers: correlates of drug type and use status. J Pain Symptom manage. 1994;9:462–473. doi: 10.1016/0885-3924(94)90203-8. [DOI] [PubMed] [Google Scholar]
- 11.Compton P, Charuvastra VC, Kintaudi K, Ling W. Pain Responses in Methadone-Maintained Opioid Abusers. J. Pain Symptom Manage. 2000;20:237–245. doi: 10.1016/s0885-3924(00)00191-3. [DOI] [PubMed] [Google Scholar]
- 12.Compton P, Charuvastra VC, Ling W. Pain Intolerance in Opioid-Maintained Former Opiate Addicts: Effect of Long-Acting Maintenance Agent. Drug Alcohol Depend. 2001;63:139–146. doi: 10.1016/s0376-8716(00)00200-3. [DOI] [PubMed] [Google Scholar]
- 13.Dinnerstein AJ, Blitz B, Lowenthal M. Effects of aspirin on detection and tolerance of electric shock. J Appl Physiol. 1965;20:1052–5. doi: 10.1152/jappl.1965.20.5.1052. [DOI] [PubMed] [Google Scholar]
- 14.Doverty M, White JM, Somogyi AA, Bochner F, Ali R, Ling W. Hyperalgesic responses in methadone maintenance patients. Pain. 2001a;90:91–6. doi: 10.1016/s0304-3959(00)00391-2. [DOI] [PubMed] [Google Scholar]
- 15.Doverty M, Somogyi AA, White JM, Bochner F, Beare CH, Menelaou A, Ling W. Methadone maintenance patients are cross-tolerant to the antinociceptive effects of morphine. Pain. 2001b;93:155–63. doi: 10.1016/S0304-3959(01)00306-2. [DOI] [PubMed] [Google Scholar]
- 16.Eckhardt K, Li S, Ammon S, Schanzle G, Mikus G, Eichelbaum M. Same incidence of adverse drug events after codeine administration irrespective of the genetically determined differences in morphine formation. Pain. 1998;76:27–33. doi: 10.1016/s0304-3959(98)00021-9. [DOI] [PubMed] [Google Scholar]
- 17.Fishbain DA, Cole B, Lewis JE, Gao J, Rosomoff RS. Do opioids induce hyperalgesia in humans? An evidence-based structured review. Pain Med. 2009;10:829–39. doi: 10.1111/j.1526-4637.2009.00653.x. [DOI] [PubMed] [Google Scholar]
- 18.Hay JL, White JM, Bochner F, Somogyi AA, Semple TJ. Rounsefell: Hyperalgesia in Opioid-Managed Chronic Pain and Opioid-Dependent Patients. J Pain. 2009;10:316–322. doi: 10.1016/j.jpain.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Ho A, Dole VP. Pain perception in drug-free and in methadone-maintained human ex-addicts. Proc Soc Exp Biol Med. 1979;162:392–395. doi: 10.3181/00379727-162-40689. [DOI] [PubMed] [Google Scholar]
- 20.Koppert W, Schmelz M. The impact of opioid-induced hyperalgesia for postoperative pain. Best Pract Res Clin Anaesthesiol. 2007;21:65–83. doi: 10.1016/j.bpa.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 2011;14:145–61. [PubMed] [Google Scholar]
- 22.Li X, Angst MS, Clark JD. A murine model of opioid-induced hyperalgesia. Brain Res Mol Brain Res. 2001;86:56–62. doi: 10.1016/s0169-328x(00)00260-6. [DOI] [PubMed] [Google Scholar]
- 23.Liebmann PM, Lehofer M, Moser M, Hoehn-Saric R, Legl T, Pernhaupt G, Schauenstein K. Persistent analgesia in former opiate addicts is resistant to blockade of endogenous opioids. Biol Psychiatry. 1997;42:962–4. doi: 10.1016/S0006-3223(97)00337-5. [DOI] [PubMed] [Google Scholar]
- 24.Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;8:CD002209. doi: 10.1002/14651858.CD002209. [DOI] [PubMed] [Google Scholar]
- 25.Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2008;16:CD002207. doi: 10.1002/14651858.CD002207.pub3. [DOI] [PubMed] [Google Scholar]
- 26.McLellan AT, Luborsky L, O’Brien CP, Woody GE. An improved diagnostic instrument for substance abuse patients: The Addiction Severity Index. J Nervous Mental Dis. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Newshan G. Pain management in the addicted patient: Practical considerations. Nurs Outlook. 2000;48:81–85. doi: 10.1016/s0029-6554(00)90007-1. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen CS, Staud R, Price DDJ. Individual differences in pain sensitivity: measurement, causation, and consequences. Pain. 2009;10:231–7. doi: 10.1016/j.jpain.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Peles E, Schreiber S, Gordon J, Adelson M. Significantly higher methadone dose for methadone maintenance treatment (MMT) patients with chronic pain. Pain. 2005;113:340–6. doi: 10.1016/j.pain.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 30.Peles E, Schreiber S, Hetzroni T, Adelson M, Defrin R. The differential effect of methadone dose and of chronic pain on pain perception of former heroin addicts receiving methadone maintenance treatment. J Pain. 2011 Jan;12(1):41–50. doi: 10.1016/j.jpain.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 31.Potter JS, Prather K, Weiss RD. Physical pain and associated clinical characteristics in treatment-seeking patients in four substance use disorder treatment modalities. Am J Addict. 2008;17:121–5. doi: 10.1080/10550490701862902. [DOI] [PubMed] [Google Scholar]
- 32.Prosser JM, Steinfeld M, Cohen LJ, Derbyshire S, Eisenberg DP, Cruciani RA, Galynker II. Abnormal heat and pain perception in remitted heroin dependence months after detoxification from methadone-maintenance. Drug Alcohol Depend. 2008;95:237–44. doi: 10.1016/j.drugalcdep.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Pud D, Cohen D, Lawental E, Eisenberg E. Opioids and abnormal pain perception: New evidence from a study of chronic opioid addicts and healthy subjects. Drug Alcohol Depend. 2006;82:218–223. doi: 10.1016/j.drugalcdep.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Rosenblum A, Joseph H, Fong C, Kipnis S, Cleland C, Portenoy RK. Prevalence and characteristics of chronic pain among chemically dependent patients in methadone maintenance and residential treatment facilities. JAMA. 2003;289:2370–8. doi: 10.1001/jama.289.18.2370. [DOI] [PubMed] [Google Scholar]
- 35.Ruscheweyh R, Stumpenhorst F, Knecht S, Marziniak M. Comparison of the cold pressor test and contact thermode-delivered cold stimuli for the assessment of cold pain sensitivity. J Pain. 2010;11:728–36. doi: 10.1016/j.jpain.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 36.Scimeca MM, Savage SR, Portenoy R, Lowinson J. Treatment of pain in methadone-maintained patients. Mt Sinai J Med. 2000;67:412–422. [PubMed] [Google Scholar]
- 37.Soyka M, Kranzler HR, van den Brink W, Krystal J, Möller HJ, Kasper S, WFSBP Task Force on Treatment Guidelines for Substance Use Disorders: The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of substance use and related disorders. Part 2: Opioid dependence. World J Biol Psychiatry. 2011;12:160–87. doi: 10.3109/15622975.2011.561872. [DOI] [PubMed] [Google Scholar]
- 38.Spielberger CD, Gorssuch RL, Lushene PR, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press, Inc.; 1983. [Google Scholar]
- 39.Tursky B, Watson PD, O’Connell DN. Concentric shock electrode for pain stimulation. Psychophysiology. 1965;1:296–8. doi: 10.1111/j.1469-8986.1965.tb03249.x. [DOI] [PubMed] [Google Scholar]
- 40.Wolff BB, Kantor TG, Jarvik ME, Laska E. Response of experimental pain to analgesic drugs. II. Codeine and placebo. Clin Pharmacol Ther. 1966;7:323–31. doi: 10.1002/cpt196673323. [DOI] [PubMed] [Google Scholar]