Abstract
Background
Substance P (SP) is a peptide neurotransmitter found in central and peripheral nerves. SP is involved in the control of smooth muscle, inflammation and nociception. The amino acid sequence of SP is Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH2. Five different forms of fluorescently labeled SP have recently been synthesized, in which Alexa 488, BODIPY Fl, fluorescein, Oregon Green 488 or tetramethylrhodamine has been covalently linked to SP at Lys3. Here, these novel analogs are characterized as to their ligand binding, receptor activation and fluorescence labeling properties.
Results
Competition binding studies, using radiolabeled [125I] SP, revealed that all of the labeled forms of SP, except for Alexa 488-SP, effectively competed with radiolabeled SP for binding at the rat SP receptor. With the exception of Alexa 488-SP, all of the SP analogs produced Ca++ elevations and fluorescence labeling of the SP receptor expressed in Chinese hamster ovary cells. In SP-responsive neurons, BODIPY Fl-SP and Oregon Green 488-SP were as effective as unlabeled SP in producing a reduction of the M-type K+ current. Fluorescein-SP produced variable results, while tetramethylrhodamine-SP was less potent and Alexa 488-SP was less effective on intact neurons.
Conclusions
The above results show that fluorescent labeling of SP altered the biological activity and the binding properties of the parent peptide. Oregon Green 488 and BODIPY FL-SP are the most useful fluorophores for labeling SP without affecting its biological activity. Given these results, these probes can now be utilized in further investigations of the mechanisms of SPR function, including receptor localization, internalization and recycling.
Background
Substance P (SP) is a peptide neurotransmitter that has been shown to play a role in nociception, smooth muscle control, allergic responses, inflammation and glandular secretion [1]. The amino acid sequence of SP was determined in 1970 [2] after being isolated from mammalian gastrointestinal tract in 1931 [3]. SP acts as an agonist at the SP receptor (SPR), known as the neurokinin-1 receptor (NK1) in mammalian systems. SP activation of the SPR, a G-protein-coupled receptor, has a variety of effects in the nervous system including inhibition of the M-type K+ current (IM) [4]. The mechanistic properties of the SPR have been extensively studied in receptor-expression systems. When transfected into Chinese hamster ovary (CHO) cells, activation of the SPR results in an increase in intracellular Ca++ [5], accumulation of inositol phosphates and cAMP formation [6].
The recent development of intense, photostable and pH-insensitive fluorophores, along with improvements in optical detection systems, has led to fluorophore labeling of many pharmacological agents. Fluorescent probes can be used to directly label ligand-binding sites without the use of radioactivity or antibodies. Receptor-labeling with a fluorophore-conjugated agonist provides advantages over these conventional methods. The production of antibodies to the receptor protein is not required, as the labeled agonist will bind directly to the receptor. Fluorescence rather than radioactivity can detect the labeled ligand, which provides more information on the localization of the receptor upon SP activation. In addition, labeled agonists can be used in live cells. Therefore, the use of fluorescently labeled agents may allow a more extensive investigation into various receptor functions.
Molecular Probes, Inc. (Eugene, OR) has recently synthesized five fluorophore-conjugated analogs of SP as potential tools for direct labeling of the SPR. Alexa 488, BODIPY Fl, fluorescein, Oregon Green 488 and tetramethylrhodamine have been conjugated to the third amino acid of SP, Lys3. The amine group of Lys provides a convenient reactive group for labeling SP without altering its original amino acid sequence. Alexa 488, BODIPY Fl, fluorescein and Oregon Green 488 are green fluorophores, while tetramethylrhodamine is a red fluorophore.
We have compared the receptor activation and labeling of five newly synthesized fluorescent analogs of SP. Each of the probes has been tested for: 1) ability to bind to the receptor, 2) receptor activation in both a heterologous expression system and in native neurons and 3) fluorescence labeling of the receptor. This study provides an extensive characterization of the new SP derivatives that will provide a basis for future studies involving the fluorescent conjugates. Oregon Green 488 was found to be the most useful fluorophore for labeling SP without altering its biological activity, whereas Alexa 488 drastically altered the binding and activation of SP.
Results
Spectra properties of fluorescent analogs
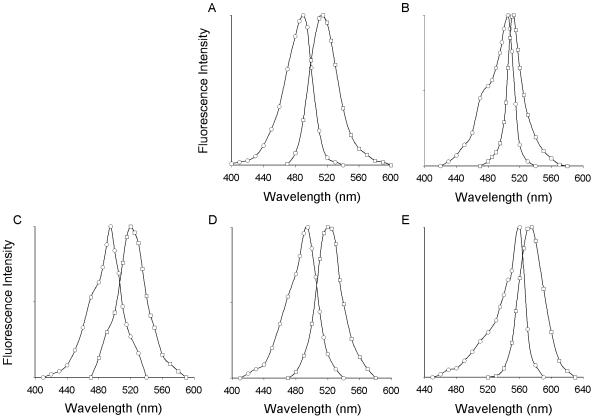
The fluorescent properties of a fluorophore can change when the fluorophore is attached to another molecule, in this case a peptide. Furthermore, the conditions under which fluorescence is measured, such as pH, ionic strength and buffer composition, can affect fluorescence. The absorption and emission spectra of the fluorescent SP analogs were assessed to determine their fluorescence under experimental conditions used here. As shown in Fig. 2, the peak excitations of Alexa 488-SP, BODIPY Fl-SP, fluorescein-SP, Oregon Green 488-SP and tetramethylrhodamine-SP were 490 nm, 505 nm, 495 nm, 495 nm and 560 nm, respectively. The peaks of the emission curves were 515 nm, 510 nm, 520 nm, 520 nm and 575 nm, respectively.
Figure 2.
Spectral analysis. The excitation and emission spectra was determined for (A) Alexa 488-SP, (B) BODIPY Fl-SP, (C) fluorescein-SP, (D) Oregon Green 488-SP and (E) tetramethylrhodamine-SP. See results for peak data.
Epifluorescence microscopy
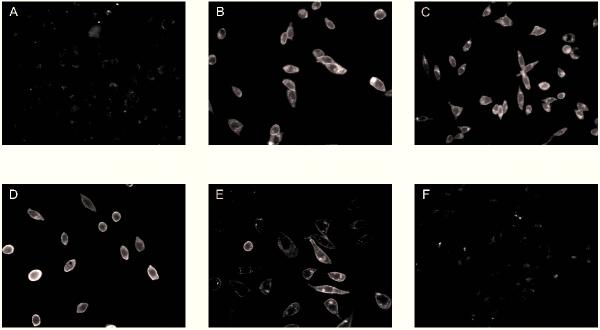
Direct-labeling of the SPR with fluorescent ligands like those analyzed in this study would offer advantages over current methods of receptor labeling. Some of these advantages include the ability to label live cells and the ability to conduct receptor labeling without antibodies or radioactive ligands. Therefore, an important characteristic of the fluorescent SP analogs is their ability to bind to the SPR and to fluoresce under physiological conditions. In order to observe the direct-labeling of the SP conjugates to the SPR, CHO cells expressing the rat SPR (rSPR), were incubated with the SP analogs (100 nM) for 2 hrs at 4°C, to prevent internalization of the receptor, rinsed and fixed. Under these conditions, all of the labeled forms of SP, except for Alexa 488-SP, produced bright membrane staining (Fig. 3). Observation of transfected cells incubated with phosphate buffered saline (PBS) in the absence of labeled SP conjugate showed that the transfected cells had some intracellular auto-fluorescence (Fig. 3, F). The fluorophore-treated cells also show some dull intracellular fluorescence, similar to the auto-fluorescence seen in the unstained control cells. The intensity of the staining produced by the green fluorophores was compared by quantitative analysis. The intensities of the fluorescence after staining with BODIPY Fl-SP, fluorescein-SP and Oregon Green 488-SP were significantly greater (p < 0.0001) than the intensity of the fluorescence of cells treated with Alexa 488-SP. Tetramethylrhodamine-SP also produced intense membrane staining. The intensity of the staining with tetramethylrhodamine-SP could not be directly compared to the others due to its different excitation and emission wavelengths.
Figure 3.
Fluorescence microscopy. CHO cells expressing the rSPR were stained with (A) Alexa 488-SP, (B) BODIPY Fl-SP, (C) fluorescein-SP, (D) Oregon Green 488-SP, (E) tetramethylrhodamine-SP and (F) PBS in the absence of a fluorophore. The intensity to area ratio of Alexa 488-SP was significantly less than the other green fluorophores. However a significant difference was not seen between BODIPY Fl-SP, fluorescein-SP or Oregon Green 488-SP.
The specificity of the fluorescence staining was revealed by the finding that unlabeled SP effectively prevented the fluorescent staining of rSPR-transfected CHO cells. The fluorescence of these cells was similar to that of transfected cells incubated in PBS alone (Fig. 3, F) in that they possessed some intracellular auto-fluorescence but lacked membrane staining.
Application of the labeled analogs to untransfected CHO cells did not produce membrane staining (data not shown). Only intracellular auto-fluorescence, comparable to that observed with transfected cells incubated with PBS in the absence of labeled SP (Fig. 3, F), was seen in cells lacking the rSPR.
SP receptor binding
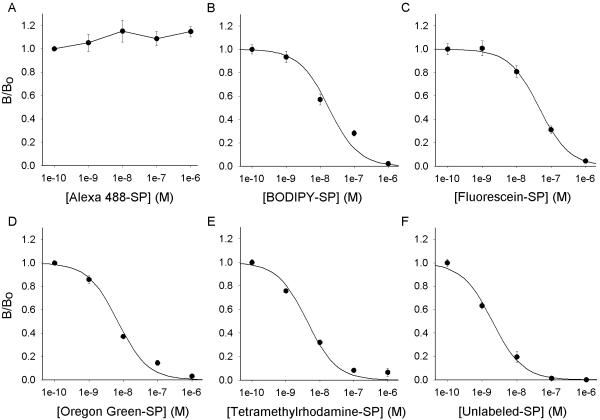
An important determinant of the usefulness of the SP conjugates is their ability to bind to and activate the SPR. Therefore, the ability of each of the fluorescent SP analogs to compete with [125I] SP binding to the SPR was assessed using a heterologous expression system, rSPR-expressing CHO cells. The results are shown in Fig. 4. The concentration of fluorescent analog required to inhibit the binding of radiolabeled SP by 50 % (IC50) was calculated from the binding studies. The rank order of potency for the competing peptides was: unlabeled SP (2.0 nM) > tetramethylrhodamine-SP (4.2 nM) > Oregon Green 488-SP (6.4 nM) > BODIPY-SP (18.0 nM) > fluorescein-SP (44.5 nM) >>> Alexa 488-SP. Alexa 488-SP (Fig. 4, A) did not compete with [125I] SP for binding at the rSPR, therefore an IC50 value could not be determined.
Figure 4.
Binding of conjugated and unconjugated SP to the rSPR. Competition binding of rSPR transfected CHO cells was performed with 50 pM [125I] SP and increasing concentrations (1 pM, 100 pM, 1 nM, 100 nM and 1 μM) of each fluorescent SP analog. The calculated IC50 values for BODIPY Fl-SP, fluorescein-SP, Oregon Green 488-SP, tetramethylrhodamine-SP and unconjugated SP are 18.0 nM, 44.5 nM, 6.39 nM, 4.20 nM and 1.98 nM, respectively. Note that Alexa 488-SP was unable to displace the radioisotope. Each data point represents an n of at least 4 from 2 or 3 separate experiments.
Two-way analysis of variance followed by Student-Newman-Keuls test showed that there was a dose-dependent effect (p < 0.001) on the binding of unlabeled SP and the SP analogs to the rSPR. Statistical analysis also demonstrated that the competition binding curves generated by the SP conjugates were significantly different than the unlabeled SP binding curve (p < 0.001). Further analysis showed that, with the exception of the binding curves produced by tetramethylrhodamine-SP and Oregon Green 488-SP, a significant difference was seen between the binding curves of the different SP conjugates (p < 0.05).
Calcium measurements
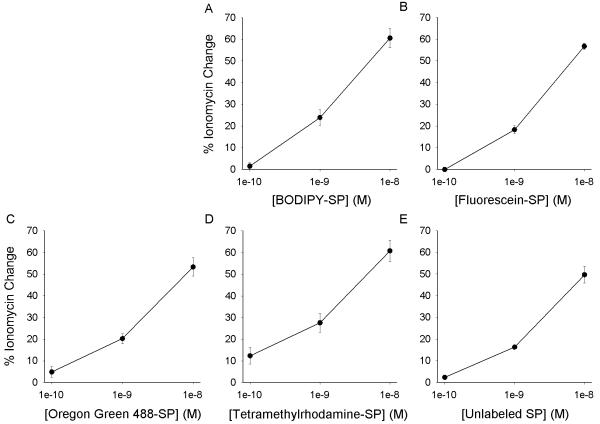
Activation of the SPR expressed in CHO cells results in an elevation in intracellular Ca++ [7]. The biological activity of the SP conjugates was tested by examining their ability to elicit Ca++ elevations. Dose-response curves for the labeled SP analogs and for unlabeled SP to produce an increase in intracellular Ca++ in CHO cells transfected with the rSPR are shown in Fig. 5.
Figure 5.
Calcium dose response data for the effects of conjugated and unconjugated SP at the rSPR expressed in CHO cells. Values are the peak Ca++ elevation produced by each drug expressed as the % of the ionomycin response for that coverslip. ANOVA showed significant dose-dependency, but no difference between Ca++ responses produced by the drugs was shown. Each data point represents the average of at least 5 experiments. Alexa 488-SP is not graphed since it did not elicit specific Ca++ responses at the rSPR.
None of the labeled SP analogs produced Ca++ elevations (n = 3) when added to untransfected CHO cells at a concentration of 10 nM (data not shown). When the fluorescent derivatives were added at a concentration of 100 nM they produced an increase in the fluorescence signal due to the inherent fluorescence of the probes. This could also be seen in the absence of cells. Due to this interference, the activity of the fluorescent probes to produce Ca++ elevations could not be tested at concentrations higher than 10 nM.
Alexa 488-SP produced the most prominent increase in non-specific fluorescence in the absence of cells. When 1 nM or 10 nM Alexa 488-SP was added the fluorophore produced signals similar in intensity to those observed when this analog was added to transfected or untransfected CHO cells (data not shown). To determine if Alexa 488-SP could specifically produce a receptor-mediated Ca++ response, the average of the fluorescence signal produced by Alexa 488-SP in the absence of cells was subtracted from the fluorescent signal obtained when Alexa 488-SP was added to SPR-expressing CHO cells. The results showed that Alexa 488-SP did not activate the SPR to produce a Ca++ response. However, the other four SP analogs were able to elicit Ca++ responses in rSPR-expressing CHO cells.
Statistical analysis, two-way analysis of variance followed by Student-Newman-Keuls test, demonstrated that unlabeled SP and the SP analogs, except Alexa 488-SP, elicited dose-dependent Ca++ elevations (p < 0.001). Analysis also showed that there was not a significant difference (p > 0.05) in Ca++ elevations generated by unlabeled SP or the various SP conjugates, with the exception of Alexa 488-SP which did not elicit a calcium response.
Effects of SP analogs on single neurons
The data thus far show that the labeled forms of SP, with the exception of Alexa 488-SP, can bind to and activate the receptor in a heterologous expression system. It is also of importance to determine the activity of the labeled ligands in a cell that endogenously expresses the SPR. The principal neurons of bullfrog sympathetic ganglia express a SPR that has been well characterized with regard to its structure and function. The bullfrog sympathetic ganglia SPR (bfSPR) has 69% homology to the rSPR at the amino acid level. Perhaps most importantly, nine of the ten residues that have been demonstrated to be important for SP binding to mammalian receptors are conserved in the bullfrog receptor. The effects of SP and other tachykinins at the bfSPR have been previously well characterized [8, 9, 5]. The primary biological response following activation of the ganglionic SPR is inhibition of the M-type K+ current, IM.
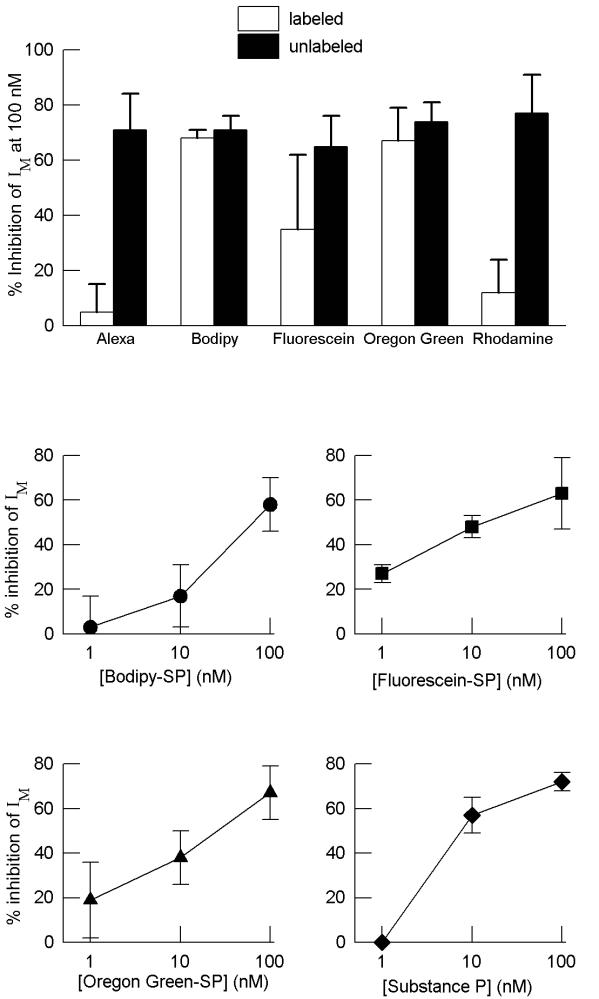
SP produces a dose-dependent inhibition of IM [9]. The responses of a given neuron to a given form of labeled SP have been compared to the responses of those same neurons to unlabeled SP at a concentration of 100 nM (Fig. 6). At this concentration SP typically elicits a 70 % depression of IM. BODIPY Fl-SP and Oregon Green 488-SP produced an inhibition of IM similar to that of unlabeled SP. Fluorescein-SP was variable in its ability to inhibit the current, being effective on only two of the four cells tested. At a concentration of 100 nM, neither Alexa 488-SP nor tetramethylrhodamine-SP inhibited IM; thus they were further tested at a concentration of 1 μM. At this concentration tetramethylrhodamine-SP inhibited IM by 57 ± 33 %, (n = 4), however Alexa 488-SP did not inhibit IM. Dose-response curves for the analogs that inhibited IM and for unlabeled SP are shown in the plots in Fig. 6. A dose-dependency was observed for the effects of BODIPY Fl-SP, fluorescein-SP and Oregon Green 488-SP.
Figure 6.
Top panel - Comparison of the effects of conjugated and unconjugated SP on single neurons. The bars show the percent inhibition of IM produced by labeled or unlabeled SP at a concentration of 100 nM. Both drugs were applied to the same cell. Alexa 488-SP and tetramethylrhodamine-SP did not inhibit IM, fluorescein-SP only inhibited the current 50% of the time, where as BODIPY Fl-SP and Oregon Green 488- SP produced inhibitions similar to unlabeled SP. Bottom panel - IM inhibition dose response curve for labeled and unlabeled SP. Data is expressed as % IM inhibition. Each point represents an average of 4 experiments, except fluorescein-SP, which is only an average of 2 experiments. A dose-dependency was observed for each drug that inhibited the IM.
Discussion
A number of previous studies have demonstrated that fluorescently labeled ligands are useful probes for studying ligand-receptor interactions [10, 11, 12]. Improved fluorophores, better optics and the increased sensitivity of detection systems have led to an increased usage of fluorescently labeled ligands. It is of vital importance that additions of fluorescent groups do not alter the normal functioning of the ligand, for example, by changing the binding affinity or by altering the receptor activation properties of the ligand. If the fluorescent group does alter the properties of the ligand, it is important that these alterations be understood so that the results obtained with the labeled ligand can be correctly interpreted. In this study, we have examined the fundamental consequences of labeling the neuropeptide SP with five different fluorescent probes by comparing the effects of the labeled compounds with those of the parent compound, SP.
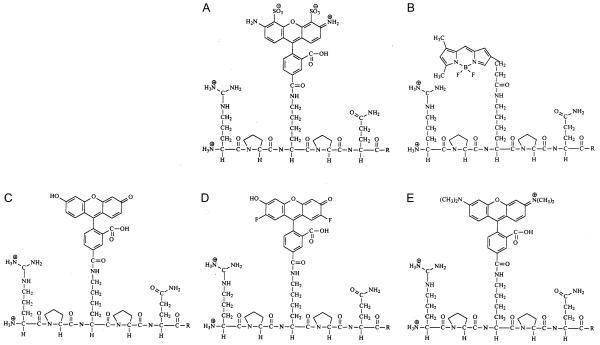
All five of the fluorescent probes were modified at the Lys3 position with aromatic groups. Alexa 488 and tetramethylrhodamine add negative and positive charges, respectively, to SP. The remaining fluorophores, BODIPY Fl, fluorescein and Oregon Green 488, do not alter the charge. With regard to size, BODIPY Fl is the smallest of the fluorophores added, whereas Alexa 488 and tetramethylrhodamine are the largest of the fluorophores.
The most significant alteration of the activity of SP was produced when Alexa 488 was added to SP. The experiments repeatedly showed that Alexa 488-SP does not posses the same characteristics as unlabeled SP. Alexa 488-SP did not compete with [125I] SP in the competition binding assay. Consistent with the binding data, this SP analog did not stain rSPR-transfected CHO cells. Furthermore, Alexa 488-SP did not exhibit functional activity in two different assays. It failed to elicit a Ca++ response in rSPR-expressing CHO cells and failed to produce IM inhibition at the bfSPR, both of which are characteristic effects mediated by SP.
Like Alexa 488, tetramethylrhodamine also adds a charge to SP, but tetramethylrhodamine-SP generated an IC50 that was the most similar to that of unlabeled SP. This SP analog also produced intense staining and typical Ca++ responses at the rSPR. However, tetramethylrhodamine-SP was ineffective at inhibiting IM at a 100 nM concentration, but did so upon increasing the concentration to 1 μM. Therefore, tetramethylrhodamine-SP is capable of binding to the SPR and activating Ca++ responses, but has a decreased potency for inhibiting IM.
Lys3 of SP has been shown to interact with the NH2-terminal extracellular tail (residues 1-21) of the mouse SPR [13]. Though residues 1-21 of the mouse SPR are primarily conserved in the rSPR, only 5 of the 21 residues are conserved in the bullfrog SPR. Given the importance of Lys3 in the mouse SPR, it is therefore conceivable that an alteration in the charge of the parent compound, SP, as is the case with Alexa 488-SP and tetramethylrhodamine-SP, may cause a change in the typical actions of SP. The addition of Alexa 488 causes a greater change in the charge of SP, which might inhibit proper ligand-receptor interaction and thus hinder binding of the SP analog to the SPR. Tetramethylrhodamine also alters the charge of SP, but unlike Alexa 488, the fluorophore is positively charged. Perhaps the addition a single positive charge to SP by tetramethylrhodamine does not disrupt proper ligand/receptor interactions as drastically as the multiple negative charges that Alexa 488 adds to SP. Therefore, the less drastic charge alteration made by tetramethylrhodamine may allow the mechanistic characteristics of SP to be preserved, albeit with a decreased potency to inhibit IM.
Although the structure of fluorescein is very similar to that of Oregon Green 488 (2', 7'-difluorofluorescein), fluorescein-SP did not behave in the same manner as Oregon Green 488-SP or unlabeled SP. Of the conjugates that competed with [125I] SP, fluorescein-SP had the lowest binding affinity as compared to unlabeled SP. The analog was also variable in its ability to inhibit IM at the bfSPR. However, it consistently produced Ca++ elevations and membrane staining at the rSPR expressed in CHO cells.
BODIPY Fl-SP and Oregon Green 488-SP produced inhibition of IM and Ca++ elevations similar to that produced by unlabeled SP. Thus, labeling with either of these two fluorophores did not alter the functional activity of SP at the SPR in either a heterologous expression system or native cells. These SP analogs also brightly stained the membranes of rSPR transfected CHO cells. The IC50 values of BODIPY Fl-SP and Oregon Green 488-SP in competition binding studies were greater than that of unlabeled SP. When compared to unlabeled SP, Oregon Green 488-SP and BODIPY FL-SP had a two-fold and ten-fold increase in binding affinity, respectively. Interestingly, neither BODIPY Fl nor Oregon Green 488 is charged.
It is interesting to note that although there was a significant difference between the binding curves of the fluorescent derivatives of SP and unlabeled SP, there was not a statistically significant in the peptides' ability to produce dose-dependent Ca++ elevations. These might be explained by the differences in the mechanisms that underlie the measurements made in the different experiments. The binding experiments measure the actions of the peptides at the receptor. Ca++ fluxes indicate additional mechanisms downstream of the receptor, including second messenger activation. The data may reflect the fact that receptor binding and signal activation are not strictly correlated in G-protein coupled systems.
The evidence presented here suggests that four of the analogs, BODIPY Fl-SP, fluorescein-SP, Oregon Green 488-SP and tetramethylrhodamine-SP, are acting specifically at the SPR. Binding analysis showed that the above conjugates compete with [125I] SP at the rSPR. These four analogs only produced Ca++ responses in cells transfected with the SPR; they had no effect in non-transfected cells. Similarly, membrane staining was observed in transfected CHO cells, but not in untransfected cells.
Molecular Probes introduced and investigated seven Alexa dyes, including Alexa 488, and reported that the dyes were more fluorescent and more photostable than their counterparts [14]. In their study, Alexa 488 was compared to fluorescein. The investigators conjugated the Alexa fluorophores and other similar compounds, such as fluorescein, to different proteins to compare brightness and stability. They showed that Alexa 488 was brighter and more stable than fluorescein when conjugated to biotin binding proteins, goat anti-mouse IgG and its F(ab')2 fragments. However, they did not test for alterations in binding affinities or biological activity after the addition of the fluorophores.
Another study has examined the effects of fluorescent conjugation on opioid peptide activity [15]. Alexa 488 and BODIPY TR were used to label dermorphin, deltrophin, TIPP (Try-Tic-Phe-Phe) and endomorphin by adding a cysteine to each peptide at the C-terminus, followed by conjugation of the fluorophores to the cysteine residue. The investigators found that the dermorphin and deltorphin analogs had an increased binding affinity for the μ- and δ-opioid receptors, respectively, and that the biological activities of parent compounds were preserved. However, when Alexa 488 was conjugated to TIPP and endomorphin, the binding affinities of the parent compounds were decreased.
Buku et al. [16] synthesized two fluorescent probes for the vasotocin receptor using fluorescein and tetramethylrhodamine. These investigators had previously conjugated the fluorophores to the seventh position of vasotocin, a nonapeptide, and found that it had biological activity, but that the biological activity was reduced when compared to the native compound [17]. Therefore, the investigators conjugated the fluorophores to the ninth position and found that the analog was 10-fold more potent than the analog with the fluorophores at the seventh position. Thus, they concluded that by moving the bulky fluorophore to the end of vasotocin, and not altering an amino acid involved in binding and activation of the vasotocin receptor minimized the reduction in biological activity of the probes.
In summary, our results along with those from other labs show that, in some cases, conjugation of a specific fluorescent label can be added to a peptide without altering the activity of the parent compound. In other cases, however, the activity of the original compound may be drastically altered. Therefore, the biological activity of each novel fluorophore conjugate must be analyzed. Analysis of labeled compounds should include assessment of binding and biological activity as well as brightness and stability.
Conclusions
The results of our study show that there are dramatic differences in the function of SP labeled at the Lys3 position with different fluorophores. Alexa 488, which was the largest and added the most charged groups to SP, was unable to label the SPR and altered the biological activity of SP in live and cultured cells. Oregon Green 488 and BODIPY Fl, which are smaller and uncharged, appear to be the most useful fluorophores for labeling SP without altering the normal functions of SP at the SPR regardless of the expression system. These results suggest that Oregon Green 488-SP and BODIPY Fl-SP would be the most useful fluorescent ligands for future studies of the SPR.
Materials and Methods
Labeled peptides
SP labeled with Alexa 488 (S-13426), BODIPY Fl (S-13425), fluorescein (S-13424), Oregon Green 488 (S-13427) or tetramethylrhodamine (S-13428) on Lys3 were purchased from Molecular Probes (Eugene, OR), product numbers in parenthesis. The chemical formulae for the labeled compounds are shown in Fig. 1.
Figure 1.
Fluorescent SP analog structures. A) Alexa 488-SP, B) BODIPY Fl-SP, C) fluorescein-SP, D) Oregon Green 488-SP and E) tetramethylrhodamine-SP. The first 5 amino acids of SP (Arg1-Pro2-Lys3-Pro4-Gln5) are shown, with each of the fluorophores conjugated to Lys3. R = Gln6-Phe7-Phe8-Gly9-Leu10-Met11-NH2. Alexa 488 is the most charged structure, BODIPY Fl is the smallest, and Alexa 488 and tetramethylrhodamine are the largest.
Spectra
Fluorophore spectral analysis was performed on a Perkin Elmer 512 Spectrofluorometer (Foster City, CA). Alexa 488-SP, BODIPY Fl-SP, fluorescein-SP, Oregon Green 488-SP and tetramethylrhodamine-SP were analyzed at a concentration of 10 nM, using PBS, pH 7.4, as a solvent.
Epifluorescence microscopy
CHO cells stably transfected with the cDNA of the rSPR were obtained from Dr. James Krause and maintained as previously described [18]. Transfected CHO cells were cultured overnight on 3-well HTC super cured glass slides (Cel-Line Associates, Inc., Newfield, NJ). The following day, the slides were rinsed with PBS. Two of the three wells from each slide were stained with 100 nM of one of the SP conjugates. The third well of each slide served as a negative control, in that the cells were incubated with PBS in the absence of a SP analog. Slides were incubated for 2 hrs at 4°C, to prevent internalization of the receptor and thus provide membrane staining. The cells were then rinsed and fixed with paraformaldehyde (2 %) (Fisher Scientific, Pittsburgh, PA) at 4°C for 20 min. Slides were viewed under a Nikon Eclipse E600FN fluorescent microscope (Fryer Company, Inc., Cincinnati, OH). The green fluorophores were excited using a FITC (fluorescein isothiocyanate) filter set (excitation 465-495 nm, dichroic 505LP, emission 515-555 nm). The red fluorophore was excited using a TRITC (tetramethylrhodamine isothiocyanate) filter set (excitation 528-553 nm, dichroic 565LP, emission 600-660 nm). Pictures were taken with a SPOT camera (Diagnostic Instruments, Inc., Sterling Heights, MI). All digital images of the green fluorophores were taken at a constant gain (16) and exposure time (0.7 s) to allow for direct comparison between cells and experiments. Pictures of tetramethylrhodamine-SP, the red fluorophore, were taken at a gain of 8 and a 0.4 s exposure time.
The specificity of the fluorescent staining to the rSPR was analyzed in two ways. First, incubating untransfected CHO cells with 100 nM of each analog assessed non-specific staining. Secondly, staining of rSPR-transfected CHO cells was performed in the presence of an excess of unlabeled SP. Transfected cells were pretreated with 1 μM unlabeled SP for 1 hr at 4°C. Then, the cells were rinsed and incubated with 1 μM unlabeled SP and 100 nM labeled SP for 1 hr at 4°C. The cells were rinsed, fixed and viewed as described above.
Image Analysis
Epifluorescence images from the wells containing only conjugated SP (100 nM) were analyzed using Scion Image (Scion Corporation, Frederick, MD). For each fluorophore, except tetramethylrhodamine, a line was drawn around the outside of the cell membrane of 10 cells (5 cells/experiment) to create a boundary; the area and pixel intensity were then measured within each boundary. Background, as determined by measuring the intensity and area of 10 control cells incubated with PBS in the absence of a fluorophore, was subtracted. Cell area was used to normalize between cells, thus the ratio of intensity to area was calculated for the treated and untreated cells.
Receptor binding
Radioligand competition binding assays utilizing CHO cells stably expressing the rSPR were performed with Bolton-Hunter [125I] SP (NEN, Boston, MA) at a concentration of 50 pM. Fluorescein-SP, Alexa 488-SP, BODIPY Fl-SP, fluorescein-SP, Oregon Green 488-SP or tetramethylrhodamine-SP was added at increasing concentrations, from 100 pM to 1 μM, to compete with the [125I] SP. In order to reach equilibrium, rSPR-expressing CHO cells (100,000 cells/well) were incubated in a pre-wetted Millipore Multiscreen 96 well BV filtration plate (France) with both radiolabeled and fluorescently labeled SP at 4°C for 2 hrs. Peptides and cells were prepared in Tris Buffered Saline Binding Buffer: 50 mM Tris, 120 mM NaCl, 0.2 mg/ml bacitracin, 20 μg/ml leupeptin, 20 μg/ml chymostatin, 0.1 % bovine serum albumin, pH 7.4. Following incubation, cells were filtered and washed using a Millipore vacuum manifold (France). Filters were punched and analyzed using a Packard Cobra II series auto-gamma counter (Meriden, CT) to determine counts per minute (cpm).
Non-specific binding was specified as the counts obtained with 1 μM unlabeled SP in the presence of radiolabeled SP. Statistical analysis showed that there was not a significant difference between the fluorescent conjugates and unlabeled SP when 1 μM of SP or its derivatives was present with 50 pM [125I] SP. In each experiment, specific binding was determined by subtracting nonspecific binding from the original cpm measurements. The data were normalized by calculating B/Bo for each fluorophore, where B is the cpm of [125I] SP specifically bound in the presence of non-radioactive SP and Bo is the cpm of [125I] SP specifically bound in the presence of 100 pM fluorescently labeled SP. The data were plotted as a function of competitor concentration vs. B/Bo. Sigmoidal (Hill, 3 parameter) regression was then performed on the data to determine the IC50, the concentration of fluorescent analog required to inhibit the binding of radiolabeled SP by 50 %, for each SP analog.
Calcium measurements
Dose-response curves for the ability of the SP analogs to produce Ca++elevations upon binding to the SPR were obtained as previously described [5]. Briefly, transfected CHO cells expressing the rSPR were cultured onto coverslips until ∼ 90% confluent. The coverslips were incubated in a Ca++-free solution containing fura-PE3 (AM) and pluronic acid (Texas Fluorescence Labs, Austin, TX) for 30 min., washed, then incubated in a Ca++-containing solution for 30 min. A CAF-110 Intracellular Ion Analyzer (Jasco Corp., Tokyo, Japan) was used to measure the fura- PE3 fluorescence emission. Cytosolic Ca++ concentrations were measured by taking the ratio of fluorescence emission at 510 nm by excitation at 340 and 380 nm. Dose-response curves were obtained by measuring the Ca++ responses produced by adding various concentrations (1 pM, 100 nM, 1 nM and 10 nM) of the labeled probes. Ionomycin (Sigma, St. Louis, MO) was added at a concentration of 10 μM at the end of each experiment to obtain the maximal Ca++ elevation for that coverslip. Ca++ responses were measured by subtracting the peak of each response from the baseline. Responses are expressed as a fraction of the ionomycin (maximum) response.
Two experiments were carried out to serve as negative controls. In the first control experiment, untransfected CHO cells were utilized to determine if any of the SP conjugates were eliciting Ca++ responses through a pathway independent of rSPR activation. Each of the SP isoforms was added to untransfected CHO cells at a concentration of 10 nM and the signals, if any, were recorded. If a SP conjugate caused an increase in Ca++ at 10 nM, then 0.1 and 1 nM concentrations for that fluorophore were also tested.
The second control experiment measured fluorescence produced by the SP analogs themselves, in the absence of cells. Each of the SP fluorophores was added to the Ca++ solution in the absence of cells at a concentration of 10 nM and increases in fluorescence, if any, were recorded. If a response was observed at a 10 nM concentration, then 0.1 and 1 nM concentrations were also tested for that SP analog. If a SP analog produced a Ca++ elevation in either control assay, then the average (n of 3) for the differences between the baseline and peak Ca++ signals for that concentration was calculated. The average was then subtracted from the Ca++ responses observed when that SP analog was added to transfected and untransfected CHO cells at that concentration.
Electrophysiology
Single neurons were dissociated from bullfrog sympathetic ganglia as described previously [19]. Briefly, ganglia were dissected, treated with enzymes and then stored in growth medium at 4°C for 1 to 3 days. The ganglia were triturated to release single neurons for daily use.
Whole cell recordings of isolated neurons were conducted at room temperature, ∼ 21°C. Drugs were applied by single cell superfusion [20] and the bath constantly perfused separately with extracellular solution. Whole cell recordings were made with electrodes with resistances of 0.25-1 MΩ when filled with intracellular solution.
The compositions of solutions for electrophysiology are shown in mM unless otherwise noted. Intracellular (or recording electrode) solution: KCl 120, MgCl2 2, HEPES 10, K4BAPTA 1, ATP 1.15, GTP 0.4, pH 6.8 (with KOH). Extracellular solution: NaCl 118, KCl 2.4, CaCl2 1.8, MgCl2 1.8, sodium pyruvate 5, glucose 5, HEPES 10, TTX 0.0003, pH 7.4 (with NaOH). Growth medium: NaCl 118, KCl 2.4, creatine 5.7, glucose 5, sodium pyruvate 5, 100× MEM vitamins 10 ml/L, penicillin 100 U/ml, streptomycin 100 μg/ml, 50× MEM essential amino acids 20 ml/L, 100× MEM non-essential amino acids 10 ml/L, HEPES 20, pH 7.4 (with NaOH).
IM was monitored by 500 ms pulses from a holding potential of -30 mV to -50 mV every 8 s. The recordings were filtered at 1 kHz and stored on magnetic tape. The IM relaxations were sampled on line at 2.4 kHz. IM was measured as the amplitude of the current tail following a voltage step from -50 mV to -30 mV [21]. To quantify the inhibition of IM, the current before drug and at the maximum inhibition during each response were measured and the percentage of inhibition of IM was calculated.
Statistical Analysis
The percent of ionomycin data from the Ca++ elevation experiments and the B/B0 data from the binding experiments were both subjected to two-way ANOVA analysis, with the independent variables being fluorescent conjugate and dose. One-way ANOVA analysis was used to describe the intensity to area measurements from the fluorescent images, except tetramethylrhodamine. These data, like the data obtained from the binding assay and Ca++ analysis, were analyzed for variance followed by the Student-Newman-Keuls test.
Acknowledgments
Acknowledgements
We thank Drs. Todd Green and Gary Meszaros for many helpful discussions and for generosity in sharing of reagents. This work was supported by grant NS25999 from the National Institute of Neurological Diseases and Stroke to MAS, by a Summer Undergraduate Research Fellowship from the American Society for Pharmacology and Experimental Therapeutics to VJB and by a graduate research fellowship from Marshall University and Kent State University to VJB.
Contributor Information
Vicki J Bennett, Email: vjbennet@neoucom.edu.
Mark A Simmons, Email: simmons@neoucom.edu.
References
- Otsuka M, Yoshioka K. Neurotransmitter functions of mammalian tachykinins. Physiol Rev. 1993;73:229–308. doi: 10.1152/physrev.1993.73.2.229. [DOI] [PubMed] [Google Scholar]
- Chang MM, Leeman SE. Isolation of a sialogogic peptide from bovine hypothalamic tissue and its characterization as substance P. J Biol Chem. 1970;245:4784–4790. [PubMed] [Google Scholar]
- Von Euler US, Gaddum JH. An unidentified depressor substance in certain tissue extracts. J Physiol. 1931;72:74–86. doi: 10.1113/jphysiol.1931.sp002763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams PR, Brown DA. Substance P inhibits the M-current in bullfrog sympathetic neurones. Br J Pharmac. 1983;79:330–333. doi: 10.1111/j.1476-5381.1983.tb11004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrine SA, Whitehead TL, Hicks RP, Szarek JL, Krause JE, Simmons MA. Solution structures in SDS micelles and functional activity at the bullfrog substance P receptor of ranatachykinin peptides. J Med Chem. 2000;43:1741–1753. doi: 10.1021/jm000093v. [DOI] [PubMed] [Google Scholar]
- Torrens Y, Beaujouan JC, Saffroy M, Glowinski J, Tence M. Functional coupling of the NK1 tachykinin receptor to phospholipase D in Chinese hamster ovary cells and astrocytoma cells. J Neurochem. 1998;70:2091–2098. doi: 10.1046/j.1471-4159.1998.70052091.x. [DOI] [PubMed] [Google Scholar]
- Li HS, Leeman SE, Slack BE, Hauser G, Saltsman WS, Krause JE, Blusztajn JK, Boyd ND. A substance P (neurokinin-1) receptor mutant carboxyl-terminally truncated to resemble a naturally occurring receptor isoform displays enhanced responsiveness and resistance to desensitization. PNAS. 1997;94:9475–9480. doi: 10.1073/pnas.94.17.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akasu T, Ishimatsu M, Yamada K. Tachykinins cause inward current through NK1 receptors in bullfrog sensory neurons. Brain Res. 1996;713:160–167. doi: 10.1016/0006-8993(95)01506-X. [DOI] [PubMed] [Google Scholar]
- Simmons MA, Brodbeck RM, Karpitskiy VV, Schneider CR, Neff DPA, Krause JE. Molecular characterization and functional expression of a substance P receptor from the sympathetic ganglion of Rana catesbeiana. Neurosci. 1997;79:1219–1229. doi: 10.1016/S0306-4522(97)00027-4. [DOI] [PubMed] [Google Scholar]
- Heithier H, Hallmann D, Boege F, Reiländer H, Dees C, Jaeggi KA, Arndt-Jovin D, Jovin TM, Helmreich EJM. Synthesis and properties of fluorescent β-adrenoceptor ligands. Biochem. 1994;33:9126–9134. doi: 10.1021/bi00197a015. [DOI] [PubMed] [Google Scholar]
- Tota MR, Daniel S, Sirotina A, Mazina DE, Fong TM, Longmore J, Strader CD. Characterization of a fluorescent substance P analog. Biochem. 1994;33:13079–13086. doi: 10.1021/bi00248a017. [DOI] [PubMed] [Google Scholar]
- Turcatti G, Zoffmann S, Lowe J, Drozda S, Chassaing G, Schwartz T, Chollet A. Characterization of non-peptide antagonist and peptide agonist binding sites of the NK1 receptor with fluorescent ligands. J Biol Chem. 1997;272:21167–2117. doi: 10.1074/jbc.272.34.21167. [DOI] [PubMed] [Google Scholar]
- Li YM, Marnerakis M, Stimson ER, Maggio JE. Mapping peptide-binding domains of the substance P (NK-1) receptor from P388D1 cells with photolabile agonists. J Biol Chem. 1995;270:1213–1220. doi: 10.1074/jbc.270.3.1213. [DOI] [PubMed] [Google Scholar]
- Panchuk-Voloshina N, Haugland RP, Bishop-Stewart J, Bhalgat MK, Millard PJ, Mao F, Leung W, Haugland RP. Alexa dyes, a series of new fluorescent dyes that yield exceptionally bright, photostable conjugates. J Histochem Cytochem. 1999;47:1179–1188. doi: 10.1177/002215549904700910. [DOI] [PubMed] [Google Scholar]
- Arttamangkul S, Alvarez-Maubecin V, Thomas G, Williams JT, Grandy DK. Binding and internalization of fluorescent opioid peptide conjugates in living cells. Mol Pharm. 2000;58:1570–1580. doi: 10.1124/mol.58.6.1570. [DOI] [PubMed] [Google Scholar]
- Buku A, Masur S, Eggena P. Synthesis and characterization of fluorescein- and tetramethylrhodamine-labeled probes for vasotocin receptors. Am J Physiol. 1989;257:E804–808. doi: 10.1152/ajpendo.1989.257.6.E804. [DOI] [PubMed] [Google Scholar]
- Eggena P, Buku A. Synthesis and characterization of a long-acting fluorescent analogue of vasotocin. Biol Cell. 1989;66:1–6. doi: 10.1016/0248-4900(89)90144-5. [DOI] [PubMed] [Google Scholar]
- Raddatz R, Crankshaw CL, Snider RM, Krause JE. Similar rates of phosphatidylinositol hydrolysis following activation of wild-type and truncated rat neurokinin-1 receptors. J Neurochem. 1995;64:1183–1191. doi: 10.1046/j.1471-4159.1995.64031183.x. [DOI] [PubMed] [Google Scholar]
- Simmons MA, Mather RJ. Selectivity of the effects of guanosine-5'-O-(2-thiodiphosphate) on agonist inhibition of the M current in amphibian sympathetic neurons. J Neurosci. 1991;11:2130–2134. doi: 10.1523/JNEUROSCI.11-07-02130.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons MA, Mather RJ. Intracellular guanosine-5'-O-(2-thiodiphosphate) alters the dynamics of receptor-mediated responses in bullfrog sympathetic neurons. Mol Pharm. 1992;41:527–534. [PubMed] [Google Scholar]
- Simmons MA, Schneider CR, Krause JE. Regulation of the responses to gonadotropin releasing hormone, muscarine, and substance P in sympathetic neurons by changes in cellular constituents and intracellular application of peptide fragments of the substance P receptor. J Pharm Exp Ther. 1994;271:581–589. [PubMed] [Google Scholar]