Abstract
Imbalance between reactive oxygen species generation and antioxidant capacity induces a condition known as oxidative stress which is implicated in numerous pathological processes. In this study we evaluated whether natural zeolites chabazite/phillipsite/analcime may affect the levels of different antioxidant enzymes (gluthatione peroxidase, superoxide dismutase, gluthatione reductase), total antioxidant status and oxidative stress in 25 clinically healthy men, both non-smokers and smokers. Measurements were performed on whole blood or on plasma samples before (T0) and after 4-weeks zeolites intake (T1). At T1, gluthatione peroxidase, superoxide dismutase and gluthatione reductase increased compared to T0 levels, both considering all subjects as joint and after subdivision in non-smokers and smokers. Differently, a reduction in total antioxidant status was observed at T1. Anyway, total antioxidant status resulted higher than the reference values in both groups at each time point. A decrease in lipid peroxidation, a major indicator of oxidative stress assessed by monitoring thiobarbituric acid reactive substances, was also observed in all subjects at T1. Our results suggested that chabazite/phillipsite/analcime may help to counteract oxidative stress in apparently healthy subjects exposed to different oxidative stress risk factors, such as smoking, thus representing a particular kind of food with potential antioxidant properties.
Keywords: gluthatione reductase, gluthatione peroxidase, superoxide dismutase, total antioxidant status, zeolites
Introduction
Natural zeolites are vulcanic hydrated micro porous crystals with defined structures based on AlO4 and SiO4 tetrahedral building blocks connected through oxygen atoms and with an ”open” structure which can accommodate a wide variety of positive ions. Due to their structure, zeolites exhibit versatile adsorptive, cation exchanger, dehydrating-rehydrating and catalytic properties.(1) Some zeolites are already used in medicine as antidiarrheal, antibacterial, antifungal drugs and glucose absorbent. Moreover, previous studies also indicated that the zeolite clinoptilolite exerts anticancerogenic and antioxidant effects.(2–4)
Reactive oxygen species (ROS) are highly reactive molecules which may be both important mediators of some physiological functions and also potential prooxidants. Imbalance between ROS generation and antioxidant capacity induces a condition known as oxidative stress which may play a major role in the initiation and progression of numerous pathologies including cardiovascular dysfunction associated with vascular disease, hyperlipidemia, diabetes mellitus, hypertension and ischemia/reperfusion injury. The potential damage caused by an excess of ROS is controlled by a series of antioxidant defence mechanisms and, among them, a key protective role is played by the antioxidant enzymes gluthatione (GSH) peroxidase, superoxide dismutase (SOD) and GSH reductase.(5)
Presently, the antioxidant role attributed to zeolites, and specifically to clinoptilolite, is based on the ability to reduce lipid peroxidation, free radicals levels and also to increase total antioxidant status (TAS) in serum.(2–4)
Due to their key protective role in preventing or slowing down oxidative damage, antioxidant enzymes could be potential target of zeolites action too. To this aim, in the present study we evaluated whether chabazite/phillipsite/analcime, tipical zeolites of the central-souther of Italy, may affect the levels of GSH peroxidase, SOD, GSH reductase and TAS in a group of clinically healthy men, both non-smokers and smokers. Furthermore, since lipid peroxidation is a major indicator of oxidative stress, thiobarbituric acid reactive substances (TBARS) were measured for screening and monitoring lipid peroxidation in our subjects.
Materials, Subjects and Methods
Materials
The fine powder of natural zeolites from central Italy (near Bolsena lake) was obtained by tribomechanical micronization and sterilization at 100°C for 24 h (Geomedical srl, Viterbo, Italy). The zeolitic-tuff used contained approximately 33.7% chabazite [CaAl2SiO12×6(H2O)], 17.0% phillipsite [(K,Na,Ca)1-2(Si,Al)8O16×6(H2O)] and 6.1% analcime [NaAlSi2O6×H2O]. Other components were: sanidine (18%), plagioclase (14.6%) and muscovite mica (10.6%). No traces of clinoptilotite were found. The total zeolitic content of the tuff was about 56.8%. The cation-exchange capacity and specific mass were 400 meq/100 g and 1.26 N/m3, respectively. Specific surface area was 1.77 m2/g. Particle size analysis showed a granulometry less then 1 µm for 15% of the particles, 2 µm for 20% of the particles, 5 µm for 40% of the particles, 15 µm for 80% of the particles and 100 µm for 100% of the particles.
Subjects
In this study, 25 clinically healthy male (40 ± 3 years; age mean ± standard deviation (SD)) were enrolled. Subjects gave informed consent to the examination protocol, conducted in accordance with the Declaration of Helsinki and approved by the institutional committee. These subjects were following a typical mediterranean diet and leading a non-sedentary life style. Of these, 12 were non-smokers and 13 were smokers (more then 20 cigarettes/day). Subjects received for 4 weeks, twice a day, 1/2 h before breakfast and dinner, 3 g of chabazite/phillipsite/analcime as powder suspension dissolved in water. EDTA-anti-coagulated blood samples were taken before (T0) and at the end of the 4 weeks (T1). Plasma samples were obtained after blood centrifugation at 1800 g for 15 min at room temperature and stored at −20°C until assay.
Biochemical assays
GSH peroxidase, SOD, GSH reductase and TAS were measured or on whole blood or on plasma samples by specific kits from Randox Laboratories Ltd. (Crumlin, Belfast, UK) on the semi-automated analyzer for colorimetric and turbidimetric assays RX MonzaTM (Randox Laboratories Ltd.) following manufacture’s instructions. The reference values for the parameters in healthy subjects were: 4171–10881 U/L of whole blood for GSH peroxidase, 164–240 U/ml of whole blood for SOD, 33–77 U/l of plasma for GSH reductase, 1.3–1.7 mmol/l of plasma for TAS. Reported maximum intra- and inter-assay coefficients of variations were 3.79% and 4.90% for GSH peroxidase, 3.58% and 7.07% for SOD, 2.58% and 4.32% for GSH reductase, 3.08% and 3.75% for TAS, respectively. TBARS test was performed using the method described by Richard et al.(6) Briefly, after the reaction of thiobarbituric acid with malondialdehyde, the product was extracted in butanol and was measured spectrofluorometrically (excitation: 532 nm, emission 553 nm, slit 10 mm). TBARS levels on plasma were expressed as nmol/ml.
Statistical analysis
Box-and-whisker diagrams were used to represent data. The smallest observation (sample minimum), lower quartile (25th), median (50th), upper quartile (75th), and largest observation (sample maximum) are shown. The normality of data distribution was assessed by the Kolmogorov-Smirnoff test. Comparisons between groups were performed using Student’s two-tailed t test and a p value<0.05 was considered significant. Data were analyzed by GraphPad Software Inc. (San Diego, CA).
Results
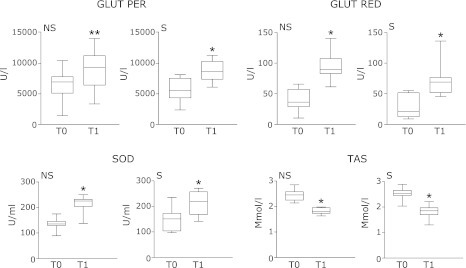
GSH peroxidase, SOD, GSH reductase and TAS levels were measured before (T0) and after 4-weeks zeolites intake (T1). At T0, no statistically significant differences were observed for GSH peroxidase, SOD, GSH reductase and TAS levels between non-smokers and smokers subjects, although GSH peroxidase and GSH reductase mean values appeared more elevated in the non-smoking group. Among the analyzed parameters, TAS levels resulted higher than the normal values in both groups, whereas all the other parameters were within the normal reference ranges. At T1, GSH peroxidase, GSH reductase and SOD resulted increased compared to basal levels, both considering all subjects as joint and after subdivision in the two sub-groups, non-smokers and smokers (Table 1 and Fig. 1). Differently from the other parameters, a statistically significant reduction in TAS levels was observed at T1. Anyway, TAS levels remained higher than the reference values in both groups (Table 1 and Fig. 1). As previously observed at T0, also at T1 GSH peroxidase, SOD and TAS levels were similar in both groups. Differently from T0, instead, a major increased in GSH reductase levels was observed in non-smoking compared to smoking subjects (94.98 ± 20.17 U/ml ± SD vs 70.41 ± 22.88 U/ml ± SD; p<0.001).
Table 1.
Levels of gluthatione peroxidase (GLUT PER), superoxide dismutase (SOD), glutathione reductase (GLUT RED), total antioxidant status (TAS) and thiobarbituric acid reactive substance (TBARS) in subjects before (T0) and after 4-weeks zeolites consume (T1). Data are mean ± standard deviation (SD) values
| Whole Subjects (n = 25) |
Non-Smoking (n = 12) |
Smoking (n = 13) |
||||
|---|---|---|---|---|---|---|
| T0 | T1 | T0 | T1 | T0 | T1 | |
| GLUT PER (U/l) | 6118.44 ± 2227.30 | 8924.64 ± 2479.2** | 6489.80 ± 2526.4 | 9024.00 ± 3187.10* | 5775.60 ± 1950.90 | 8832.90 ± 1721.30** |
| GLUT RED (U/l) | 34.69 ± 18.68 | 82.20 ± 24.60** | 40.38 ± 17.86 | 94.98 ± 20.17** | 29.45 ± 18.53 | 70.41 ± 22.88** |
| SOD (U/ml) | 141.14 ± 33.11 | 214.14 ± 39.54** | 135.64 ± 21.87 | 214.62 ± 32.87** | 146.23 ± 11.42 | 213.69 ± 46.22** |
| TAS (mmol/l) | 2.49 ± 0.22 | 1.82 ± 0.18** | 2.44 ± 0.22 | 1.82 ± 0.12** | 2.54 ± 0.21 | 1.82 ± 0.23** |
| TBARS (nmol/ml) | 2.70 ± 0.60 | 1.60 ± 0.30** | 2.10 ± 0.30 | 1.50 ± 0.30** | 3.30 ± 0.20 | 1.60 ± 0.30** |
*p<0.05 vs T0; **p<0.001 vs T0. GLUT PER: gluthatione peroxidase; GLUT RED: gluthatione reductase; SOD: superoxide dismutase; TAS: total antioxidant status; TBARS: thiobarbituric acid reactive substance.
Fig. 1.
Levels of gluthatione (GSH) peroxidase, superoxide dismutase (SOD), GSH reductase and total antioxidant status (TAS) in non-smoking (NS) and smoking (S) subjects before (T0) and after 4-weeks zeolites consume (T1). The smallest observation (sample minimum), lower quartile (25th), median (50th), upper quartile (75th), and largest observation (sample maximum) are shown. *p<0.05 vs T0; **p<0.001 vs T0.
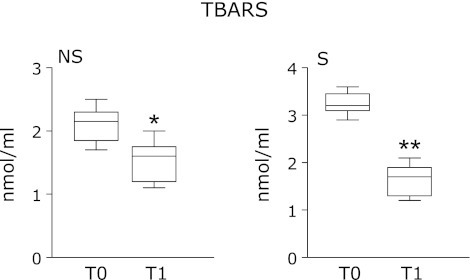
TBARS were measured for screening and monitoring lipid peroxidation which is considered as a major indicator of oxidative stress. As shown in Table 1 and Fig. 2, at T0 plasma TBARS levels are significantly lower in non-smokers than in smokers subjects (2.1 ± 0.3 nmol/ml ± SD vs 3.3 ± 0.2 nmol/ml ± SD; p<0.05). At T1, TBARS resulted decreased compared to T0, both considering all subjects as joint (T0: 2.7 ± 0.6 nmol/ml ± SD; T1: 1.6 ± 0.3 nmol/ml ± SD; p<0.001) and after subdivision in the two sub-groups, non-smokers (1.5 ± 0.3 nmol/ml ± SD; p<0.05) and smokers (1.6 ± 0.3 nmol/ml ± SD; p<0.01). No statistically significance difference was found between the two groups.
Fig. 2.
Levels of thiobarbituric acid reactive substances (TBARS) in non-smoking (NS) and smoking (S) subjects before (T0) and after 4-weeks zeolites consume (T1). The smallest observation (sample minimum), lower quartile (25th), median (50th), upper quartile (75th), and largest observation (sample maximum) are shown. *p<0.05 vs T0; **p<0.001 vs T0.
Discussion
In the present study we observed that 4-weeks chabazite/phillipsite/analcime intake in clinically healthy subjects, both non-smokers and smokers, increased the levels of GSH peroxidase, SOD and GSH reductase, three antioxidant enzymes able to remove ROS, and decreased TBARS, a major indicator of lipid peroxidation utilized for monitoring oxidative stress, thus preventing or slowing down oxidative damage. Anthropologists have always maintained that our ancestor ingested, to cure themselves, different varieties of rocks taken from the surrounding environment. This diet behavior has profound biological bases, bonded to organic need to take the mineral elements necessary for life. Since today developed societies are exposed to a wide variety of exogenous and endogenous stress factors that may influence the generation of ROS and antioxidant activity, the use of integrators and particular kind of food, like zeolites, may help to eliminate free radicals and toxic substances from the body. In addition to the previously suggested antioxidant properties of some zeolites,(3,4) in the present study we observed that the intake of chabazite/phillipsite/analcime, three zeolites widespread in many volcanoclastic depositis in the central-southern of Italy, gave a significant protection against oxidative stress by increasing the levels of important antioxidant enzymes and reducing oxidative stress. These zeolites have been used in our study for the following reasons: they are the most abundant zeolites of Italian soil, they have an overall cation-exchange capacity of 400 meq/100 g and, finally, they have not been previously studied as medicinal food relative to their antioxidant potential. The exchange capacity, which indicates the ability to release beneficial elements while capturing and binding others, has been indicated as an important requirement for the therapeutic application of zeolites. For this reason, due to a cation-exchange capacity of 400 meq/100 g, which is greater than that of other zeolites (about 200 meq for the most used and studied clinoptilolite), chabazite/phillipsite/analcime may have the requirements to be more effective then other zeolites.
The subjects included in the study were both non-smoking and smoking. Previous studies which evaluated the effect of smoking on the levels of antioxidant enzymes reported different conclusions, since both significant and not significant differences in the activities of these antioxidant enzymatic systems have been described.(7–10) Although at T0 we observed that GSH peroxidase and GSH reductase mean values were lower in the smoking group, however we did not observe any statistically significant difference. After chabazite/phillipsite/analcime consume, increased levels of all the three antioxidant enzymes were observed in both groups. These data thus may indicate that chabazite/phillipsite/analcime are able to increase physiological mechanisms against oxidative stress in healthy subjects exposed to different risk factors of oxidative stress, such as smoking, and may be considered as protective agents against oxidative stress.
The decrement of TAS levels at T1 seems to be in contrast with previous studies which indicated that zeolites intake may help to increase TAS.(3,4) One possible explanation is that our subjects are healthy and daily following a typical mediterranean diet rich in natural antioxidants which contribute to increase TAS to levels higher than the normal range. Thus, it is possible that after ingestion, chabazite/phillipsite/analcime remove not only toxins and biological waste but also absorb other molecules. Although chabazite/phillipsite/analcime reduced TAS, however, it is to be noted that it remained higher than normal values. In addition, the observation that TBARS were reduced after zeolites intake suggests that zeolites do not aggravate oxidative stress. On the contrary a general improvement was observed in both groups (non-smoking and smoking). Future studies considering other categories of subjects following different diets and also patients affected by pathologies characterized by increased oxidative stress, will be necessary to better clarify the effect of chabazite/phillipsite/analcime on TAS.
At now, the mechanisms mediating zeolites antioxidant effects are not fully understood and since orally-administered zeolites are not absorbed into the blood it is possible that their in vivo effect may be due to an indirect interaction with biochemical systems, such as toxin and biological waste removal from the gut, activation of the immune system via the mucosal associated intestinal lymphoid tissue and also by increasing the bioavailability of mineral elements, which are important co-factors for the enzymes.(2,11–13)
In conclusion, our results strongly indicated that chabazite/phillipsite/analcime may help to counteract the effects of oxidative stress by increasing the levels of antioxidant enzymes and reducing oxidative stress in apparently healthy subjects exposed to different risk factors of oxidative stress. Future studies will be necessary to better clarify which mechanisms mediate the observed zeolites effects and will be helpful to better identify some other potential applications of such compounds also in fields like cardiovascular prevention and atherosclerosis.
Acknowledgment
Founds Research Contract N. 13387, year 2010, Università degli Studi di Milano. We thank the volunteers of clinical study. Professor Diego Gatta (Dipartimento di Scienze della Terra, Università degli Studi di Milano) is kindly acknowledged for his help in discussing the chemical properties of zeolites.
Abbreviations
- GSH
gluthatione
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TAS
total antioxidant status
- TBARS
thiobarbituric acid reactive substances
References
- 1.Mumpton FA. La roca magica: uses of natural zeolites in agriculture and industry. Proc Natl Acad Sci USA. 1999;96:3463–3470. doi: 10.1073/pnas.96.7.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zarkovic N, Zarkovic K, Kralj M, et al. Anticancer and antioxidative effects of micronized zeolite clinoptilolite. Anticancer Res. 2003;23:1589–1595. [PubMed] [Google Scholar]
- 3.Ivkovic S, Deutsch U, Silberbach A, Walraph E, Mannel M.Dietary supplementation with the tribomechanically activated zeolite clinoptilolite in immunodeficiency: effects on the immune system Adv Ther 2004; 21: 135–147 [DOI] [PubMed] [Google Scholar]
- 4.Grancarić AM, Tarbuk A, Kovaček I. Nanoparticles of activated natural zeolite on textiles for protection. CI&CEQ. 2009;15:203–210. [Google Scholar]
- 5.Zawadzka-Bartczak E, Kopka L, Gancarz A. Antioxidative enzyme profiles in fighter pilots. Aviat Space Environ Med. 2003;74:654–658. [PubMed] [Google Scholar]
- 6.Richard MJ, Arnaud J, Jurkovitz C, et al. Trace elements and lipid peroxidation abnormalities in patients with chronic renal failure. Nephron. 1991;57:10–15. doi: 10.1159/000186208. [DOI] [PubMed] [Google Scholar]
- 7.Volkovová K, Beno I, Staruchová M, Bobek P, Mekinová D, Tatara M. Antioxidative enzyme activity in the blood of healthy persons. Bratisl Lek Listy. 1996;97:134–138. [PubMed] [Google Scholar]
- 8.Andersen HR, Nielsen JB, Nielsen F, Grandjean P. Antioxidative enzyme activities in human erythrocytes. Clin Chem. 1997;43:562–568. [PubMed] [Google Scholar]
- 9.Bolzán AD, Bianchi MS, Bianchi NO. Superoxide dismutase, catalase and glutathione peroxidase activities in human blood: influence of sex, age and cigarette smoking. Clin Biochem. 1997;30:449–454. doi: 10.1016/s0009-9120(97)00047-7. [DOI] [PubMed] [Google Scholar]
- 10.Bogdanska JJ, Korneti P, Todorova B. Erythrocyte superoxide dismutase, glutathione peroxidase and catalase activities in healthy male subjects in Republic of Macedonia. Bratisl Lek Listy. 2003;104:108–114. [PubMed] [Google Scholar]
- 11.Karube-Harada A, Sugino N, Kashida S, et al. Induction of manganese superoxide dismutase by tumour necrosis factor-alpha in human endometrial stromal cells. Mol Hum Reprod. 2001;7:1065–1072. doi: 10.1093/molehr/7.11.1065. [DOI] [PubMed] [Google Scholar]
- 12.Pavelić K, Hadzija M, Bedrica L, et al. Natural zeolite clinoptilolite: new adjuvant in anticancer therapy. J Mol Med (Berl) 2001;78:708–720. doi: 10.1007/s001090000176. [DOI] [PubMed] [Google Scholar]
- 13.Pavelić K, Katic M, Sverko V, et al. Immunostimulatory effect of natural clinoptilolite as a possible mechanism of its antimetastatic ability. J Cancer Res Clin Oncol. 2002;128:37–44. doi: 10.1007/s00432-001-0301-6. [DOI] [PubMed] [Google Scholar]