Abstract
Background
Norwood outcomes vary across centers, and a relationship between center volume and outcome has been previously described. It is unclear whether this volume-outcome relationship exists across all levels of patient risk or holds true for all centers. We evaluated the impact of patient risk status on the relationship between center volume and outcome, and the extent to which differences in center volume account for between-center variation in outcome.
Methods
Infants in the Society of Thoracic Surgeons Congenital Heart Surgery Database undergoing the Norwood operation (2000–2009) were included. Mortality associated with annual Norwood volume overall and across patient pre-operative risk tertiles was evaluated in multivariable analysis. We also estimated the proportion of between-center variation in mortality explained by center volume.
Results
The cohort included 2557 infants from 53 centers (n=34 centers: 0–10 Norwood cases/year, n=13 centers: 11–20 cases/year, n=6 centers: >20 cases/year). Unadjusted in-hospital mortality was 22%. In multivariable analysis, lower center volume was associated with higher mortality [OR in low vs. high volume centers 1.54, 95% CI 1.02–2.32, p=0.04]. The volume-outcome relationship did not differ across pre-operative risk tertiles (p=0.7). Norwood volume explained an estimated 14% of the between-center variation in mortality observed, and significant between-center variation in mortality remained after adjusting for volume (p<0.001).
Conclusion
Center volume is modestly associated with outcome following the Norwood operation independent of patient risk status. However, this relationship explains only a portion of the between-center variation in mortality in this cohort.
Keywords: CHD, Norwood, outcomes
Introduction
While outcomes following the Norwood operation have improved over the past 3 decades with refinement in surgical technique and advances in peri-operative care, morbidity and mortality remain significant and outcomes vary widely from center-to-center (1–4). Previous studies have suggested that center surgical volume is an important factor associated with outcome following the Norwood operation (4–9). More recent studies have called the volume-outcome relationship into question, but have been limited by the use of administrative data, and including only patients enrolled in certain studies in volume estimates rather than evaluating total Norwood volume (7,10). In addition, it is unknown whether the volume-outcome relationship exists across all levels of patient risk or only applies to high risk patients (11).
We utilized a large multi-center registry to evaluate the association of center Norwood volume with in-hospital mortality, across varying levels of patient pre-operative risk. We also evaluated the extent to which differences in center volume account for between-center variation in outcome.
Methods
Data Source
The Society of Thoracic Surgeons Congenital Heart Surgery Database contains operative, peri-operative, and outcomes data on >180,000 children undergoing heart surgery since 1998, and currently represents nearly three quarters of all US centers performing pediatric heart surgery (12). Data on all children undergoing heart surgery at participating centers are entered into the database. Data quality and reliability are assured through intrinsic verification of data (for example, identification and correction of potential data entry errors regarding patient weight based on comparison to the range of expected values), along with a formal process of in-person site visits and data audits conducted by a panel of independent data quality personnel and pediatric cardiac surgeons at 5 randomly chosen institutions each year (13). The Duke Clinical Research Institute serves as the data warehouse for the STS Databases. This study was approved by the Duke University Institutional Review Board with waiver of consent, and also approved by the STS Access and Publications Committee.
Patient Population
A total of 78 US centers submitted data on at least one Norwood operation to the database from 2000–2009. Centers with >15% missing data for any study variable (n=16) and centers who performed <5 Norwood operations during the entire study period (n=9) were excluded leaving 53 centers available for analysis. While the STS Database contains nearly complete data for the standard core data fields required to calculate in-hospital mortality, not all centers submit complete data for other variables and it is standard practice to exclude centers with >15% missing data for key study variables, in order to maximize data integrity and minimize missing data (14). From the included centers, patients with missing data on pre-operative factors or mortality (n=32 patients) were then excluded. Of note, overall in-hospital mortality was similar in the included cohort vs. all patients with available mortality data undergoing the Norwood operation in the STS Database during this time period (22% vs. 21%).
Data collection
All patients undergoing the Norwood operation were included in the study regardless of underlying anatomy, and characterized by type of single ventricle: right dominant, left dominant, or undifferentiated (15). The presence of other secondary lesions such as total anomalous pulmonary venous return was also collected, along with the presence of any non-cardiac/genetic abnormality, pre-operative length of stay, and other pre-operative factors including mechanical ventilatory or circulatory support, shock, arrhythmia, and neurologic deficit as defined in the STS Database (16). The primary outcome was in-hospital mortality. Center characteristics included geographic region and average annual volume of Norwood operations.
Statistical Analysis
Study variables were described using standard summary statistics. Missing data were rare; pre-operative non-cardiac/genetic abnormality was the only variable with missing data (0.7%). For this variable, missing was imputed to none. Center Norwood volume was categorized for descriptive purposes based on the distribution of the data as: 0–10, 11–20, and >20 cases per year, such that there was an approximately equal number of patients in each group. Patient characteristics overall and in each volume category were described. Outcomes unadjusted for patient characteristics were compared across volume groups using logistic regression accounting for within-center correlation.
Multivariable logistic regression was performed to evaluate the association of center Norwood volume with in-hospital mortality adjusting for patient pre-operative characteristics. Volume was analyzed both as a continuous (log-transformed) and categorical (as defined above) variable. The method of generalized estimating equations (GEE) was utilized to account for correlation between outcomes of patients at the same center. All models were adjusted for year of surgery, age, weight, sex, dominant ventricle, diagnosis of total anomalous pulmonary venous return, pre-operative length of stay, the presence of any non-cardiac/genetic abnormality, and pre-operative shock, mechanical ventilatory or circulatory support, arrhythmia, complete atrioventricular block, or neurologic deficit. Adjusted odds ratios and 95% confidence intervals are reported.
In order to display the relationship between center volume and mortality graphically, the adjusted mortality rate at each center was plotted in order of increasing center volume. Adjusted mortality rates were calculated as: (observed mortality rate/expected mortality rate) * (overall sample observed mortality rate), where the expected mortality rate at each center was obtained from multivariable logistic regression models adjusting for patient characteristics as noted above.
The association of center volume with outcome across different levels of patient pre-operative risk was also evaluated. For this analysis, any pre-operative factor found to be associated with mortality with a p-value of 0.1 or less was entered into a multivariable logistic regression model. These included sex, weight <2.5 kg, dominant ventricle (right vs. non-right), diagnosis of total anomalous pulmonary venous return, prolonged pre-operative length of stay (>75th percentile for cohort), any non-cardiac/genetic abnormality, and pre-operative shock, mechanical ventilatory or circulatory support, arrhythmia, complete atrioventricular block, or neurologic deficit. The estimated model coefficients for each variable were calculated, representing the degree of risk associated with each variable. A risk score was then calculated for each patient summing the risk associated with all of the patient’s pre-operative risk factors. The patients were then divided into low, middle, and high risk categories based on their total risk score. With these three risk groups defined, a GEE model was fit with the interaction of risk group and volume category. We tested whether the effect of volume on outcome varied across the risk groups.
Finally, we evaluated the percent of between-center variation in mortality explained by center volume using hierarchical models with center-level random effects. Models with and without center volume as a main effect were fitted and the difference in estimated variance calculated. We also tested whether there was still significant between-center variation in mortality even after accounting for center volume. All analyses were performed using SAS version 9.2 (SAS Institute Inc, Cary, NC). A p-value <0.05 was considered statistically significant.
Results
Patient and center characteristics
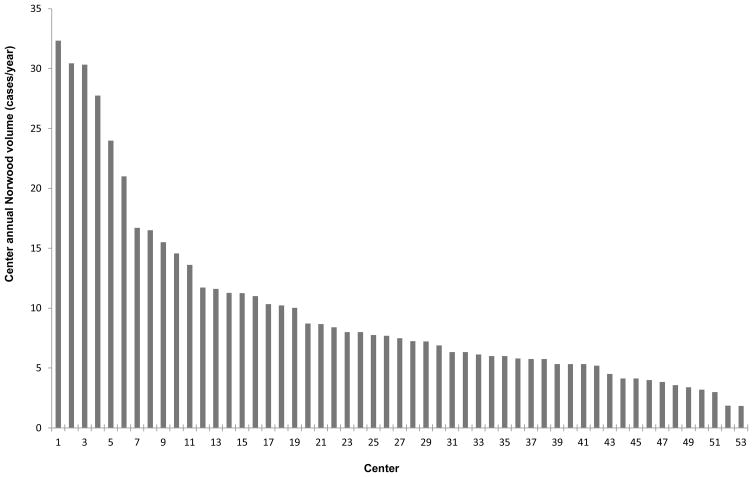
A total of 2557 infants undergoing the Norwood operation from 53 centers (45% South, 27% Midwest, 17% West, 11% Northeast) were included. Analysis of center volume revealed 34 centers with 0–10 Norwood cases/year, 13 centers with 11–20 Norwood cases/year, and 6 centers with >20 Norwood cases/year (Figure 1). The median annual number of Norwood cases/year for the overall cohort was 7.5 (interquartile range 5.3–11.3). Center annual Norwood volume was highly correlated with overall volume of pediatric cardiac cases (r=0.9).
Figure 1. Distribution of Center Annual Norwood Volume.
Patient characteristics are displayed in Table 1. Age, weight and proportion with a right dominant single ventricle were similar between center volume categories. Lower volume centers tended to have a greater proportion of patients with non-cardiac/genetic abnormalities, and pre-operative shock, while higher volume centers had a greater proportion with pre-operative mechanical ventilation (Table 1).
Table 1.
Patient Characteristics
| Overall (n=2557) | Center Volume (Norwood cases/year) | |||
|---|---|---|---|---|
| 0–10 (n=971) | 11–20 (n=760) | >20 (n=826) | ||
| Age, days | 6 (4–9) | 6 (5–10) | 6 (4–9) | 5 (3–7) |
| Weight, kg | 3.18 (2.80–3.50) | 3.10 (2.79–3.50) | 3.20 (2.80–3.56) | 3.20 (2.81–3.50) |
| Weight <2.5 kg | 247 (9.7%) | 100 (10.3%) | 60 (7.9%) | 87 (10.5%) |
| Gender, male | 1488 (58.2%) | 551 (56.8%) | 443 (58.3%) | 494 (59.8%) |
| Non-cardiac/genetic abnormality | 508 (19.9%) | 230 (23.7%) | 147 (19.3%) | 131 (15.9%) |
| Diagnosis | ||||
| Right dominant | 2293 (89.7%) | 865 (89.1%) | 676 (89.0%) | 752 (91.0%) |
| Left dominant | 203 (7.9%) | 78 (8.0%) | 65 (8.6%) | 60 (7.3%) |
| Undifferentiated | 61 (2.4%) | 28 (2.9%) | 19 (2.5%) | 14 (1.7%) |
| TAPVR | 33 (1.3%) | 17 (1.8%) | 7 (0.9%) | 9 (1.1%) |
| Other pre-operative factors | ||||
| Mechanical ventilatory support | 1021 (39.9%) | 378 (38.9%) | 292 (38.4%) | 351 (42.5%) |
| Mechanical circulatory support | 20 (0.8%) | 11 (1.1%) | 6 (0.8%) | 3 (0.4%) |
| Shock | 170 (6.7%) | 81 (8.3%) | 46 (6.1%) | 43 (5.2%) |
| Arrhythmia | 66 (2.6%) | 28 (2.9%) | 13 (1.7%) | 25 (3.0%) |
| Complete AV block | 5 (0.2%) | 1 (0.1%) | 2 (0.3%) | 2 (0.2%) |
| Neurologic deficit | 33 (1.3%) | 20 (2.1%) | 7 (0.9%) | 6 (0.7%) |
| LOS > 7 days | 532 (20.8%) | 268 (27.6%) | 182 (24.0%) | 82 (9.9%) |
Data are displayed as frequencies and percentages or median and interquartile range. For definitions of specific variables, see the STS Congenital Database Full Specifications (http://www.sts.org/documents/pdf/Congenital_DataSpecs_250.pdf) (16).
TAPVR = total anomalous pulmonary venous return, LOS = length of stay, AV=atrioventricular
Post-operative outcomes
Overall unadjusted in-hospital mortality was 22%. In unadjusted analysis, lower center volume was significantly associated with higher mortality [27% mortality (0–10 cases/year), 21% mortality (11–20 cases/year), and 18% mortality (>20 cases/year), p=0.037]. After adjustment for patient characteristics in multivariable analysis, lower center volume remained significantly associated with higher in-hospital mortality when evaluated as a continuous and categorical variable (Table 2).
Table 2.
Adjusted Post-operative Outcomes
| In-hospital mortality | OR (95% CI) | p-value |
|---|---|---|
| Center Volume (Norwood cases/year) | ||
| Volume as continuous variable* | 1.17 (1.01–1.35) | 0.04 |
| Volume as categorical variable | ||
| 0–10 | 1.54 (1.02–2.32) | 0.04 |
| 11–20 | 1.27 (0.80–1.99) | 0.31 |
| >20 | Reference | |
Adjusted odds ratios (OR) and 95% confidence intervals (CI) are displayed both for center volume as a continuous variable and categorical variable. The data displayed for center volume as a continuous variable represent the OR and 95% CI associated with a decrease in center volume by two-fold.
Patients were then categorized based on pre-operative risk factors as described above. Mortality rates in the low, middle, and high risk groups were 14%, 21%, and 33%, respectively. The relationship between center Norwood volume and mortality did not vary significantly across the risk groups (p=0.70). In other words, the relationship between volume and outcome (ie. lower volume related to higher mortality) was similar for all risk groups.
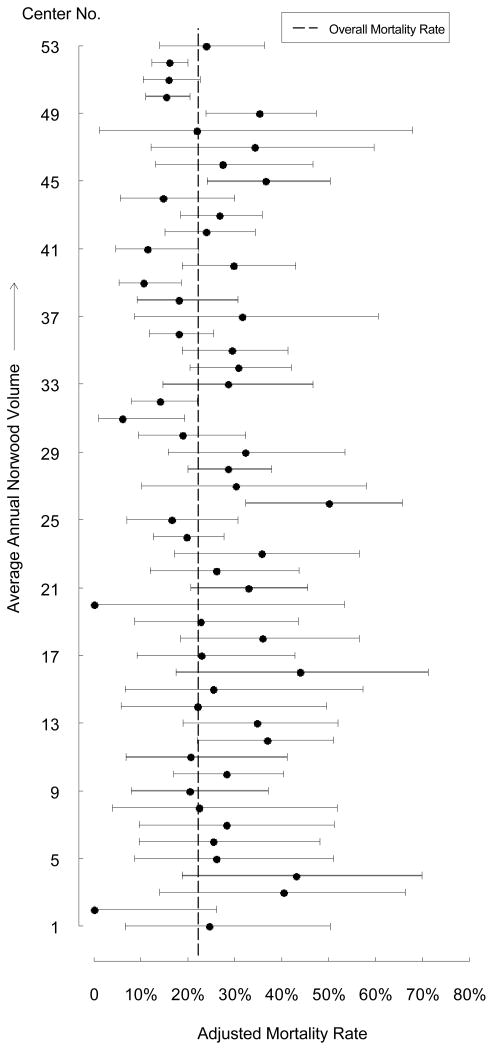
Finally, variation in center-specific outcome was evaluated graphically, which demonstrated that the volume-outcome relationship did not hold true across all centers (Figure 2). There were some middle volume centers with adjusted mortality rates comparable to higher volume centers, and some higher volume centers with mortality rates similar to those in the lower volume groups. Center Norwood volume explained an estimated 14% of the between-center variation in mortality observed in this cohort, and after adjusting for center volume, significant between-center variation in mortality remained (p<0.001).
Figure 2. Adjusted Mortality Rate Displayed by Increasing Center Volume.
The adjusted mortality rate (and 95% confidence interval) for each center is displayed in order of increasing center volume. The overall mortality rate in the entire cohort is represented by the dashed line. As center Norwood volume increases, a greater proportion of center mortality estimates are located to the left of the vertical line (overall mortality rate in entire cohort), indicating lower adjusted mortality rates, or better outcomes. However, this relationship does not hold true for all centers. In particular, there are some middle volume centers (ie. center 31&39) with mortality rates comparable to higher volume centers, and some higher volume centers (ie. center 45&49) with mortality rates similar to those in the lower volume groups.
Discussion
We found that center volume was modestly associated with outcome following the Norwood operation independent of patient risk status. This relationship does not hold true for all centers, and center volume explains only a portion of between-center variation in outcome.
Several previous studies have evaluated the volume-outcome relationship in children undergoing heart surgery, including analyses of New York and California state databases and the Nationwide Inpatient Sample, and found higher mortality rates at lower volume centers (7–9). Using the same clinical database in our study, Welke et al. also found an inverse association between case volume and mortality that became increasingly important as case complexity increased (4). A relationship between volume and outcome has also been reported specifically for patients undergoing the Norwood operation. Hirsch and colleagues evaluated 624 Norwood patients in the Kids’ Inpatient Database and found a significant inverse association between volume and mortality (35% in low volume vs. 17% in high volume centers) (5). Checchia et al. reported similar results analyzing 801 patients in the Pediatric Health Information Systems Database (6).
However, there are limitations to using administrative data in this type of analysis. Administrative datasets rely upon International Classification of Diseases, 9th revision (ICD-9) diagnosis and procedure codes from the hospital bill to identify patients of interest. There is no ICD-9 code for the Norwood operation, such that a combination of other diagnosis and procedure codes must be used, the validity of which is unknown. The present study confirms the relationship (although modest) between Norwood volume and outcome in >2500 patients in a large clinical registry. These findings differ from a recent Congenital Heart Surgeons Society (CHSS) study which did not find a significant relationship between volume and outcome (10). However, volume estimates in the CHSS study were based on the number of patients from each center enrolled in a CHSS cohort of patients with aortic atresia or stenosis undergoing the Norwood operation (rather than the overall number of patients at each center undergoing the Norwood operation), patients were enrolled in an earlier era compared with the present analysis, and long-term mortality rather than in-hospital mortality was examined (10). These and other differences make it difficult to directly compare these two studies.
Few previous studies have evaluated whether the volume-outcome relationship varies by patient pre-operative risk status. The CHSS study found that some institutions were able to “neutralize” certain risk factors such as low birth weight (10). Studies in the adult cardiac surgery literature have reported conflicting results as to whether the relationship between hospital volume and mortality in patients undergoing coronary artery bypass grafting (CABG) is applicable to all patients regardless of surgical risk, or only applies to high risk patients (17,18). Data from the present study suggest that for patients undergoing the Norwood operation, the relationship between volume and outcome does not vary across different levels of patient pre-operative risk.
Based on studies showing a relationship between volume and outcome in the adult cardiac surgery population, the Leapfrog Group has recommended that patients and payors should choose hospitals with an annual CABG volume of >450 cases (19). Should similar policy for pediatric heart surgery be considered? Our analysis suggests that this may not be the optimal approach. First, while we did find a significant relationship between Norwood volume and outcome, this relationship was modest at best and did not hold true across all centers. Some middle volume hospitals had similar outcomes to high volume hospitals, and some high volume hospitals had outcomes similar to those in the lower volume groups. Second, we found that center Norwood volume accounted for only a small proportion of the overall between-center variation in outcome which exists for this operation. Thus, these data would suggest that the use of center volume alone as a quality metric for the Norwood operation may not be justified. In their analysis of patients undergoing all types of pediatric cardiac surgery, Welke and colleagues reported similar findings, and concluded that volume alone was a poor discriminator of mortality (7).
The use of center-specific risk adjusted outcome as a quality metric may be more informative than relying upon center volume alone. This approach also requires several important considerations. It has previously been shown that adjustment for both surgical case complexity as well as patient specific factors is important when comparing outcomes across centers (20–22). In addition, due to the relatively rarity of most congenital heart lesions, the low case volumes for any individual procedure performed at one center make statistically meaningful comparisons difficult (23). Thus, it is often necessary to evaluate several years of data rather than a one year period. Finally, combining individual procedures into groups of operations of similar surgical risk may also facilitate analysis (20).
Reducing variation in outcome across sites may potentially be addressed through two different mechanisms. First, regionalization of care, or selective referral of patients to high performing centers, may improve outcome. A previous study has suggested that selective referral of patients in California from low-medium to high volume hospitals could theoretically reduce mortality for children undergoing heart surgery, with an estimated 83 deaths avoided during a three year period (24). Regionalization of care for pediatric heart surgery in Europe has already taken place. In Sweden, care was centralized to two centers with the lowest mortality in 1993, and 30 day national mortality rates were reduced from 9.5% to 1.9% (25). Whether or not regionalization of care for children undergoing heart surgery in the United States is feasible or even desirable remains under debate. Alternative strategies include the development of evidence-based best practice guidelines, and design and implementation of quality assessment and improvement initiatives. Few previous studies have evaluated variation in care across centers and how practice variation may be associated with outcome (26). Further study of differences in peri-operative management strategies, hospital structural and process measures, and training and availability of personnel is necessary to identify best practices and further our understanding of areas to target in quality improvement initiatives.
Limitations
The limitations of this study are primarily related to the nature of the STS Database. While the Database is currently the largest pediatric heart surgery registry in North America, not all centers participate. In addition, while data for standard core data fields are nearly complete, not all centers submit complete data for all variables captured by the Database, and thus, are not included in the analysis. Thus, our results may not be generalizable to all US centers. In addition, we included patients undergoing the Norwood operation regardless of underlying anatomy; thus the center volume numbers in our analysis will be slightly higher compared with analyses restricted to patients with hypoplastic left heart syndrome. Although we were able to include many patient pre-operative risk factors in our analysis of patient risk status, not all potential risk factors were collected in the Database during the study period, including gestational age (although we were able to adjust for weight at surgery), the anatomic subtype of hypoplastic left heart syndrome (ie. mitral stenosis/atresia or aortic stenosis/atresia), size of the ascending aorta, and presence of a restrictive atrial septum, though pre-operative shock and mechanical ventilation are captured in the database (and accounted for in our analysis) and may be related to the latter. Longer pre-operative length of stay may also be a surrogate measure for a more complicated pre-operative course, and was accounted for in our analysis. Despite these limitations, we were able to successfully classify our study population into tertiles of increasing risk based on available pre-operative risk factors. In addition, during the study period the source of pulmonary blood flow (modified Blalock-Tausig shunt vs. right ventricle-to-pulmonary artery conduit) was not specified in the Database (this variable was subsequently added in 2010). Therefore we were unable to account for this in our analysis. However, we were able to adjust for year of surgery in our models, with use of the right ventricle-to-pulmonary artery conduit becoming more prevalent in recent years. In addition, while the database does contain specific definitions for all variables, this does not exclude variation in coding across centers. Finally, the Database currently does not capture information regarding personnel, or hospital structural or process measures, therefore we are unable to evaluate the relationship of these factors to center volume or outcome.
Conclusions
This multi-center analysis suggests a modest association of center volume with outcome following the Norwood operation independent of patient risk status. However, this relationship does not hold true for all centers and center volume explains only a portion of between-center variation in outcome. Thus, the use of center volume alone as a quality metric for the Norwood operation may not be justified, and center-specific risk adjusted outcome may be more appropriate.
Footnotes
Disclosures
This study was supported by an American Heart Association Mid-Atlantic Affiliate Clinical Research Award (PI: Pasquali).
Dr. Pasquali: Grant support, National Heart, Lung, and Blood Institute (1K08HL103631-01)
Dr. J Jacobs: Chair, Society of Thoracic Surgeons Congenital Heart Surgery Database Task Force.
Dr. Peterson: Principal Investigator, Society of Thoracic Surgeons National Databases Analytic Center.
References
- 1.Norwood WI, Lang P, Casteneda AR, Campbell DN. Experience with operations for hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 1981;82:511–519. [PubMed] [Google Scholar]
- 2.Graham EM, Bradley SM, Atz AM. Perioperative management of hypoplastic left heart syndrome. Expert Opin Pharmacother. 2005;6:687–93. doi: 10.1517/14656566.6.5.687. [DOI] [PubMed] [Google Scholar]
- 3.Pearl JM, Nelson DP, Schwartz SM, Manning PB. First-stage palliation for hypoplastic left heart syndrome in the twenty-first century. Ann Thorac Surg. 2002;73(1):331. doi: 10.1016/s0003-4975(01)02720-5. [DOI] [PubMed] [Google Scholar]
- 4.Welke KF, O’Brien SM, Peterson ED, et al. The complex relationship between pediatric cardiac surgical case volumes and mortality rates in a national clinical database. J Thorac Cardiovasc Surg. 2009;137:1133–1140. doi: 10.1016/j.jtcvs.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch JC, Gurney JG, Donohue JE, et al. Hospital mortality for Norwood and Arterial Switch Operations as a function of institution volume. Pediatr Cardiol. 2008;29:713–717. doi: 10.1007/s00246-007-9171-2. [DOI] [PubMed] [Google Scholar]
- 6.Checchia PA, McCollegan M, Daher N, et al. The effect of surgical case volume on outcome after the Norwood procedure. J Thorac Cardiovasc Surg. 2005;129:754–759. doi: 10.1016/j.jtcvs.2004.07.056. [DOI] [PubMed] [Google Scholar]
- 7.Welke KF, Diggs BS, Karamlou T, Ungerleider RM. The relationship between hospital surgical case volumes and mortality rates in pediatric cardiac surgery: a national sample, 1988–2005. Ann Thorac Surgery. 2008;89:889–896. doi: 10.1016/j.athoracsur.2008.04.077. [DOI] [PubMed] [Google Scholar]
- 8.Hannan EL, Racz M, Kavey R, et al. Pediatric cardiac surgery: the effect of hospital and surgeon volume on in-hospital mortality. Pediatrics. 1998;101:963–969. doi: 10.1542/peds.101.6.963. [DOI] [PubMed] [Google Scholar]
- 9.Bazzani LG, Marcin JP. Case volume and mortality in pediatric cardiac surgery patients in California, 1998–2003. Circulation. 2007;115:2652–2659. doi: 10.1161/CIRCULATIONAHA.106.678904. [DOI] [PubMed] [Google Scholar]
- 10.Karamlou T, McCrindle BW, Blackstone EH, et al. Lesion-specific outcomes in neonates undergoing congenital heart surgery are related predominantly to patient and management factors rather than institution or surgeon experience: A Congenital Heart Surgeons Society Study. J Thorac Cardiovasc Surg. 2010;139:569–577. doi: 10.1016/j.jtcvs.2008.11.073. [DOI] [PubMed] [Google Scholar]
- 11.Ashburn DA, McCrindle BW, Tchervenkiv CI, et al. Outcomes after the Norwood operation in neonates with critical aortic stenosis or aortic valve atresia. J Thorac Cardiovasc Surg. 2003;125:1070–1082. doi: 10.1067/mtc.2003.183. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs ML, Mavroudis C, Jacobs JP, Tchervenkov CI, Pelletier GJ. Report of the 2005 STS Congenital Heart Surgery Practice and Manpower Survey: A Report from The STS Work Force on Congenital Heart Surgery. Ann Thorac Surg. 2006;82:1152–1158. doi: 10.1016/j.athoracsur.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 13.Clarke DR, Breen LS, Jacobs ML, et al. Verification of data in congenital cardiac surgery. Cardiol Young. 2008;18 (Suppl2):177–187. doi: 10.1017/S1047951108002862. [DOI] [PubMed] [Google Scholar]
- 14.Johnson JN, Jaggers J, Li S, et al. Center variation and outcomes associated with delayed sternal closure following stage 1 palliation for hypoplastic left heart syndrome. J Thorac and Cardiovasc Surg. 2010;139:1205–1210. doi: 10.1016/j.jtcvs.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs JP, O'Brien SM, Chai PJ, Morell VO, Lindberg HL, Quintessenza JA. Management of 239 patients with hypoplastic left heart syndrome and related malformations from 1993 to 2007. Ann Thorac Surg. 2008;85:1691–1696. doi: 10.1016/j.athoracsur.2008.01.057. [DOI] [PubMed] [Google Scholar]
- 16. [Accessed February 1, 2011];STS Congenital Database Full Specifications. http://www.sts.org/documents/pdf/Congenital_DataSpecs_250.pdf.
- 17.Nallamothu BK, Saint S, Ramsey SD, et al. The role of hospital volume in coronary artery bypass grafting: is more always better? J Am Coll Cardiol. 2001;38:1923–1930. doi: 10.1016/s0735-1097(01)01647-3. [DOI] [PubMed] [Google Scholar]
- 18.Wu C, Hannan EL, Ryan TJ, et al. Is the impact of hospital and surgeon volumes on the in-hospital mortality rate for coronary artery bypass graft surgery limited to patients at high risk? Circulation. 2004;110:784–789. doi: 10.1161/01.CIR.0000138744.13516.B5. [DOI] [PubMed] [Google Scholar]
- 19.Leapfrog Group. [Accessed February 2, 2011];Evidence-Based Hospital Referral. Available at: http://www.leapfroggroup.org/media/file/FactSheet_EBHR.pdf.
- 20.O’Brien SM, Clarke DR, Jacobs JP, et al. An empirically based tool for analyzing mortality associated with congenital heart surgery. J Thorac Cardiovasc Surg. 2009;138:1139–1153. doi: 10.1016/j.jtcvs.2009.03.071. [DOI] [PubMed] [Google Scholar]
- 21.Jenkins KJ, Gauvreau K, Newburger JW, Spray TL, Moller JH, Iezzoni LI. Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg. 2002;123:110–118. doi: 10.1067/mtc.2002.119064. [DOI] [PubMed] [Google Scholar]
- 22.Lacour-Gayet FG, Clarke D, Jacobs JP, et al. The Aristotle Score: A Complexity-Adjusted Method to Evaluate Surgical Results. Eur Journal of Cardiothorac Surg. 2004;25:911–924. doi: 10.1016/j.ejcts.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 23.Welke KF. Interpreting congenital heart disease outcomes: What do available metrics really tell us? World Journal for Pediatric and Congenital Heart Surgery. 2010;1:194–198. doi: 10.1177/2150135110372530. [DOI] [PubMed] [Google Scholar]
- 24.Chang RR, Klitzner TS. Can regionalization decrease the number of deaths for children who undergo cardiac surgery? A theoretical analysis. Pediatrics. 2002;109:173–181. doi: 10.1542/peds.109.2.173. [DOI] [PubMed] [Google Scholar]
- 25.Lundstrom NR, Berggren H, Bjorkhem G, Jogi P, Sunnegardh J. Centralization of pediatric heart surgery in Sweden. Pediatr Cardiol. 2000;21:353–357. doi: 10.1007/s002460010079. [DOI] [PubMed] [Google Scholar]
- 26.Burstein DS, Jacobs JP, Sheng S, et al. Care models in congenital heart surgery and associated outcomes. Pediatrics. 2011;127(6):e1482–e1489. doi: 10.1542/peds.2010-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]