Abstract
Annatto has been identified as carotenoids that have antioxidative effects. It is well known that one of the key elements in the development of diabetic complications is oxidative stress. The immune system is especially vulnerable to oxidative damage because many immune cells, such as neutrophils, produce reactive oxygen species and reactive nitrogen species as part of the body’s defense mechanisms to destroy invading pathogens. Reactive oxygen species/reactive nitrogen species are excessively produced by active peripheral neutrophils, and may damage essential cellular components, which in turn can cause vascular complications in diabetes. The present study was undertaken to evaluate the possible protective effects of annatto on the reactive oxygen species and nitric oxide (NO) inhibition in neutrophils from alloxan-induced diabetic rats. Adult female rats were divided into six groups based on receiving either a standard diet with or without supplementation of annatto extract or beta carotene. All animals were sacrificed 30 days after treatment and the neutrophils were isolated using two gradients of different densities. The reactive oxygen species and NO were quantified by a chemiluminescence and spectrophotometric assays, respectively. Our results show that neutrophils from diabetic animals produce significantly more reactive oxygen species and NO than their respective controls and that supplementation with beta carotene and annatto is able to modulate the production of these species. Annatto extract may have therapeutic potential for modulation of the balance reactive oxygen species/NO induced by diabetes.
Keywords: diabetes, annatto, neutrophils, reactive oxygen species, nitric oxide
Introduction
Oxidative stress is defined as an imbalance in prooxidants and antioxidants, which results in macromolecular damage and disruption of redox signaling and control pathways.(1) There is a growing amount of evidence that, in diabetes, the excess generation of highly reactive free radicals, which is largely due to hyperglycemia, causes oxidative stress, further accelerating the development and progression of the disease and its complications.(2,3)
The immune system is especially vulnerable to oxidative damage because many immune cells, such as neutrophils, produce reactive oxygen species (ROS) and reactive nitrogen species (RNS) as part of the body’s defense mechanisms to destroy invading pathogens.(4) It is well known that the function of neutrophils is altered in diabetes; one of the major functional changes in neutrophils in diabetes is the increased generation of extracellular superoxide and ROS. The chronic hyperglycemia resulting from poorly controlled diabetes can prime neutrophils and monocytes, resulting in an exaggerated inflammatory response and tissue damage.(5,6) Neutrophils are also capable of the production and release of nitric oxide (NO).(7,8) The functional role of NO depends on both its concentration and its association with other biological molecules and proteins. NO and ROS can act separately or combine to form the highly toxic peroxynitrite.(9) Peroxynitrite is a potentially toxic species that has a different set of targets.(10) Antioxidant systems prevent the uncontrolled formation of free radicals and reactive oxygen species or inhibit their reactions with biological structures.(11) The efficiency of this defense mechanism is altered in diabetes.(12) The protective effects of exogenously administered antioxidants have been extensively studied in various animal models of diabetes in recent years, and the beneficial effects of numerous antioxidants, mainly from plant sources, have been demonstrated.(13)
The carotenoids constitute a family of pigmented compounds that are synthesized by plants and microorganisms;(14) they are responsible for scavenging free radicals and therefore act as antioxidants under conditions of reduced oxygen in vitro and in vivo. A wealth of scientific evidence links the antioxidant properties of carotenoids with their beneficial effects on chronic diseases including diabetes.(15) In this context, Bixa orellana, a shrub native to tropical America, is a rich source of orange-red pigments that have been widely used by the food color industry. These pigments are commercially known as annatto (E160b), and their main colored component is bixin (C25H30O4).(16) After ingestion of annatto, bixin levels reach high concentrations in human plasma and are completely cleared in 8 h.(17) Thus, the bixin present in processed foods may be an important nutritional factor that can promote human health. Some of the components of annatto have been identified as carotenoids that have antioxidative and anticarcinogenic effects.(18,19) However, the effects of annatto on oxidative stress in diabetic models have not been completely investigated. Therefore, the objective of this study was to assess the production of ROS and NO in neutrophils from diabetic rats treated with β-carotene (reference compound) and an annatto extract. The results of this work provide new perspectives on the use of annatto as a possible modulator in the production these species in neutrophils of diabetic rats.
Materials and Methods
Chemical reagents
The chemical reagents, including alloxan (2,4,5,6-tetraoxypyrimidine; 5,6-dioxyuracil), luminol (5-amino-2,3-dihydro-1,4-phthalazinedione), diphenylene iodonium (DPI), Zymosan (ZC3b) and β-carotene (synthetic trans-β-carotene type I – C9750) were purchased from Sigma-Aldrich (St. Louis, MO). Leukopaque and Monopaque gradients were obtained from Bion LTDA, Brazil.
Animals
The Laboratory of Experimental Nutrition from the Federal University of Ouro Preto (UFOP) provided the female albino Fisher rats used in the experiment; the animals were approximately 13 weeks old and weighed about 192 g. All animals were kept in collective cages (four per cage) placed in an environment with the temperature maintained at 22–28°C and a photoperiod of 12 h; they received either a modified standard rat diet (AIN-93M) Reeves et al.,(20) or a modified standard rat diet with supplementation and water ad libitum. This work was carried out in accordance with the international standards of animal protection and with the ethical principles of the Brazilian College of Animal Experimentation(21) and was approved by the Ethics Committee on Animal Use (CEUA) of UFOP (OF 01/2009 and OF 011/2009).
Diabetes induction
To induce diabetes, we treated rats intraperitoneally with 150 mg/kg alloxan (ALX) dissolved in 0.2 mL NaCl (0.01 M, pH 4.5). The control animals received an intraperitoneal injection of NaCl. Three days after administration of ALX, blood samples were collected to confirm the development of diabetes. Animals with glucose levels above 400 mg/dL (22.24 mmol/L) were considered diabetic. Biochemical analyses of plasma glucose concentrations and glycosylated hemoglobin content were performed using commercially available spectrophotometric assays (Labtest, Minas Gerais, Brazil and Katal, Minas Gerais, Brazil, respectively).
Diets and experimental design
Forty-eight rats were distributed into six groups according to the treatment they received. The control (C) and diabetic (D) groups were fed the modified standard diet AIN-93M, the control + annatto extract (CAn) and diabetic + annatto extract (DAn) groups received the standard diet including 0.09% annatto extract, and the control + β-carotene (Cβcar) and diabetic + β-carotene (Dβcar) groups received the standard diet including 0.1% β-carotene. The length of treatment was 30 days. In this experiment, we used a macerated, mechanically extracted form of annatto seed (known commercially as bixin pie) that was kindly provided by the company Corantec Dye Natural Ltda, Sao Paulo, Brazil. This mash was termed annatto extract (An). The concentration of bixin in the annatto extract was 2.17 gramas/100 g. The composition of experimental diets was presented on Table 1.
Table 1.
Compositions of experimental diets (gramas/Kg)
| Composition | Standard diet modified AIN-93M | Standard diet with supplementation of β-carotene | Standard diet with supplementation of annatto extract |
|---|---|---|---|
| Choline | 2.5 | 2.5 | 2.5 |
| Vitamin mixture1 | 10.0 | 10.0 | 10.0 |
| Mineral mixture2 | 35.0 | 35.0 | 35.0 |
| Cellulose | 50.0 | 50.0 | 50.0 |
| Casein | 140.0 | 140.0 | 140.0 |
| Soybean oil | 40.0 | 40.0 | 40.0 |
| Cornstarch | 722.5 | 721.5 | 721.6 |
| β-carotene | — | 1.0 | — |
| Annatto extract | — | — | 0.9 |
| Energy content (kcal/kg)3 | 3810 | 3806 | 3806.4 |
1Vitamin and 2Mineral mixture followed the recommendation of the Association of Official Analytical Chemists.(22) 3Conversion factors: protein: 4 kcal/g, fat: 9 kcal/g, carbohydrate: 4 kcal/g.
Isolation of polymorphonuclear leukocytes
Blood was obtained by bleeding of the brachial plexus and was collected in heparinized tubes. The neutrophils were then isolated using two gradients of different densities, Monopaque (d = 1.08) and Leukopaque (d = 1.12), according to the procedure of Bicalho et al.(23) with minor modifications. The cell viability of each sample was determined by trypan blue exclusion and was always greater than 95%.
In vitro test
In this experimental procedure, 1 × 106 neutrophils in 1 mL of PBS were incubated with 500 µL of luminol (10−4 M) for 10 min in siliconized glass tubes. The photons emitted were recorded at every minute for 1 min using the internal printer of the luminometer. After running the reaction for the initial 10 min, 100 µL of extract annatto (0.1 µg/100 µL) or β-carotene (0.1 µg/100 µL) was added. For all tests, the final volume was adjusted to 700 µL with PBS (pH 7.35). The chemiluminescence was recorded for 30 min.
In vivo tests
Quantification of ROS
To evaluate the generation of ROS, we performed a chemiluminescence assay based on the amplification of luminol during the activation of phagocytic cells as described by Chaves et al.(24) Luminol reacts with ROS generated by neutrophils to produce an excited aminophtalate anion that emits light when returning to ground state.(25) It is well established that luminol measures O2•− (26) although to detect ROS in general.(27)
Briefly, neutrophils (1 × 106 cells in Hank’s solution, pH 7.35) were incubated with 500 µL of 10−4 M luminol for 30 min in siliconized tubes. The photons emitted during this period were recorded every minute using an internal luminometer printer. Values were expressed as RLU/30 min.
Quantification of nitric oxide
The quantification of nitric oxide was performed by nitrite determination in granulocytes (1 × 106/mL) using commercially available spectrophotometric assays (BioAssay systems, CA).
In ex-vivo test
In this experimental procedure, 1 × 106 neutrophils in 1 mL of PBS were incubated with 500 µL of luminol (10−4 M) for 10 min in siliconized glass tubes. A luminol [Sigma Co.] stock solution was made by dissolving 1.77 mg of luminol in 1.0 mL of dimethyl sulfoxide (DMSO) to give a final concentration of 10−2 M. Before use, this solution was further diluted to 10−4 M in PBS (pH 7.4). The photons emitted were recorded at every minute for 10 min using the internal printer of the luminometer. After running the reaction for the initial 10 min, 100 µL of DPI (10−4 M) or 50 µL of opsonized zymosan particles (13 mg/mL) was added. For all tests, the final volume was adjusted to 700 µL with PBS (pH 7.35). The chemiluminescence measurements were performed in a luminometer (Lumat, LB 9507, Berthold, Germany). The chemiluminescence was recorded for 30 min, which allowed observation of the peak. The results were expressed as relative light units/30 min (RLU/30 min).
Opsonization of zymosan particles
Zymosan derived from the cell wall of Saccharomyces cerevisiae is rich in both b-glucan and mannan.(28) It has been widely used as a model fungal particle to study immune responses conducted by different innate and adaptive immunity cells.
Zymosan was opsonized by adding 900 µL of PBS (pH 7.4) to 100 µL of zymosan (13 mg/mL) as described in the study by Nogueira Machado et al.(29) The solution was then centrifuged for 2 min at 200 × g. The supernatant was discarded and the pellet was resuspended in 200 µL of fresh autologous serum and 800 µL of Hank’s (pH 7.35). This solution was incubated for 30 min in a 37°C water bath and shaken at 10 min intervals. Subsequently, the solution was centrifuged again for 2 min at 200 × g; the supernatant was discarded, and the pellet was resuspended in 500 µL of PBS. The volume of opsonized zymosan with serum (ZC3b) used for the experiments was 50 µL.
Statistical analysis
The data were expressed as means ± SD. All data were analyzed by D’Agostino & Pearson omnibus normality test. Data (Table 2) with normal distribution were analyzed by one-way analysis of variance (ANOVA), and differences were considered statistically significant for p<0.05. Tukey’s post-hoc test was used to determine the differences among the groups. For the remaining analysis we chose to use Student’s unpaired t test. Tests were performed with GraphPad Prism version 4.00 for Windows (San Diego, CA).
Table 2.
Evaluation of body weights (initial and final), plasma glucose and glycosylated hemoglobin levels in non-diabetic and diabetic rats that were left untreated or treated with annatto extract and β-carotene
| Initial body weight (g) | Final body weight (g) | Glucose (mmol/L) | Glycosylated hemoglobin (%) | |
|---|---|---|---|---|
| C | 191.0 ± 15.1 | 245.7 ± 14.6 (a) | 6.30 ± 0.14 (c) | 3.01 ± 0.51 (e) |
| CAn | 193.0 ± 14.7 | 237.1 ± 24.3 (a) | 8.09 ± 0.45 (c) | 2.81 ± 0.27 (e) |
| Cβcar | 190.2 ± 12.9 | 241.5 ± 18.5 (a) | 6.41 ± 0.31 (c) | 3.14 ± 0.59 (e) |
| D | 191.2 ± 3.5 | 151.9 ± 16.8 (b) | 25.68 ± 1.89 (d) | 6.19 ± 0.64 (f) |
| DAn | 192.5 ± 10.6 | 159.7 ± 21.9 (b) | 25.32 ± 1.66 (d) | 5.71 ± 0.47 (f) |
| Dβcar | 191.4 ± 9.1 | 164.5 ± 15.3 (b) | 24.77 ± 1.90 (d) | 5.67 ± 0.63 (f) |
The data are presented as the mean ± SD (n = 8). Different superscript letters in the same column indicate statistically significant differences between groups (p≤0.05) as determined by one-way ANOVA and Tukey test. C: Control, CAn: Control + 0.09% annatto extract treatment, Cβcar: Control + 0.1% β-carotene treatment, D: Diabetic, DAn: Diabetic + 0.09% annatto extract treatment, Dβcar: Diabetic + 0.1% β-carotene treatment.
Results
Evaluation of body weight, plasma glucose and glycosylated hemoglobin
Table 2 shows that there was no significant difference in initial weight among the groups. However, we observed a significant reduction in final weight in the animals in the diabetic groups (D, DAn and Dβcar) compared to the control groups (C, CAn and Cβcar). The animals in the diabetic groups also showed increased final blood glucose levels and glycosylation of hemoglobin when compared to the animals in the control groups. Treatment with annatto extract or β-carotene failed to improve the glycemic profiles of these animals.
Evaluation of ROS production in neutrophils
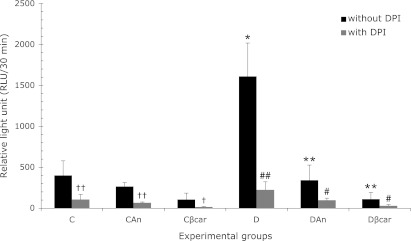
In vitro, the results presented in Table 3 show that isolated neutrophils incubated with annatto extract and β-carotene did not alter ROS production compared to basal metabolism of these cells. In vivo, the results presented in Fig. 1 show that neutrophils from diabetic animals produce significantly more ROS than their respective controls. Our results demonstrated that treatment with annatto extract and β-carotene significantly decreased ROS production in diabetic animals in 79% and 93%, respectively, indicating that annatto extract and β-carotene were effective at modulating ROS generation. To determine the role of NADPH oxidase in this process, we evaluated ROS production in neutrophils incubated with DPI, an inhibitor of NADPH oxidase. The results show that the DPI was able to significantly inhibit ROS production in all groups.
Table 3.
Evaluation of production of reactive oxygen species (ROS) in neutrophils incubated with annatto extract and β-carotene
| Assay condition | Reactive oxygen species (RLU/min) |
|---|---|
| (1) Neutrophil + PBS | 239.8 ± 40.3 N.S. |
| (2) Neutrophil + Annatto | 227.8 ± 36.5 N.S. |
| (3) Neutrophil + β-carotene | 207.3 ± 32.8 N.S. |
The data are presented as the mean ± SD (n = 5). (1, 2) and (1, 3) indicate not significant differences (p≥0.05) as determined by Student’s unpaired t test.
Fig. 1.
Annatto extract and β-carotene treatment decreased reactive oxygen species (ROS) production in neutrophils from non-diabetic and diabetic rats. The data are presented as the means ± SD. C: Control (n = 8), CAn: Control + 0.09% annatto extract treatment (n = 7), Cβcar: Control + 0.1% β-carotene treatment (n = 8), D: Diabetic (n = 6), DAn: Diabetic + 0.09% annatto extract treatment (n = 10), Dβcar: Diabetic + 0.1% β-carotene treatment (n = 9). *p<0.0001 compared to C, **p<0.0001 compared to D, †† p<0.01 compared to ROS in C and CAn, † p<0.05 compared to ROS in Cβcar, ##p = 0.0008 compared to ROS D, #p<0.03 compared to ROS in DAn and Dβcar.
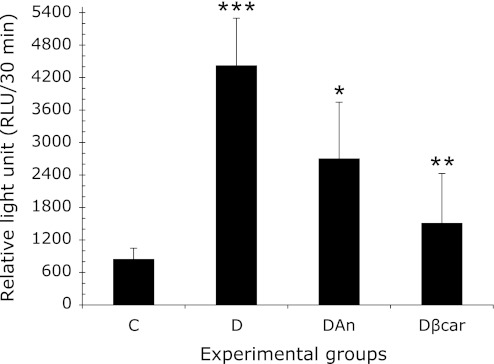
The results presented in Fig. 2 show that neutrophils from diabetic animals incubated with ZC3b particles produce significantly more ROS (4426.7 ± 872.2) than their respective controls (847.0 ± 201.5). However, when neutrophils from diabetic rats treated with annatto extract (2706.2 ± 978.9) and β-carotene (1513.5 ± 914.5) were incubated with ZC3b particles, there was a significant reduction in ROS release compared to untreated cells.
Fig. 2.
Annatto extract and β-carotene decreased reactive oxygen species (ROS) production in neutrophils from diabetic rats after incubation with ZC3b particles. The data are presented as the means ± SD. C: Control (n = 8), D: Diabetic (n = 6), DAn: Diabetic + 0.09% annatto extract treatment (n = 10), Dβcar: Diabetic + 0.1% β-carotene treatment (n = 9). ***p<0.0005 compared to C, *p<0.05 compared to D; **p<0.001 compared to D.
Evaluation of NO production in neutrophils
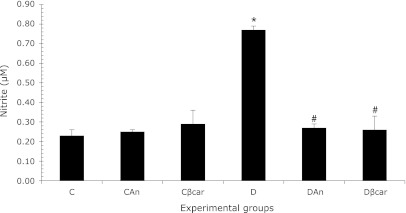
The results in Fig. 3 show that neutrophils from diabetic animals produce significantly more NO (0.77 ± 0.02) than their respective controls (0.23 ± 0.03). However, results demonstrated that treatment with annatto extract (0.27 ± 0.02) and β-carotene (0.26 ± 0.07) significantly decreased NO production in treated versus untreated diabetic animals, indicating that annatto extract and β-carotene were effective at modulating nitric oxide generation. The treatment did not alter nitric oxide production in neutrophils from control rats.
Fig. 3.
Annatto extract and β-carotene decreased nitric oxide (NO) production in neutrophils from diabetic rats. The data are presented as the means ± SD. C: Control (n = 4), CAn: Control + 0.09% annatto extract treatment (n = 4), Cβcar: Control + 0.1% β-carotene treatment (n = 6), D: Diabetic (n = 4), DAn: Diabetic + 0.09% annatto extract treatment (n = 6), Dβcar: Diabetic + 0.1% β-carotene treatment (n = 6). *p<0.0001 compared to C, #p<0.0001 as compared to D.
Evaluation of balance ROS/NO
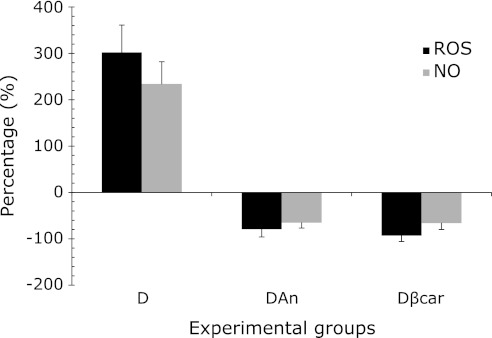
We conducted a joint assessment of ROS and NO; for this experiment, we analyzed the percentage of activation or inhibition of both of these species in neutrophils from treated or untreated diabetic rats. The results presented in Fig. 4 show that the increased production of ROS in neutrophils from diabetic rats is accompanied by increased NO production. Treatment with β-carotene and annatto extract is capable of reducing both species. These results together suggest a balance between ROS and NO.
Fig. 4.
Evaluation of ROS/NO balance in neutrophils from treated or untreated diabetic rats. The data are presented as the means ± SD. D: Diabetic (ROS/NO), DAn: Diabetic + 0.09% annatto extract treatment (ROS/NO), Dβcar: Diabetic + 0.1% β-carotene treatment (ROS/NO).
Discussion
The annatto dye is prepared by stirring annatto seeds in water and used to color butter and cheese; it is also widely used in Latin America to color rice and other foods.(30) Few studies have examined the hypoglycemic effects of annatto, and in most these studies the experimental model used was the dog.(31) Fernandes et al.,(32) show that the continuous ingestion of annatto extract or norbixin caused hyperglycemia in rats. In our experimental model, annatto was unable to alter the body weights, plasma glucose levels or glycosylated hemoglobin levels (Table 2) of diabetic animals. It is well known that one of the key elements in the development of diabetic complications is oxidative stress.(33) Previous studies in vascular endothelial cells and phagocytic leukocytes have linked the generation of superoxide and other ROS and the ensuing enhanced oxidative stress with hyperglycemia.(34,35) Although multiple sources of ROS may be involved in diabetes, a major source of ROS that contributes to inflammatory lesions is the neutrophil NADPH oxidase.(36) Activated polymorphonuclear neutrophils produce and release a variety of ROS such as the superoxide anion (O2•−) and hydrogen peroxide (H2O2). It is assumed that a plasma membrane-bound NADPH oxidase complex catalyzes a one-electron reduction of oxygen to O2•−, which is then converted into H2O2 spontaneously or via the action of superoxide dismutase. The NADPH oxidase present in neutrophils is a multi-protein complex that comprises the following: 1) a membrane-bound flavocytochrome b composed of two subunits (p91phox and p22phox); 2) the cytosolic proteins p47phox, p67phox, and p40phox; and 3) the small G proteins Rac 2 and Rap A1.(37) Upon neutrophil activation some of the cytosolic proteins, mainly p47 phox, are phosphorylated, allowing the translocation of the complex composed of all the cytosolic proteins to the membrane, where they interact with flavocytochrome b.(38,39) Fig. 1 shows a significant increase in ROS production in neutrophils from diabetic rats; these results are consistent with work by Ayilavarapu et al.,(40) who demonstrated that neutrophils from poorly controlled diabetic subjects generate significantly higher amounts of superoxide compared with healthy controls.(5,41) However, our results showed, for the first time, that treatment with the annatto extract or with β-carotene was effective at reducing ROS production in the neutrophils of diabetic rats. To test our hypothesis that the NADPH oxidase complex is involved in the induction of ROS in neutrophils from diabetic rats, we conducted an experiment with DPI, an inhibitor of NADPH oxidase. Our results demonstrate that DPI was able to significantly inhibit ROS production in neutrophils from both control and diabetic rats; however, this inhibition was more pronounced in the rats with the disease. These data demonstrate the participation of the NADPH oxidase complex in the exacerbation of ROS production-induced diabetes.
Neutrophils are the first effector cells recruited to the site of infection; there, they internalize, kill and digest bacteria and fungi. Binding of ligands to receptors such as complement receptor 3 (CR3) and Fcg on neutrophils leads to phagocytosis by a process that involves actin polymerization and insertion of new membrane. After phagosome formation, effector mechanisms are activated to kill and digest particles; these mechanisms include the production of reactive oxygen species (ROS) and oxidized halides and the release of granule enzymes.(10) The incubation of neutrophils from diabetic rats with opsonized particles of zymosan induced a significant increase in the production of ROS. The ZC3b particles bind both the FcR and CR receptors. This activation leads to the generation of various ROS by these cells. However, pre-treatment with annatto was able to reduce ROS production in neutrophils from diabetic rats incubated with zymosan. These results lead us to conclude that the likely mechanism of action of the annatto extract is a regulation of gene expression or activity altered NADPH oxidase. Neutrophils isolated from rats treated with the annatto extract and incubated with zymozan were less responsive in ROS production, probably because NADPH oxidase was partially inhibited.
Moreover, the results (in vitro) presented in Table 3 leads us to refute the hypothesis that the annatto extract exerts a direct scavenger of reactive species produced by neutrophils, since neutrophils incubated with the annatto extract and beta carotene was not able to reduce ROS production when compared to basal metabolism these cells. Some literature data show that carotenoids may decreases O2•− production stimulated by NADPH oxidase.(42)
Activated polymorphonuclear leukocytes (PMNs) produce not only ROS but also NO,(43,44) which subsequently reacts with a O2•− to yield peroxynitrite anions (ONOO−).(45) It has been observed that NO-derived peroxynitrite is an important mediator of free radical-dependent toxicity because of its strong oxidizing effects.(46) All of these radicals and their reactive derivatives are also released into extracellular spaces, where they indiscriminately break down biological macromolecules and, thus, exacerbate the injury to surrounding tissue.(47) Our results showed an increase in NO production induced by diabetes; however, in neutrophils from rats treated with β-carotene or annatto, there is a decrease in NO production. In the experiment depicted in Fig. 4, we performed a comparative analysis of the balance between ROS and NO and found that in neutrophils from diabetic rats the increase in ROS production is accompanied by increased production of NO. In these cells, treatment with β-carotene or annatto was able to simultaneously reduce ROS and NO production. The increased production of ROS and RNS by activated neutrophils and the concomitant decrease in antioxidant defensive capacity give rise to an oxidant/antioxidant imbalance that leads to oxidative stress.(47) Neutrophils are specialized for the release of ROS, nitric oxide (NO) and proteolytic enzymes. Both the superoxide anion radical and nitric oxide generate secondary reactive oxygen species (ROS) and reactive nitrogen species (RNS). Physiologically, ROS/RNS formation performs an essential microbicidal function. Although the formation of reactive species is desirable for the host’s defense, overproduction of these species can damage the body’s own cells, cause tissue injury, and contribute to the development of a number of serious diseases. Thus, the modulation of their production is important for treatment of immune and inflammatory diseases.(45,48–50) The antioxidant properties of annatto extract were also confirmed by further analysis the activity of superoxide dismutase (SOD), which indicated that animals treated with annatto extract (80.81 ± 1.92 inhibition rate, %) showed an increase in SOD activity than animals diabetics (71.07 ± 1.75 inhibition rate, %) (results not shown). Based on these observations, annatto extract may have therapeutic potential for modulation of ROS/NO in alloxan-induced diabetic rats.
Acknowledgments
This research was supported by the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) – Processo APQ-02832-09, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Universidade Federal de Ouro Preto (UFOP), Brazil. We are grateful to Dr. José Augusto Nogueira-Machado for the density gradients (Leucopaque and Monopaque).
Abbreviations
- ALX
alloxan
- An
annatto extract
- C
control group
- CAn
control + annatto extract group
- Cβcar
control + β-carotene group
- D
diabetic group
- DAn
diabetic + annatto extract group
- DPI
diphenylene iodonium
- Dβcar
diabetic + β-carotene group
- NO
nitric oxide
- PMNs
polymorphonuclear leukocytes
- RLU
relative light unit
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- ZC3b
opsonized zymosan
Conflicts of Interests
The authors declare no conflicts of interest that are relevant to this article.
References
- 1.Sies H, Jones DP.Fink G.Encyclopedia of Stress. Vol 3. Elsevier; 2007, 45–48. [Google Scholar]
- 2.Rösen P, Nawroth PP, King G, Möller W, Tritschlev HJ, Packer L. The role of oxidative stress in the onset and progression of diabetes and its complication: a summary of a Congress Series sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab Res Rev. 2001;17:189–212. doi: 10.1002/dmrr.196. [DOI] [PubMed] [Google Scholar]
- 3.Wolff SP. Diabetes mellitus and free radicals. Free radicals, transition metals and oxidative stress in the aetiology diabetes mellitus and complication. Br Med Bull. 1993;49:642–652. doi: 10.1093/oxfordjournals.bmb.a072637. [DOI] [PubMed] [Google Scholar]
- 4.Marin DP, Bolin AP, Macedo RCS, Sampaio SC, Otton R. Ros production in neutrophils from alloxan-induced diabetic rats treated in vivo with astaxanthin. Int Immunopharmacol. 2011;11:103–109. doi: 10.1016/j.intimp.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Hand WL, Hand DL, Vasquez Y. Increased polymorphonuclear leukocyte respiratory burst function in type 2 diabetes. Diabetes Res Clin Pract. 2007;76:44–50. doi: 10.1016/j.diabres.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 6.Omori K, Ohira T, Uchida Y, et al. Priming of neutrophil oxidative burst in diabetes requires preassembly of the NADPH oxidase. J Leukoc Biol. 2008;84:292–301. doi: 10.1189/jlb.1207832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marletta MA, Yoon PS, Iyengar R, Leaf CD, Wishnok JS. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1998;27:8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- 8.Hibbs JB, Jr., Taintor RR, Vavrin Z, Rachlin EM. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988;157:87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- 9.Ardestani A, Yazdanparast R, Jamshidi Sh. Therapeutic effects of Teucrium polium extract on oxidative stress in pancreas of streptozotocin-induced diabetic rats. J Med Food. 2008;11(3):525–532. doi: 10.1089/jmf.2006.0230. [DOI] [PubMed] [Google Scholar]
- 10.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991;266(7):4244–4250. [PubMed] [Google Scholar]
- 11.Chaudière J, Ferrari-Iliou R. Intracellular antioxidants: from chemical to biochemical mechanisms. Food Chem Toxicol. 1999;37:949–962. doi: 10.1016/s0278-6915(99)00090-3. [DOI] [PubMed] [Google Scholar]
- 12.Wohaieb SA, Godin DV. Alteration in free radical tissue-defense mechanisms in streptozotocin-induced diabetes in rats. Effects of insulin treatment. Diabetes. 1987;36:1014–1018. doi: 10.2337/diab.36.9.1014. [DOI] [PubMed] [Google Scholar]
- 13.Osawa T, Kato Y. Protective role of antioxidative food factors in oxidative stress caused by hyperglycemia. Ann NY Acad Sci. 2005;1043:440–451. doi: 10.1196/annals.1333.050. [DOI] [PubMed] [Google Scholar]
- 14.Rao AV, Rao LG. Carotenoids and human health. Pharmacol Res. 2007;55:207–216. doi: 10.1016/j.phrs.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Krinsky NI, Johnson EJ. Carotenoid actions and their relation to health and disease. Mol Aspects Med. 2005;26:459–516. doi: 10.1016/j.mam.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Júnior AC, Asad LM, Oliveira EB, Kovary K, Asad NR, Felzenszwalb I. Antigenotoxic and antimutagenic potential of an annatto pigment (norbixin) against oxidative stress. Genet Mol Res. 2005;4(1):94–99. [PubMed] [Google Scholar]
- 17.Levy LW, Regalado E, Navarrete S, Watkins RH. Bixin and norbixin in human plasma: determination and study of the absorption of a single dose of annatto food color. Analyst. 1997;122:977–980. doi: 10.1039/a701304c. [DOI] [PubMed] [Google Scholar]
- 18.Kiokias S, Gordon MH. Dietary supplementation with a natural carotenoid mixture decreases oxidative stress. Eur J Clin Nutr. 2003;57:1135–1140. doi: 10.1038/sj.ejcn.1601655. [DOI] [PubMed] [Google Scholar]
- 19.Reddy MK, Alexander-Lindo RL, Nair MG. Relative inhibition of lipid peroxidation, cyclooxygenase enzymes, and human tumor cell proliferation by natural food colors. J Agric Food Chem. 2005;53:9268–9273. doi: 10.1021/jf051399j. [DOI] [PubMed] [Google Scholar]
- 20.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 21.Colégio Brasileiro de Experimentação Animal . Princípios éticos na experimentação animal do Colégio Brasileiro de Experimentação Animal. São Paulo: COBEA; 1991. [Google Scholar]
- 22.Association of Official Analytical Chemists . Official methods of analysis. Washington, DC: Association of Official Analytical Chemists; 1980. [Google Scholar]
- 23.Bicalho HM, Gontijo MC, Nogueira-Machado JA. A simple technique for simultaneous human leuckocytes separation. J Immunol Methods. 1981;40:115–116. doi: 10.1016/0022-1759(81)90087-9. [DOI] [PubMed] [Google Scholar]
- 24.Martins Chaves, Rocha-Vieira E, Pereira dos Reis A, de Lima e Silva R, Gerzstein NC, Nogueira-Machado JA. Increase of reactive oxygen (ROS) and nitrogen (RNS) species generated by phagocyting granulocytes related to age. Mech Ageing Dev. 2000;119:1–8. doi: 10.1016/s0047-6374(00)00153-6. [DOI] [PubMed] [Google Scholar]
- 25.Fäldt J, Ridell M, Karlsson A, Dahlgren C. The phagocyte chemiluminescence paradox: luminol can act as an inhibitor of neutrophil NADPH-oxidase activity. Luminescence. 1999;14(3):153–160. doi: 10.1002/(SICI)1522-7243(199905/06)14:3<153::AID-BIO534>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 26.Rinaldi M, Moroni P, Paape Mj, Bannerman DD. Evaluation of assays for the measurement of bovine neutrophil reactive oxygen species. Vet Immunol Immunopathol. 2007;115:107–125. doi: 10.1016/j.vetimm.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 27.Freitas M, Lima JL, Fernandes E. Optical probes for detection and quantification of neutrophils’ oxidative burst. A review. Anal Chim Acta. 2009;649(1):8–23. doi: 10.1016/j.aca.2009.06.063. [DOI] [PubMed] [Google Scholar]
- 28.Di Carlo FJ, Fiore JV. On the composition of zymosan. Science. 1958;127:756–757. doi: 10.1126/science.127.3301.756-a. [DOI] [PubMed] [Google Scholar]
- 29.Nogueira-Machado JA, Lima e Silva FC, Mares-Guia ML, Costa DC, Chaves MM. Discrimination between granulocytes from type I and type II diabetes patients by calorimetry. Thermochimica Acta. 2003;395:115–120. [Google Scholar]
- 30.Oboh G, Akomolafe TL, Adefegha SA, Adetuyi AO. Inhibition of cyclophosphamide-induced oxidative stress in rat brain by polar and non-polar extracts of Annatto (Bixa orellana) seeds. Exp Toxicol Pathol. 2011;63:257–262. doi: 10.1016/j.etp.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Russell KR, Morrison EY, Ragoobirsingh D. The effect of annatto on insulin binding properties in the dog. Phytother Res. 2005;19:433–436. doi: 10.1002/ptr.1650. [DOI] [PubMed] [Google Scholar]
- 32.Fernandes AC, Almeida CA, Albano F, et al. Norbixin ingestion did not induce any detectable DNA breakage in liver and kidney but caused a considerable impairment in plasma glucose levels of rats and mice. J Nutr Biochem. 2002;13:411–420. doi: 10.1016/s0955-2863(02)00177-8. [DOI] [PubMed] [Google Scholar]
- 33.Rösen P, Nawroth PP, King G, Möller W, Tritschler HJ, Packer L. The role of oxidative stress in the onset and progression of diabetes and its complications: a summary of a Congress Series sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab Res Rev. 2001;17:189–212. doi: 10.1002/dmrr.196. [DOI] [PubMed] [Google Scholar]
- 34.Bellin C, de Wiza, Wiernsperger NF, Rösen P. Generation of reactive oxygen species by endothelial and smooth muscle cells: influence of hyperglycemia and metformin. Horm Metab Res. 2006;38:732–739. doi: 10.1055/s-2006-955084. [DOI] [PubMed] [Google Scholar]
- 35.Ding Y, Kantarci A, Hasturk H, Trackman PC, Malabanan A, Van Dyke TE. Activation of RAGE induces elevated O2•− generation by mononuclear phagocytes in diabetes. J Leukoc Biol. 2007;81:520–527. doi: 10.1189/jlb.0406262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shurtz-Swirski R, Sela S, Herskovits AT, et al. Involvement of peripheral polymorphonuclear leukocytes in oxidative stress and inflammation in type 2 diabetic patients. Diabetes Care. 2001;24:104–110. doi: 10.2337/diacare.24.1.104. [DOI] [PubMed] [Google Scholar]
- 37.Forman HJ, Torres M. Redox signaling in macrophages. Mol Aspects Med. 2001;22:189–216. doi: 10.1016/s0098-2997(01)00010-3. [DOI] [PubMed] [Google Scholar]
- 38.Klinka M, Jastrzembska K, Bednarska K, Banasikb M, Sulowska Z. Effect of nitric oxide donors on NADPH oxidase signaling pathway in human neutrophils in vitro. Immunobiology. 2009;214:692–702. doi: 10.1016/j.imbio.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 39.Brandes RP, Kreuzer J. Vascular NADPH oxidases: molecular mechanisms of activation. Cardiovasc Res. 2005;65:16–27. doi: 10.1016/j.cardiores.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 40.Ayilavarapu S, Kantarci A, Fredman G, et al. Diabetes-induced oxidative stress is mediated by Ca2+ independent phospholipase A2 in neutrophils. J Immunol. 2010;184(3):1507–1515. doi: 10.4049/jimmunol.0901219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta A, Tripathi AK, Tripathi RL, Madhu SV, Banerjee BD. Advanced glycosylated end products mediated activation of polymorphonuclear neutrophils in diabetes mellitus and associated oxidative stress. Indian J Biochem Biophys. 2007;44:373–378. [PubMed] [Google Scholar]
- 42.Monroy-Ruiz J, Sevilla MÁ, Carrón R, Montero MJ. Astaxanthin-enriched-diet reduces blood pressure and improves cardiovascular parameters in spontaneously hypertensive rats. Pharmacology Res. 2011;63:44–50. doi: 10.1016/j.phrs.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt HHHW, Seifert R, Böhme E. Formation and release of nitric oxide from human neutrophils and HL-60 cells induced by a chemotactic peptide, platelet activating factor and leukotriene B4. FEBS Lett. 1989;244:357–360. doi: 10.1016/0014-5793(89)80562-9. [DOI] [PubMed] [Google Scholar]
- 44.Stolarek R, Kula P, Kurmanowska Z, Nowak D. Effect of various agonists on nitric oxide generation by human polymorphonuclear leukocytes. Int J Clin Lab Res. 1998;28:104–109. doi: 10.1007/s005990050028. [DOI] [PubMed] [Google Scholar]
- 45.Ren J, Chung SH. Anti-inflammatory effect of alpha-linolenic acid and its mode of action through the inhibition of nitric oxide production and inducible nitric oxide synthase gene expression via NF-kappaB and mitogen-activated protein kinase pathways. J Agric Food Chem. 2007;55:5073–5080. doi: 10.1021/jf0702693. [DOI] [PubMed] [Google Scholar]
- 46.Radi R, Cosgrove TP, Beckman JS, Freeman BA. Peroxynitrite-induced luminol chemiluminescence. Biochem J. 1993;290:51–57. doi: 10.1042/bj2900051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braga PC, Sambataro G, Dal Sasso M, Culici M, Alfieri M, Nappi G. Antioxidant effect of sulphurous thermal water on human neutrophil bursts: chemiluminescence evaluation. Respiration. 2008;75:193–201. doi: 10.1159/000107976. [DOI] [PubMed] [Google Scholar]
- 48.Levine SJ. Bronchial epithelial cell-cytokine interactions in airway inflammation. J Investig Med. 1995;43:241–249. [PubMed] [Google Scholar]
- 49.Müns G, Rubinstein I, Singer P. Phagocytosis and oxidative burst of granulocytes in the upper respiratory tract in chronic and acute inflammation. J Otolaryngol. 1995;24:105–110. [PubMed] [Google Scholar]
- 50.Lojek A, Pecivova J, Macickova T, Nosal R, Papezikova I, Ciz M. Effect of carvedilol on the production of reactive oxygen species by HL-60 cells. Neuro Endocrinol Lett. 2008;29:779–783. [PubMed] [Google Scholar]