Abstract
AIM: To evaluate the oncological outcomes of transanal local excision and the need for immediate conventional reoperation in the treatment of patients with high risk T1 rectal cancers.
METHODS: Twenty five high risk T1 rectal cancers treated by transanal local excision at the Guangdong General Hospital were analyzed retrospectively. Twelve patients received transanal local excision and 13 patients underwent subsequent immediate surgical rescue after transanal local excision within 4 wk. Differences in the local recurrence rates and 5-year overall survival rates between the two groups were analyzed. The prognostic value of immediate conventional reoperation for high risk T1 rectal cancers was also evaluated.
RESULTS: The median follow-up period was 62 mo. The local recurrence rates after transanal local excision for high risk T1 rectal cancer were 50%. By immediate conventional reoperation, the local recurrence rates were significantly reduced to 7.7%. The difference between these two groups was statistically significant (P = 0.030). Kaplan-Meier survival analysis showed a trend for decreased 5-year overall survival rates for patients treated by transanal local excision compared with immediate conventional reoperation (63% vs 89%).
CONCLUSION: Transanal local excision cannot be considered sufficient treatment for patients with high risk T1 rectal cancers. Immediate conventional reoperation should be performed if the pathology of the local excision is high risk.
Keywords: Rectal cancer, Transanal local excision, Immediate reoperation, Local recurrence, Overall survival
INTRODUCTION
With en bloc excision of the primary tumor and mesorectal lymph nodes, the abdominoperineal resection and low anterior resection have been considered as the gold standard treatments for rectal cancers because these operations led to excellent oncological outcomes with a significant decrease in local recurrence and a trend for improved overall survival[1-4]. However, the main disadvantages of these radical procedures include significant mortality and morbidity, as well as the necessity of permanent colostomy that may not be warranted for early rectal cancers which may be treated with local excision[5,6]. With less intraoperative blood loss[7], shorter length of hospital stay[8,9], lower postoperative mortality and morbidity[10,11], excellent maintenance of function[12,13] and avoidance of permanent colostomy[14,15], the benefits of local excision compared to radical surgery are significant. However, local excision carries the unavoidable risk of leaving untreated potential disease in the mesorectum and cannot provide adequate nodal staging because mesorectal lymph nodes are not removed and are therefore not pathologically assessed.
Selecting appropriate patients who can be treated by local excision without compromising oncological outcomes is a prerequisite for accepting local excision as a curative therapy. However, specific patient selection criteria remain incompletely defined. The role of local excision as a curative therapy in the treatment of patients with T1 rectal cancers is still controversial[16-18]. There is increasing evidence to suggest that local excision should be restricted to patients with low risk T1 rectal cancers[5,6,11,19]. In these strictly selected patients, local excision may be an acceptable alternative with equivalent oncological outcomes to radical surgery. In the treatment of patients with high risk T1 rectal cancers, the oncological adequacy of local excision has not been universally accepted and the efficacy of immediate conventional reoperation after local excision remains unclear. Therefore, the main objectives of this study were to evaluate the oncological outcomes of transanal local excision and the need for immediate surgical rescue in the treatment of patients with high risk T1 rectal cancers.
MATERIALS AND METHODS
Data of 25 patients with high risk T1 rectal cancers treated by transanal local excision were analyzed retrospectively. There were 14 men and 11 women, ranging in age from 43 to 87 years, with a median age of 63 years. The lesions were located 2-7 cm from the anal verge, with a median distance of 4 cm. The median tumor diameter was 3 (1-5) cm (Table 1). Immediate conventional reoperation (abdominoperineal resection or low anterior resection) was recommended for patients with high risk T1 rectal cancers. Therefore, 13 patients underwent subsequent surgical rescue after transanal local excision within 4 wk. However, 5 patients (4 patients were classified ASA score IV and 1 patient ASA score V) were unable to tolerate radical resection due to medical comorbidities and 7 patients would have required abdominoperineal resection but were opposed to permanent colostomy. These 12 patients only received transanal local excision. Thus, patients were divided into two groups: Group A (immediate conventional reoperation after transanal local excision) and Group B (transanal local excision). There were no significant differences according to age, gender, tumor location and tumor diameter between the two groups.
Table 1.
Clinical characteristics of the study group
| Clinical characteristics | |
| Surgical procedure | |
| Transanal local excision alone | 12 |
| Immediate reoperation | 13 |
| Gender | |
| Male | 14 |
| Female | 11 |
| Age (yr) | |
| Median | 63 |
| Range | 43-87 |
| Tumor location (cm) | |
| Median distance from the anal verge | 4 |
| Range | 2-7 |
| Tumor size (cm) | |
| Median | 3 |
| Range | 1-5 |
In this study, preoperative assessment included digital rectal examination, proctoscopy, chest X-ray, abdominal computed tomography (CT) scan, endorectal ultrasound (ERUS) and measurement of serum carcinoembryonic antigen (CEA) levels. ERUS was performed preoperatively in all the patients to assess the invasion depth and lymph node status. Abdominal CT scan was used to exclude distant metastases. The clinical stage of the tumors was I stage (T1N0M0). None of these patients received preoperative chemotherapy or radiotherapy. In Group A, two patients were identified with lymph node metastases after radical resection. These two patients were up-staged (IIIA stage, T1N1M0) and received postoperative adjuvant chemotherapy. In Group B, all patients received postoperative adjuvant chemoradiation because of these high risk features.
Transanal local excision was performed under general anesthesia using either the dorsal lithotomy or prone jackknife position. The lesions were removed using electrocautery to perform a full-thickness excision in all cases. The excised tumor specimens were pinned and oriented before submitting it to the pathologist. Histopathological observations, including depth of tumor invasion, margin status, histological grade and presence or absence of lymphovascular invasion, were performed whenever possible. In this study, histopathological examination confirmed that there were 18 poorly differentiated tumors. The surgical margin was positive in 11 cases and lymphovascular invasion was detected in 7 cases (Table 2). Tumors with poor differentiation or positive margin or lymphovascular invasion were defined as high risk tumors.
Table 2.
Histopathological characteristics of the patients between two groups
| Histopathological characteristics | Group A | Group B |
| Poorly differentiated | 1 | 6 |
| Poorly differentiated + positive margin | 5 | 2 |
| Positive margin | 3 | 1 |
| Lymphovascular invasion | 1 | 2 |
| Poorly differentiated + lymphovascular invasion | 3 | 1 |
Group A: Immediate conventional reoperation after transanal local excision; Group B: Transanal local excision alone.
Patients were followed at 3 mo intervals during the first postoperative year, biannually the second postoperative year and annually thereafter. Digital rectal examination, chest X-ray, abdominal ultrasound and measurement of serum CEA levels were performed at each patient visit. Additional postoperative surveillance, including abdominopelvic CT scan and colonoscopy, was performed annually. Local recurrence was defined as any tumor recurrence within the true pelvis.
The difference of local recurrence rates between the two groups was tested by the Fishers Exact Test. Mean survival time and 5-year overall survival rates were evaluated by Kaplan-Meier survival analysis and log-rank test was used to assess the statistical significance. A value of P < 0.05 was considered statistically significant.
RESULTS
In total, 25 patients with high risk T1 rectal cancers were treated by transanal local excision. In Group A, 8 patients underwent low anterior resection and 5 patients were offered abdominoperineal resection. Immediate surgical rescue was performed within 4 wk. Two patients were identified with lymph node metastases after radical resection. These two patients were up-staged (from I stage to IIIA stage). There was no postoperative mortality or severe complications in both groups.
The median follow-up period was 62 (14-140) mo. In Group A, 1 patient was found with local recurrence and unresectable lung metastases at 42 mo post-surgery. The patient received chemoradiotherapy only and died of the disease 10 mo later. In Group B, 6 patients had disease recurrence, of which 3 were local recurrence only, 2 local recurrence and hepatic metastases, and 1 local recurrence and lung metastases. Among the 3 patients who developed local recurrence only, 2 patients were able to have a successful salvage surgery to complete resection of their disease recurrence and were alive with no evidence of disease at the last follow up. The other patient underwent colostomy due to obstruction at 30 mo post-surgery and died of the disease 16 mo later. Three patients who developed local recurrence and distant recurrence died at 28, 35 and 38 mo post-surgery, respectively (Table 3). In total, local recurrence rates after transanal local excision alone for patients with high risk T1 rectal cancer were 50% (6 of 12 cases). By immediate conventional reoperation, the local recurrence rates were significantly reduced to 7.7% (1 of 13 cases). The difference between these two groups was statistically significant (P = 0.030).
Table 3.
Histopathological characteristics of patients with tumor recurrence
| Tumor differentiation | Margin status | Lymphovascular invasion | Group | Type of recurrence | Salvage therapy | Follow up (mo) | Remarks |
| Poor | Negative | Positive | A | Rectum lung | Chemoradiotherapy1 | 52 | Dead |
| Poor | Positive | Negative | B | Rectum liver | Chemotherapy1 | 38 | Dead |
| Poor | Positive | Negative | B | Rectum | APR | 52 | Alive |
| Poor | Negative | Positive | B | Rectum lung | Chemoradiotherapy1 | 28 | Dead |
| Moderate | Positive | Negative | B | Rectum | LAR | 48 | Alive |
| Moderate | Negative | Positive | B | Rectum liver | Chemotherapy1 | 35 | Dead |
| Well | Negative | Positive | B | Rectum | Colostomy1 | 46 | Dead |
Palliative. Group A: Immediate conventional reoperation after transanal local excision; Group B: Transanal local excision alone. APR: Abdominoperineal resection; LAR: Low anterior resection.
In this study, 4 patients with lymphovascular invasion developed tumor recurrences and all these recurrences were UICC IV. All these patients died of the disease within 4 years postoperatively. In Group B, 3 patients with positive margins were detected with disease recurrence. About 66.7% (2 of 3 cases) of these patients were able to have a successful salvage surgery and acquire acceptable oncological results (Table 3).
Kaplan-Meier survival analysis showed a trend for improvement in mean survival time (130.22 ± 9.22 mo, 95% CI: 112.15-148.29 mo vs 96.09 ± 13.58 mo, 95% CI: 69.48-122.70 mo) of the patients following immediate reoperation after transanal local excision over the patients treated by transanal local excision alone. Five-year overall survival rates of the patients in Group A were as high as 89%, while that of the patients in Group B were only 63%. However, the differences between these two groups were not statistically significant (log-rank, P = 0.126) (Figure 1).
Figure 1.
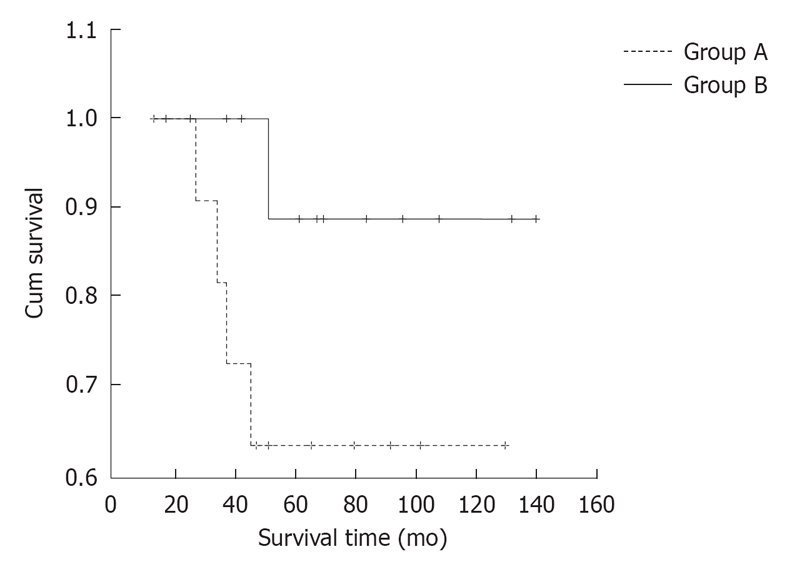
Survival time and overall survival rates for high risk T1 rectal cancers. Group A: Patients treated by transanal local excision alone; Group B: Patients underwent immediate conventional reoperation after transanal local excision.
DISCUSSION
The challenge in treating rectal cancers is selecting the proper approach for the appropriate patient. With excellent oncological outcomes, the anterior resection and abdominoperineal resection have been regarded as curative therapies for rectal cancers until now. However, as stated above, these operations are accompanied by significant mortality and morbidity, as well as the risk of permanent colostomy, which have led surgeons to search for less invasive, safer alternatives that yield similar oncological outcomes[20]. Compared to radical surgery, the benefits of local excision are clear. Postoperative complications are low, maintenance of function is excellent and permanent colostomy is avoided. Over the past three decades, the use of local excision for T1 rectal cancers has dramatically increased[21]. However, controversy also exists about whether local excision compromises the oncological outcomes of patients with T1 rectal cancers. Although limited available prospective trials revealed that oncological outcomes of the patients with T1 rectal cancers treated by local excision were comparable to that observed after radical surgery[22-24], multiple retrospective studies demonstrated that relatively high local recurrence rates were observed in the patients who underwent local excision for T1 rectal cancers[25]. Much of the apparent discrepancy is due to patient selection, which is far more rigid in prospective trials. It has been universally accepted that optimal candidates for local excision alone include mobile, low-lying, node negative on ERUS, occupying 40% or less of the rectal circumference, low risk (well to moderately differentiated, without lymphovascular invasion or microscopic involvement of the surgical margin) T1 rectal cancers. However, the oncological adequacy of local excision in the treatment of patients with high risk T1 rectal cancers lacks consensus and the efficacy of immediate surgical rescue after local excision remains unclear. Therefore, the main purpose of our study was to evaluate the oncological outcomes of transanal local excision for the patients with high risk T1 rectal cancers. The prognostic value of immediate conventional reoperation after transanal local excision was also evaluated.
In our study, local recurrence rates of patients with high risk T1 rectal cancers treated by transanal local excision alone were 50% (6 of 12 cases), considerably higher than those previously reported for radical surgery. What is the reason for the high local recurrence rates in our study Firstly, unfavorable histopathological features may be a possible explanation for the high local recurrence rates. Gopaul et al[26] reported that the incidence of local recurrence was significantly associated with histological grade of differentiation and margin status. It should be noted that clear margins are critical for transanal local excision. In our study, patients with positive margins after transanal local excision developed disease recurrence. However, clear margins cannot be wholly obtained by transanal local excision. Secondly, the presence of unresected regional lymph node metastases may be another major cause of local recurrence after transanal local excision. The operation cannot provide adequate nodal staging since it does not remove mesorectal lymph nodes, which will be positive in up to 18% of unselected T1 rectal cancers[27,28]. Among thirteen patients who underwent radical resection after transanal local excision in our study, two patients (15.4%) were identified with lymph node metastases. These two patients were up-staged. Thirdly, the possible reason is the shedding and implantation of tumor cells into the surgical excision site that may contribute to local recurrence[29]. Therefore, irrigation of the surgical field prior to closure is recommended in order to improve local control after local excision.
Borschitz et al[19] reported that immediate reoperation after local excision of T1 rectal cancers with unfavorable histological finding could avoid local recurrences. However, awaiting recurrences would lead to bad oncological outcomes with high local recurrences and low survival rates. In our study, we found the local recurrence rates were significantly decreased to 7.7% (1 of 13 cases, P = 0.030) by immediate conventional reoperation. We also found a trend for decreased 5-year overall survival rates for patients treated by transanal local excision compared with immediate conventional reoperation (63% vs 89%). The results showed that the significant increase in local recurrence and the trend for decreased overall survival were insufficient to accept transanal local excision as curative therapy for patients with high risk T1 rectal cancers. By immediate conventional reoperation, the local recurrence rates could be significantly reduced and overall survival rates could be improved to a level similar to initial radical surgery. Therefore, we conclude that transanal local excision could not be considered sufficient treatment for patients with high risk T1 rectal cancers. Immediate conventional reoperation should be performed if the pathology of the local excision is high risk. For patients who are unable to undergo radical surgery or decline a permanent colostomy, transanal local excision is also an acceptable alternative. However, patients should be preoperatively informed of the increased risk of local recurrence and possible need for further salvage surgery.
COMMENTS
Background
The challenge in treating rectal cancers is selecting the proper approach for the appropriate patient. With excellent oncological outcomes, the anterior resection and abdominoperineal resection have been regarded as curative therapies for rectal cancers until now. However, these operations are accompanied by significant mortality and morbidity, as well as the risk of permanent colostomy, which have led surgeons to search for less invasive, safer alternatives that yield similar oncological outcomes. Compared to radical surgery, the benefits of local excision are clear. Postoperative complications are low, maintenance of function is excellent and permanent colostomy is avoided. Over the past three decades, the use of local excision for T1 rectal cancers has dramatically increased. However, controversy also exists about whether local excision compromises the oncological outcomes of patients with T1 rectal cancers.
Research frontiers
There is increasing evidence to suggest that local excision should be restricted to patients with low risk T1 rectal cancers. In these strictly selected patients, local excision may be an acceptable alternative, with equivalent oncological outcomes to radical surgery.
Innovations and breakthroughs
The oncological adequacy of local excision in the treatment of patients with high risk T1 rectal cancers lacks consensus and the efficacy of immediate surgical rescue after local excision remains unclear. Therefore, the main purpose of our study was to evaluate the oncological outcomes of transanal local excision for the patients with high risk T1 rectal cancers. The prognostic value of immediate conventional reoperation after transanal local excision was also evaluated.
Applications
In this study, the authors conclude that transanal local excision cannot be considered sufficient treatment for patients with high risk T1 rectal cancers. Immediate conventional reoperation should be performed if the pathology of the local excision is high risk.
Terminology
Tumors with poor differentiation or positive margin or lymphovascular invasion were defined as high risk tumors.
Peer review
The authors evaluated the oncological outcomes of transanal local excision and the need for immediate conventional reoperation in the treatment of patients with high risk T1 rectal cancers. This manuscript will be interesting for the readers.
Footnotes
Supported by The Guangdong WST Foundation of China, No. 2000112736580706003
Peer reviewers: Antonio Macrì, Associate Professor, Department of Human Pathology, General Surgery Unit, University of Messina, Via Consolare Valeria, 98125 Messina, Italy; Imtiaz Ahmed Wani, MD, Amira Kadal, Srinagar, Kashmir 190009, India; John Griniatsos, MD, Assistant Professor, Department of Surgery, University of Athens, Medical School, 1st LAIKO Hospital, 17 Agiou Thoma str, GR 115-27, Athens, Greece
S- Editor Wang JL L- Editor Roemmele A E- Editor Zheng XM
References
- 1.Silberfein EJ, Kattepogu KM, Hu CY, Skibber JM, Rodriguez-Bigas MA, Feig B, Das P, Krishnan S, Crane C, Kopetz S, et al. Long-term survival and recurrence outcomes following surgery for distal rectal cancer. Ann Surg Oncol. 2010;17:2863–2869. doi: 10.1245/s10434-010-1119-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Law WL, Chu KW. Anterior resection for rectal cancer with mesorectal excision: a prospective evaluation of 622 patients. Ann Surg. 2004;240:260–268. doi: 10.1097/01.sla.0000133185.23514.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiappa A, Biffi R, Zbar AP, Luca F, Crotti C, Bertani E, Biella F, Zampino G, Orecchia R, Fazio N, et al. Results of treatment of distal rectal carcinoma since the introduction of total mesorectal excision: a single unit experience, 1994-2003. Int J Colorectal Dis. 2005;20:221–230. doi: 10.1007/s00384-004-0670-9. [DOI] [PubMed] [Google Scholar]
- 4.Bernardshaw SV, Øvrebø K, Eide GE, Skarstein A, Røkke O. Treatment of rectal cancer: reduction of local recurrence after the introduction of TME - experience from one University Hospital. Dig Surg. 2006;23:51–59. doi: 10.1159/000093494. [DOI] [PubMed] [Google Scholar]
- 5.Blackstock W, Russo SM, Suh WW, Cosman BC, Herman J, Mohiuddin M, Poggi MM, Regine WF, Saltz L, Small W, et al. ACR Appropriateness Criteria: local excision in early-stage rectal cancer. Curr Probl Cancer. 2010;34:193–200. doi: 10.1016/j.currproblcancer.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Bretagnol F, Rullier E, George B, Warren BF, Mortensen NJ. Local therapy for rectal cancer: still controversial. Dis Colon Rectum. 2007;50:523–533. doi: 10.1007/s10350-006-0819-4. [DOI] [PubMed] [Google Scholar]
- 7.Suppiah A, Maslekar S, Alabi A, Hartley JE, Monson JR. Transanal endoscopic microsurgery in early rectal cancer: time for a trial. Colorectal Dis. 2008;10:314–327; discussion 327-329. doi: 10.1111/j.1463-1318.2007.01448.x. [DOI] [PubMed] [Google Scholar]
- 8.Koebrugge B, Bosscha K, Ernst MF. Transanal endoscopic microsurgery for local excision of rectal lesions: is there a learning curve. Dig Surg. 2009;26:372–377. doi: 10.1159/000257228. [DOI] [PubMed] [Google Scholar]
- 9.Lebedyev A, Tulchinsky H, Rabau M, Klausner JM, Krausz M, Duek SD. Long-term results of local excision for T1 rectal carcinoma: the experience of two colorectal units. Tech Coloproctol. 2009;13:231–236. doi: 10.1007/s10151-009-0521-3. [DOI] [PubMed] [Google Scholar]
- 10.Tarantino I, Hetzer FH, Warschkow R, Zünd M, Stein HJ, Zerz A. Local excision and endoscopic posterior mesorectal resection versus low anterior resection in T1 rectal cancer. Br J Surg. 2008;95:375–380. doi: 10.1002/bjs.6133. [DOI] [PubMed] [Google Scholar]
- 11.Nastro P, Beral D, Hartley J, Monson JR. Local excision of rectal cancer: review of literature. Dig Surg. 2005;22:6–15. doi: 10.1159/000084345. [DOI] [PubMed] [Google Scholar]
- 12.Geisler DP. Local treatment for rectal cancer. Clin Colon Rectal Surg. 2007;20:182–189. doi: 10.1055/s-2007-984862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Touzios J, Ludwig KA. Local management of rectal neoplasia. Clin Colon Rectal Surg. 2008;21:291–299. doi: 10.1055/s-0028-1089945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saito N, Ono M, Sugito M, Ito M, Morihiro M, Kosugi C, Sato K, Kotaka M, Nomura S, Arai M, et al. Early results of intersphincteric resection for patients with very low rectal cancer: an active approach to avoid a permanent colostomy. Dis Colon Rectum. 2004;47:459–466. doi: 10.1007/s10350-003-0088-4. [DOI] [PubMed] [Google Scholar]
- 15.Grimard L, Stern H, Spaans JN. Brachytherapy and local excision for sphincter preservation in T1 and T2 rectal cancer. Int J Radiat Oncol Biol Phys. 2009;74:803–809. doi: 10.1016/j.ijrobp.2008.08.075. [DOI] [PubMed] [Google Scholar]
- 16.Endreseth BH, Myrvold HE, Romundstad P, Hestvik UE, Bjerkeset T, Wibe A. Transanal excision vs. major surgery for T1 rectal cancer. Dis Colon Rectum. 2005;48:1380–1388. doi: 10.1007/s10350-005-0044-6. [DOI] [PubMed] [Google Scholar]
- 17.Madbouly KM, Remzi FH, Erkek BA, Senagore AJ, Baeslach CM, Khandwala F, Fazio VW, Lavery IC. Recurrence after transanal excision of T1 rectal cancer: should we be concerned. Dis Colon Rectum. 2005;48:711–719; discussion 719-721. doi: 10.1007/s10350-004-0666-0. [DOI] [PubMed] [Google Scholar]
- 18.Paty PB, Nash GM, Baron P, Zakowski M, Minsky BD, Blumberg D, Nathanson DR, Guillem JG, Enker WE, Cohen AM, et al. Long-term results of local excision for rectal cancer. Ann Surg. 2002;236:522–529; discussion 529-530. doi: 10.1097/00000658-200210000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borschitz T, Gockel I, Kiesslich R, Junginger T. Oncological outcome after local excision of rectal carcinomas. Ann Surg Oncol. 2008;15:3101–3108. doi: 10.1245/s10434-008-0113-x. [DOI] [PubMed] [Google Scholar]
- 20.Stamos MJ, Murrell Z. Management of early rectal T1 and T2 cancers. Clin Cancer Res. 2007;13:6885s–6889s. doi: 10.1158/1078-0432.CCR-07-1150. [DOI] [PubMed] [Google Scholar]
- 21.You YN, Baxter NN, Stewart A, Nelson H. Is the increasing rate of local excision for stage I rectal cancer in the United States justified: a nationwide cohort study from the National Cancer Database. Ann Surg. 2007;245:726–733. doi: 10.1097/01.sla.0000252590.95116.4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell AH, Harris J, Rosenberg PJ, Sause WT, Fisher BJ, Hoffman JP, Kraybill WG, Byhardt RW. Anal sphincter conservation for patients with adenocarcinoma of the distal rectum: long-term results of radiation therapy oncology group protocol 89-02. Int J Radiat Oncol Biol Phys. 2000;46:313–322. doi: 10.1016/s0360-3016(99)00440-x. [DOI] [PubMed] [Google Scholar]
- 23.Steele GD, Herndon JE, Bleday R, Russell A, Benson A, Hussain M, Burgess A, Tepper JE, Mayer RJ. Sphincter-sparing treatment for distal rectal adenocarcinoma. Ann Surg Oncol. 1999;6:433–441. doi: 10.1007/s10434-999-0433-5. [DOI] [PubMed] [Google Scholar]
- 24.Greenberg JA, Shibata D, Herndon JE, Steele GD, Mayer R, Bleday R. Local excision of distal rectal cancer: an update of cancer and leukemia group B 8984. Dis Colon Rectum. 2008;51:1185–1191; discussion 1191-1194. doi: 10.1007/s10350-008-9231-6. [DOI] [PubMed] [Google Scholar]
- 25.Mellgren A, Sirivongs P, Rothenberger DA, Madoff RD, García-Aguilar J. Is local excision adequate therapy for early rectal cancer. Dis Colon Rectum. 2000;43:1064–1071; discussion 1071-1074. doi: 10.1007/BF02236551. [DOI] [PubMed] [Google Scholar]
- 26.Gopaul D, Belliveau P, Vuong T, Trudel J, Vasilevsky CA, Corns R, Gordon PH. Outcome of local excision of rectal carcinoma. Dis Colon Rectum. 2004;47:1780–1788. doi: 10.1007/s10350-004-0678-9. [DOI] [PubMed] [Google Scholar]
- 27.Nascimbeni R, Burgart LJ, Nivatvongs S, Larson DR. Risk of lymph node metastasis in T1 carcinoma of the colon and rectum. Dis Colon Rectum. 2002;45:200–206. doi: 10.1007/s10350-004-6147-7. [DOI] [PubMed] [Google Scholar]
- 28.Blumberg D, Paty PB, Guillem JG, Picon AI, Minsky BD, Wong WD, Cohen AM. All patients with small intramural rectal cancers are at risk for lymph node metastasis. Dis Colon Rectum. 1999;42:881–885. doi: 10.1007/BF02237095. [DOI] [PubMed] [Google Scholar]
- 29.Gimbel MI, Paty PB. A current perspective on local excision of rectal cancer. Clin Colorectal Cancer. 2004;4:26–35; discussion 36-37. doi: 10.3816/ccc.2004.n.007. [DOI] [PubMed] [Google Scholar]


