Abstract
Oxytocin is important to social behavior and emotion regulation in humans. Oxytocin’s role derives in part from its effect on memory performance. More specifically, previous research suggests that oxytocin facilitates recognition of social (e.g., faces), but not of non-social stimuli (e.g., words, visual objects). We conducted the first within-subject study to this hypothesis in a double-blind, placebo-controlled design. We administered oxytocin (24 IU) and placebo (saline) in two separate sessions and in randomized order to healthy men. To obtain a baseline measure for session-dependent memory effects, which are caused by proactive interference, an additional group of male subjects in each session received placebo unbeknownst to them and the experimenter. After administration, participants studied faces and houses. Exactly one day after each study session, participants were asked to make memory judgments of new and old items. In the first study-test session, participants administered with oxytocin showed reduced recollection of previously studied faces and houses. Oxytocin also interacted with proactive-interference effects. By impeding memory in the first session, it reduced proactive interference in the second. But oxytocin contributed additionally to the memory-reducing effect of proactive interference when administered in the second session. These results demonstrate that oxytocin can have a memory-impairing effect on both social and non-social visual objects. The present study also emphasizes the necessity of including a non-treated, baseline group in within-subject designs when investigating oxytocin’s effects on human memory.
Keywords: oxytocin, recognition memory, remember/know, faces, visual objects, within-subject
1. Introduction
The neuropeptide oxytocin is important for learning, memory, and behavioral regulation in nonhuman mammals and humans. Oxytocin is produced and released by the magnocellular neurons of the paraventricular and supraoptic nuclei of the hypothalamus. It is also synthesized in peripheral tissues like the placenta, uterus, corpus luteum, testis, and heart. Oxytocin’s dual functions as a central neurotransmitter and a peripheral hormone, however, do not have a direct relationship (MacDonald & MacDonald, 2010). Central oxytocin receptors are found throughout the brain in many structures important for information processing and memory, including the hippocampus, amygdala, striatum, hypothalamus, nucleus accumbens, and midbrain (Gimpl & Fahrenholz, 2001). The role oxytocin plays in these central nervous regions have been investigated in nonhuman mammals for more than four decades (e.g., Insel, 2010; Lee et al., 2009, for review). Research on human behavior has grown rapidly in recent years (see Fehm-Wolfsdorf et al., 1991; Heinrichs & Domes, 2008; MacDonald & MacDonald, 2010, for reviews) and shown that oxytocin affects human social behavior, emotion regulation, social cognition, and memory. The focus of the present study is on oxytocin’s effect on memory, which has been found to be diverse in lab animals but especially in humans.
In lab animals, oxytocin facilitates social recognition, which is defined as a reduction in the time that is spent investigating a conspecific during the second encounter as compared to the first. The effects of oxytocin on social recognition in nonhuman mammals have been shown in two ways. First, oxytocin injections in various regions of the brain facilitated social recognition (Ferguson et al., 2002; Lee et al., 2009). Second, knock-out animals that lack oxytocin genes required for the development of oxytocin receptors demonstrate a deficit in social recognition (Ferguson et al., 2000; Lee et al., 2009). This deficit can be temporarily restored by a single injection of oxytocin before the initial encounter of a conspecific (Ferguson et al., 2000). However, not all studies on oxytocin and social recognition have confirmed oxytocin’s memory-enhancing effect. Some studies have instead suggested an interaction of the oxytocin dose with memory (Bielsky & Young, 2004). Other studies, which investigated types of memory other than social recognition (Bohus et al., 1978; De Wied et al., 1991; Dubrovsky et al., 2002; Engelmann et al., 1996; Wu & Yu, 2004), reported both memory-facilitating and memory-impairing effects, suggesting that the behavioral test type and the brain area under investigation modulate oxytocin-induced effects (Engelmann et al., 1996).
Oxytocin has also been shown to have diverse effects on memory in humans. It has been found to facilitate memory for faces, which are considered social stimuli (Guastella et al., 2008; Rimmele et al., 2009; Savaskan et al., 2008). When administered before the study phase of a recognition memory experiment, oxytocin enhanced memory performance for faces and was therefore suggested to enhance memory-encoding processes (Guastella et al., 2008; Rimmele et al., 2009). Savaskan et al. (2008) administered oxytocin directly after the study phase but 24 hours before the test phase and still found a memory-enhancing effect. They therefore suggested that oxytocin might not only enhance encoding but also consolidation processes. These positive effects of oxytocin on face memory, however, have not been consistently found and might depend on the emotional expressions of the faces. For example, some studies reported no effect or a small memory-impairing effect of oxytocin on neutral (Bruins et al., 1992; Ferrier et al., 1980; Guastella et al., 2008) or happy faces (Savaskan et al., 2009), whereas other studies did not find any dependency on emotional expressions (Rimmele et al., 2009). Studies that used non-social stimuli (i.e., words) found either a memory-impairing effect or none at all (Bruins et al., 1992; Fehm-Wolfsdorf et al., 1984, 1988; Ferrier et al., 1980; Di Simplicio et al., 2009; Heinrichs et al., 2004).
Based on the complex picture of oxytocin’s effects on human memory it has been suggested that oxytocin might facilitate memory for social but not for non-social stimuli (Lee et al., 2009; Rimmele et al., 2009; Savaskan et al., 2009). The only study that directly compared memory performance for faces and non-social, visual objects (Rimmele et al., 2009), found a selective memory-enhancing effect for faces. But this result may have been influenced by the fact that the non-social condition, which consisted of houses, landscapes, and art sculptures, contained a heterogeneous set of items and was thus easier to remember than the homogenous face condition.
The aim of the present study was to investigate oxytocin-induced effects on memory for faces and a homogenous non-social class of objects (houses). The study is the first to use a within-subject design to control for individual differences in general memory and other personality traits, which could potentially lead to group differences and thus confound drug/placebo effects in between-subject designs. As in some previous studies (Guastella et al., 2008; Rimmele et al., 2009), a remember/know paradigm was used to investigate the influence of oxytocin on the two proposed sub-processes of recognition memory: familiarity (i.e., an item feels familiar) and recollection (i.e., an item is remembered with details from the study episode) (Yonelinas, 2002).
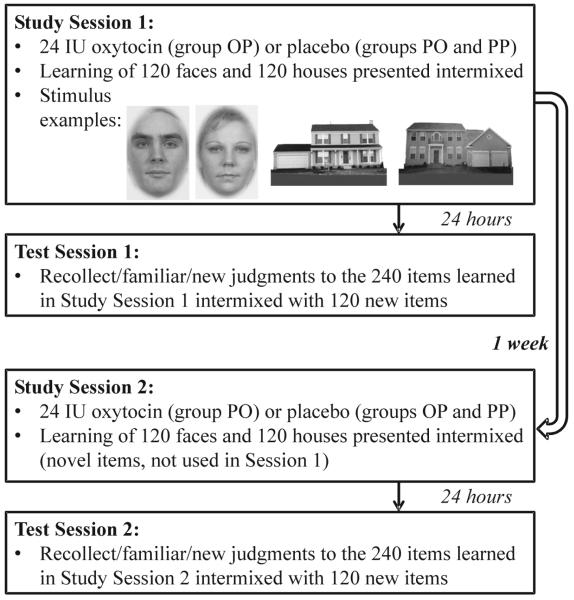
The double-blind, placebo-controlled study consisted of two study-test sessions for each participant. Please see Figure 1 for a schematic of the experimental procedure. In each study session, oxytocin or placebo was administered. Drug/placebo administration was counterbalanced across sessions within subjects. After substance administation, participants were asked to study a series of faces and houses, which were shown intermixed within blocks. Exactly 24 hours after the study sessions, recognition memory was tested. Participants were shown studied and new items. For each item, they were asked to decide whether the stimulus was new, “familiar,” or if they “recollect” it. Recollection was explained as remembering that an item is old and in addition retrieving details from the experience studying this item like a thought that came to mind during study. Familiarity was explained as being sure that an item is old but not being able to retrieve any details about the study episode. Exactly one week after the first study session, participant returned for their second study-test session, which followed the same procedure as the first. This time, participants received either oxytocin or placebo depending on their administration in the first study-test session (i.e., if they had received oxytocin first, they received placebo now).
Figure 1.
Schematic of the experimental design and examples for stimuli. Group abbreviations refer to the administrated nasal spray (O for oxytocin, P for placebo) and indicate the order in which it was received (e.g., OP indicates that oxytocin was received in Study Session 1 and placebo in Study Session 2).
The study was initially conducted such that subjects randomly received either oxytocin in the first study-test session and placebo in the second, or placebo in the first and oxytocin in the second. The results indicated that oxytocin-induced effects on memory performance were modulated by the study-test session in which oxytocin was administered. This was very likely due to interactions of oxytocin with proactive interference, which refers to the phenomenon whereby previously studied material (i.e., from the first study-test session) interferes with the learning of new material at a later point in time (i.e., in the second study-test session). The initial design with just two subject groups made it impossible to disentangle this interaction of oxytocin and proactive interference because it confounded the experimental factors drug/placebo with study-test session as seen in previous within-subject designs (Tops & Wijers, 2011; Wirth et al., 2011). To elucidate the observed interaction of oxytocin with proactive interference, an additional subject group was included in the study. All participants in that group received placebo in both the first and second study-test session. But to maintain double-blindness as well as the expectation of receiving oxytocin, this administration scheme was unknown to the subjects and the experimenter. The present study thus investigated not only how oxytocin influenced memory performance for social (faces) and non-social (houses) visual objects, but also the effects of a single administration of oxytocin within repeated study-test sessions that used novel social and non-social objects.
2. Results
2.1. Performance measurement
Raw “recollect” responses were interpreted as “recollected” hits (to old items) and false alarms (to new items). The raw “familiar” condition cannot be taken as a direct reflection of familiarity because these responses are contingent on non-recollection. Using the so-called independent remember-know procedure (IRK, Yonelinas, 2002), familiarity hits and false alarms were calculated as the probability for responding “familiar” to an item provided that the item was not given a “recollect” response (i.e., for hit rates and false alarms, respectively, IRK “familiar” = “familiar”/(1-“recollect”)). Discrimination indices of recollection and familiarity were estimated separately as hits minus false alarms using “recollect” and IRK “familiar” responses. Qualitatively similar results were obtained using d’ rather than hits minus false alarms as a discrimination measure. Overall recognition-memory accuracy was assessed as d’ by summarizing “familiar” and “recollect” judgments into “old” responses. We also computed the response bias, c, using the sum of “familiar” and “recollect” judgments as “old” responses.
2.2. Statistical analysis
Effects of oxytocin and repeated study-test sessions were analyzed in mixed model analyses of variance (ANOVA) with repeated measures on the within-subject factors test session (1 and 2) and stimulus category (faces and houses) and the between-subject factor group (OP: oxytocin first/placebo second, PO: placebo first/oxytocin second, PP: placebo first/placebo second). For analyses of control variables, the same ANOVAs were calculated but the factor stimulus category was excluded. Post-tests that followed up on significant main effects or interactions were Bonferroni-corrected for multiple comparisons. Cohen’s d or eta-squared (η2), indicating the proportion (between 0—none and 1—all) of variance in the dependent variables accounted for by the variation in the independent variable, are provided for all analyses.
2.3. Control variables
None of the included control variables showed any systematic variation across the three experimental groups. No significant differences between the OP, PO, and PP groups were found in attention (accuracies and reaction times of the Continuous Performance Test, all ps > .48), wakefulness (wakefulness scale of the MDMQ, all ps > .20), or mood (positive and negative affect scales of the PANAS, all ps > .53), all of which were measured before drug/placebo administration and just before the peak of the oxytocin concentration (35 min after administration, Born et al. 2002). No group differences were found for the frequencies or reaction times of attractiveness ratings to faces and houses in the study phase (all ps > .10). At the end of Test Session 2, subjects were unable to identify the order in which they had received oxytocin and placebo (p > .72) as indicated by a χ2 test.
2.4. Memory performance
Table 1 shows memory performance for all three experimental groups in Test Sessions 1 and 2 as measured by d’, response bias c, hits and false alarms for “recollect” and IRK “familiarity” judgments as well as discrimination indices of recollection and familiarity measured as hits minus false alarms for “recollect” and IRK “familiar” judgments, respectively. A separate group x test session x stimulus category ANOVA was run on each of the dependent measures shown in Table 1. Only significant effects are described below. Thus, any non-reported effects were not significant. In general, measures of recollection, discrimination index and hit rates, showed the most reliable effects. These results are highlighted in Figures 2 and 3, respectively.
Table 1.
Recognition memory performance for faces and houses in the first and second test session for all three experimental groups (OP for oxytocin first/placebo second, PP for placebo in first and second session, PO for placebo first/oxytocin second). Shown are means and standard deviations in parenthesis.
| Faces | Houses | ||||||
|---|---|---|---|---|---|---|---|
| OP | PP | PO | OP | PP | PO | ||
| d’ | Session 1 | 1.54 (0.78) |
1.95 (0.65) |
2.05 (0.83) |
0.65 (0.30) |
0.76 (0.35) |
0.89 (0.37) |
| Session 2 | 1.28 (0.88) |
1.60 (0.65) |
1.61 (0.72) |
0.56 (0.25) |
0.78 (0.33) |
0.64 (0.20) |
|
| Response bias c | Session 1 | 0.04 (0.54) |
0.00 (0.56) |
0.00 (0.32) |
−0.19 (0.50) |
−0.61 (0.39) |
−0.37 (0.30) |
| Session 2 | −0.02 (0.50) |
−0.08 (0.45) |
0.13 (0.44) |
−0.30 (0.51) |
−0.53 (0.39) |
−0.40 (0.51) |
|
| Recollection1 | Session 1 | 0.31 (0.13) |
0.44 (0.17) |
0.53 (0.23) |
0.15 (0.09) |
0.19 (0.08) |
0.25 (0.10) |
| Session 2 | 0.28 (0.15) |
0.34 (0.12) |
0.35 (0.21) |
0.19 (0.11) |
0.20 (0.10) |
0.20 (0.10) |
|
| Hits “recollect” | Session 1 | 0.36 (0.13) |
0.48 (0.19) |
0.56 (0.24) |
0.28 (0.14) |
0.31 (0.12) |
0.35 (0.17) |
| Session 2 | 0.33 (0.16) |
0.39 (0.14) |
0.39 (0.23) |
0.30 (0.18) |
0.30 (0.13) |
0.31 (0.21) |
|
| False alarms “recollect” | Session 1 | 0.05 (0.05) |
0.04 (0.06) |
0.03 (0.05) |
0.13 (0.10) |
0.11 (0.09) |
0.10 (0.10) |
| Session 2 | 0.05 (0.08) |
0.05 (0.07) |
0.04 (0.08) |
0.12 (0.15) |
0.10 (0.09) |
0.11 (0.15) |
|
| IRK Familiarity1 | Session 1 | 0.38 (0.10) |
0.46 (0.15) |
0.43 (0.17) |
0.17 (0.09) |
0.20 (0.13) |
0.24 (0.14) |
| Session 2 | 0.33 (0.24) |
0.41 (0.19) |
0.37 (0.15) |
0.13 (0.07) |
0.22 (0.09) |
0.15 (0.09) |
|
| Hits IRK “familiar” | Session 1 | 0.62 (0.15) |
0.65 (0.13) |
0.59 (0.17) |
0.56 (0.19) |
0.75 (0.13) |
0.65 (0.12) |
| Session 2 | 0.61 (0.17) |
0.66 (0.18) |
0.57 (0.13) |
0.58 (0.19) |
0.72 (0.13) |
0.63 (0.17) |
|
|
False alarms IRK “familiar” |
Session 1 | 0.24 (0.22) |
0.19 (0.19) |
0.16 (0.12) |
0.39 (0.18) |
0.54 (0.14) |
0.41 (0.17) |
| Session 2 | 0.28 (0.22) |
0.17 (0.17) |
0.19 (0.16) |
0.45 (0.17) |
0.50 (0.13) |
0.48 (0.17) |
|
Recollection and IRK Familiarity are calculated as hits minus false alarms for the corresponding memory judgments.
Figure 2.
Discrimination index for recollection measured as hits minus false alarms for faces and houses in Test Sessions 1 and 2 for all three experimental groups (OP for oxytocin first/placebo second, PP for placebo in first and second session, PO for placebo first/oxytocin second). Error bars indicate standard error.
Figure 3.
Hit rates of accurate “recollect” judgments for studied (i.e., old) faces and houses in Test Sessions 1 and 2 for all three experimental groups (OP for oxytocin first/placebo second, PP for placebo in first and second session, PO for placebo first/oxytocin second). Error bars indicate standard error.
Memory performance was generally more accurate for faces than for houses. This was seen in all measures reported in Table 1, Fs(1,13) > 21.8, ps < .001, η2s = 0.63, apart from hit rates of IRK “familiar” judgments.
Memory performance was also generally better in Test Session 1 than 2, which we attribute to proactive interference. This was seen in all measures reported in Table 1, Fs(1,13) > 7.5, ps < .05, η2s = 0.36, apart from the false alarms for “recollect” judgments and the hit rates of IRK “familiar” judgments. For measures of overall recognition (d’) and recollection (discrimination index and hit rates), this main effect of test session was further qualified by a stimulus category x test session interaction, Fs(1,13) > 7.1, ps < .05, η2s = 0.35, which indicated that memory performance for faces but not for houses was systematically lower in Test Session 2.
Memory performance marginally differed across the three experimental groups for the discrimination index of recollection, F(2,26) = 2.8, p = .08, η2= 0.18. These differences across groups were only significant in Test Session 1, F(2,26) = 6.4, ps < .05, η2s = 0.33. In Test Session 1, participants who had received oxytocin (i.e., OP group) showed significantly less accurate recollection than participants who had received placebo (i.e., the PO and PP groups, which did not differ from one another, p > .36), F(1,13) = 12.3, p < .01, η2 = 0.49, for the comparison of OP with PO and F(1,13) = 6.3, p < .05, η2 = 0.33 for the comparison of OP with PP, respectively.
Finally, memory performance, in particular recollection, was differently affected by repeated testing in the three experimental groups, indicated by a group x test session interaction found for the discrimination index of recollection, F(2,26) = 8.4, p < .01, η2= 0.39, and for hit rates of “recollect” judgments, F(2,26) = 7.1, p < .01, η2= 0.35. These group differences in proactive interference between Test Session 1 and 2 are depicted in Figures 4 and 5. Statistics are given for the discrimination index of recollection (Fig. 4) and hit rates of “recollect” judgments (Fig. 5), respectively. Recollection performance declined between Test Sessions 1 and 2 for the PO group, Fs(1,13) = 31.7 and 23.0, ps < .001, η2s = 0.71 and 0.64, and the PP group, Fs(1,13) = 5.3 and 7.7, ps < .05, η2s = 0.29 and 0.37, but not for the OP group, ps > .96 and .85. PO and PP also showed test session x stimulus category interactions, Fs(1,13) > 4.1, ps < .05, η2s > 0.22, respectively, but note that the three-way interactions category x test session x group were not significant in the overall ANOVAs. Recollection of faces, but not houses, was significantly reduced in the second test session as compared to the first for PO, ts(13) = 6.6 and 6.8, ps < .001, Cohen’s ds = .82 and .72, and to a lesser extent for PP, ts(13) = 3.4 and 2.5, ps < .05, Cohen’s ds = .68 and .54, but not for OP, ps > .26. For houses, similar although non-significant patterns of results were found (Fig. 4 and 5).
Figure 4.
Effects of test session measured as performance in Test Session 1 minus Test Session 2 for the discrimination index of recollection (i.e., hits minus false alarms) for faces and houses for all three experimental groups (OP for oxytocin first/placebo second, PP for placebo in first and second session, PO for placebo first/oxytocin second). Error bars indicate standard error.
Figure 5.
Effects of test session measured as performance in Test Session 1 minus Test Session 2 for the hit rates of accurate “recollect” judgments for studied (i.e., old) faces and houses for all three experimental groups (OP for oxytocin first/placebo second, PP for placebo in first and second session, PO for placebo first/oxytocin second). Error bars indicate standard error.
For the response bias, no main effects or interactions were found apart from the main effect of stimulus category reported above. Thus, neither oxytocin nor repeated testing influenced the response bias.
3. Discussion
This is the first double-blind, placebo-controlled, within-subjects study to investigate the effects of oxytocin on recognition memory for homogenous groups of social (faces) and non-social (houses) stimuli, and the second study to directly contrast the effect of oxytocin on memory for social and non-social visual objects (following Rimmele et al., 2009). We found a moderate memory-impairing effect of oxytocin for both faces and houses. With regard to the two component-processes of recognition memory (Yonelinas, 2002), recollection but not familiarity was affected by oxytocin. Specifically, oxytocin lowered the correct and detailed recollection of previously studied (i.e., old) faces and houses but did not affect overall recognition discrimination (d’), response bias, familiarity, or memory judgments to new items. No differences in attention, wakefulness, or mood, which could explain the observed memory-related effects, were found following oxytocin and placebo administration. The observed moderate memory-impairing effect therefore appears to represent an influence of oxytocin on memory processes not specific to the social or non-social nature of the stimuli.
Evidence for the memory-impairing effect was found in between-group as well as within-group analyses. For the discrimination index of recollection, trends for a main effect of group and a group x test session interaction were found. In the first test session, subjects who had received oxytocin (i.e., group OP) while studying faces and houses showed subsequently lower recollection, than subjects who were administered with placebo (i.e., groups PO and PP; Fig. 2). As with all between-subject analyses, it is possible that this difference between the subject groups was driven by individual differences in general memory such that the oxytocin-group might have had generally lower memory performance than the placebo-groups independent of oxytocin administration. Individual differences, however, cannot explain the present findings because oxytocin selectively influenced differences in memory performance between Test Session 1 and 2 (i.e., session effects). Session effects represent within-group comparisons and thus control for individual differences.
Session effects, found in the discrimination index of recollection and the hit rates for “recollect” judgments, provided further, within-subject evidence for the memory impairing effect of oxytocin. No significant session effects (Cohen’s d of 0.21 and 0.20 for both the discrimination index of recollection and hit rates for “recollect” judgments) were found for the OP group, who received oxytocin in the first session. The largest session effects (Cohen’s d of 0.82 and 0.72, respectively) were found for the PO group, who received oxytocin in the second session. The PP group never received oxytocin and represents a baseline measure for the session effect. This group showed medium session effects (Cohen’s d of 0.68 and 0.54, respectively), which were smaller than those of the PO group1. The present patterns of session effects (Fig. 4 and 5) suggest that oxytocin can prevent proactive interference when administered in the first study-test session (i.e., in the OP group). In this session, oxytocin impairs memory and leads to fewer stored memory-representations. Subsequently, in the second study-test session, less proactive interference is observed because the reduced number of memory representations from the first study-test session interferes less with the new learning material of the second study-test session. The PP group, which never received oxytocin, showed a significant effect of proactive interference because stored memory representations from the first study-test session interfered with the learning of new material in the second session. Finally, the PO group showed the largest session effects because this group was not only affected by proactive interference (similarly as the PP group) but also by the administration of oxytocin in the second study-test session, which further reduced memory performance (Fig. 4 and 5). This result cannot be explained by individual differences between PP and PO because both groups had similar memory performance in the first session. Taken together, the observed session effects suggest that oxytocin impaired memory, in the first session for OP and in the second session for PO. For OP, it prevented proactive interference in the second study-test session. But for PO, it impeded memory performance in the second study-test session in addition to the impairing effect of proactive inference as seen in PP.
Possible mechanisms for the observed memory-impairing effect are the detrimental influences of oxytocin on the hippocampus and amygdala, both of which are involved in the recollection of social and non-social memories (Diana et al., 2007; Spaniol et al., 2009) and which have been shown to be affected by oxytocin administration. In rats, for example, oxytocin infusion into the ventricles induced long-term depression in the dentate gyrus (Dubrovsky et al., 2002). In humans, oxytocin administered as nasal spray is assumed to bind to receptors in the amygdala, and thereby reduces amygdala activity and associative memory consolidation (Domes et al., 2007a,b; Heinrichs & Gaab, 2007; Kirsch et al., 2005; Pitman et al., 1993). Animal studies also showed that the effects of oxytocin on memory are mediated by the amygdala (Ferguson et al., 2001).
The present results of memory-impairing effects of oxytocin on faces and houses differ from previous findings of memory-enhancing effects for faces (Guastella et al., 2008; Savaskan et al., 2008; Rimmele et al., 2009). It could be argued that this is because faces in the present study had neutral or weakly smiling expressions, which were shown to be associated with either no effect of oxytocin (Bruins et al., 1992; Guastella et al., 2008; Savaskan et al., 2009) or a memory-impairing effect (Ferrier et al., 1980). However, other studies have shown that oxytocin-induced effects do not depend on facial expressions (Rimmele et al., 2009) or that oxytocin can enhance memory for neutral (Savaskan et al., 2009) or happy faces (Guastella et al., 2008). Differences in facial expressions are thus not a sufficient explanation for the present memory-impairing effects.
By finding a memory-impairing effect on both social and non-social stimuli, the present study differs from Rimmele et al., 2009, the only other study that directly compared effects of oxytocin on memory for social and non-social stimuli. Kirsch et al. (2005), which investigated amygdala activation to fearful faces and threatening scenes in a matching task, also reported identical oxytocin-induced effects on social and non-social stimuli. Together with the present study, these findings suggest that the distinction between memory for social and non-social stimuli (Savaskan et al., 2008; Lee et al., 2009; Rimmele et al., 2009) is not sufficient to explain the inconsistent findings of oxytocin-induced effects on human memory.
The present moderate memory-impairing results are in accord with those studies that found no effect or a memory-impairing effect of oxytocin on both non-social and social stimuli (Bruins et al., 1992; Di Simplicio et al., 2009; Fehm-Wolfsdorf et al., 1984, 1988; Ferrier et al., 1980; Heinrichs et al., 2004). The present results are also consistent with reports of amnestic effects of oxytocin on consolidation and retrieval in rats (Bielsky and Young, 2004; Bohus et al., 1978; De Wied et al., 1991; Dubrovsky et al., 2002). It is outside the scope of this study to solve the apparent inconsistency of oxytocin-induced effects on human memory. Future research on this issue should explore the multiple factors currently being discussed as possibly moderating the effects of oxytocin (Bartz et al., 2011; De Dreu et al. 2011; Ophir et al., 2009; Rodrigues et al., 2009), including such aspects of the individual as sex, age, personality traits, ability differences, or genetic variations of the oxytocin receptor gene, as well as such aspects of the testing situation as stimulus sex, task difficulty, time of day of testing, or task instructions.
The present study is the first to use a within-subject design to investigate the effects of oxytocin on recognition memory. Along with previous considerations about within-subject manipulations of drug/placebo administrations (Tops & Wijers, 2011; Wirth et al., 2011), the present study confirms the importance of including a control condition, in which subjects do not receive the drug, to be able to interpret possible interactions of the drug/placebo administration with repeated testing2. This is especially important when memory performance is the dependent variable of interest because of the known effect of proactive interference on memory. It is possible to avoid the influence of proactive interference by using between-subject designs. But, instead of generally dismissing within-subject designs for the study of drug effects on memory, it may be advisable for future within-subject memory-experiments to decrease proactive inference by increasing the inter-session interval. Still, within-subject designs with narrow intervals between study-test sessions appear to be especially relevant for the study of drugs, like oxytocin, which have been considered as potential treatment for various psychiatric conditions (Heinrichs et al., 2009, for review; Pitman et al., 1993). Such treatments would be administered in an environment that is comprised of repeated learning and testing situations, for which within-subject designs might be a surrogate.
To conclude, the present study provided evidence both from between-subject and within-subject analyses that oxytocin can impair memory nonspecifically for social and non-social visual objects. The detailed recollection of previously studied faces and houses was found to be compromised when items were learned under oxytocin. No evidence was found for an influence of oxytocin on familiarity processes or memory judgments to new items (i.e., false alarms). This study also emphasizes the necessity of including a non-treated, baseline group in within-subject designs when investigating oxytocin’s effects on human memory. With the inclusion of this group, we were able to show that oxytocin interacts with proactive interference and provided further evidence for the memory-impairing effect of oxytocin on faces and houses.
4. Experimental Procedure
4.1. Subjects
Forty-two healthy, young, right-handed, non-smoking adult undergraduates from the University of Colorado Boulder volunteered in this study. As in many previous oxytocin studies, females were excluded because of possible side effects and ethical considerations (Campbell, 2008; Guastella et al., 2008; Rimmele et al., 2009). Studies that included male and female participants reported no sex differences in the effects of oxytocin on various cognitive tasks including face memory (e.g., Alvares et al., 2010; Guastella et al., 2009; Savaskan et al., 2008). None of our participants had ever been diagnosed with any neurological, psychiatric, or medical illness or was on any medication, as determined in a self-report interview by a trained experimenter. The study was approved by the Institutional Review Board and the Scientific Advisory Research Committee of the University of Colorado and was conducted in accordance with the Declaration of Helsinki. All participants gave written informed consent and were paid for participation.
4.2. Design
In a placebo-controlled, double-blind, within-subject design, two study-test sessions were conducted for all subjects.14 men (aged 18-29 years, M = 22.3, SD = 3.4) were randomly selected to receive oxytocin in the first study-test session and placebo in the second. Another 14 men (aged 18-28 years, M = 22.1, SD = 3.2) received placebo in the first session and then oxytocin. Analysis of these two groups of subjects indicated that oxytocin interacted with session effects likely caused by proactive interference. To further elucidate this finding, an additional group of 14 subjects (aged 18-28 years, M = 21.1, SD = 3.3) was run under exactly the same conditions (i.e., double-blind, two study-test sessions) as the two previous groups. This additional group, however, received placebo in each session to obtain a baseline for the proactive interference effects. To maintain double-blindness as well as the expectation of receiving oxytocin, the administration of placebo in each session was made unbeknownst to the subjects and experimenter. There were no significant age differences across the three groups (p > .56) or for any pair-wise comparisons (p > .30). No participant reported any adverse side effects.
4.3. Stimuli
360 grayscale Caucasian faces (Color FERET database, Phillips et al., 2000) and 360 grayscale American houses (courtesy of Alumit Ishai, Ishai et al., 1999) were used as stimuli. Faces were presented in an elliptic mask that hid hair, ears, and clothing (Fig. 1). All faces had neutral or weakly smiling facial expressions. Half of the faces were female3. Houses were edited to show no background (i.e., sky or clouds; Fig. 1). In the first study-test session, 120 faces and 120 houses served as encoding material, and 60 faces and 60 houses served as distracters. In the second study-test session, the same numbers of completely new stimuli were used. In a pilot study, a separate group of men (N = 26, M = 20.6, SD = 3.0 years) completed two recognition-memory experiments to determine the recognition difficulty of each item. Based on the pilot data, two sets of target and distracter stimuli were created for faces and houses. All sets within a given stimulus category were exactly matched for recognition difficulty. One set of stimuli was used in the first study-test session for half of each of the three groups of participants; the same set was used in the second study-test session for the other half of the participants. This order was reversed for the other set of stimuli. The order of stimulus sets was also counterbalanced within all three groups of subjects.
4.4. Procedure
The study consisted of two procedurally identical study-test sessions (Fig. 1). The interval between study sessions was exactly one week for all subjects. The delay between study and test sessions was exactly 24 hours for all subjects. Study and test sessions were conducted between 9 am and 4 pm. Time of day of testing did not vary significantly across the three subject groups (all ps for pairwise comparisons > .52). Participants were instructed to abstain from beverages with caffeine or alcohol 24 hours before the study days and to maintain a regular sleep-wake cycle two nights before and during the study days, with sleep periods between about 11 pm and 7 am.
On the study days, participants received 24 IU of oxytocin (Syntocinon Spray; Novartis; three puffs per nostril; each puff with 4 IU of oxytocin) or a placebo (saline nasal spray, three puffs per nostril) intranasally. This dose of oxytocin was chosen because the same dose was used in previous studies on oxytocin’s effects on memory (Di Simplicio et al., 2009; Guastella et al., 2008; Heinrichs et al., 2004; Savaskan et al., 2008; Rimmele et al., 2009). Forty minutes after administration, when central nervous oxytocin levels reached the plateau of their highest concentration (Born et al., 2002), participants studied 120 faces and 120 houses intermixed in 12 blocks. To promote retention until the next day, the study list was repeated once in a different random order, yielding a total of 24 blocks. In each run, each picture was presented for two seconds on a light gray background in the middle of a 17-inch monitor. Participants were told to memorize all stimuli as well as possible and to make attractiveness ratings (1 = very unattractive to 7 = very attractive) on a computer keyboard to foster memory encoding. To keep the presentation time for all items constant, participants were instructed to respond after the stimulus had disappeared. One second after their response, the next stimulus was presented. The study experiment lasted about 45 minutes.
Possible oxytocin-related changes in attention (assessed with a computerized Continuous Performance Task, CPT), wakefulness (assessed with the wakefulness scale of an English version of the Multidimensional Mood Questionnaire, MDMQ, Steyer et al., 1997), and mood (assessed with the Positive and Negative Affect Scale, PANAS, Watson et al., 1988) were tracked over the course of the study session. Measurements were taken right before drug/placebo administration and right before the start of the study lists (35 minutes after drug/placebo administration).
On the test days, exactly 24 hours after each study session, participants returned to complete the recognition test. All 240 studied items, intermixed with 60 new faces and 60 new houses, were tested in 12 blocks. Each stimulus was presented for two seconds. The response options then appeared below the stimulus, and participants were asked, without time limit, to make memory judgments for each item by pressing the corresponding key on a computer keyboard. They were told to judge the items as “recollected” when they could remember the presented item together with specific details about learning this item in the study session (such as a thought that came to mind or something that happened in the room), as “familiar” when they knew they had seen the item in the study session but could not remember any details from the study episode, and as “new” when they thought they had never seen the item before (Rajaram, 1993; Woodruff et al., 2006). Before the recognition experiment, participants practiced to make recollect/familiar judgments to verify, as judged by the experimenter, that they fully understood the differences between the meanings of these memory judgments.
A self-report questionnaire of the participants’ beliefs about the order in which they had received oxytocin and placebo was completed at the end of the second recognition session.
Highlights.
First within-subject, placebo-controlled study on oxytocin and memory
Oxytocin reduces accurate recollection but not familiarity
Oxytocin impedes memory for studied social and non-social objects
Oxytocin modulates the effects of proactive interference
Acknowledgements
Funding for this research was provided by NIH Grant MH64812, NIH/NCRR Colorado CTSI Grant Number UL1 RR025780 to the Clinical Translational Research Center at the University of Colorado, NSF grant #SBE-0542013 to the Temporal Dynamics of Learning Center (an NSF Science of Learning Center), and a James S. McDonnell Foundation grant to the Perceptual Expertise Network. Contents are the authors’ sole responsibility and do not necessarily represent official views of any funding agencies.
Portions of the research in this paper use the Color FERET database of facial images collected under the FERET program (Phillips et al., 2000). We thank Elizabeth Connick for help with the clinical setup of the study, Patrick White, Debra Coady, and Mary Dang for research assistance. We thank Marlene Behrmann, Suzy Scherf, and Jim Tanaka, for helpful discussions.
Footnotes
For the discrimination index of recollection and hit rates for “recollect” judgments, the session effects of the PP group were significantly smaller than those for the PO group, Fs (1,13) = 6.8 and 4.8, ps < .05, η2s = 0.34 and 0.27, respectively.
In fact, a complete design would not only have involved an additional group that received placebo in both test sessions but also a group that would have received oxytocin in both sessions. In the present study, we decided against this option for practical reasons. Thus, the investigation of effect of repeated administration of oxytocin remains an open question for future research.
No significant statistical effects or interactions of oxytocin with the sex of the stimulus face were found.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvares GA, Hickie IB, Guastella AJ. Acute effects of intranasal oxytocin on subjective and behavioral responses to social rejection. Exp. Clin. Psychopharmacol. 2010;18:316–321. doi: 10.1037/a0019719. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends in Cogn. Sci. 2011;15:301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Young LJ. Oxytocin, vasopressin, and social recognition mammals. Peptides. 2004;25:1565–1574. doi: 10.1016/j.peptides.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Bohus M, Kovàcs GL, De Wied D. Oxytocin, vasopressin and memory: opposite effects on consolidation and retrieval processes. Brain. Res. 1978;157:414–417. doi: 10.1016/0006-8993(78)90052-5. [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat. Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Bruins J, Hijman R, Van Ree JM. Effect of a single dose of des-glycinamide-[Arg8]vasopressin or oxytocin on cognitive processes in young healthy subjects. Peptides. 1992;13:461–468. doi: 10.1016/0196-9781(92)90075-e. [DOI] [PubMed] [Google Scholar]
- Campbell A. Attachment, aggression, and affiliation: the role of oxytocin in female social behavior. Biol. Psychol. 2008;77:1–10. doi: 10.1016/j.biopsycho.2007.09.001. [DOI] [PubMed] [Google Scholar]
- De Dreu CKW, Greer LL, Van Kleef GA, Shalvi S, Handgraaf MJJ. Oxytocin promoted human ethnocentrism. Proc. Natl. Acad. Sci. USA. 2011;108:1262–1266. doi: 10.1073/pnas.1015316108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wied D, Elands J, Kovàcs GL. Interactive effects of neurohypophyseal neuropeptides with receptor antagonists on passive avoidance behavior: mediation by a cerebral neurohypophyseal hormone receptor. Proc. Natl. Acad. Sci. USA. 1991;88:1494–1498. doi: 10.1073/pnas.88.4.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends in Cogn. Sci. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Di Simplicio M, Massey-Chase R, Cowen P, Harmer C. Oxytocin enhances processing of positive versus negative emotional information in healthy male volunteers. J. Psychopharmacol. 2009;23:241–248. doi: 10.1177/0269881108095705. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Gläscher J, Büchel C, Braus DF, Herpertz S. Oxytocin attenuates amygdala response to emotional faces regardless of valence. Biol. Psychiatry. 2007a;62:1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz S. Oxytocin improves “mind-reading” in humans. Biol. Psychiatry. 2007b;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Dubrovsky B, Harris J, Gijsbers K, Tatarinov A. Oxytocin induces long-term depression on the rat dentate gyrus: possible ATPase and ectoprotein kinase mediation. Brain Res. Bull. 2002;58:141–147. doi: 10.1016/s0361-9230(01)00748-1. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Wotjak CT, Neumann I, Ludwig M, Landgraf R. Behavioral consequences of intracerebral vasopressin and oxytocin: focus on learning and memory. Neurosci. Biobehav. Rev. 1996;20:341–358. doi: 10.1016/0149-7634(95)00059-3. [DOI] [PubMed] [Google Scholar]
- Fehm-Wolfsdorf G, Born J. Behavioral effects of neurohypophyseal peptides in healthy volunteers: 10 years of research. Peptides. 1991;12:1399–1406. doi: 10.1016/0196-9781(91)90226-f. [DOI] [PubMed] [Google Scholar]
- Fehm-Wolfsdorf G, Bachholz G, Born J, Voigt K, Fehm HL. Vasopressin but not oxytocin enhances cortical arousal: an integrative hypothesis on behavioral effects of neurohypophyseal hormones. Psychopharmacology. 1988;94:496–500. doi: 10.1007/BF00212844. [DOI] [PubMed] [Google Scholar]
- Fehm-Wolfsdorf G, Born J, Voigt KM, Fehm HL. Human memory and neurohypophyseal hormones: opposite effects of vasopressin and oxytocin. Psychoneuroendocrinology. 1984;9:285–292. doi: 10.1016/0306-4530(84)90007-6. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J. Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat. Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Insel TR. The neuroendocrine basis of social recognition. Front. Neuroendocrinol. 2002;23:200–224. doi: 10.1006/frne.2002.0229. [DOI] [PubMed] [Google Scholar]
- Ferrier BM, Kennett DJ, Devlin MC. Influence of oxytocin on human memory processes. Life Sci. 1980;27:2311–2317. doi: 10.1016/0024-3205(80)90499-3. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol. Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Carson DS, Dadds MR, Mitchell PB, Cox RE. Does oxytocin influence the early detection of angry and happy faces. Psychoneuroendocrinology. 2009;34:220–225. doi: 10.1016/j.psyneuen.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Mathews F. Oxytocin enhances the encoding of positive social memories in humans. Biol. Psychiatry. 2008;64:256–258. doi: 10.1016/j.biopsych.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Domes G. Neuropeptides and social behavior: effects of oxytocin and vasopressin in humans. Prog. Brain Res. 2008;170:337–350. doi: 10.1016/S0079-6123(08)00428-7. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Gaab J. Neuroendocrine mechanisms of stress and social interaction: implications for mental disorders. Curr. Opin. Psychiatry. 2007;20:158–162. doi: 10.1097/YCO.0b013e3280146a13. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Meinlschmidt G, Wippich W, Ehlert U, Hellhammer DH. Selective amnesic effects of oxytocin on human memory. Physiol. Behav. 2004;83:31–38. doi: 10.1016/j.physbeh.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Front. Neuroendocrin. 2009;30:548–557. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Insel TR. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron. 2010;65:768–779. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishai A, Ungerleider LG, Martin A, Schouten JL, Haxby JV. Distributed representation of objects in the human ventral visual pathway. Proc. Natl. Acad. Sci. USA. 1999;96:9379–9384. doi: 10.1073/pnas.96.16.9379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. J. Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H-J, Macbeth AH, Pagani JH, Young WS. Oxytocin: the great facilitator of life. Prog. Neurobiol. 2009;88:127–151. doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald K, MacDonald TM. The peptide that binds: a systematic review of oxytocin and its prosocial effects in humans. Harv. Rev. Psychiat. 2010;18:1–21. doi: 10.3109/10673220903523615. [DOI] [PubMed] [Google Scholar]
- Ophir AG, Zheng D-J, Eans S, Phelps SM. Social investigation in a memory task relates to natural variation in septal expression of oxytocin receptor and vasopressin receptor 1a in prairie voles microtus ochrogaster) Behav. Neurosci. 2009;123:979–991. doi: 10.1037/a0016663. [DOI] [PubMed] [Google Scholar]
- Phillips PJ, Moon H, Rizvi SA, Rauss PJ. The FERET evaluation methodology for face recognition algotithms. IEEE T. Pattern Anal. 2000;22:1090–1104. [Google Scholar]
- Pitman RK, Orr SP, Lasko NB. Effects of intranasal vasopressin and oxytocin on physiologic responding during personal combat imagery in Vietnam veterans with posttraumatic stress disorder. Psychiatry Res. 1993;48:107–117. doi: 10.1016/0165-1781(93)90035-f. [DOI] [PubMed] [Google Scholar]
- Rajaram S. Remembering and knowing: two means of access to the personal past. Mem. Cogn. 1993;21:89–102. doi: 10.3758/bf03211168. [DOI] [PubMed] [Google Scholar]
- Rimmele U, Hediger K, Heinrichs M, Klaver P. Oxytocin makes a face in memory familiar. J. Neurosci. 2009;29:38–42. doi: 10.1523/JNEUROSCI.4260-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues SM, Saslow LR, Garcia N, John OP, Keltner D. Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proc. Natl. Acad. Sci. USA. 2009;106:21437–21441. doi: 10.1073/pnas.0909579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savaskan E, Ehrhardt R, Schulz A, Walter M, Schächinger H. Post-learning intranasal oxytocin modulates human memory for facial identity. Psychoneuroendocrinology. 2008;33:368–374. doi: 10.1016/j.psyneuen.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Davidson PSR, Kim ASN, Han H, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: Meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47:1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Steyer R, Schwenkmezger P, Notz P, Eid M. Der Mehrdimensionale Befindlichkeitsbogen MDBF) Hogrefe; Göttingen: 1997. [Google Scholar]
- Tops M, Wijers AA. Re: “The effect of cortisol on emotional responses depends on order of cortisol and placebo administration in a within-subject design” by Wirth et al. Psychoneuroendocrinology. 2011;36:1097–1098. doi: 10.1016/j.psyneuen.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Pers. Social Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wirth MM, Scherer SM, Hoks RM, Abercrombie HC. The effect of cortisol on emotional responses depends on order of cortisol and placebo administration in a within-subject design. Psychoneuroendocrinology. 2011;36:945–954. doi: 10.1016/j.psyneuen.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff CC, Hayama HR, Rugg MD. Electrophysiological dissociation of the neural correlates of recollection and familiarity. Brain Res. 2006;1100:125–135. doi: 10.1016/j.brainres.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: a review of 30 years of research. J. Mem. Lang. 2002;46:441–517. [Google Scholar]