Abstract
Stanniocalcin 1 and stanniocalcin 2 are two glycoprotein hormones, which act as calcium phosphate-regulating factor on intestine and kidney. We have previously reported that stanniocalcin 2 expression is positively and negatively controlled by 1,25(OH)2D3 and parathyroid hormone in renal proximal tubular cells. However, it has been unclear whether they regulate the stanniocalcin 1 gene expression. In this study, we identified the opossum stanniocalcin 1 cDNA sequence. The opossum stanniocalcin 1 amino acid sequence had 83% homology with human stanniocalcin 1, and has a conserved putative N-linked glycosylation site. Real-time PCR analysis using opossum kidney proximal tubular (OK-P) cells revealed that the mRNA levels of stanniocalcin 1 gene is up-regulated by both 1,25(OH)2D3 and parathyroid hormone in dose-dependent and time-dependent manners. We also demonstrated that the stanniocalcin 1 expression was increased in parathyroid hormone injected rat kidney. Furthermore, the mRNA expression of stanniocalcin 1 and stanniocalcin 2 were oppositely regulated by phorbol 12,13-myristic acetate, a specific PKC activator. Interestingly, the up-regulation of stanniocalcin 1 gene by 1,25(OH)2D3 and phorbol 12,13-myristic acetate were not prevented in the presence of actinomycin D, an RNA synthesis inhibitor. These results suggest that the stanniocalcin 1 gene expression is up-regulated by 1,25(OH)2D3 and parathyroid hormone through mRNA stabilization in renal proximal tubular cells.
Keywords: stanniocalcin 1; gene regulation analysis; 1,25-dihydroxy vitamin D3 [1,25(OH)2D3]; parathyroid hormone; opposum kidney proximal tubular cells
Introduction
Stanniocalcin (STC) is a glycoprotein hormone first identified from the corpuscles of Stannius in bony fish, endocrine glands that are embryologically derived from the kidney tubules.(1,2) An increase in plasma ionized calcium levels is the principal stimulus for secretion and one of the primary roles of STC in fishes is preventing the development of hypercalcemia.(3) Indeed, fish STC acts on gill, gut and kidney as a calcium and phosphate-regulating hormone.(3–5) Fish and mammals express two stanniocalcin genes, STC1 and STC2.(6) STC1 and STC2 contain N-glycosylation sites, a signal peptide sequence of ~24 amino acids.
Localization of STC1 expression in kidney was examined by in situ hybridization and immunohistochemistry. In rat kidney, the STC1 gene was expressed in the collecting ducts and distal nephron segments.(7) Other reports, however, showed STC1 protein expression in the proximal tubules (proximal straight tubules).(8) Indeed, it has been known that STC1 stimulates phosphate reabsorption in the small intestine and proximal tubules of the kidney.(9,10) We have previously identified human STC2 gene and observed that the STC2 gene is widely expressed in mice. We also examined the STC2 mRNA expression in hypophosphatemic (Hyp) mice, which is a model of human X-linked hypophosphatemic vitamin D-resistant rickets (XLH) and found it was down-regulated in many organs, and human STC2 decreases phosphate uptake activity and human type IIa sodium-dependent phosphate co-transporter (human NaPi-2a) gene promoter activity in opossum renal proximal tubular cell line (OK cells).(11) Our recent work reported the identification of opossum STC2 gene and observed that its expression and secretion in OK cells were positively and negatively controlled by 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] and parathyroid hormone (PTH).(12) On the other hand, renal STC1 mRNA expression is increased by calcium and 1,25(OH)2D3 in rat and its renal expression are also up-regulated in alpha klotho mutant mice indicating hyperphosphatemia, hypercalcemia and hypervitaminosis D.(13) However, there is no evidence the regulation of STC1 expression by 1,25(OH)2D3 and PTH in renal proximal tubular cells in vitro or whether PTH can affect on its renal expression in vivo.
In the present study, we identified the opossum STC1 cDNA sequence and investigated the regulation of STC1 gene expression by 1,25(OH)2D3 and PTH in renal proximal tubular cells.
Materials and Methods
Cell culture and treatments
Opossum kidney proximal tubular (OK-P) cells were a gift from Dr. Judith A. Cole (Department of Biology, University of Memphis, Memphis, TN). OK-P cells were cultured as described previously.(12) 1,25(OH)2D3 was obtained from Solvay Pharmaceuticals (Marietta, GA). 22-oxacalcitriol (OCT) and 2β-(3-hydroxypropoxy) calcitriol (ED-71) were kindly provided by Chugai Pharmaceutical Co., Ltd. (Tokyo, Japan). Human parathyroid hormone (1–34 fragment) (PTH), 8-bromo-cyclic AMP (8b-cAMP), phorbol 12,13-myristic acetate (PMA) and actinomycin D (ActD) were purchased from Sigma-Aldrich. After reaching 100% confluence, OK-P cells were treated with each reagent at different concentrations and times.
cDNA isolation and DNA sequence analysis
Opossum STC1 gene information was obtained from a BLAST search on the UCSC Genome Bioinformatics website (http://www.genome. ucsc.edu/) with the amino acid sequence of human STC1. On the basis of this information, oligonucleotide primers specific to the STC1 cDNA sequence were synthesized. The sequences of the upstream and downstream primers were 5'-TCAGAGAATGCT CCACAACTCC-3', and 5'-CTGCCTCGGTTACTCGCTCT-3' respectively. Opossum STC1 cDNA containing an open reading frame was amplified by RT-PCR using total RNA isolated from OK-P cells. The amplification products were purified and subcloned into a pGEM-T Easy vector (Promega), and manually sequenced using the dye terminator cycle sequencing method (Applied Biosystems, Foster City, CA). The cDNA sequences of the opossum STC1 gene have been deposited in GenBank (http://www.ncbi.nlm.nih.gov) under the accession number AB622590.
Reverse transcription (RT)-PCR and quantitative real-time RT-PCR
The cells were homogenized in RNA iso plus reagent (Takara Bio Inc., Shiga, Japan). After phenol/chloroform extraction and 2-propanol precipitation, total RNA was dissolved in RNase-free water. First-strand cDNA was synthesized from 2.5 µg of total RNA, primed with oligo (dT) using the MMLV-reverse transcriptase (Invitrogen, San Diego, CA). Two µl of the cDNA was then used for subsequent PCR, with 20–30 cycles of amplification, and then PCR products were separated by electrophoresis using 2% agarose gels. For PCR amplification, the primer sequences were: opossum STC1, 5'-CAGAGAATGCTCCAC AACTCCGCC-3' and 5'-CTGCAGAGCAGTGTTGAGGCAT CGG-3'; opossum STC2, 5'-ATGCCCTGAATGGTAAAGCCC ATG-3' and 5'-CACGTAGGGTTCATGCAGTAGCAGATC-3'; opossum β-actin, 5'-CTGACCCTGAAGTACCCCATTGAACA-3' and 5'-CTGGGGTGTTGAAGGTCTCAAACATG-3'; rat STC1, 5'-CCAAGGTCTTCCTTGCCATT-3' and 5'-TGCTGCAAACA T TGAGCTTG-3'; rat β-actin, 5'-GTCCCAGTATGCCTCTGG TCGTAC-3' and 5'-CCACGCTCGGTCAGGATCTTCATG-3'. RT-PCR was performed with a PCR system (ASTEC, Fukuoka, Japan) using Go Taq Master Mix (Promega, Madison, WI). Real-time PCR analysis was performed using the LightCyclerTM (Roche Diagnostics, Tokyo, Japan). The prepared first-strand cDNA was PCR amplified using Light Cycler fast start DNA master SYBR Premix Ex Taq (Takara Bio Inc., Shiga, Japan) in a 20 µl reaction volume, with 4 pmol of each primer. β-actin was used as the internal control. The amplification program was 95°C for 10 s followed by 50 cycles of 95°C for 10 s, 60°C for 15 s, and 72°C for 15 s. Amplified products were then analyzed using a melting curve, which confirmed the presence of a single PCR product in all reaction (apart from negative control). The products were quantified by fit-point analysis, and results for STC1 and STC2 were normalized to those of β-actin.
Experimental animals
Eight-week-old Sprague-Dawley (SD) male rats were purchased from Japan SLC, Inc. (Shizuoka, Japan). Rats were maintained with 12 h light: 12 h darkness cycles with free access to regular rat chow and water under pathogen-free conditions. The breeding and handling of all animals in these experiments was approved by the Animal Experimentation Committee of the University of Tokushima. Rats were fed a low-phosphate diet (0.02% Pi and 0.6% Ca) for 3 days.(14) Then, human-PTH (1–34) (10 µg/100 g body weight) was injected into the jugular vein. After treatment for 0.5 h, the rats were sacrificed.
Western blot analysis
The whole kidney was homogenized in lysis buffer containing 1% Triton X-100, 1% Na deoxycholate, 0.1% SDS, 10 mM Tris-HCl, pH 8.0, 140 mM NaCl. Protein samples were electrophoresed on 12% SDS/PAGE and transferred to PVDF membrane (Immobilon-P, Millipore). The membrane were treated with diluted anti-STC1 antibody (1:1000) (Santa Cruz) and anti-NaPi-2a antibody (1:5000). Mouse anti-actin monoclonal antibody (Sigma) was used as an internal control. Horseradish peroxidase (HRP)-labeled anti-IgG (Bio-Rad) was utilized as the secondary antibody, and signal were detected using the ECL plus system (GE Healthcare, Buckinghamshire, UK).
Statistical analysis
Data are expressed as means ± SD. Statistical significance between groups was determined using ANOVA followed by post hoc testing using Fisher’s protected least significant difference (PLSD). p<0.05 was considered to be significant.
Results
Identification of opossum STC1 amino acid sequences
We first identified opossum STC1 cDNA using information obtained from the UCSC genome database website. The opossum STC1, human STC1 sequences and opossum STC2 were then aligned. We assessed STC1 in opossum and human, and STC1 and STC2 in opossum. The results presented in Fig. 1A showed that opossum STC1 amino acid sequence had 83.7% homology with human STC1, and conserved 11 cysteine residues and a putative N-linked glycosylation site as well as human STC1. We next compared the amino acid sequence of opossum STC1 with that of opossum STC2. Although it was seen at 10 conserved cysteine residues and a putative N-linked glycosylation site, it had only 35.3% homology.
Fig. 1.
Identification of opossum STC1 amino acid sequences. Opossum STC1 cDNA was cloned by RT-PCR analysis using information obtained from the UCSC genome database website. The amino acid sequences of opossum STC1 were then aligned with (A) human STC1 and (B) opossum STC2. The underlined sequence indicated the predicted signal peptide region. The N-glycosylation site is shown as a closed triangle. The conserved cysteine residues are indicated by black dots.
Regulation of STC1 mRNA expressions by 1,25(OH)2D3 in opossum kidney cells
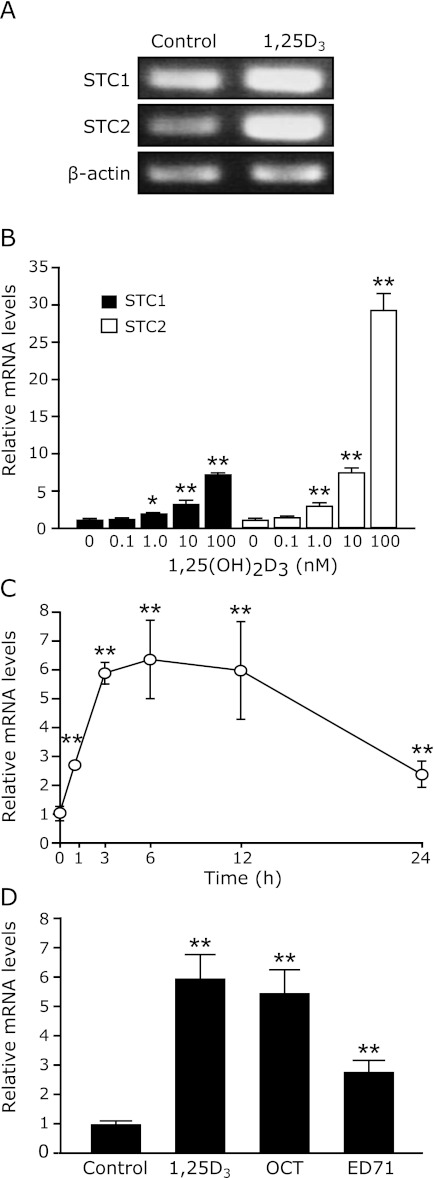
In our previous study, we identified opossum STC2 and demonstrated the STC2 gene expression in OK-P cells was up-regulated by 1,25(OH)2D3 in transcriptional control, but PTH and PMA as the PKC activator down-regulated its expression.(12) In this experiment, we investigated about the regulation of STC1 gene by 1,25(OH)2D3, PTH and PMA, and compared with the STC2 gene expression. Using Reverse Transcript-PCR and Real time PCR, we now investigated whether the STC1 mRNA expression in OK-P cells is controlled by 1,25(OH)2D3 and PTH. As shown in Fig. 2A, 1,25(OH)2D3 increased both the mRNA levels of STC1 and STC2 in OK-P cells. Real-time quantitative PCR analyses revealed that 1,25(OH)2D3 increases the STC1 mRNA levels in OK-P cells in a dose- and time-dependent manner (Fig. 2 B and C). However, the efficacy of 1,25(OH)2D3 for the STC1 gene was 10-folds less than STC2 gene (Fig. 2B). The up-regulation of STC1 gene expression by 1,25(OH)2D3 was evident at 1 h and reached its peak at 6 h, with a 7-folds increase at the time (Fig. 2C). In addition, we found the STC1 mRNA expression is also up-regulated by OCT and ED-71, which is 1,25(OH)2D3 analogs (Fig. 2D).
Fig. 2.
Effects of 1,25(OH)2D3 on the STC1 mRNA expressions in OK-P cells. When OK-P cells reached confluence, the cells were treated with the control (final concentration of ethanol, 0.1%) or (A) 100 nM 1,25D3 for 6 h, or multiple concentrations of (B) 1,25D3 for 6 h, or (C) 100 nM 1,25D3 for time course study. (D) The cells were also incubated with the control, 100 nM 1,25D3, OCT or ED-71 for 6 h. RNA was extracted from cells at the indicated time points, and RT-PCR or real-time RT-PCR was performed to evaluate STC1 and STC2 expression. Results were normalized to the mRNA expression level of β-actin. The data are represented as the means ± SD (n = 3). *p<0.05, **p<0.001 compared with control.
Up-regulation of STC1 mRNA expression by PTH in vitro and in vivo
We then determined whether PTH affects on the STC1 mRNA expression in OK-P cells. 6 h treatment with PTH in OK-P cells significantly increased the levels of STC1 mRNA, but decreased that of STC2 mRNA (Fig. 3A). The opposite effects of PTH on the mRNA expression of STC1 and STC2 were observed in time-dependent manners (Fig. 3B). In addition, we confirmed the effects of PTH on renal STC1 expression in vivo. As shown in Fig. 3C, the renal mRNA levels of STC1 was significantly increased at both periods of 0.5 h and 3 h after PTH injection. It has been well-known that the renal NaPi-2a expression is down-regulated by PTH.(15–17) Therefore, we also investigated the renal NaPi-2a expression as a good PTH target gene. Western blot analysis showed that PTH increased the protein levels of STC1, but decreased that of NaPi-2a (Fig. 3D).
Fig. 3.
Effects of PTH on the STC1 expression in OK-P cells and rat kidney. (A, B) OK-P cells were incubated with the control (final concentration of ethanol, 0.1%) and 100 nM PTH for 6 h or different periods of time. The mRNA levels of STC1 and STC2 in OK-P cells were determined by RT-PCR and real-time PCR analysis. (C, D) SD male rats were fed a low-phosphate diet for 3 days, low-phosphate diet contained 0.02% Pi and 0.6% Ca, and then 10 µg human PTH (1–34)/100 g body weight was injected into the jugular vein. After treatment for 0.5 h and 3 h, the mRNA expression levels of STC1 in kidney were quantified by real-time PCR. Results were normalized to the mRNA expression level of β-actin. Values are means ± SD (n = 3–5). *p<0.05, **p<0.001 compared with control. (D) The renal protein levels of STC1 and NaPi-2a were measured by western blot analysis. β-actin was used as an internal control.
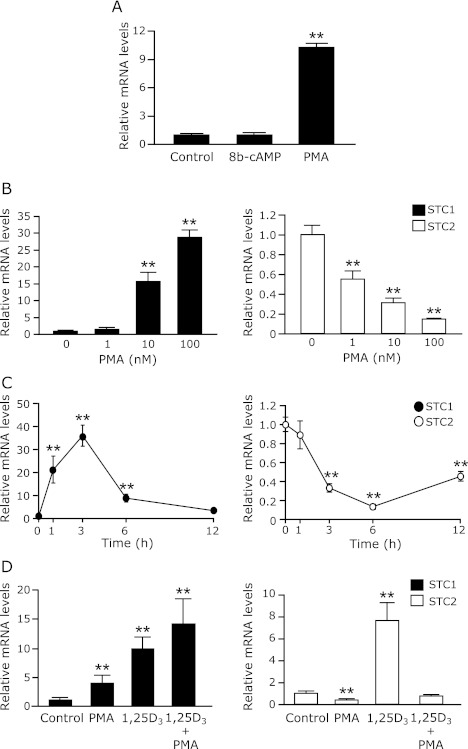
PMA stimulates STC1 mRNA expression in OK-P cells
To gain further understanding of the mechanisms involved in the regulation of the STC1 gene by PTH, we observed that the effects of 8-brommo-cyclic AMP as a PKA activator and PMA as a PKC activator on the STC1 mRNA expression in OK-P cells. As showed in Fig. 4A, the STC1 mRNA levels were significantly increased by PMA, but not 8-brommo-cyclic AMP. Dose- and time-dependent experiments showed that PMA oppositely controlled the mRNA levels of STC1 and STC2 gene in OK-P cells as well as PTH (Fig. 4 B and C). Furthermore, PMA and 1,25(OH)2D3 additively increased the STC1 mRNA levels, however, PMA completely suppressed 1,25(OH)2D3-induced STC2 mRNA expression (Fig. 4D).
Fig. 4.
Effects of PMA on the STC1mRNA expression in OK-P cells. (A) OK-P cells were treated with the control or 100 nM 8-bro-cAMP, a specific PKA activator, or PMA, a specific PKC activator for 3 h. OK-P cells were also treated with (B) the different doses of PMA for 3 h, or (C) 100 nM PMA for time course study, or (D) 100 nM PMA for 3 h or 100 nM 1,25(OH)2D3 for 6 h. After treatment with 1,25(OH)2D3 and/or PMA, the cells were harvested and total RNA was extracted. The mRNA levels of STC1 and STC2 were determined by real-time PCR analysis. Results are expressed as the means ± SD (n = 3). *p<0.05, **p<0.01 compared with vehicle.
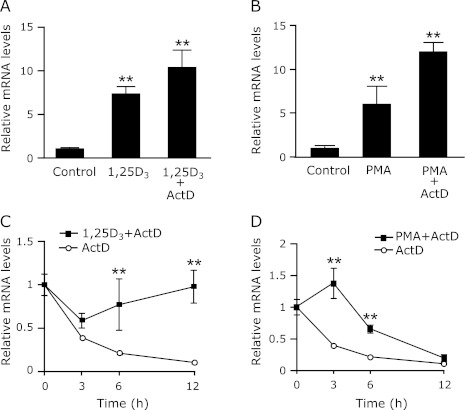
Stabilization of STC1 mRNA by 1,25(OH)2D3 and PMA in OK-P cells
Our previous study using ActD indicated that the opossum STC2 gene expression was up-regulated by 1,25(OH)2D3 at transcriptional levels.(12) Surprisingly, pretreatment with ActD did not suppressed 1,25(OH)2D3 or PMA-induced STC1 mRNA expression in the OK-P cells (Fig. 5 A and B). To determine if the elevation in STC1 mRNA was associated with enhanced mRNA stability, we performed a standard mRNA decay assay. ActD was added to OK-P cells to prevent any new gene transcription, and STC1 mRNA levels were monitored over the following 12-h period in cells treated with ActD only and in cells treated with ActD plus either 1,25(OH)2D3 or PMA. As shown in Fig. 5 C and D, the half-life of STC1 mRNA in ActD-treated cells was about 2.5 h; 1,25(OH)2D3 and PMA treatments increased the half-life ~2.5-fold (to 12 h) and ~3-fold (6 h), respectively. These results indicate that both 1,25(OH)2D3 and PMA induce STC1 mRNA levels primarily by inhibiting its decay.
Fig. 5.
Stabilization of the STC1 mRNA by 1,25(OH)2D3 and PMA in OK-P cells. OK-P cells were pretreated with 100 nM ActD, then, the cells were incubated (A) 100 nM 1,25(OH)2D3 for 6 h or (B) 100 nM PMA for 3 h. OK-P cells were treated with (C) 100 nM 1,25(OH)2D3 for 6 h or (D) 100 nM PMA for 3 h, followed by incubation with 1000 nM ActD for different periods of time. The mRNA expression levels of STC1 were determined by real-time PCR analysis. Results are expressed as the means ± SD (n = 3). **p<0.01 compared with vehicle.
Discussion
The present study characterized a cDNA that encodes STC1 in opossum and investigated the effects of 1,25(OH)2D3 and PTH on STC1 mRNA levels in renal proximal tubular cells. Fig. 1A indicated that the opossum STC1 amino acid sequence have showed 83.7% homology with human STC1. More importantly, the deduced amino acid sequence of opossum STC1 has features perfectly conserved with the 11 cysteine residues and the unique N-linked glycosylation site of human STC1.(18–20) Although opossum STC1 showed a lower sequence identity of 35.3% sequence homology with opossum STC2, we found that the position of key cysteine residues and the N-linked glycosylation site are strictly conserved between opossum STC1 and opossum STC2 (Fig. 1B). These results consisted with our previous report for the identification of human STC2 cDNA sequence and the homology with human STC1.(11) It has been assumed that STC1 and STC2 have overlapping functions because of their similarities in primary amino acid sequence.(20,21) However, human STC1 inhibits calcium transport activity on gill in fish, but not human STC2.(3,22) Furthermore, high-affinity STC1-binding are observed on both the plasma membrane and mitochondria in nephron epitherial cells and liver hepatocytes, but, STC2 is unable or weakly capable of displacing STC1 in receptor binding assays.(23–28) These evidences strongly suggest that the biological roles of STC1 in mammals may differ from those of STC2.
We have previously reported STC2 expression is positively and negatively regulated by 1,25(OH)2D3 and PTH in the renal proximal tubular cells.(12) RT-PCR and real-time PCR analysis indicated that both STC1 and STC2 mRNA expressions were increased by 1,25(OH)2D3. These results could support previous in vivo experiments that 1,25(OH)2D3 could increase the STC1 expression in kidney.(13,29,30) Interestingly, we also found that PTH stimulated the STC1 mRNA expression in OK-P cells and rat kidney. In contrast, the STC2 gene expression in OK-P cells was down-regulated by PTH as well as our previous experiment.(12) Furthermore, we confirmed the decrease of NaPi-2a protein levels at 0.5 h after PTH treatment (Fig. 3D). Although hyperphosphatemia or hypercalcemia or calcitriol increases the STC1 gene expression,(13,29,30) we characterized that 1,25(OH)2D3 and PTH directly up-regulates the STC1 gene in real proximal tubular cells. Because 1,25(OH)2D3 and PTH have been well-known as major hormones regulating calcium and phosphate homeostasis in kidney and intestine,(31) therefore, our finding in this study about the up-regulation of STC1 gene by 1,25(OH)2D3 and PTH may be able to understand the physiological significance of STC1 as a calcium/phosphate-regulating hormone.
PTH binds to the PTH/PTH-related protein receptor on kidney cells and generates multiple second messengers including cAMP (which activates PKA), diacylglycerol (which activates PKC), inositol trisphosphate, and increased levels of intracellular calcium.(32,33) To determine the stimulatory mechanism of PTH on STC1 expression, we examined the signaling pathway of PKA and PKC using 8-bromo-cAMP and PMA, respectively. As shown in Fig. 4 B and C, PMA increased the STC1 mRNA levels, but decreased the STC2 mRNA levels in dose- and time-dependent manners. These data suggest that PTH up-regulates the STC1 expression mediated through the PKC activation in renal proximal tubular cells. Recently study has been reported that the STC1 expression in kidney is induced by hypertonicity such as NaCl or mannitol, and the activation of PKC, phospholipase C (PLC) and inositol 1,4,5-trisphosphate (IP3) is involved in the hypertonicity-dependent STC1 gene regulation.(34) More interestingly, they showed that the hypertonicity increased the STC1 mRNA levels in the cortex, but not in the inner medulla.(34) In fact, we also observed that after 3 h treatment with PTH in rat significantly increased the mRNA levels of STC1 in the cortex kidney but not in the medullar kidney (data not shown).
We finally examined whether or not the stability of the STC1 mRNA could be altered by treatment with 1,25(OH)2D3 and PMA. In Fig. 5 A and B, surprisingly there was no observation the suppression of the 1,25(OH)2D3 and PMA induced STC1 mRNA expression by ActD. Moreover, both of 1,25(OH)2D3 and PMA increased the stability of STC1 mRNA in the presence of ActD (Fig. 5 C and D). It has been reported that 1,25(OH)2D3 stabilizes the osteocalcin mRNA in ROS 17/2.8 cells and c-fms mRNA in HL-60 cells,(35,36) 5-lipoxygenase mRNA in the human monocytic cell line Mono Mac 6.(37) In this study, we can not explain how 1,25(OH)2D3 increases the STC1 mRNA in OK-P cells, however, there is an evidence that the stimulatory effect of Ca2+ on STC gene expression is due to mRNA stabilization in primary cultured trout corpuscles of Stannius cells. We now believe that the analysis of not only the promoter region but also the 3'-untranslated region of the STC1 gene is necessary to understand the molecular mechanism of STC1 gene regulation.
Importantly, the genome-wide association studies in the chronic kidney disease (CKD) patients has been identified STC1 and NaPi-2a (SLC34A1) as one of susceptibility loci in association with estimated glomerular filtration rate.(38,39) Recent study has also reported that STC1 has anti-inflammatory and renal protective actions in a model of anti-glomerular basement membrane glomerulonephritis.(40) Thus the suitable control of STC1 gene expression in kidney might be able to contribute provide both prevention and treatment for CKD.
In summary, we have identified a novel STC1 amino acid sequence in opossum and demonstrated that STC1 gene is directly up-regulation by 1,25(OH)2D3 and PTH in renal proximal tubular OK-P cells, and PTH can increase its renal expression in vivo. Furthermore, we have shown that 1,25(OH)2D3 and PMA regulates the STC1 gene in post-transcriptional levels.
Acknowledgments
This work was supported by the Ministry of Education, Science, Sports and Culture of Japan [grant numbers 16790526 (to H.Y.), 13470013 (to E.T.)]; and by the Human Nutritional Science on Stress Control 21st Century Center of Excellence Program (COE).
We thank Tomohiro Kagawa, Mari Tajiri, Nozomi Yokoyama (Department of Clinical Nutrition, Institute of Health Biosciences, University of Tokushima Graduate School, Tokushima, Japan) for technical assistance and Dr. Ken-Ichi Miyamoto (Department of Molecular Nutrition, Institute of Health Biosciences, University of Tokushima Graduate School, Tokushima, Japan) and Dr. Beate Lanske (Department of Developmental Biology, University of Harvard School of Dental Medicine, Boston, MA) for helpful discussions and comments.
Conflicts of Interest
The authors have nothing to disclose.
References
- 1.Butkus A, Roche PJ, Fernley RT, et al. Purification and cloning of a corpuscles of Stannius protein from Anguilla australis. Mol Cell Endocrinol. 1987;54:123–133. doi: 10.1016/0303-7207(87)90149-3. [DOI] [PubMed] [Google Scholar]
- 2.Wagner GF, Hampong M, Park CM, Copp DH. Purification, characterization, and bioassay of teleocalcin, a glycoprotein from salmon corpuscles of Stannius. Gen Comp Endocrinol. 1986;63:481–491. doi: 10.1016/0016-6480(86)90149-8. [DOI] [PubMed] [Google Scholar]
- 3.Lafeber FP, Flik G, Wendelaar Bonga SE, Perry SF. Hypocalcin from Stannius corpuscles inhibits gill calcium uptake in trout. Am J Physiol. 1988;254:R891–R896. doi: 10.1152/ajpregu.1988.254.6.R891. [DOI] [PubMed] [Google Scholar]
- 4.Lu M, Wagner GF, Renfro J. Stanniocalcin stimulates phosphate reabsorption by flounder renal proximal tubule in primary culture. Am J Physiol. 1994;267:R1356–R1362. doi: 10.1152/ajpregu.1994.267.5.R1356. [DOI] [PubMed] [Google Scholar]
- 5.Sundell K, Björnsson BT, Itoh H, Kawauchi H. Chum salmon (Oncorhynchus keta) stanniocalcin inhibits in vitro intestinal calcium uptake in Atlantic cod (Gadus morhua) J Comp Physiol B. 1992;162:489–495. doi: 10.1007/BF00264807. [DOI] [PubMed] [Google Scholar]
- 6.Varghese R, Wong CK, Deol H, Wagner G, DiMattia G. Comparative analysis of mammalian stanniocalcin genes. Endocrinology. 1998;139:4714–4725. doi: 10.1210/endo.139.11.6313. [DOI] [PubMed] [Google Scholar]
- 7.Haddad M, Roder S, Olsen HS, Wagner GF. Immunocytochemical localization of stanniocalcin cells in the rat kidney. Endocrinology. 1996;137:2113–2117. doi: 10.1210/endo.137.5.8612555. [DOI] [PubMed] [Google Scholar]
- 8.Wong CK, Ho MA, Wagner GF. The co-localization of stanniocalcin protein, mRNA and kidney cell markers in the rat kidney. J Endocrinol. 1998;158:183–189. doi: 10.1677/joe.0.1580183. [DOI] [PubMed] [Google Scholar]
- 9.Madsen KL, Tavernini MM, Yachimec C, et al. Stanniocalcin: a novel protein regulating calcium and phosphate transport across mammalian intestine. Am J Physiol. 1998;274:G96–G102. doi: 10.1152/ajpgi.1998.274.1.G96. [DOI] [PubMed] [Google Scholar]
- 10.Wagner GF, Vozzolo BL, Jaworski E, et al. Human stanniocalcin inhibits renal phosphate excretion in the rat. J Bone Miner Res. 1997;12:165–171. doi: 10.1359/jbmr.1997.12.2.165. [DOI] [PubMed] [Google Scholar]
- 11.Ishibashi K, Miyamoto K, Taketani Y, et al. Molecular cloning of a second human stanniocalcin homologue (STC2) Biochem Biophys Res Commun. 1998;250:252–258. doi: 10.1006/bbrc.1998.9300. [DOI] [PubMed] [Google Scholar]
- 12.Takei Y, Yamamoto H, Masuda M, Sato T, Taketani Y, Takeda E. Stanniocalcin 2 is positively and negatively controlled by 1,25(OH)2D3 and PTH in renal proximal tubular cells. J Mol Endocrinol. 2009;42:261–268. doi: 10.1677/JME-08-0161. [DOI] [PubMed] [Google Scholar]
- 13.Yahata K, Mori K, Mukoyama M, et al. Regulation of stanniocalcin 1 and 2 expression in the kidney by klotho gene. Biochem Biophys Res Commun. 2003;310:128–134. doi: 10.1016/j.bbrc.2003.08.131. [DOI] [PubMed] [Google Scholar]
- 14.Segawa H, Kaneko I, Takahashi A, et al. Growth-related renal type II Na/Pi cotransporter. J Biol Chem. 2002;277:19665–19672. doi: 10.1074/jbc.M200943200. [DOI] [PubMed] [Google Scholar]
- 15.Taketani Y, Segawa H, Chikamori M, et al. Regulation of type II renal Na+-dependent inorganic phosphate transporters by 1,25-dihydroxyvitamin D3. Identification of a vitamin D-responsive element in the human NAPi-3 gene. J Biol Chem. 1998;273:14575–14581. doi: 10.1074/jbc.273.23.14575. [DOI] [PubMed] [Google Scholar]
- 16.Tenenhouse HS. Regulation of phosphorus homeostasis by the type iia na/phosphate cotransporter. Annu Rev Nutr. 2005;25:197–214. doi: 10.1146/annurev.nutr.25.050304.092642. [DOI] [PubMed] [Google Scholar]
- 17.Nashiki K, Taketani Y, Takeichi T, et al. Role of membrane microdomains in PTH-mediated down-regulation of NaPi-IIa in opossum kidney cells. Kidney Int. 2005;68:1137–1147. doi: 10.1111/j.1523-1755.2005.00505.x. [DOI] [PubMed] [Google Scholar]
- 18.Roch GJ, Sherwood NM. Genomics reveal ancient forms of stanniocalcin in amphioxus and tunicate. Integr Comp Biol. 2010;50:86–97. doi: 10.1093/icb/icq010. [DOI] [PubMed] [Google Scholar]
- 19.Chang AC, Reddel RR. Identification of a second stanniocalcin cDNA in mouse and human: stanniocalcin 2. Mol Cell Endocrinol. 1998;141:95–99. doi: 10.1016/s0303-7207(98)00097-5. [DOI] [PubMed] [Google Scholar]
- 20.Moore EE, Kuestner RE, Conklin DC, et al. Stanniocalcin 2: characterization of the protein and its localization to human pancreatic alpha cells. Horm Metab Res. 1999;31:406–414. doi: 10.1055/s-2007-978764. [DOI] [PubMed] [Google Scholar]
- 21.Yoshiko Y, Aubin JE. Stanniocalcin 1 as a pleiotropic factor in mammals. Peptides. 2004;25:1663–1669. doi: 10.1016/j.peptides.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 22.Wagner GF, Dimattia GE. The stanniocalcin family of proteins. J Exp Zool A Comp Exp Biol. 2006;305:769–780. doi: 10.1002/jez.a.313. [DOI] [PubMed] [Google Scholar]
- 23.McCudden CR, James KA, Hasilo C, Wagner GF. Characterization of mammalian stanniocalcin receptors: mitochondrial targeting of ligand and receptor for regulation of cellular metabolism. J Biol Chem. 2002;277:45249–45258. doi: 10.1074/jbc.M205954200. [DOI] [PubMed] [Google Scholar]
- 24.McCudden CR, Majewski A, Chakrabarti S, Wagner GF. Co-localization of stanniocalcin-1 ligand and receptor in human breast carcinomas. Mol Cell Endocrinol. 2004;213:167–172. doi: 10.1016/j.mce.2003.10.042. [DOI] [PubMed] [Google Scholar]
- 25.Paciga M, McCudden CR, Londos C, DiMattia GE, Wagner GF. Targeting of big stanniocalcin and its receptor to lipid storage droplets of ovarian steroidogenic cells. J Biol Chem. 2003;278:49549–49554. doi: 10.1074/jbc.M307302200. [DOI] [PubMed] [Google Scholar]
- 26.Sazonova O, James KA, McCudden CR, Segal D, Talebian A, Wagner GF. Stanniocalcin-1 secretion and receptor regulation in kidney cells. Am J Physiol Renal Physiol. 2008;294:F788–F794. doi: 10.1152/ajprenal.00553.2007. [DOI] [PubMed] [Google Scholar]
- 27.Hasilo CP, McCudden CR, Gillespie JR, et al. Nuclear targeting of stanniocalcin to mammary gland alveolar cells during pregnancy and lactation. Am J Physiol Endocrinol Metab. 2005;289:E634–E642. doi: 10.1152/ajpendo.00098.2005. [DOI] [PubMed] [Google Scholar]
- 28.Luo CW, Kawamura K, Klein C, Hsueh AJ. Paracrine regulation of ovarian granulosa cell differentiation by stanniocalcin (STC) 1: mediation through specific STC1 receptors. Mol Endocrinol. 2004;18:2085–2096. doi: 10.1210/me.2004-0066. [DOI] [PubMed] [Google Scholar]
- 29.Honda S, Kashiwagi M, Ookata K, Tojo A, Hirose S. Regulation by 1alpha,25-dihydroxyvitamin D3 of expression of stanniocalcin messages in the rat kidney and ovary. FEBS Lett. 1999;459:119–122. doi: 10.1016/s0014-5793(99)01225-9. [DOI] [PubMed] [Google Scholar]
- 30.Ookata K, Tojo A, Onozato M, Kashiwagi M, Honda S, Hirose S. Distribution of stanniocalcin 1 in rat kidney and its regulation by vitamin D3. Exp Nephrol. 2001;9:428–435. doi: 10.1159/000052642. [DOI] [PubMed] [Google Scholar]
- 31.Beckerman P, Silver J. Vitamin D and the parathyroid. Am J Med Sci. 1999;317:363–369. doi: 10.1097/00000441-199906000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Muff R, Fischer JA, Biber J, Murer H. Parathyroid hormone receptors in control of proximal tubule function. Annu Rev Physiol. 1992;54:67–79. doi: 10.1146/annurev.ph.54.030192.000435. [DOI] [PubMed] [Google Scholar]
- 33.Tovey SC, Goraya TA, Taylor CW. Parathyroid hormone increases the sensitivity of inositol trisphosphate receptors by a mechanism that is independent of cyclic AMP. Br J Pharmacol. 2003;138:81–90. doi: 10.1038/sj.bjp.0705011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turner J, Xiang FL, Feng Q, Wagner GF. The renal stanniocalcin-1 gene is differentially regulated by hypertonicity and hypovolemia in the rat. Mol Cell Endocrinol. 2011;331:150–157. doi: 10.1016/j.mce.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 35.Mosavin R, Mellon WS. Posttranscriptional regulation of osteocalcin mRNA in clonal osteoblast cells by 1,25-dihydroxyvitamin D3. Arch Biochem Biophys. 1996;332:142–152. doi: 10.1006/abbi.1996.0326. [DOI] [PubMed] [Google Scholar]
- 36.Biskobing DM, Fan D, Rubin J. c-fms mRNA is regulated posttranscriptionally by 1,25(OH)2D3 in HL-60 cells. Calcif Tissue Int. 1997;61:205–209. doi: 10.1007/s002239900324. [DOI] [PubMed] [Google Scholar]
- 37.Rådmark O, Zhang YY, Hammarberg T, et al. 5-lipoxygenase: structure and stability of recombinant enzyme, regulation in Mono Mac 6 cells. Adv Prostaglandin Thromboxane Leukot Res. 1995;23:1–9. [PubMed] [Google Scholar]
- 38.Köttgen A, Glazer NL, Dehghan A, et al. Multiple loci associated with indices of renal function and chronic kidney disease. Nat Genet. 2009;41:712–717. doi: 10.1038/ng.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Köttgen A, Pattaro C, Böger CA, et al. New loci associated with kidney function and chronic kidney disease. Nat Genet. 2010;42:376–384. doi: 10.1038/ng.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang L, Garcia G, Lou Y, et al. Anti-inflammatory and renal protective actions of stanniocalcin-1 in a model of anti-glomerular basement membrane glomerulonephritis. Am J Pathol. 2009;174:1368–1378. doi: 10.2353/ajpath.2009.080476. [DOI] [PMC free article] [PubMed] [Google Scholar]