Abstract
Obstructive sleep apnea (OSA) and diabetes mellitus are both highly prevalent disorders. There has been a recent recognition of an association between insulin resistance and sleep apnea. Continuous positive airway pressure (CPAP) has emerged as an effective therapy for treatment of OSA and has been shown to positively influence numerous pathophysiological factors that contribute to cardiovascular risk. There is emerging data that explores the influence of CPAP therapy, insulin sensitivity and glycemic control. In the current review, we examine this literature critically and formulate a synopsis that summarizes the current knowledge in this field.
Keywords: Obstructive sleep apnea, Continuous positive airway pressure, Diabetes, Metabolic syndrome
INTRODUCTION
Obstructive sleep apnea (OSA) is characterized by episodic and repetitive upper airway narrowing during sleep that leads to a well recognized clinical syndrome of snoring and excessive daytime sleepiness[1]. OSA has been independently associated with hypertension and cardiovascular disease[2]. A high prevalence rate is being increasingly recognized, particularly in patients with metabolic syndrome, and several studies have suggested an independent association between OSA and insulin resistance (IR) and glycemic control[3-11].
EPIDEMIOLOGY
A recent study from France followed 806 elderly subjects over 7 years and found an independent association between sleep apnea and metabolic syndrome, even after correcting for age, gender and obesity[11]. A prospective study from Japan that spanned five years, examined nocturnal oximetry in over 4000 patients and calculated that a multivariable-adjusted hazard ratio (95% CI) for developing type 2 diabetes was 1.69 (1.04-2.76) among those with moderate to severe nocturnal intermittent hypoxia[12]. A Veterans Affairs based observational cohort study examined 1233 consecutive patients referred for evaluation of OSA, of whom 544 were free from pre-existing diabetes. At a median follow-up time of 2.7 years, they found a multivariable-adjusted hazard ratio of 1.43 per quartile for incident diabetes[13]. In a case control study from Japan, Kono et al[14] found that OSA severity as assayed by apnea-hypopnea index (AHI) was a strong predictor of a number of metabolic syndrome parameters, such as hypertension, hyperglycemia and dyslipidemia, while body mass index and lowest arterial oxygen saturation during sleep did not. A British study involving patients with type 2 diabetes and the use of structured questionnaires and overnight oximetry found a 23% prevalence of OSA[15]. The Wisconsin sleep study found an increased incidence of diabetes in patients with OSAS, but the significance of OSAS disappeared after accounting for obesity[16]. In examining results from the Sleep Heart Health study involving 2656 subjects, Punjabi et al[9] found a relationship between OSA severity as measured by both AHI and degree of sleep-related oxygen desaturations and IR. More recently, the same investigators used an intravenous glucose tolerance test in a cohort of patients with sleep apnea and without diabetes mellitus and found that OSA, independent of adiposity, is associated with impairments in insulin sensitivity, glucose effectiveness and pancreatic β-cell function[17]. Similarly, in a study involving 270 subjects referred for polysomnography who did not have known diabetes mellitus, Ip et al[18] found that there was a strong association of OSA and insulin resistance in both obese and non-obese subjects and that both AHI and minimum oxygen saturation were independent determinants of insulin resistance.
PATHOPHYSIOLOGY
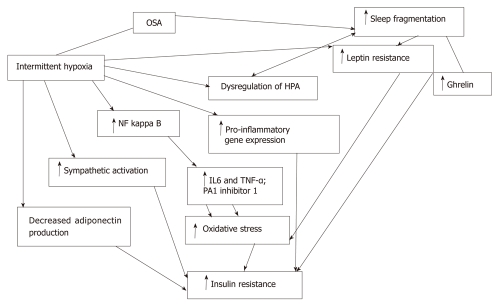
A variety of putative pathogenetic mechanisms have been described and studied that explores the interplay between intermittent hypoxia, sleep fragmentation and insulin resistance (Figure 1).
Figure 1.
Flow diagram demonstrating interplay between intermittent hypoxia and sleep fragmentation, and insulin resistance. OSA: Obstructive sleep apnea; NFκB: Nuclear factor kappa B; PA1: Plasminogen activator inhibitor 1; HPA: Hypothalamopituitary axis; IL: Interleukin; TNFα: Tumor necrosis factor-alpha; ISI: Insulin sensitivity index.
Hypoxia has been shown to induce a multitude of effects on adipocytes, including inflammatory activation, transcription of genes regulated by the hypoxia inducible factor-1 and endoplasmic reticulum stress[19,20]. Adiponectin is an important adipokine with protective effects against insulin resistance. At least two studies have examined the behavior of adipocytes exposed to in vitro intermittent hypoxia and have shown less adiponectin production, despite a significant upregulation of adiponectin mRNA expression[21,22]. Sleep disruption is an additional, potentially important mechanism by which OSA may affect metabolism[23]. In healthy subjects, sleep restriction was associated with IR, increased appetite and craving for carbohydrates[24,25]. Disruption of normal sleep architecture has also been shown to induce a pro-inflammatory state, with increased release of interleukin (IL)-6 and tumor necrosis factor (TNF)-α by circulating monocytes[26]. Sleep apnea has also been postulated to lead to dysregulation of the hypothalamo-pituitary axis and this may well play a central role in modulating insulin resistance and a predisposition to diabetes mellitus[27]. Nocturnal awakenings have been shown to be associated with pulsatile cortisol release[28] and autonomic activation.
EFFECT OF CPAP THERAPY ON IR
Continuous positive airway pressure (CPAP) has emerged as an effective therapy for OSA. In view of the evidence that OSA can lead to insulin resistance and abnormality in glucose metabolism, studies have been done by several investigators to see if CPAP therapy, in addition to eliminating apnea, hypopnea, desaturation and sympathetic surge in turn, can lead to improvement in insulin resistance and blood glucose control. We will undertake a review of the literature as it pertains to the effect of CPAP in blood sugar control and insulin resistance.
EFFECT OF CPAP ON GLUCOSE METABOLISM
A multitude of studies have explored the influence of CPAP therapy on blood sugar control. Many of these have had issues with study design, including the lack of a control group and small sample size. These studies are summarized in Table 1.
Table 1.
Effect of continuous positive airway pressure on blood glucose control
| Study | Study design/ cohort | Sample size | Control group | Outcome/ measurements | Study duration | Conclusions | +/- |
| Stoohs et al[29] | OSA patients | 5 | None | Fasting glucose and insulin | 2 mo | No change in either fasting or nocturnal insulin level | _ |
| Increase in nocturnal and fasting glucose | |||||||
| Saini et al[30] | OSA patients BMI 32.7 ± 2.3 Kg/m2 | 8 | None | Glucose and insulin every 10 min interval during sleep | 1 night | Mean insulin and glucose did not differ between pre treatment and treatment night | - |
| Davies et al[31] | OSA patients | 10 | Matched control | Fasting insulin, lipid profile | 3 mo | No change in insulin level with CPAP | - |
| Brooks et al[32] | OSA patients with BMI 42.7 ± 4.3 kg/m2 | 10 | None | Hyperinsulinemic euglycemic clamp | 4 mo | Improvement in insulin responsiveness seen | + |
| Cooper et al[33] | OSA patients | 6 | None | Insulin and c-peptide sample every hour and glucose sample every 30 min during sleep | 1 night | No changes in glucose, insulin and C-peptide with CPAP treatment | - |
| Saarelainen et al[34] | OSA patients | 7 | None | Hyperinsulinemic euglycemic clamp | 3 mo | No change in insulin responsiveness | _ |
| Pierzchala et al[35] (article in Polish) | Type 1 and type 2 diabetes patients with OSA | 30 | None | Blood glucose | 6 mo | Better blood glucose control | + |
| Chin et al[36] | OSA patients | 22 | OSA patients | Oral glucose tolerance test with insulin measurement | 6 mo | No change in glucose and insulin level except in patients who have lost weight | _ |
| Ip et al[37] | OSA patients | 30 | Matched 30 non-OSA control | Fasting glucose and insulin | 6 mo | No change in fasting glucose and insulin seen (decrease in Leptin and triglyceride was seen) | _ |
| Smurra et al[38] | 16 OSA patients; 10 from endocrine clinic and 6 other OSA patients | 16 | None | Oral glucose tolerance test in 10 patients and hyperinsulinemic euglycemic clamp in 6 patients | 2 mo | No change in mean glycemia, insulin level or insulin responsiveness was seen | _ |
| Harsch et al[39] | OSA patients | 40 | None | Hyperinsulinemic euglycemic clamp | 3 mo | Improvement in insulin sensitivity at day 2 and 3 mo, in patient with BMI < 30, than in patients with BMI > 30 | + |
| Harsch et al[40] | Type 2 diabetes patients with OSA | 9 | None | Hyperinsulinemic euglycemic clamp | 3 mo | Insulin sensitivity was unchanged after 2 days, but significantly improved after 3 mo; glycemic control was unaffected after 3 mo | +/- |
| Babu et al[41] | Type 2 diabetes patients with OSA | 25 | None | HBA1c and post prandial blood glucose | 3 mo | Decrease in HBA1c and postprandial am glucose level | + |
| Czupryniak et al[42] | Non diabetic OSA patients | 9 | None | Continuous glucose monitoring, plasma insulin, HOMA-IR | 1 night | Mean blood glucose, fasting insulin and HOMA-IR were significantly higher with CPAP treatment | - |
| Hassaballa et al[43] | Type 2 diabetes and OSA (retrospective) | 38 | None | HBA1c | Approx. 3 mo | Decrease in HBA1c was seen with CPAP therapy | + |
| Lindberg et al[44] | OSA patients | 28 | Matched control without OSA | HOMA and fasting insulin | 6 mo | Decrease in insulin resistance and fasting insulin | + |
| West et al[45] | Type 2 diabetes and OSA | 42 | Randomized, double blind | HOMA, hyperinsulinaemic euglycemic clamp, HBA1c, highly sensitive C-reactive protein | 3 mo | No change in glycemic control or insulin resistance | _ |
| Coughlin et al[46] | OSA patients | 34 | Randomized placebo-controlled blinded crossover trial | Insulin, fasting glucose, HOMA-IR | 6 wk | No change in glucose or insulin resistance | _ |
| Pallayova et al[47] | Type 2 diabetes with OSA | 14 | None | Continuous glucose monitoring | Several d | Reduction in nocturnal glucose variability and improved overnight glucose control | + |
| Wang et al[48] | Type 2 diabetes and OSA | 30 | None | HOMA | 7 d | Improve ISI | + |
| Dawson et al[49] | Type 2 diabetes with OSA | 20 | None | Continuous glucose monitoring | Average 41 d (26-96 d) | Decrease in sleeping blood glucose seen | + |
| Steiropoulos et al[50] | Diabetes with OSA | 56 | None | HBA1c, fasting glucose, insulin level, HOMA-IR | 6 mo | Only patients with CPAP use > 4 h/night showed decrease in HBA1c | +/- |
| Wei et al[51] | OSA patients | 11 | None | Fasting blood glucose, plasma insulin, HOMA-IR | 4 d | Decrease in blood glucose and increase in insulin sensitivity seen | + |
| Oktay et al[52] | OSA and metabolic syndrome | 20 | None | Fasting blood glucose | 1 yr | No difference in blood glucose seen | _ |
| Lam et al[53] | OSA patients | 61 (30 control and 31 study group) | Sham CPAP | Short insulin tolerance test | 12 wk | Improvement in insulin sensitivity seen only in subjects with BMI ≥ 25 | +/- |
| Garcia et al[54] | Obese OSA patients | 20 | None | OGTT, insulin level, Gherlin, adiponectin, leptin | 6 mo | Increase insulin and IR; gherlin decrease, whereas leptin and adiponectin remains unchanged | - |
| Shpirer et al[55] | OSA patients | 30 | None | HBA1c | 3-5 mo | Decrease in HBA1c in severe OSA patients | + |
HOMA-IR: Homeostatic model assessment of insulin resistance; HBA1c: Glycosylated Hemoglobin; ISI: Insulin sensitivity index; OGTT: Oral glucose tolerance test; BMI: Body mass index; OSA: Obstructive sleep apnea; CPAP: Continuous positive airway pressure.
CONCLUSION
There is a strong association between OSA and diabetes mellitus. A multitude of pathophysiological perturbations have been demonstrated both in vitro and in vivo that demonstrate a close interrelationship, including inflammatory mediators of oxidative stress, as well as leptin resistance and hypothalamo-pituitary axis dysregulation. These effects are mediated secondary to both the effect of intermittent hypoxia as well as sleep fragmentation. Treatment with nightly CPAP leads to a resolution of both these behaviors and has been shown to be effective, not only in resolving daytime sleepiness, but also improving cardiovascular mortality. The data on the impact, if any, and its magnitude on glycemic control are neither convincing nor clear. The few randomized trials that have explored this impact have had mixed results, moreover confounded by the influence of morbid obesity as well as lack of optimal CPAP compliance. Future research is needed to clarify both the downstream mechanisms that stem from sleep fragmentation and the oxidative stress of intermittent hypoxia, as well as the impact of complete resolution of OSA with adequate CPAP compliance (adequately powered and randomized controlled design) on the metabolic profile of patients with OSA and diabetes mellitus in both obese and non-obese cohorts.
Footnotes
Peer reviewer: Dr. Armin Rashidi, Department of Internal Medicine, Eastern Virginia Medical School, 825 Fairfax Avenue, Ste 410, Norfolk, VA 23507, United States
S-Editor Wu X L- Editor Roemmele A E- Editor Wu X
References
- 1.Stradling JR, Davies RJ. Sleep. 1: Obstructive sleep apnoea/hypopnoea syndrome: definitions, epidemiology, and natural history. Thorax. 2004;59:73–78. doi: 10.1136/thx.2003.007161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lattimore JD, Celermajer DS, Wilcox I. Obstructive sleep apnea and cardiovascular disease. J Am Coll Cardiol. 2003;41:1429–1437. doi: 10.1016/s0735-1097(03)00184-0. [DOI] [PubMed] [Google Scholar]
- 3.Coughlin SR, Mawdsley L, Mugarza JA, Calverley PM, Wilding JP. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J. 2004;25:735–741. doi: 10.1016/j.ehj.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 4.Tasali E, Mokhlesi B, Van Cauter E. Obstructive sleep apnea and type 2 diabetes: interacting epidemics. Chest. 2008;133:496–506. doi: 10.1378/chest.07-0828. [DOI] [PubMed] [Google Scholar]
- 5.Lévy P, Bonsignore MR, Eckel J. Sleep, sleep-disordered breathing and metabolic consequences. Eur Respir J. 2009;34:243–260. doi: 10.1183/09031936.00166808. [DOI] [PubMed] [Google Scholar]
- 6.Strohl KP, Novak RD, Singer W, Cahan C, Boehm KD, Denko CW, Hoffstem VS. Insulin levels, blood pressure and sleep apnea. Sleep. 1994;17:614–618. doi: 10.1093/sleep/17.7.614. [DOI] [PubMed] [Google Scholar]
- 7.Stoohs RA, Facchini F, Guilleminault C. Insulin resistance and sleep-disordered breathing in healthy humans. Am J Respir Crit Care Med. 1996;154:170–174. doi: 10.1164/ajrccm.154.1.8680675. [DOI] [PubMed] [Google Scholar]
- 8.Resnick HE, Redline S, Shahar E, Gilpin A, Newman A, Walter R, Ewy GA, Howard BV, Punjabi NM. Diabetes and sleep disturbances: findings from the Sleep Heart Health Study. Diabetes Care. 2003;26:702–709. doi: 10.2337/diacare.26.3.702. [DOI] [PubMed] [Google Scholar]
- 9.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160:521–530. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 10.Punjabi NM, Polotsky VY. Disorders of glucose metabolism in sleep apnea. J Appl Physiol. 2005;99:1998–2007. doi: 10.1152/japplphysiol.00695.2005. [DOI] [PubMed] [Google Scholar]
- 11.Assoumou HG, Gaspoz JM, Sforza E, Pichot V, Celle S, Maudoux D, Kossovsky M, Chouchou F, Barthelemy JC, Roche F. Obstructive sleep apnea and the metabolic syndrome in an elderly healthy population: the SYNAPSE cohort. Sleep Breath. 2011:Epub ahead of print. doi: 10.1007/s11325-011-0593-y. [DOI] [PubMed] [Google Scholar]
- 12.Muraki I, Tanigawa T, Yamagishi K, Sakurai S, Ohira T, Imano H, Kitamura A, Kiyama M, Sato S, Shimamoto T, et al. Nocturnal intermittent hypoxia and the development of type 2 diabetes: the Circulatory Risk in Communities Study (CIRCS) Diabetologia. 2010;53:481–488. doi: 10.1007/s00125-009-1616-0. [DOI] [PubMed] [Google Scholar]
- 13.Botros N, Concato J, Mohsenin V, Selim B, Doctor K, Yaggi HK. Obstructive sleep apnea as a risk factor for type 2 diabetes. Am J Med. 2009;122:1122–1127. doi: 10.1016/j.amjmed.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kono M, Tatsumi K, Saibara T, Nakamura A, Tanabe N, Takiguchi Y, Kuriyama T. Obstructive sleep apnea syndrome is associated with some components of metabolic syndrome. Chest. 2007;131:1387–1392. doi: 10.1378/chest.06-1807. [DOI] [PubMed] [Google Scholar]
- 15.West SD, Nicoll DJ, Stradling JR. Prevalence of obstructive sleep apnoea in men with type 2 diabetes. Thorax. 2006;61:945–950. doi: 10.1136/thx.2005.057745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172:1590–1595. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Punjabi NM, Beamer BA. Alterations in Glucose Disposal in Sleep-disordered Breathing. Am J Respir Crit Care Med. 2009;179:235–240. doi: 10.1164/rccm.200809-1392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165:670–676. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 19.Ye J, Gao Z, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab. 2007;293:E1118–E1128. doi: 10.1152/ajpendo.00435.2007. [DOI] [PubMed] [Google Scholar]
- 20.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901–911. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 21.Chen B, Lam KS, Wang Y, Wu D, Lam MC, Shen J, Wong L, Hoo RL, Zhang J, Xu A. Hypoxia dysregulates the production of adiponectin and plasminogen activator inhibitor-1 independent of reactive oxygen species in adipocytes. Biochem Biophys Res Commun. 2006;341:549–556. doi: 10.1016/j.bbrc.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Magalang UJ, Cruff JP, Rajappan R, Hunter MG, Patel T, Marsh CB, Raman SV, Parinandi NL. Intermittent hypoxia suppresses adiponectin secretion by adipocytes. Exp Clin Endocrinol Diabetes. 2009;117:129–134. doi: 10.1055/s-2008-1078738. [DOI] [PubMed] [Google Scholar]
- 23.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11:163–178. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 25.Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30:1145–1152. doi: 10.1093/sleep/30.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–1762. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 27.Buckley TM, Schatzberg AF. On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J Clin Endocrinol Metab. 2005;90:3106–3114. doi: 10.1210/jc.2004-1056. [DOI] [PubMed] [Google Scholar]
- 28.Späth-Schwalbe E, Gofferje M, Kern W, Born J, Fehm HL. Sleep disruption alters nocturnal ACTH and cortisol secretory patterns. Biol Psychiatry. 1991;29:575–584. doi: 10.1016/0006-3223(91)90093-2. [DOI] [PubMed] [Google Scholar]
- 29.Stoohs RA, Facchini FS, Philip P, Valencia-Flores M, Guilleminault C. Selected cardiovascular risk factors in patients with obstructive sleep apnea: effect of nasal continuous positive airway pressure (n-CPAP) Sleep. 1993;16:S141–S142. doi: 10.1093/sleep/16.suppl_8.s141. [DOI] [PubMed] [Google Scholar]
- 30.Saini J, Krieger J, Brandenberger G, Wittersheim G, Simon C, Follenius M. Continuous positive airway pressure treatment. Effects on growth hormone, insulin and glucose profiles in obstructive sleep apnea patients. Horm Metab Res. 1993;25:375–381. doi: 10.1055/s-2007-1002123. [DOI] [PubMed] [Google Scholar]
- 31.Davies RJ, Turner R, Crosby J, Stradling JR. Plasma insulin and lipid levels in untreated obstructive sleep apnoea and snoring; their comparison with matched controls and response to treatment. J Sleep Res. 1994;3:180–185. doi: 10.1111/j.1365-2869.1994.tb00126.x. [DOI] [PubMed] [Google Scholar]
- 32.Brooks B, Cistulli PA, Borkman M, Ross G, McGhee S, Grunstein RR, Sullivan CE, Yue DK. Obstructive sleep apnea in obese noninsulin-dependent diabetic patients: effect of continuous positive airway pressure treatment on insulin responsiveness. J Clin Endocrinol Metab. 1994;79:1681–1685. doi: 10.1210/jcem.79.6.7989475. [DOI] [PubMed] [Google Scholar]
- 33.Cooper BG, White JE, Ashworth LA, Alberti KG, Gibson GJ. Hormonal and metabolic profiles in subjects with obstructive sleep apnea syndrome and the acute effects of nasal continuous positive airway pressure (CPAP) treatment. Sleep. 1995;18:172–179. [PubMed] [Google Scholar]
- 34.Saarelainen S, Lahtela J, Kallonen E. Effect of nasal CPAP treatment on insulin sensitivity and plasma leptin. J Sleep Res. 1997;6:146–147. doi: 10.1046/j.1365-2869.1997.00034.x. [DOI] [PubMed] [Google Scholar]
- 35.Pierzchała W, Ograbek M. [Sleep apnea syndrome in patients with diabetes. Effectiveness of treatment for respiratory assistance at night with continuous positive airway pressure] Wiad Lek. 1998;51:166–172. [PubMed] [Google Scholar]
- 36.Chin K, Shimizu K, Nakamura T, Narai N, Masuzaki H, Ogawa Y, Mishima M, Nakamura T, Nakao K, Ohi M. Changes in intra-abdominal visceral fat and serum leptin levels in patients with obstructive sleep apnea syndrome following nasal continuous positive airway pressure therapy. Circulation. 1999;100:706–712. doi: 10.1161/01.cir.100.7.706. [DOI] [PubMed] [Google Scholar]
- 37.Ip MS, Lam KS, Ho C, Tsang KW, Lam W. Serum leptin and vascular risk factors in obstructive sleep apnea. Chest. 2000;118:580–586. doi: 10.1378/chest.118.3.580. [DOI] [PubMed] [Google Scholar]
- 38.Smurra M, Philip P, Taillard J, Guilleminault C, Bioulac B, Gin H. CPAP treatment does not affect glucose-insulin metabolism in sleep apneic patients. Sleep Med. 2001;2:207–213. doi: 10.1016/s1389-9457(00)00079-4. [DOI] [PubMed] [Google Scholar]
- 39.Harsch IA, Schahin SP, Radespiel-Tröger M, Weintz O, Jahreiss H, Fuchs FS, Wiest GH, Hahn EG, Lohmann T, Konturek PC, et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2004;169:156–162. doi: 10.1164/rccm.200302-206OC. [DOI] [PubMed] [Google Scholar]
- 40.Harsch IA, Schahin SP, Brückner K, Radespiel-Tröger M, Fuchs FS, Hahn EG, Konturek PC, Lohmann T, Ficker JH. The effect of continuous positive airway pressure treatment on insulin sensitivity in patients with obstructive sleep apnoea syndrome and type 2 diabetes. Respiration. 2004;71:252–259. doi: 10.1159/000077423. [DOI] [PubMed] [Google Scholar]
- 41.Babu AR, Herdegen J, Fogelfeld L, Shott S, Mazzone T. Type 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Arch Intern Med. 2005;165:447–452. doi: 10.1001/archinte.165.4.447. [DOI] [PubMed] [Google Scholar]
- 42.Czupryniak L, Loba J, Pawlowski M, Nowak D, Bialasiewicz P. Treatment with continuous positive airway pressure may affect blood glucose levels in nondiabetic patients with obstructive sleep apnea syndrome. Sleep. 2005;28:601–603. doi: 10.1093/sleep/28.5.601. [DOI] [PubMed] [Google Scholar]
- 43.Hassaballa HA, Tulaimat A, Herdegen JJ, Mokhlesi B. The effect of continuous positive airway pressure on glucose control in diabetic patients with severe obstructive sleep apnea. Sleep Breath. 2005;9:176–180. doi: 10.1007/s11325-005-0033-y. [DOI] [PubMed] [Google Scholar]
- 44.Lindberg E, Berne C, Elmasry A, Hedner J, Janson C. CPAP treatment of a population-based sample--what are the benefits and the treatment compliance? Sleep Med. 2006;7:553–560. doi: 10.1016/j.sleep.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 45.West SD, Nicoll DJ, Wallace TM, Matthews DR, Stradling JR. Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax. 2007;62:969–974. doi: 10.1136/thx.2006.074351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coughlin SR, Mawdsley L, Mugarza JA, Wilding JP, Calverley PM. Cardiovascular and metabolic effects of CPAP in obese males with OSA. Eur Respir J. 2007;29:720–727. doi: 10.1183/09031936.00043306. [DOI] [PubMed] [Google Scholar]
- 47.Pallayova M, Donic V, Tomori Z. Beneficial effects of severe sleep apnea therapy on nocturnal glucose control in persons with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2008;81:e8–11. doi: 10.1016/j.diabres.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 48.Wang H, Wang L, Liu J. [The effect of short-time continuous positive airway pressure treatment on insulin sensitivity in patients with obstructive sleep apnea-hypopnea syndrome and type 2 diabetes] Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2008;22:597–599. [PubMed] [Google Scholar]
- 49.Dawson A, Abel SL, Loving RT, Dailey G, Shadan FF, Cronin JW, Kripke DF, Kline LE. CPAP therapy of obstructive sleep apnea in type 2 diabetics improves glycemic control during sleep. J Clin Sleep Med. 2008;4:538–542. [PMC free article] [PubMed] [Google Scholar]
- 50.Steiropoulos P, Papanas N, Nena E, Tsara V, Fitili C, Tzouvelekis A, Christaki P, Maltezos E, Bouros D. Markers of glycemic control and insulin resistance in non-diabetic patients with Obstructive Sleep Apnea Hypopnea Syndrome: does adherence to CPAP treatment improve glycemic control? Sleep Med. 2009;10:887–891. doi: 10.1016/j.sleep.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 51.Wei CY, Wang HL, Li J, Dong XS, An P, Ji LN, Wang F, Han F. [Effect of continuous positive airway pressure upon 24 h changes of blood glucose level in patients with obstructive sleep apnea hypopnea syndrome and type 2 diabetes] Zhonghua Yi Xue Za Zhi. 2009;89:2686–2689. [PubMed] [Google Scholar]
- 52.Oktay B, Akbal E, Firat H, Ardiç S, Kizilgun M. CPAP treatment in the coexistence of obstructive sleep apnea syndrome and metabolic syndrome, results of one year follow up. Acta Clin Belg. 2009;64:329–334. doi: 10.1179/acb.2009.051. [DOI] [PubMed] [Google Scholar]
- 53.Lam JC, Lam B, Yao TJ, Lai AY, Ooi CG, Tam S, Lam KS, Ip MS. A randomised controlled trial of nasal continuous positive airway pressure on insulin sensitivity in obstructive sleep apnoea. Eur Respir J. 2010;35:138–145. doi: 10.1183/09031936.00047709. [DOI] [PubMed] [Google Scholar]
- 54.Garcia JM, Sharafkhaneh H, Hirshkowitz M, Elkhatib R, Sharafkhaneh A. Weight and metabolic effects of CPAP in obstructive sleep apnea patients with obesity. Respir Res. 2011;12:80. doi: 10.1186/1465-9921-12-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shpirer I, Rapoport MJ, Stav D, Elizur A. Normal and elevated HbA1C levels correlate with severity of hypoxemia in patients with obstructive sleep apnea and decrease following CPAP treatment. Sleep Breath. 2012;16:461–466. doi: 10.1007/s11325-011-0525-x. [DOI] [PubMed] [Google Scholar]