Abstract
Background
Biodegradable hydrogels can deliver therapeutic payloads with great potentials in endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) to yield improvements in efficacy and foster mucosal regeneration.
Objective
To assess the efficacy of an injectable drug eluting elastomeric polymer (iDEEP) as a submucosal injection material.
Design
Comparative study among 3 different solutions using material characterization tests, ex vivo and in vivo porcine models.
Setting
Academic hospital.
Interventions
30 gastric submucosal cushions were achieved with saline (0.9%), sodium hyaluronate (0.4%), and iDEEP (n = 10) in ex vivo porcine stomachs. Four porcine gastric submucosal cushions were then performed in vivo using iDEEP.
Main outcome measurements
Maximum injection pressure, Rebamipide release rate, submucosal elevation duration, and assessment of in vivo efficacy by en bloc resection.
Results
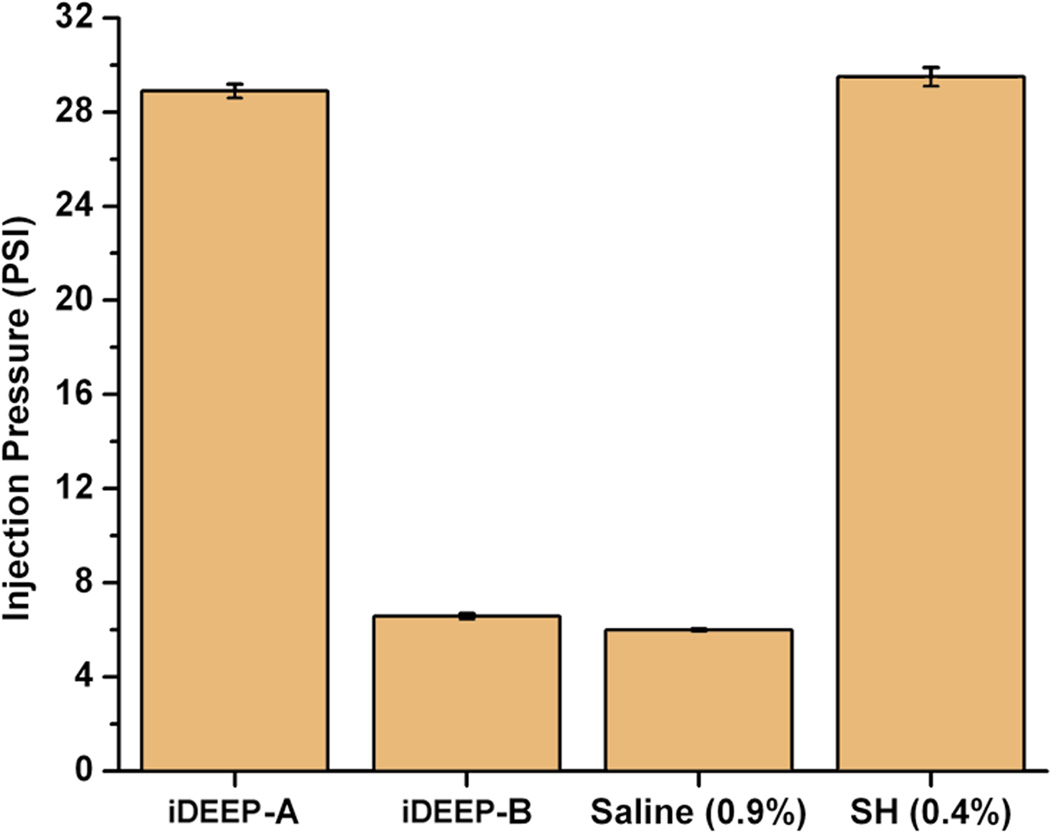
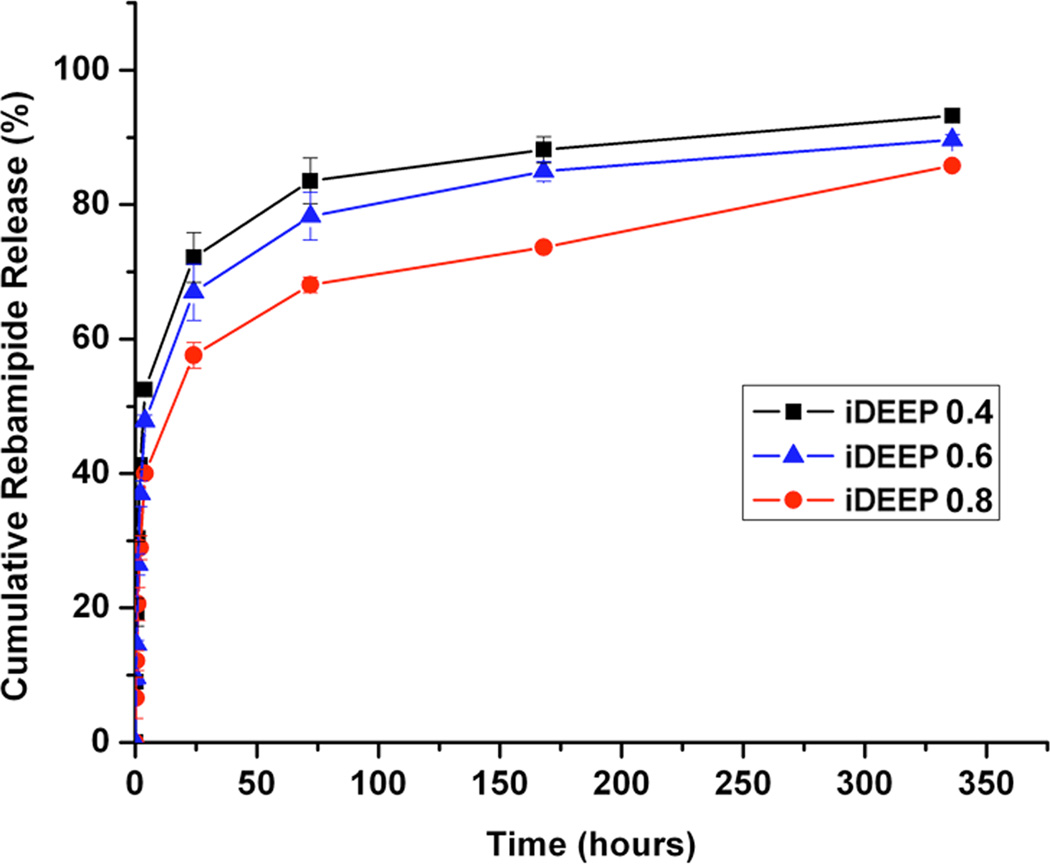
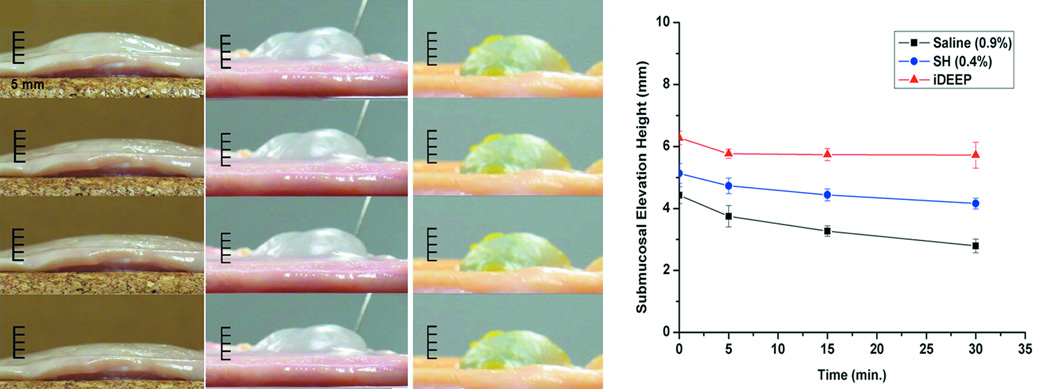
No significant difference in injection pressures between iDEEP (28.9 ± 0.3 PSI) and sodium hyaluronate (29.5 ± 0.4 PSI, P > .05) was observed. iDEEP gels displayed a controlled release of Rebamipide up to 2 weeks in vitro. The elevation height of iDEEP (5.7 ± 0.5 mm) was higher than saline (2.8 ± 0.2 mm, P < .01) and SH (4.2 ± 0.2 mm, P < .05). All EMR procedures were successfully performed after injection of iDEEP, and a large gel cushion was noted after the resection procedure.
Limitations
Benchtop, ex vivo, and non-survival pig study.
Conclusions
A novel injection solution was evaluated for endoscopic resection. These results suggest that iDEEP may provide a significant step towards the realization of an ideal EMR and ESD injection material.
Introduction
Endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) are minimally invasive procedures to remove early malignant lesions limited to the superficial layers of the gastrointestinal tract.1, 2 In order to improve efficacy and safety, EMR and ESD techniques require the injection of a solution underneath the mucosa into the submucosal space.3 Although numerous injection solutions have been proposed and tested, saline or diluted epinephrine with saline is the most commonly used in clinic due to its low cost and ease of use, but is hampered by rapid dispersion within the submucosal plane resulting in the need for repeated injections.4 In order to improve submucosal lift durations, sodium hyaluronate (SH) is currently being studied owing to its high viscosity, ease of injection, and ability to provide long lasting submucosal lift durations.5–8 However, high costs and concerns for tumor stimulation limit its large-scale use.3, 9
Recent research has indicated a paradigm shift towards the development of mucosal resection injection solutions, which rely on gel formation to provide extended submucosal lift durations.10 Photocrosslinkable chitosan and thermoresponsive poloxamers have been recently reported for EMR with great enthusiasm, but are limited by administration difficulties.11, 12 For example, the liquid to gel transformation using photoinitiated free radical polymerization requires the use of an ultraviolet light, which may be difficult in hard-to-reach areas, and thermoresponsive polymers have been shown to clog inside long delivery tools at normal body temperature.12, 13
We have recently reported on the development of biodegradable elastomeric hydrogel, poly (ethylene glycol maleate citrate) (PEGMC), which has been shown to have excellent cyto-/tissue-compatibility and controlled degradability both in vitro and in vivo for tissue engineering and drug delivery applications.14–16 Although not yet evaluated for clinical use, previous studies have shown PEGMC to elicit a minimal inflammatory response when injected subcutaneously in rats with complete material degradation within 4 weeks after implantation. The leachable and degradation products of PEGMC hydrogels were evaluated in vitro with NIH 3T3 fibroblasts and was found to be comparable to currently US Food and Drug Administration (FDA) approved materials, poly(ethylene glycol diacrylate) (PEGDA) hydrogel.14 The ability to be injected using minimally invasive methods and deliver therapeutics in a controlled manner has prompted the investigation of PEGMC as a new EMR injection solution. Unlike previous materials, the liquid to gel transformation of PEGMC can provide sustained mucosal lift without administration difficulties, and the controlled release of Rebamipide,17 which stimulates prostaglandin generation and improves the speed of ulcer healing, from the biodegradable gel can potentially aid in mucosal regeneration after resection. The purpose of this study is to evaluate the efficacy and safety of a PEGMC based injectable drug eluting elastomeric polymer (iDEEP), which aims to address the limitations of previous solutions.
Materials and Methods
All chemicals were purchased from Sigma Aldrich (St. Louis, MO). PEGMC with different citric acid: maleic anhydride monomer ratios were synthesized as previously described.14 To prepare the iDEEP Part A Component (iDEEP-A), PEGMC was dissolved in deionized water (20 wt.-%), and combined with poly (ethylene glycol diacrylate) (12 wt.-%), and tetramethylethylenediamine (0.5 wt.-%). The iDEEP Part B Component (iDEEP-B) was prepared by dissolving ammonium persulfate redox initiator (0.25 wt.-%) in deionized water. Combining the Part A and B solutions in a 2:1 ratio, respectively, produced iDEEP gels.
To assess the ease of injection, maximum injection pressures were evaluated using a 25-gauge endoscopic needle (US Endoscopy, Mentor, OH), digital manometer (Cole-Parmer, Vernon Hills, IL), and syringe pump (KD Scientific, Holliston, MA) connected to a 3-way luer lock stopcock delivered at 5 mL/minute. To determine the Rebamipide release rate, Rebamipide (1 mM) was mixed with various iDEEP-A solutions, and combined with the iDEEP-B to form crosslinked gels. The drug-loaded gels were then incubated in phosphate buffered saline (37 °C; pH 7.4), and Rebamipide release was determined using high performance liquid chromatography (HPLC) (Waters, Milford, MA). The upper third of porcine stomachs were used for all ex vivo studies due to the resemblance with the human stomach in thickness and histology. The gastric specimens were obtained immediately after sacrifice, cut into 5 × 5 cm squares, and fixed onto a corkboard. Using a 2.5 mL syringe and 25-gauge needle, 1 mL of each solution was injected tangentially into the submucosa through the mucosal surface. Mucosal elevation height was quantitatively determined from photographs using Image J Analysis software. For the in vivo model, 4 EMR procedures were performed in the stomach of a porcine specimen using a 25-gauge catheter injection needle. All solutions were mixed with methylene blue (0.5/10 mL of solution) for visualization. An “en bloc” resection of the elevated mucosa was performed with a hook-knife and polypectomy snare, and recorded with endoscopic photographs.
The results are expressed as the mean ± standard deviation (n = 10). Statistical significance between two sets of data was calculated using a two-tail Student’s t-test, and non-parametric one-way ANOVA tests were performed where appropriate. Data was taken to be significant when a P value < .05 was obtained.
Results
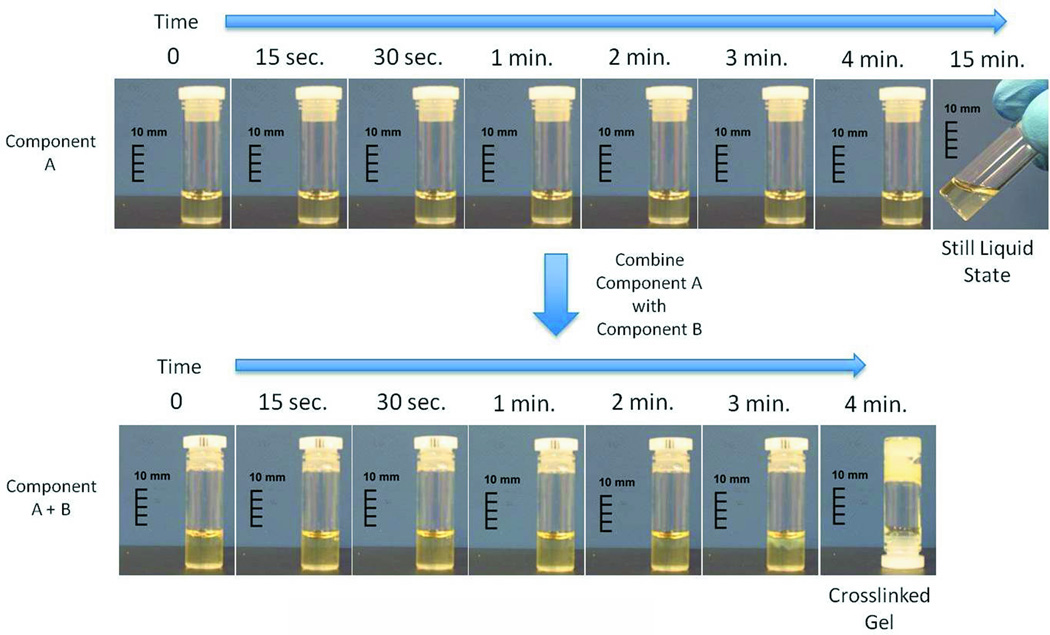
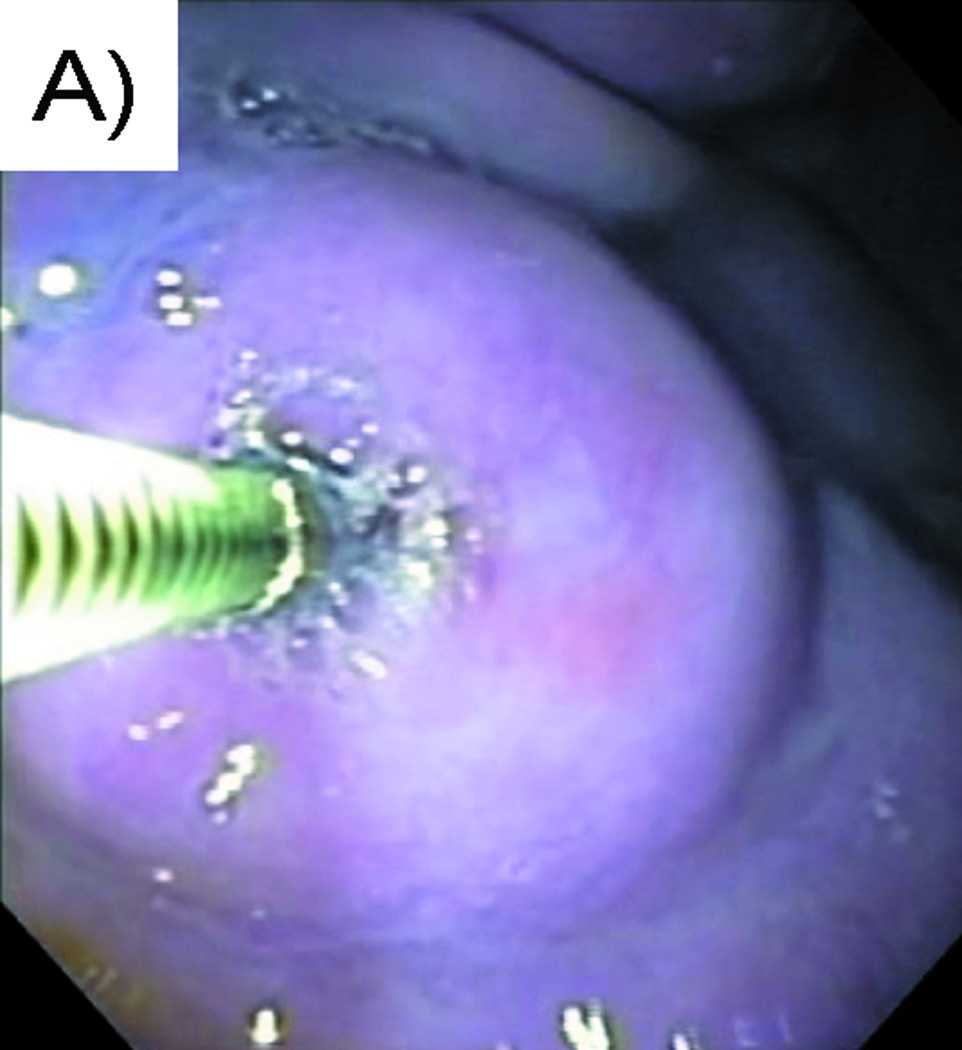
Liquid to gel transformation occurred within 4 minutes of combining the iDEEP Part A and B Components (Fig. 1). No significant difference in the pressures developed during injection was observed between iDEEP-A (28.9 ± 0.3 PSI) and SH (29.5 ± 0.4 PSI, P > .05). Injection pressures of iDEEP-B were much lower (6.6 ± 0.1 PSI) and were found comparable to that of saline solutions (6.0 ± 0.1 PSI, P > .05). Rebamipide release studies from iDEEP showed an initial burst release at 4 hours for all iDEEP-A compositions. After the initial burst release, a controlled release for up to 2 weeks was observed, and could be controlled with the iDEEP-A monomer ratios (Fig. 2). In the ex vivo study (Fig. 3), iDEEP displayed the highest submucosal elevation heights at all time points. After 30 minutes, iDEEP displayed extended lift durations (5.7 ± 0.5 mm) with higher submucosal elevations over saline (2.8 ± 0.2 mm, P < .01) and SH (4.2 ± 0.2 mm, P < .05). In the preliminary in vivo study, the iDEEP-A was easily injected into the porcine stomach to create submucosal elevation (Fig. 4A). Using the same injection needle, the iDEEP-B was injected to produce a soft biodegradable gel underneath the mucosa (Fig. 4B). No electrocautery settings changes were needed to perform the procedure.
Figure 1.
Photographic representation of the liquid to gel transformation of the iDEEP. Gel transformation only occurs after the A and B Components are combined.
Figure 2.
A) Maximum injection pressures of the tested solutions, and B) in vitro Rebamipide release profiles from iDEEP gels.
Figure 3.
Photographic images depicting the chronological changes in the submucosal elevation of A) saline (0.9%), B) sodium hyaluronate (0.4%), and C) iDEEP (30%) from top to bottom in turn 1, 5, 15, and 30 minutes after injection using porcine gastric samples ex vivo. D) Graphical representation of the chronological changes in the submucosal elevation.
Figure 4.
Endoscopic views of A) Contained vertical submucosal elevation after injection of the iDEEP Part A and B solution, and B) Mucosal defect post-iDEEP EMR resection reveals a solidified soft biodegradable gel.
Discussion
EMR and ESD are minimally invasive endoscopic procedures now accepted worldwide as a treatment modality in the removal dysplastic and early malignant lesions limited to the superficial layers of the gastrointestinal tract.1, 2 Unfortunately, the EMR/ESD procedure has been historically limited by the short submucosal lift durations of the available injection solutions, which have been constrained by two design avenues: the osmolarity or viscosity of a solution is responsible for the lifting properties the material.18 The recent introduction of injectable materials, which use a liquid to gel transformation, has shown promise in providing extended submucosal lift durations. However, many of these gel-forming materials are plagued by administration difficulties, which further complicate the procedure. In review of the recent progress in the development of EMR solutions, the ideal injection solution should be cost-effective, widely available, easily injectable, biocompatible, biodegradable, able to provide prolonged submucosal lift durations, and able to aid in mucosal healing after the resection process in order to have clinical relevance.4, 8
In this study, we have developed a novel injectable drug eluting elastomeric biodegradable polymer, which aims to meet all the requirements of an ideal EMR solution and overcome the limitations of previous solutions. As shown in Figure 1, iDEEP uses both viscosity and gel formation through redox initiated crosslinking to overcome the limitations of previous designs. A water-soluble iDEEP-A, which is more viscous than saline, will remain a viscous liquid until combined with the water-soluble iDEEP-B to produce a soft biodegradable hydrogel. Dividing the system into two separate components offers a huge advantage over previous designs in that the surgeon can precisely control the gel setting location and time and avoid premature gelling inside the delivery tools. In addition, the utilization of a redox initiated crosslinking mechanism does not require the use of additional equipment such as UV light for the gel formation to occur. The above criteria have all been developed with the cost-effectiveness of the system in mind. Although the iDEEP system is more expensive than saline solution, it is roughly 33 times less when compared with hyaluronic acid solution formulations (Hyalgan, $66/mL), and 2.5 times less when compared with a 0.4% hyaluronic acid solution (MucoUp, $5/mL), which is commercially available in Japan.12, 19
A higher viscosity liquid typically translates into a greater force required to inject the solution through small caliber delivery tools, which may produce unwanted administration difficulties. To ensure the iDEEP components were easily injectable, we have assessed the maximum pressures developed using real life conditions. Injection of the iDEEP-A component through a 25-gauge endoscopic needle greater difficulty in achieving a constant flow when compared with saline solution, but was found comparable to SH. iDEEP-B solutions were even easier to inject showing similar injection pressures to that of normal saline. The localized and controlled delivery of Rebamipide,8 a mucosal protective and ulcer healing drug shown to stimulate prostaglandin generation, may improve the speed of ulcer healing to aid in the management of EMR-induced damages. The in vitro Rebamipide release from iDEEP gels displayed an initial burst release followed by a sustained release for up to two weeks, and could be controlled through polymer monomer ratios. The developed polymers are also capable of incorporating hemostatic and/or anti-neoplastic drugs to assist mucosal resection and treatment.
In the ex vivo studies, all the submucosal cushions created with iDEEP were more durable than those performed with saline and SH at all time points. No significant changes in iDEEP cushion height were observed after 5 minutes due to gel formation. To minimize any discrepancies and limitations of an ex vivo study, all specimens were obtained within the first hour of the animal’s death, and all tests were performed at constant temperature of 37 °C to minimize any tissue changes. To evaluate the efficacy of iDEEP, standard EMR procedures were performed in vivo using a live porcine stomach model. The iDEEP-A was easily injected using standard delivery tools, and was able to create an adequate submucosal cushion. Using the same injection needle, the iDEEP-B solution was then injected into the same location without any clogging inside the delivery tool. After 5 minutes of iDEEP-B injection, the en bloc resection of the elevated mucosa revealed a soft biodegradable gel underneath the mucosa to provide protection for the underlying muscle layer from electrocautery damage. The presence of the iDEEP gel did not complicate the resection procedure or require any changes to the electrocautery settings. Although the iDEEP gel cannot be removed entirely after the EMR procedure, previous studies have shown complete biodegradation of the hydrogel, excellent tissue compatibility, and minimal inflammation.14 We also believe that the remaining material left behind after the resection procedure can be used to delivery therapeutics, and promote regeneration of the damaged mucosa. Although mucosal regeneration was not evaluated in this study, long term in vivo degradation and mucosal regeneration in porcine stomachs using survival animal models with detailed pathological review will be the focus of future studies.
In conclusion, iDEEP is a cost-effective, readily available, and easily injectable two-component solution, which allows for biodegradable gel formation under the submucosal space without complex administration difficulties and can potentially aid in mucosal regeneration through controlled therapeutic delivery. iDEEP displayed long lasting cushion elevations over other frequently used injection solutions, and performed well in EMR procedures in vivo. Although standard EMR techniques are a relatively quick and easy procedures, our iDEEP solution is potentially very useful in EMR for relatively large lesions that need repeated resections and submucosal injections, and for ESD, which is a long-lasting, high-end endoscopic resection technique for gastrointestinal neoplasm involving higher risk of perforation.20 These results suggest that iDEEP may provide a significant step towards the realization of an ideal injection material for EMR and ESD. Although the in vivo resection procedures in this study were only used to determine preliminary efficacy of the iDEEP system, further comparative long-term studies in living animals with pathological review are needed to confirm the efficacy, depth of resection ability, and submucosal regeneration of the iDEEP.
Acknowledgements
This work was supported in part by a R21 award (EB009795) and a R01 award (1R01EB012575-01A1) from the National Institute of Biomedical Imaging and Bioengineering (NIBIB), and a National Science Foundation (NSF) CAREER award 0954109.
Acronyms
- ESD
endoscopic submucosal dissection
- EMR
endoscopic mucosal resection
- iDEEP
injectable drug eluting elastomeric polymer
- HPLC
high performance liquid chromatography
- PEGMC
poly (ethylene glycol maleate citrate)
- SH
sodium hyaluronate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We have no conflict of interest to disclose
References
- 1.Bures J, Kopacova M, Kvetina J, et al. Different solutions used for submucosal injection influenced early healing of gastric endoscopic mucosal resection in a preclinical study in experimental pigs. Surg Endosc. 2009;23:2094–2101. doi: 10.1007/s00464-008-0207-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coda S, Lee SY, Gotoda T. Endoscopic Muscosal Resection and Endoscopic Submucosal Dissection as Treatements for Early Gastrointestinal Cancers in Western Countries. Gut and Liver. 2007;1:12–21. doi: 10.5009/gnl.2007.1.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujishiro M, Yahagi N, Kashimura K, et al. Comparison of various submucosal injection solutions for maintaining mucosal elevation during endoscopic mucosal resection. Endoscopy. 2004;36:579–583. doi: 10.1055/s-2004-814517. [DOI] [PubMed] [Google Scholar]
- 4.Kantsevoy SV, Adler DG, Conway JD, et al. Endoscopic mucosal resection and endoscopic submucosal dissection. Gastrointest Endosc. 2008;68:11–18. doi: 10.1016/j.gie.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto H, Yube T, Isoda N, et al. A novel method of endoscopic mucosal resection using sodium hyaluronate. Gastrointest Endosc. 1999;50:251–256. doi: 10.1016/s0016-5107(99)70234-8. [DOI] [PubMed] [Google Scholar]
- 6.Lee SH, Cho WY, Kim HJ, et al. A new method of EMR: submucosal injection of a fibrinogen mixture. Gastrointest Endosc. 2004;59:220–224. doi: 10.1016/s0016-5107(03)02689-0. [DOI] [PubMed] [Google Scholar]
- 7.Uraoka T, Fujii T, Saito Y, et al. Effectiveness of glycerol as a submucosal injection for EMR. Gastrointest Endosc. 2005;61:736–740. doi: 10.1016/s0016-5107(05)00321-4. [DOI] [PubMed] [Google Scholar]
- 8.Hyun JJ, Chun HR, Chun HJ, et al. Comparison of the characteristics of submucosal injection solutions used in endoscopic mucosal resection. Scand J Gastroenterol. 2006;41:488–492. doi: 10.1080/00365520500325994. [DOI] [PubMed] [Google Scholar]
- 9.Matsui Y, Inomata M, Izumi K, et al. Hyaluronic acid stimulates tumor-cell proliferation at wound sites. Gastrointest Endosc. 2004;60:539–543. doi: 10.1016/s0016-5107(04)01890-5. [DOI] [PubMed] [Google Scholar]
- 10.Eun SH, Cho JY, Jung IS, et al. Effectiveness of sodium alginate as a submucosal injection material for endoscopic mucosal resection in animal. Gut Liver. 2007;1:27–32. doi: 10.5009/gnl.2007.1.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayashi T, Matsuyama T, Hanada K, et al. Usefulness of photocrosslinkable chitosan for endoscopic cancer treatment in alimentary tract. J Biomed Mater Res B Appl Biomater. 2004;71:367–372. doi: 10.1002/jbm.b.30099. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Esparrach G, Shaikh SN, Cohen A. Efficacy of a reverse-phase polymer as a submucosal injection solution for EMR: a comparative study (with video) Gastrointest Endosc. 2009;69:1135–1139. doi: 10.1016/j.gie.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 13.Ifkovits JL, Burdick JA. Review: photopolymerizable and degradable biomaterials for tissue engineering applications. Tissue Eng. 2007;13:2369–2385. doi: 10.1089/ten.2007.0093. [DOI] [PubMed] [Google Scholar]
- 14.Gyawali D, Nair P, Zhang Y, et al. Citric acid-derived in situ crosslinkable biodegradable polymers for cell delivery. Biomaterials. 2010;31:9092–9105. doi: 10.1016/j.biomaterials.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gyawali D, Tran RT, Guleserian KJ, et al. J Biomater Sci Polym Ed. 2010;21:1761–1782. doi: 10.1163/092050609X12567178204169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tran RT, Thevenot P, Gyawali D, et al. Synthesis and characterization of a biodegradable elastomer featuring a dual crosslinking mechanism. Soft Matter. 2010;6:2449–2461. doi: 10.1039/C001605E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim YJ, Cheon JH, Lee SK, et al. Rebamipide may be comparable to H2 receptor antagonist in healing iatrogenic gastric ulcers created by endoscopic mucosal resection: a prospective randomized pilot study. J Korean Med Sci. 2010;25:583–588. doi: 10.3346/jkms.2010.25.4.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polymeros D, Kotsalidis G, Triantafyllou K, et al. Comparative performance of novel solutions for submucosal injection in porcine stomachs: An ex vivo study. Dig Liver Dis. 2010;42:226–229. doi: 10.1016/j.dld.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida N, Naito Y, Kugai M, et al. Efficacy of hyaluronic acid in endoscopic mucosal resection of colorectal tumors. J Gastroen Hepatol. 2011;26:286–291. doi: 10.1111/j.1440-1746.2010.06505.x. [DOI] [PubMed] [Google Scholar]
- 20.Oyama T, Tomori A, Hotta K, et al. Endoscopic Submucosal Dissection of Early Esophogeal Cancer. Clin Gastroenterol Hepatol. 2005;3(7 Suppl 1):S67–S70. doi: 10.1016/s1542-3565(05)00291-0. [DOI] [PubMed] [Google Scholar]