Abstract
Alzheimer’s disease is a devastating neurodegenerative disorder, the most common among the dementing illnesses. Acetaminophen has gaining importance in neurodegenerative diseases by attenuating the dopaminergic neurodegeneration in Caenorhabditis elegans model, decreasing the chemokines and the cytokines and increasing the anti apoptotic protein such as Bcl-2 in neuronal cell culture. The low concentration acetaminophen improved the facilitation to find the hidden platform in Morris Water Maze Test. Also some data suggest that acetaminophen could contribute in neurodegeneration. The present study was aimed to evaluate the effect of acetaminophen against colchicine induced cognitive impairment and oxidative stress in wistar rats. The cognitive learning and memory behaviour was assessed using step through passive avoidance paradigm and acetylcholine esterase activity. The parameters of oxidative stress were assessed by measuring the malondialdehyde, reduced glutathione and catalase levels in the whole brain homogenates. There was a significant memory improvement in the rats received acetaminophen treatment and it has also decreased the acetylcholine esterase enzyme level, confirming its nootropic activity. Acetaminophen neither increases nor decreases the reduced glutathione and catalase in the whole brain homogenates, showing that acetaminophen is devoid of any adverse effect on brain antioxidant defense system.
Keywords: Alzheimer’s disease, Acetaminophen, Learning and memory, colchicine
Introduction
Alzheimer’s disease (AD) is a devastating neurodegenerative disorder and its pathological hallmarks are senile plagues and neurofibrillary tangles.(1) Oxidative stress and free radicals are the central to the pathogenesis of AD by forming the neurofibrillary tangles causing irreversible cellular damage and neuronal dysfunction.(2) Impaired cortical cholinergic neurotransmission contributes to the amyloid-β (Aβ) pathology and increases the phosphorylation of tau protein.(3) The intracerebroventricular administration of colchicine in rats produces cognitive impairment and oxidative stress by disrupting the microtubule assembly and this method can be effectively used as an animal model for AD.(4–6) The most logical route toward therapeutic intervention for neurodegenerative diseases involves the identification of small molecules that have the ability to provide neuroprotection. For example, acetaminophen protects hippocampal neurons and PC12 cultures from Aβ peptide-induced oxidative stress through reduction of lipid peroxidation and by lowering cytoplasmic levels of peroxides.(7) Quinolinic acid, a neurotoxic metabolite implicated in the pathogenesis of neurodegenerative disease, is inhibited by administration of acetaminophen.(8) Acetaminophen also protects dopamingeric neurons in vitro from oxidative damage evoked by acute exposure to 6-hydroxydopamine or excessive levels of dopamine.(9) Acetaminophen has been shown to blunt neuronal apoptosis via reduction of the inflammatory transcription factor NF-κB and reduces the inflammatory protein such as chemokines and cytokines.(7) Acetaminophen also protects brain endothelial cells against oxidative stress.(10) So our aim is to determine nootropic activity of acetaminophen by measuring the acetyl cholinesterase activity in the rat brain homogenates and in vivo step through passive avoidance paradigm task and also to study its effect on brain antioxidant status in colchicine induced neurotoxicity in rats.
Materials and Methods
Animals
Wistar albino rats obtained from the Animal House of the KMCH College ofPharmacy, Coimbatore, and weighing 180–200 g. Following surgery, the animals were keptunder standard conditions of light and dark cycle with food and water ad libitum and cages with soft bedding. All the experiments were carried out between 09:00 and 15:00 h. All procedures described were reviewed and approved by the Institutional Animal Ethical Committee (IAEC).
Drug treatment
The administration of colchicine was considered as day 0. The standard drug, donepezil (2 mg/kg/day, p.o.), acetaminophen (15.1 mg/kg/day, p.o.), ascorbic acid monoglucoside (AsAG) (250 mg/kg/day, p.o.) were administered from day 1, 1 h before the experiments, until the end of the study period.
Colchicine-induced cognitive impairment
Surgery was performed according to a protocol previously described by Kumar and Gupta.(4) Colchicine was administered via the intracerebroventricular (i.c.v.) route. Briefly, the right lateral ventricle was cannulated in rats under ketamine (100 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) anaesthesia using stereotaxic coordinates, 0.6 mm posterior to the bregma, 1.8 mm right lateral and 2.7 mm below the cortical surface. Colchicine (15 µg/rat), dissolved in 15 µl of artificial cerebrospinal fluid (aCSF; in nM: NaCl 147, KCl 2.9, MgCl2 1.6, CaCl2 1.7 and dextrose 2.2), were slowly injected into the cannulated right lateral ventricle using a 20 µl Hamilton syringe and the needle was held in place for 2 min for proper dispersal of the drug from the tip. The animals were then divided into four groups of six each for treatment with aCSF, acetaminophen, AsAG and donepezil. Sham control groups were subjected to the same surgical procedure and received aCSF.
Behavioral assessment
Step-through passive avoidance apparatus.
The apparatus used for passive avoidance training consisted of two compartment box. An illuminated chamber (30 × 21 × 20 cm3) made of transparent plastic was connected by a guillotine door to the dark compartment (30 × 21 × 20 cm3) with black opaque walls and ceiling. The floors of the two compartments were constructed of stainless steel rods (3 mm in diameter, 10 mm apart) through which foot-shock could be delivered from a constant current source.
Training procedure.
All the experimental groups were first habituated to the apparatus. We placed a rat in the illuminated compartment and 10 s later the guillotine door was raised. Upon entering the dark compartment the door was closed and the rat was taken from the dark compartment into the home cage. The habituation trial was repeated after 30 min and followed by the same interval by the acquisition trial during which the guillotine door was closed and a 50 Hz, 1 mA constant current shock was applied for 2 s immediately after the animal had entered the dark compartment. In all experiments the rat was retrained in the apparatus and received a foot shock each time if it re entered the dark compartment in 120 s and if it happens for three times we excluded it from the experiment. Training was terminated when the rat remained in the light compartment for 120 consecutive seconds. All the groups were trained before day 0 and the transfer latencies were assessed in day 7, 14 and day 21.
Retention trial.
Recall of this inhibitory stimulus was evaluated on day 7, 14 and 21 post-training by returning the animals into the light compartment and recording their latency to enter the dark compartment (four paws in). If the animal had not entered to the dark compartment within 300 s, it was returned to its cage and a maximum latency of 300 s was recorded. This latency served as a measure of retention performance of the step-through avoidance response (retention latency).
Biochemical tests
Biochemical tests were carried out 24 h after the last behavioural test on day 21 following colchicine injections i.e. on day 22.
Tissue preparation.
Animals were sacrificed by decapitation and brains were removed and rinsed with ice-cold isotonic saline. Brain samples were then homogenized with 10 times (w/v) ice cold 0.1 M phosphate buffer (pH 7.4). The homogenates were centrifuged at 10,000 × g for 15 min and aliquots of supernatant were separated and used for biochemical estimation.
Measurement of catalase.
Catalase activity was measured by the method of Aebi.(11) A total of 0.1 ml of supernatant is added to cuvette containing 1.9 ml of 50 mM phosphate buffer (pH 7.0). The reaction started by the addition of 1 ml freshly prepared 30 mM H2O2. The rate of decomposition of H2O2 was measured spectrophotometrically from the changes in absorbance at 240 nm. The activity of catalase was expressed as U/mg protein.(4)
Estimation of reduced glutathione.
Glutathione was measured according to the method of Ellman.(12) The equal quantity of homogenate was mixed with 10% trichloroacetic acid and centrifuged to separate the proteins. To 0.1 ml of this supernatant, 2 ml of phosphate buffer (pH 8.4), 0.5 ml of 5,5-dithiobis (2-nitrobenzoic acid) and 0.4 ml of double distilled water were added. The mixture was vortexed and the absorbance was read at 412 nm within 15 min. The concentration of reduced glutathione was expressed as µg/g tissue.
Acetyl cholinesterase activity.
Acetyl cholinesterase activity is a marker of extended loss of the cholinergic system in the brain. The quantitative measurement of acetyl cholinesterase levels in brain was performed according to the method of Ellman et al.(13) The assay mixture contained 0.05 ml of supernatant, 3 ml of 0.01 M sodium phosphate buffer (pH 8), 0.1 ml of acetylthiocholine iodide and 0.1 ml of DTNB (Ellman reagent). The change in absorbance was measured immediately at 412 nm using spectrophotometer. Results were calculated using molar extinction coefficient of chromophore (1.36 × 104 M−1cm−1) and expressed as nmoles of acetyl choline hydrolysed/g tissue.
Lipid peroxidation.
The quantitative measurement of lipid peroxidation in brain was performed according to the method of Wills.(14) The amount of malondialdehyde, a measure of lipid peroxidation was measured by reaction with thiobarbituric acid at 532 nm using a Perkin Elmer lambda 20 spectrophotometer. The values were calculated using molar extinction coefficient of chromophore (1.56 × 105 M−1cm−1) and expressed as nmoles/g tissue.
Estimation of total protein.
The protein content of the brain homogenate was measured by biuret method using bovine serum albumin as standard.
Statistical analysis
The statistical analysis was performed by oneway ANOVA followed by Tukey’s test. p<0.05 is considered to be significant.
Results
Passive avoidance paradigm
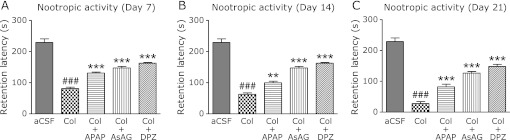
In the step through passive avoidance paradigm task on day 7, 14 and 21, significant decrease in the escape latency were found in the colchicine treated rats when compared with the rats received aCSF, whereas the rats treated with acetaminophen showed significant increase when compared with the cochicine treated rats on all the tested days. Similarly the rats treated with donepezil and AsAG also found to have significantly increased escape latency over colchicine group on day 7, 14 and 21 (Fig. 1A–C).
Fig. 1.
A–C, Results of step through passive avoidance test on days 7, 14 and 21 respectively. aCSF, rats treated with artificial cerebrospinal fluid; Col, rats treated with intracerebroventricular colchicine; Col + APAP, Col + AsAG and Col + DPZ, rats treated with intracerebroventricular colchicine followed by acetaminophen, ascorbic acid monoglucoside and donepezil respectively. Results are expressed as mean retention latency with standard error. ###p<0.001 vs Col, **p<0.01, ***p<0.001 vs aCSF (Oneway ANOVA followed by Tukey’s test).
Estimation of acetylcholine esterase enzyme
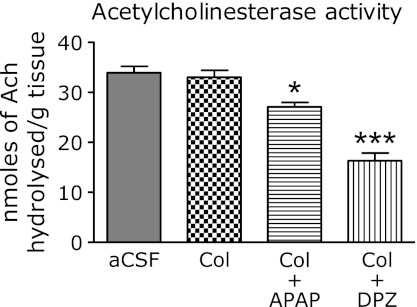
Central administration of colchicine did not impart any suppression of brain acetylcholine esterase levels as compared to the aCSF treated group, but the rats received acetaminophen had a significant decrease when compared with the colchicine and aCSF group. However the donepezil treated rats also had a significant decrease in acetylcholine esterase levels as compared to the colchicine treated rats (Fig. 2).
Fig. 2.
Determination of acetylcholinesterase activity as mentioned by nmoles of acetylcholine hydrolysed per g brain tissue. aCSF, rats treated with artificial cerebrospinal fluid; Col, rats treated with intracerebroventricular colchicine; Col + APAP and Col + DPZ, rats treated with intracerebroventricular colchicine followed by acetaminophen and donepezil respectively. Results are expressed as mean retention latency with standard error. *p<0.05, ***p<0.001 vs aCSF (Oneway ANOVA followed by Tukey’s test).
Estimation of reduced glutathione
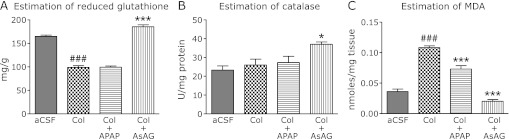
Central administration of colchicine caused a significant depletion of brain reduced glutathione as compared to the aCSF treated rats. The rats in the acetaminophen treatment group did not produce any significant change in the reduced glutathione as compared to the colchicine treated rats. The group receiving the AsAG showed a significant difference when compared with colchicine treated group (Fig. 3A).
Fig. 3.
Study of antioxidant parameters in the colchicine induced cognition impaired rats. A, estimation of reduced glutathione (mg/g tissue); B, estimation of catalase activity (Units/mg protein); C, measurement of MDA levels (nmoles/mg brain tissue). aCSF, rats treated with artificial cerebrospinal fluid; Col, rats treated with intracerebroventricular colchicine; Col + APAP and Col + AsAG, rats treated with intracerebroventricular colchicine followed by acetaminophen and ascorbic acid monoglucoside respectively. Results are expressed as mean retention latency with standard error. ###p<0.001 vs Col, ***p<0.001 vs aCSF (Oneway ANOVA followed by Tukey’s test).
Estimation of catalalse
Central administration of colchicine did not cause any depletion of brain catalase as compared to the aCSF treated rats. Similarly the rats treated with acetaminophen also did not produce any significant change in the catalase level as compared to the colchicine treated rats. Whereas the group receiving the AsAG showed a significant difference when compared with colchicine treated group (Fig. 3B).
Estimation of MDA
Treatment with colchicine caused a significant elevation in the brain MDA level as compared to the aCSF treated rats. The acetaminophen received rats showed significant reduction in MDA level as compared to the colchicine treated rats. Similarly the rats received the AsAG also showed a significant difference when compared with colchicine treated group (Fig. 3C).
Discussion
Study of anti-neuroinflammatory effects of drugs has become the major research in the treatment of AD.(15,16) The chronic administration of naproxen (20 and 40 mg/kg, p.o.) and valdecoxib (10 mg/kg, p.o.) significantly restored acetyl cholinesterase activity compared to colchicine-injected group.(17) An interesting clinical study suggests that treatment of pain with acetaminophen in nursing home residents with moderate-to-severe dementia facilitated engagement with the environment.(18) Effective management of pain can play an important part in the treatment of agitation and could reduce the number of unnecessary prescriptions for psychotropic drugs.(19) Acetaminophen use reduced pain behaviours associated with musculoskeletal pain in this sample of persons with dementia.(20) The anti-inflammatory effect of acetaminophen in AD has been reported by Landolfi et al.(21) All of these reports indicate the effectiveness of acetaminophen against behavioural protection in patients with dementia. Apart from the mechanism of pain reduction, there was an interesting report suggesting that the low concentration of acetaminophen improved the facilitation to find the hidden platform in Morris Water Maze test.(22) In the present study, intracerebroventricular administration of colchicine impaired the cognition by decreasing the escape latencies in the passive avoidance paradigm task. The chronic administration of acetaminophen (15.1 mg/kg, p.o.) had a significant difference in the escape latencies in the passive avoidance paradigm. Also acetaminophen had decreased the enzyme acetylcholine esterase in the whole brain homogenates showing that acetaminophen having nootropic activity. Thus the present study confirms the role of acetaminophen against the cognitive impairment induced by colchicine.
There are few negative reports regarding the consumption of glutathione for the metabolism of acetaminophen. Glutathione consuming effect of acetaminophen could reduce the plasma glutathione concentration. Subsequently, the plasma-derived glutathione supply to the CNS is reduced and the toxic effect of oxidative stress is amplified. New evidence suggests that glutathine cannot cross blood brain barrier(23) and thus the peripheral effect of acetaminophen on glutathione may not influence its level in the brain. In order to study the effect of acetaminophen on brain glutathione, we have measured its level in the brain homeogenate. The intracerebroventricular administration of colchicine decreased the GSH concentration in the whole brain homogenates. The decreased GSH concentration in the CNS would result in the declined defence against the oxidative stress and promote the neuron loss. The chronic administration of acetaminophen (15.1 mg/kg, p.o.) did not affect the brain GSH level, which shows that peripheral GSH is not the major source in the CNS and acetaminophen is not contributed in neurodegeneration by having any decreasing effect on GSH in the whole brain homogenates.
In addition we have measured the brain catalase levels to determine the effect of acetaminophen on brain antioxidant system. Catalase has a protective role as a hydrogen peroxide degrading enzyme. Inhibition of catalase activity with amino-triazole enhances the cytotoxicity of Alzheimer’s beta amyloid peptide suggests that catalase is involved in the protection of neuronal and non neuronal cells types.(24) Catalase was slightly inhibited (30%) in liver after acetaminophen administration in mice at a single dose of 375 mg/kg.(25) In the present study, acetaminophen (15.1 mg/kg, p.o.) neither increases nor decreases catalase in the whole brain homogenates, confirms that acetaminophen not contributed in neurodegeneration by having any decreasing effect on the enzyme catalase.
To confirm the effect of acetaminophen treatment on antioxidant defense system, we have also measured the brain MDA levels as a measure of lipid peroxidation. The rats treated with acetaminophen were protected from lipid peroxidation indicated by the low MDA levels which is comparable to the anti-lipid peroxidative effect of AsAG. These results suggest that the treatment with acetaminophen did not affect the brain antioxidant defense system and also protects it by preventing lipid peroxidation.
In summary, the present study confirms the nootropic activity of acetaminophen through increasing the escape latency and decreasing the acetyl cholinesterase activity in colchicine induced cognitive impairment in rats. And also suggesting that acetaminophen is devoid of any adverse effect on brain antioxidant system with a potential to prevent lipid peroxidation.
References
- 1.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 2.Perry G, Cash DA, Smith MA. Alzheimer disease and oxidative stress. J Biomed Biotechnol. 2002;2:120–123. doi: 10.1155/S1110724302203010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohandas E, Rajmohan V, Raghunath B. Neurobiology of Alzheimer’s disease. Indian J Psychaitry. 2009;51:55–61. doi: 10.4103/0019-5545.44908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Veerendra Kumar MH, Gupta YK. Intracerebroventricular administration of colchicine produces cognitive impairment associated with oxidative stress in rats. Pharmacol Biochem Behav. 2002;73:565–571. doi: 10.1016/s0091-3057(02)00838-9. [DOI] [PubMed] [Google Scholar]
- 5.Nakayama T, Sawada T. Involvement of microtubule integrity in memory impairment caused by colchicine. Pharmacol Biochem Behav. 2002;71:119–138. doi: 10.1016/s0091-3057(01)00634-7. [DOI] [PubMed] [Google Scholar]
- 6.Nakagawa Y, Nakamura S, Kaśe Y, Noguchi T, Ishihara T. Colchicine lesions in the rat hippocampus mimic the alterations of several markers in Alzheimer’s disease. Brain Res. 1987;408:57–64. doi: 10.1016/0006-8993(87)90358-1. [DOI] [PubMed] [Google Scholar]
- 7.Bisaglia M, Venezia V, Piccioli P, et al. Acetaminophen protects hippocampal neurons and PC12 cultures from amyloid beta-peptides induced oxidative stress and reduces NF-kappaB activation. Neurochem Int. 2002;41:43–54. doi: 10.1016/s0197-0186(01)00136-x. [DOI] [PubMed] [Google Scholar]
- 8.Maharaj H, Maharaj DS, Daya S. Acetylsalicylic acid and acetaminophen protect against oxidative neurotoxicity. Metab Brain Dis. 2006;21:189–199. doi: 10.1007/s11011-006-9012-7. [DOI] [PubMed] [Google Scholar]
- 9.Locke CJ, Fox SA, Caldwell GA, Caldwell KA. Acetaminophen attenuates dopamine neuron degeneration in animal models of Parkinson’s disease. Neurosci Lett. 2008;439:129–133. doi: 10.1016/j.neulet.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Tripathy D, Grammas P. Acetaminophen protects brain endothelial cells against oxidative stress. Microvasc Res. 2009;77:289–296. doi: 10.1016/j.mvr.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aebi H. Catalase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. Vol. II. Academic Press; New York: 1974. pp. 673–678. [Google Scholar]
- 12.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 13.Ellman GL, Courtney KD, Andres V, Jr., Feather-stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 14.Wills ED. Mechanism of lipid peroxide formation in animal tissues. Biochem J. 1996;99:667–676. doi: 10.1042/bj0990667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Liu J, Zhang Z, Bi P, Qi Z, Zhang C. Anti-neuroinflammation effect of ginsenoside Rbl in a rat model of Alzheimer disease. Neurosci Lett. 2011;487:70–72. doi: 10.1016/j.neulet.2010.09.076. [DOI] [PubMed] [Google Scholar]
- 16.Di Stefano A, Sozio P, Cerasa LS, et al. Ibuprofen and lipoic acid diamide as co-drug with neuroprotective activity: pharmacological properties and effects in beta-amyloid (1–40) infused Alzheimer’s disease rat model. Int J Immunopathol Pharmacol. 2010;23:589–599. doi: 10.1177/039463201002300221. [DOI] [PubMed] [Google Scholar]
- 17.Kumar A, Seghal N, Venketeshwara SP, Sreenivaslu PN. Differential effects of cyclooxygenase inhibitors on intracerebroventricular colchicine-induced dysfunction and oxidative stress in rat. Eur J Pharmacol. 2006;551:58–66. doi: 10.1016/j.ejphar.2006.08.076. [DOI] [PubMed] [Google Scholar]
- 18.Chibnall JT, Tait RC, Harman B, Luebbert RA. Effect of acetaminophen on behavior, well-being, and psychotropic medication use in nursing home residents with moderate-to-severe dementia. J Am Geriatr Soc. 2005;53:1921–1929. doi: 10.1111/j.1532-5415.2005.53572.x. [DOI] [PubMed] [Google Scholar]
- 19.Husebo BS, Ballard C, Sandvik R, Nilsen OB, Aarsland D. Efficacy of treating pain to reduce behavioural disturbances in residents of nursing homes with dementia: cluster randomised clinical trial. BMJ. 2011;343:d4065. doi: 10.1136/bmj.d4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elliott AF, Horgas AL. Effects of an analgesic trial in reducing pain behaviors in community-dwelling older adults with dementia. Nurs Res. 2009;58:140–145. doi: 10.1097/NNR.0b013e318199b599. [DOI] [PubMed] [Google Scholar]
- 21.Landolfi C, Soldo L, Polenzani L, et al. Inflammatory molecule release by beta-amyloid-treated T98G astrocytoma cells: role of prostaglandins and modulation by paracetamol. Eur J Pharmacol. 1998;360:55–64. doi: 10.1016/s0014-2999(98)00663-3. [DOI] [PubMed] [Google Scholar]
- 22.Ishida T, Sato T, Irifune M, Tanaka K, Nakamura N, Nishikawa T. Effect of acetaminophen, a cyclooxygenase inhibitor, on Morris water maze task performance in mice. J Psychopharmacol. 2007;21:757–767. doi: 10.1177/0269881107076369. [DOI] [PubMed] [Google Scholar]
- 23.Ye MX, Li JL. Acetaminophen and neural degeneration: is there a possible link? Med Hypotheses. 2010;74:390–391. doi: 10.1016/j.mehy.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 24.Milton NG. Inhibition of catalase activity with 3-amino-triazole enhanced the cytotoxicity of Alzheimer’s amyloid-beta peptide. Neurotoxicology. 2001;22:767–774. doi: 10.1016/s0161-813x(01)00064-x. [DOI] [PubMed] [Google Scholar]
- 25.Lores Arnaiz S, Llesuy S, Cutrín JC, Boveris A. Oxidative stress by acute acetaminophen administration in mouse liver. Free Radic Biol Med. 1995;19:303–310. doi: 10.1016/0891-5849(95)00023-q. [DOI] [PubMed] [Google Scholar]