Abstract
Our previous study demonstrates that delayed (initiated 24 hours post injury) erythropoietin (EPO) therapy for traumatic brain injury (TBI) significantly improves spatial learning. In this study, we investigated the impact of inhibition of EPO treatment-mediated neurogenesis on spatial learning after experimental TBI. Young male Wistar rats (318±7g) were subjected to unilateral controlled cortical impact injury. TBI rats received delayed EPO treatment (5,000 U/kg in saline) administered intraperitoneally once daily at 1, 2, and 3 days post injury and intracerebroventricular (icv) infusion of either a mitotic inhibitor cytosine-b-D-arabinofuranoside or vehicle (saline) for 14 days. Another 2 groups of TBI rats were treated intraperitoneally with saline and infused icv with either a mitotic inhibitor Ara-C or saline for 14 days. Animals receiving sham operation were infused icv with either Ara-C infusion or saline. Bromodeoxyuridine (BrdU) was administered to label dividing cells. Spatial learning was assessed using a modified Morris water maze test. Animals were sacrificed at 35 days after injury and brain sections stained for immunohistochemical analyses. As compared to the saline treatment, immunohistochemical analysis revealed that delayed EPO treatment significantly increased the number of BrdU-positive cells and new neurons co-stained with BrdU and NeuN (mature neuron marker) in the dentate gyrus in TBI rats. EPO treatment improved spatial learning after TBI. Ara-C infusion significantly abolished neurogenesis and spatial learning recovery after TBI and EPO treatment. Both EPO and Ara-C reduced the number of astrocytes and microglia/macrophages in the dentate gyrus after TBI. Our findings are highly suggestive for an important role of EPO-amplified dentate gyrus neurogenesis as one of the mechanisms underlying EPO therapeutic treatments after TBI, strongly indicating that strategies promoting endogenous neurogenesis may hold an important therapeutic potential for treatment of TBI.
Keywords: astrocytes, erythropoietin, microglia, neurogenesis, spatial learning, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a leading cause of mortality and morbidity in the United States, particularly among the young (Gean and Fischbein, 2010). Cognitive impairments are persistent sequelae of human TBI (Stoica and Faden, 2010). In vivo models of TBI including the controlled cortical impact (CCI) are utilized extensively to produce deficits reminiscent of those seen clinically (Marklund and Hillered, 2010). To date, there is no effective treatment identified to promote functional recovery after TBI except for routine medical intervention and care (Stein et al., 2010). Different therapeutic strategies have been investigated, including cellular, pharmaceutical and physical therapies (Jain, 2008; Xiong et al., 2009). Erythropoietin (EPO) and its receptor (EPOR), essential for erythropoiesis, are also expressed in neurons, astrocytes, and cerebral endothelial cells (Noguchi et al., 2008). Erythropoietin is a multifunctional agent with tissue protection exerting antiapoptotic, antiinflammatory, antioxidative, angiogenic, and neurotrophic properties (Cotena et al., 2008; Velly et al., 2010). EPO shows neuroprotection in animal models of stroke (Gonzalez et al., 2007; Wang et al., 2004), spinal cord injury (Celik et al., 2002; Grasso et al., 2006), concussive brain injury (Brines et al., 2000), kainate-induced seizure activity (Velly et al., 2010), and autoimmune encephalomyelitis (Cerami, 2001; Sakanaka et al., 1998). Using a CCI rat model, we have investigated the effects of the delayed administration of different doses of EPO on spatial learning recovery after TBI in rats (Meng et al., 2011). Our previous study shows that animals receiving the optimal dose of 5000 U/kg initiated 24 hours after injury exhibit a significant improvement in functional outcomes, which may be associated with EPO-mediated neurogenesis (Meng et al., 2011).
Neurogenesis occurs in two neurogenic regions of adult mammal brains: the subgranular zone in the dentate gyrus (DG) of the hippocampus and the subventricular zone in the forebrain, in which neural stem cells/neural progenitor cells (NSCs/NPCs) reside (Gage, 2010; Toni et al., 2008; Zhao et al., 2008). NSCs/NPCs have the capacity for self renewal and multiple lineage differentiation (Gage, 2010; Zhao et al., 2008). Cytosine-b-D-arabinofuranoside (Ara-C), a mitotic inhibitor that prevents cell proliferation by inhibiting DNA synthesis, has been employed to inhibit NSC/NPC proliferation and neurogenesis in vivo after stroke (Leker et al., 2007; Li et al., 2010; Zhang et al., 2004a); however, no studies to date have examined the effect of inhibition of EPO treatment-mediated neurogenesis on functional outcome after TBI. Accordingly, using a CCI-TBI rat model, we tested the idea that inhibition of neurogenesis by intracerebroventricular (icv) infusion of Ara-C would abolish neurogenesis and spatial learning mediated by delayed EPO treatment in adult rats after TBI.
Methods and materials
Experimental procedures
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Henry Ford Health System (IACUC #1125).
TBI model
We used the CCI model of TBI in rat as previously described (Dixon et al., 1991; Mahmood et al., 2004b). Young adult male Wistar rats (318 ± 7g) were anesthetized with an intraperitoneal (ip) injection of chloral hydrate (350 mg/kg b.w.). Rectal temperature was maintained at 37°C using a feedback-regulated water-heating pad. A CCI device was used to induce injury. Rats were placed in a stereotactic frame. Two 10-mm-diameter craniotomies were performed adjacent to the central suture, midway between lambda and bregma. The second craniotomy allowed for lateral movement of cortical tissue. The dura mater was kept intact over the cortex. Injury was delivered by impacting the left (ipsilateral) cortex with a pneumatic piston containing a 6-mm-diameter tip at a rate of 4 m/s and 2.5 mm of compression. Velocity was measured with a linear velocity displacement transducer.
Experimental groups and treatment
The rats were divided into 6 groups: 1) Sham+Sal group (sham-operated animals infused icv with saline, n=6); 2) Sham+Ara-C group (sham-operated animals infused icv with Ara-C, n=6); 3) TBI+Sal+Sal group (TBI rats treated ip with saline and infused icv with saline, n=8); 4) TBI+Sal+Ara-C group (TBI rats treated ip with saline and infused icv with Ara-C, n=7); 5) TBI+EPO+Sal group (TBI rats treated ip with EPO and infused icv with saline, n=7), and 6) TBI+EPO+Ara-C group (TBI rats treated ip with EPO and infused icv with Ara-C, n=7). Sham rats underwent surgery without injury. Animals in the TBI+Sal+Sal and TBI+Sal+Ara-C groups received an equal volume of saline administered ip at 24, 48 and 72 h after TBI. Animals in the TBI+EPO+Sal and TBI+EPO+Ara-C groups received EPO at an optimal dose of 5,000 U/kg body weight (Epoetin alpha, AMGEN, Thousand Oaks, CA) administered intraperitoneally at 24, 48 and 72 h after TBI. For labeling proliferating cells, bromodeoxyuridine (BrdU, 100 mg/kg; Sigma) was injected ip into rats daily for 10 days, starting 1 day after TBI. To inhibit cell proliferation and neurogenesis, animals were anesthetized ip with chloral hydrate (350 mg/kg) and placed on a stereotactic apparatus (Harvard Apparatus, Holliston, MA). A cannula was placed into the right lateral ventricle (from the Bregma: anteroposterior, −0.8 mm; lateral, 1.5 mm; depth, 3.5 mm). The cannula was sealed with dental cement and connected to a 200 µl, model 2002, Alzet pump (Alzet, Palo Alto, CA) with medical grade vinyl tubing. The minipump was implanted immediately after TBI in a subcutaneous pocket in the midscapular area of the back of the rat. The pumps were primed overnight in a 37°C saline water bath prior to insertion. Drug infusion was performed through the osmotic minipump filled with 2% Ara-C diluted in saline (TBI+Sal+Ara-C and TBI+EPO+Ara-C groups) at a rate of 0.5 µl/h for 14 days. All rats were sacrificed at 35 days after TBI for analysis of cell proliferation and neurogenesis.
Morris water maze (MWM) test
A recent version of the MWM test was used as previously described and has been found to be useful for chronic spatial memory assessment in rodents with brain injury (Choi et al., 2006; Lu et al., 2005; Meng et al., 2011; Ning et al., 2011; Xiong et al., 2011b). All animals were tested during the last 5 days (that is, 31–35 days after TBI) before they were killed. Data collection was automated using the HVS Image 2020 Plus Tracking System (US HVS Image), as described previously (Mahmood et al., 2007a). The advantage of this version of the water maze is that each trial takes on the key characteristics of a probe trial because the platform is not in a fixed location within the target quadrant (Schallert, 2006).
Tissue preparation and measurement of lesion volume
On Day 35 after TBI, rats were anesthetized with chloral hydrate administered ip and perfused transcardially with saline solution, followed by 4% paraformaldehyde in 0.1 M PBS, pH 7.4. Brains were removed and postfixed in 4% paraformaldehyde for 2 days at room temperature. The brain tissue was cut into 7 equally spaced (2-mm) coronal blocks, and it was processed for paraffin sectioning. A series of adjacent 6-µm-thick sections were cut from each block in the coronal plane, and they were stained with H & E. For lesion volume measurement, the 7 brain sections were traced by a microcomputer imaging device (Imaging Research), as previously described (Chen et al., 2005).
Immunohistochemical staining
Bromodeoxyuridine (BrdU), the thymidine analog that is incorporated into the DNA of dividing cells during S-phase, was used for labeling proliferating cells (Sigma Chemical, St. Louis, MS, USA). Measurements of BrdU-, CD 68- (marker for microglia/macrophages) and glial fibrillary acidic protein (GFAP, marker for astrocytes)-positive cells were performed on paraffin-embedded sections (6 µm thick) which were deparaffinized and rehydrated. Antigen retrieval was performed by boiling sections in 10-mM citrate buffer (pH 6.0) for 10 min. After washing with PBS, sections were incubated with 0.3% H2O2 in PBS for 10 min, blocked with 1% BSA containing 0.3% Triton-X 100 for 1 h at room temperature, and incubated with mouse anti-BrdU (dilution 1:200, Dako), mouse anti-rat CD68 monoclonal antibody (dilution 1:200; AbD Serotec, Kidlington, UK), and polyclonal rabbit anti-GFAP antibody (dilution 1:1000; Dako, Denmark) at 4°C overnight. After washing, sections were incubated with biotinylated anti–mouse antibody (dilution 1:200, Vector Laboratories, Inc.) at room temperature for 30 min. After washing, sections were incubated with an avidin-biotin-peroxidase system (ABC kit, Vector Laboratories, Inc.), visualized with diaminobenzidine (Sigma), and counterstained with hematoxylin (Xiong et al., 2011b).
Immunofluorescent staining
Newly generated neurons were identified by double labeling for BrdU and NeuN. After dehydration, tissue sections were boiled for 10 min in 10-mM citric acid buffer (pH 6). After washing with PBS, sections were incubated for 20 min in 2.4 N HCl at 37°C. Sections were then incubated for 1 h with 1% BSA containing 0.3% Triton- X-100 in PBS, followed by incubation overnight with mouse anti–NeuN antibody (dilution 1:200, Chemicon) at 4°C. Fluorescein isothiocyanate–conjugated anti–mouse antibody (dilution 1:400, Jackson ImmunoResearch) was added to sections at room temperature for 2 h. Sections were then incubated overnight with rat anti–BrdU antibody (dilution 1:200, Dako) at 4°C, followed by incubation for 2 h with Cy3-conjugated anti–rat antibody (dilution 1:400, Jackson ImmunoResearch) at room temperature. Each step was followed by three 5-min rinses in PBS. Tissue sections were mounted with Vectashield mounting medium (Vector Laboratories). Images were collected with fluorescent microscopy and merged (Xiong et al., 2011b).
Cell counting and quantitation
For quantitative measurements of BrdU-, CD 68-, GFAP-, NeuN-, and NeuN/BrdU–positive cells, we used 4 slides from each brain, with each slide containing 9 fields of view in the ipsilateral DG from the epicenter of the injury cavity (bregma −3.3 mm). The fields were digitized under the light microscope (Eclipse 80i, Nikon) at either ×200 or × 400 using a CoolSNAP color camera (Photometrics) interfaced with MetaMorph image analysis system (Molecular Devices). The immunopositive cells were calculated and divided by the measured areas and presented as numbers per square millimeter. Cell counts were performed by observers blinded to the individual treatment status of the animals. All counting was performed on a computer monitor to improve visualization and in 1 focal plane to avoid oversampling (Zhang et al., 2002). The density of CD 68-, GFAP-, BrdU-positive cells, and BrdU/NeuN–colabeled cells was calculated in the DG. We focused mainly on the ipsilateral DG and its subregions, including the subgranular zone, granular cell layer, and the molecular layer (Xiong et al., 2011b).
Statistical analysis
All data are presented as mean ± SD. Analysis of variance (ANOVA) was performed for repeated measurements of spatial learning. For lesion volume and cell counting, a one-way ANOVA followed by post hoc Student-Newman-Keuls tests were used to compare the differences between all groups. Correlation analysis of functional recovery with the number of newborn neurons was conducted by the Pearson linear correlation coefficient. Statistical significance was set at p < 0.05.
Results
EPO treatment and Ara-C infusion did not alter cortical lesion volume after TBI
In the present study, TBI caused significant cortical tissue loss. To examine whether delayed EPO treatment or continuous Ara-C infusion had the effect on tissue loss, the lesion volume was measured at 35 days post TBI. Compared with the saline treatment, delayed EPO treatment did not significantly reduce cortical lesion volume (Fig.1, p > 0.05). Ara-C infusion in the TBI+Sal+Ara-C and TBI+EPO+Ara-C groups trended to increased tissue loss but did not reach a significance compared to either the TBI+Sal+Sal or TBI+EPO+Sal groups, respectively (p > 0.05).
Fig. 1.
Effect of EPO and Ara-C on lesion volume examined 35 days after TBI. TBI significantly caused cortical lesion volume. There was no difference in the lesion volume among different TBI groups. Data represent mean ± SD. Scale bar = 3 mm. *p < 0.05 vs. TBI+Sal+Sal group. N (rats/group) = 6 (Sham+Sal); 6 (Sham+Ara-C); 8 (TBI+Sal+Sal); 7 (TBI+Sal+Ara-C); 7 (TBI+EPO+Sal); 7 (TBI+EPO+Ara-C).
Ara-C infusion significantly abolished the EPO-promoted spatial learning in TBI rats
To detect the spatial learning impairments after TBI (Fig. 2), a recent version of the MWM test was used (Xiong et al., 2011b). A higher percentage of time spent in the correct quadrant (Fig. 2a) and a lower percentage of time spent finding the hidden platform (Fig. 2b) in the Morris water maze, correlates with better spatial learning function. Ara-C infusion did not affect spatial learning in the Sham+Ara-C group compared to the Sham+Sal group, which is in line with previous reports (Koros et al., 2007; Li et al., 2008). TBI in the TBI+Sal+Sal group significantly decreased the percentage of time spent in the correct quadrant on Days 32–35 compared to the Sham+Sal group (p < 0.05). EPO treatment in the TBI+EPO+Sal group significantly increased the percentage of time spent by TBI rats in the correct quadrant on Days 33–35 compared with the TBI+Sal+Sal group (p < 0.05). However, Ara-C infusion in the TBI+Sal+Ara-C group significantly reduced the percentage of time on Days 33–35 compared to the TBI+Sal+Sal group (p < 0.05). Similarly, Ara-C infusion in the TBI+EPO+Ara-C group significantly reduced the percentage of time on Days 33–35 compared to the TBI+EPO+Sal group (p < 0.05). In addition, TBI rats in the TBI+Sal+Sal group increased the latency to find the hidden platform in the water maze during Days 32–35 after TBI compared to the Sham+Sal group (p < 0.05). Compared to the saline-treated animals in the TBI+Sal+Sal group, EPO-treated rats in the TBI+EPO+Sal group showed a significant reduction in latency to find the hidden platform on Days 32–35 post TBI (p < 0.05). Ara-C infusion in the TBI+Sal+Ara-C group significantly increased swim latency on Days 32–35 compared to saline infusion in the TBI+Sal+Sal group (p < 0.05). Furthermore, Ara-C infusion in the TBI+EPO+Ara-C group significantly increased the latency to find the hidden platform at days 32–35 compared to the TBI+EPO+Sal group (p < 0.05).
Fig. 2.
Evaluation of spatial learning by Morris water test. a: Percentage time spent in the correct quadrant. TBI significantly impaired spatial learning at Days 32–35 measured by a recent version of the water maze test compared to sham controls (p < 0.05). Delayed treatment with EPO improves spatial learning performance at Days 33–35 compared with the saline group (TBI+EPO+Sal vs TBI+Sal+Sal, p < 0.05). However, the spatial learning performance in the TBI+Sal+Ara-C group is worse than that in the TBI+Sal+Sal group at Days 33–35 (p < 0.05). The spatial learning performance in the TBI+EPO+Ara-C group is worse than that in the TBI+EPO+Sal group at Days 33–35 (p < 0.05). b: Swim latency to find the hidden platform. EPO treatment in the TBI+EPO+Sal group significantly reduced latency in finding the hidden escape platform in the Morris water maze at Days 33–35 compared to the TBI+Sal+Sal group. Ara-C infusion in the TBI+EPO+Ara-C group significantly increased the swim latency at days 32–35 compared to the TBI+EPO+Sal group (p < 0.05). c: Swim speed. There was no significant difference in swim speed among the 6 groups. Data represent mean ± SD. *p < 0.05 vs. TBI+Sal+Sal. #p < 0.05 vs. TBI+EPO+Sal. N (rats/group) = 6 (Sham+Sal); 6 (Sham+Ara-C); 8 (TBI+Sal+Sal); 7 (TBI+Sal+Ara-C); 7 (TBI+EPO+Sal); 7 (TBI+EPO+Ara-C).
However, there was no a significant difference in swim speed among all groups, indicating that motor deficits did not contribute to cognitive deficits measured by the MWM test (Fig. 2c).
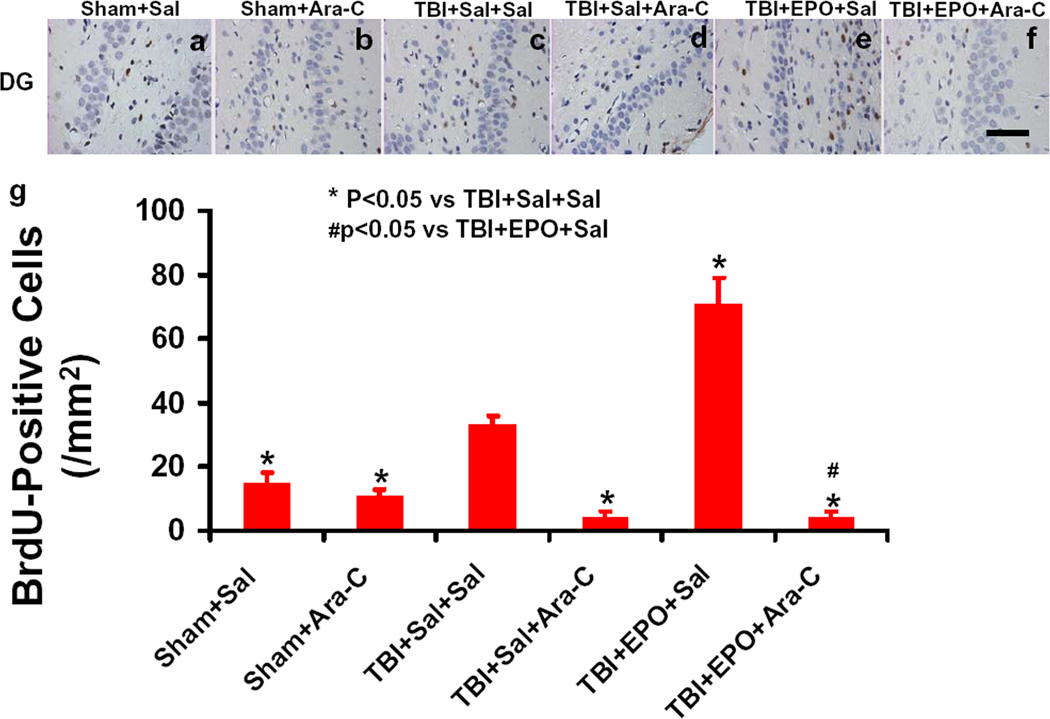
EPO significantly increased cell proliferation in the ipsilateral DG while Ara-C abolished the effect of EPO after TBI
To detect the effect of EPO and Ara-C on cell proliferation in the DG, BrdU immunostaining was performed on paraffin-embedded coronal sections. As shown in Fig.3, TBI (Fig. 3c) significantly increased the number of BrdU-positive cells (brown-stained) compared to the Sham+Sal group (Fig 3a, p < 0.05). Compared to the TBI+Sal+Sal group (Fig.3c), Ara-C in the TBI+Sal+Ara-C group (Fig.3e, p<0.05) significantly decreased the number of BrdU-positive cells. EPO treatment in the TBI+EPO+Sal (Fig.3e, p<0.05) significantly increased the number of BrdU-positive cells while Ara-C infusion significantly reduced cell proliferation in the DG in the TBI+EPO+Ara-C group (Fig.3f. p < 0.05).
Fig. 3.
BrdU-positive cells in the ipsilateral DG examined 35 days after TBI. The cells with BrdU (brown stained) that clearly localized to the nucleus (hematoxylin stained) were counted as BrdU-positive cells. TBI (c) significantly increased the number of BrdU-positive cells in the ipsilateral DG compared to sham controls (a, p < 0.05). Ara-C infusion (d) significantly reduced the number of BrdU-positive cells compared to the TBI+Sal+Sal group (p < 0.05). EPO treatment (e) significantly increased the number of BrdU-positive cells in the DG compared to the TBI+Sal+Sal group (p < 0.05). Ara-C infusion (f) significantly reduced the number of BrdU-positive cells compared to the TBI+EPO+Sal group (p < 0.05). Quantitative data on the number of BrdU-positive cells are shown in (g). Data represent mean ± SD. Scale bar = 50 µm (f). *p < 0.05 vs. TBI+Sal+Sal. #p < 0.05 vs. TBI+EPO+Sal. N (rats/group) = 6 (Sham+Sal); 6 (Sham+Ara-C); 8 (TBI+Sal+Sal); 7 (TBI+Sal+Ara-C); 7 (TBI+EPO+Sal); 7 (TBI+EPO+Ara-C).
EPO significantly increased the newborn neurons in the ipsilateral DG while Ara-C significantly abolished the effect of EPO after TBI
Newborn mature neurons were identified by double staining for BrdU (proliferating marker) and NeuN (mature neuronal marker) on paraffin-embedded coronal sections. As shown in Fig. 4, TBI (Fig. 4c) significantly increased the number of newborn neurons (arrows) compared to the Sham+Sal group (Fig 4a, p < 0.05). Compared to the TBI+Sal+Sal group (Fig.4c), Ara-C infusion significantly decreased the number of newborn neurons in the TBI+Sal+Ara-C group after TBI (Fig.4d, p < 0.05). EPO treatment in the TBI+EPO+Sal group significantly further increased the number of newborn neurons compared to the TBI+Sal+Sal group (Fig.4e, p < 0.05). Furthermore, Ara-C infusion in the TBI+EPO+Ara-C group (Fig.4f) significantly decreased the number of newborn neurons after TBI compared to the TBI+EPO+Sal group (Fig.4e, p < 0.05).
Fig. 4.
Double fluorescent staining for BrdU (red) and NeuN (green) to identify newborn neurons (yellow after merge) in the ipsilateral DG at 35 days after TBI. TBI (c) significantly increased the number of newborn neurons in the ipsilateral DG compared to sham controls (a, p < 0.05). EPO treatment (e) further significantly increased the number of newborn neurons compared to the saline group (c, p < 0.05). Ara-C infusion abolished generation of newborn neurons in the TBI+Sal+Ara-C (d) and TBI+EPO+Ara-C (f) groups (p<0.05). Newborn BrdU-positive cells (red, h) differentiate into neurons (yellow, i) expressing NeuN (green, g). Quantitative data on the number of NeuN/BrdU-colabeled cells are shown in (j). Correlation analysis of spatial learning with neurogenesis examined 35 days after TBI. The spatial learning is significantly correlated with the number of the newborn neurons generated in the DG (k, p < 0.05). Data represent mean ± SD. Scale bars = 50 µm (f); 25 µm (i). * p < 0.05 vs. TBI+Sal+Sal. #p < 0.05 vs. TBI+EPO+Sal. N (rats/group) = 6 (Sham+Sal); 6 (Sham+Ara-C); 8 (TBI+Sal+Sal); 7 (TBI+Sal+Ara-C); 7 (TBI+EPO+Sal); 7 (TBI+EPO+Ara-C).
Correlation analysis shows that the spatial learning is highly and significantly correlated with the number of the newborn neurons in the DG (Fig.4k, p < 0.001). These data strongly suggest that the EPO-amplified DG neurogenesis (i.e., increased newborn neurons), at least in part, contribute to improvement in spatial learning after TBI.
Both EPO and Ara-C significantly decreased the number of microglia/macrophages in the ipsilateral DG
Few CD68-positive microglia/macrophages were detected in the DG in the Sham+Sal group and Sham+Ara-C group (Fig.5a and 5b). Compared to the Sham+Sal group, TBI in the TBI+Sal+Sal group significantly increased the number of these cells (Fig.5c, p < 0.05). Compared to the TBI+Sal+Sal group, Ara-C infusion in the TBI+Sal+Ara-C group significantly reduced the number of CD68-positive microglia/macrophages (Fig.5d, p < 0.05). Our data show that delayed EPO treatment in the TBI+EPO+Sal group (Fig.5e, p < 0.05) significantly reduced the number of CD68-positive microglia/macrophages in the DG after TBI as compared to the TBI+Sal+Sal group. Furthermore, the number of CD68-positive microglia/macrophages in the TBI+EPO+Ara-C group (Fig. 5f) was significantly reduced compared to the TBI+EPO+Sal group (Fig.5e, p < 0.05).
Fig. 5.
CD68-positive microglia/macrophages in the ipsilateral DG examined 35 days after TBI. TBI (c) significantly increased the number of CD68-positive cells in the ipsilateral DG compared to sham controls (a, p < 0.05). Ara-C infusion (d) significantly reduced the number of CD68-positive cells compared to the TBI+Sal+Sal group (p < 0.05). EPO treatment (e) significantly decreased the number of CD68-positive cells in the DG compared to the TBI+Sal+Sal group (p < 0.05). Ara-C infusion (f) significantly reduced the number of CD68-positive cells compared to the TBI+EPO+Sal group (p < 0.05). Quantitative data on the number of CD68-positive cells are shown in (g). Data represent mean ± SD. Scale bar = 50 µm (f). *p < 0.05 vs. TBI+Sal+Sal. #p < 0.05 vs. TBI+EPO+Sal. N (rats/group) = 6 (Sham+Sal); 6 (Sham+Ara-C); 8 (TBI+Sal+Sal); 7 (TBI+Sal+Ara-C); 7 (TBI+EPO+Sal); 7 (TBI+EPO+Ara-C).
Both EPO and Ara-C significantly decreased the number of astrocytes in the ipsilateral DG
GFAP-positive astrocytes were detected in the DG in the Sham+Sal group and Sham+Ara-C group (Fig.6a and 6b). Compared to the Sham+Sal group, TBI in the TBI+Sal+Sal group significantly increased the number of astrocytes (Fig.6c, p < 0.05). Compared to the TBI+Sal+Sal group, Ara-C infusion in the TBI+Sal+Ara-C group significantly reduced the number of GFAP-positive astrocytes (Fig.6d, p < 0.05). Our data show that delayed EPO treatment in the TBI+EPO+Sal group (Fig.6e, p < 0.05) significantly reduced the number of GFAP-positive astrocytes in the DG after TBI as compared to the TBI+Sal+Sal group. Furthermore, the number of GFAP-positive astrocytes in the TBI+EPO+Ara-C group (Fig. 6f) was significantly reduced compared to the TBI+EPO+Sal group (Fig.6e, p < 0.05).
Fig. 6.
GFAP-positive astrocytes in the ipsilateral DG examined 35 days after TBI. TBI (c) significantly increased the number of GFAP-positive cells in the ipsilateral DG compared to sham controls (a, p < 0.05). Ara-C infusion (d) significantly reduced the number of GFAP-positive cells compared to the TBI+Sal+Sal group (p < 0.05). EPO treatment (e) significantly decreased the number of GFAP-positive cells in the DG compared to the TBI+Sal+Sal group (p < 0.05). Ara-C infusion (f) significantly reduced the number of GFAP-positive cells compared to the TBI+EPO+Sal group (p < 0.05). Quantitative data on the number of GFAP-positive cells are shown in (g). Data represent mean ± SD. Scale bar = 50 µm (f). *p < 0.05 vs. TBI+Sal+Sal. #p < 0.05 vs. TBI+EPO+Sal. N (rats/group) = 6 (Sham+Sal); 6 (Sham+Ara-C); 8 (TBI+Sal+Sal); 7 (TBI+Sal+Ara-C); 7 (TBI+EPO+Sal); 7 (TBI+EPO+Ara-C).
Discussion
In the present study, we demonstrate that icv infusion of mitotic inhibitor Ara-C abolishes EPO-mediated neurogenesis and spatial learning in rats after TBI, indicating an important role of EPO-amplified DG neurogenesis as one of the mechanisms underlying EPO therapeutic treatments after TBI. This finding also implies that treatment strategies enhancing adult endogenous neurogenesis may hold a significant therapeutic potential for TBI.
In this study, the TBI model we employed is a well-established unilateral CCI, which not only causes ipsilateral cortical loss but also leads to cell loss in the DG and CA3 region of the ipsilateral hippocampus (Dempsey and Raghavendra Rao, 2003; Lu et al., 2007). Impaired spatial learning and memory occur in animals after CCI (Dixon et al., 1999; Fox et al., 1998; Lu et al., 2005). Increasing evidence implies that adult neurogenesis in the DG of the hippocampus may play a critical role in learning (Castilla-Ortega et al., 2011; Clausen et al., 2005; Marin-Burgin and Schinder, 2012; Zhao et al., 2008). Our recent study demonstrates that delayed (24 h post injury) EPO treatment does not reduce cortical lesion volume but improves spatial learning in rats after CCI (Meng et al., 2011). The spatial learning function is highly correlated with the effect of EPO on reducing hippocampal cell loss and increasing neurogenesis (Meng et al., 2011). These data suggest that enhanced neurogenesis may be involved in improvement in spatial learning in TBI rats treated with EPO.
In this study, using Ara-C infusion to inhibit neurogenesis, we focused on neurogenesis of the DG and employed a modified Morris water maze test to measure spatial learning in rats after TBI and EPO treatment. Ara-C is a pyrimidine antimetabolite that prevents cell proliferation by inhibiting DNA synthesis. Ara-C has been widely used to inhibit neurogenesis in vivo (Doetsch et al., 1999; Im et al., 2010; Li et al., 2010; Yau et al., 2011; Yoshikawa et al., 2010; Zhang et al., 2004b). Our data show that delayed EPO treatment in the TBI+EPO+Sal group significantly reduces the number of GFAP-positive astrocytes and CD68-positive microglia/macrophages in the DG after TBI as compared to the TBI+Sal+Sal group, indicating the anti-inflammatory effect of EPO (Villa et al., 2003; Yatsiv et al., 2005). EPO treatment enhanced neurogenesis in the DG. It is likely that both EPO-mediated increased neurogenesis and anti-inflammatory effect on microglia/macrophages/astrocytes may contribute to improvement in spatial learning in this study. Compared to the TBI+Sal+Sal group, Ara-C infusion in the TBI+Sal+Ara-C group significantly reduces the number of GFAP-positive astrocytes and CD68-positive microglia/macrophages, abolishes generation of newborn neurons in the DG after TBI, and exacerbates spatial learning. These data suggest that endogenous neurogenesis is a physiological repair mechanism after TBI and plays a critical role in spatial learning during the spontaneous recovery after TBI. In addition, Ara-C infusion in the TBI+EPO+Ara-C group significantly abolished the beneficial effect of EPO treatment on neurogenesis and spatial learning while the effect of Ara-C on reducing the number of astrocytes and microglia/macrophages was comparable to that of EPO in the TBI+EPO+Sal group, suggesting that reducing the number of microglia/macrophages and astrocytes combined with inhibition of neurogenesis is detrimental to improving spatial learning. Taken together, our data strongly indicate that EPO treatment-amplified neurogenesis in the DG, at least in part, contributes to the improvement in spatial learning in TBI rats treated with EPO.
Neurogenesis occurs throughout life in the adult brain neurogenic regions of mammals and may play an important role in learning and memory (Toni et al., 2008; Zhao et al., 2008). Increasing evidence has shown that TBI induces cell proliferation and neurogenesis (Emery et al., 2005; Lu et al., 2005; Meng et al., 2011; Ning et al., 2011; Richardson et al., 2007; Sun et al., 2007; Xiong et al., 2011a; Xiong et al., 2011b; Zheng et al., 2011). Increased neurogenesis after TBI might function to repair brain damage and to promote functional recovery if newborn neurons are functionally integrated into existing hippocampal circuitry. To verify this possibility, Emery and colleagues demonstrate that newborn granule neurons extend their axons along the mossy fiber pathway by 2 weeks after TBI (Emery et al., 2005). Moreover, a study by (Sun et al., 2007) shows that the majority of BrdU-labeled cells become DG neurons by 10 weeks after TBI In that study, approximately 30% of newborn neurons integrate into the hippocampus and are surrounded by synaptophysin immunoreactivity, suggesting the formation of functional synapses, which is associated to cognitive recovery (Sun et al., 2007). If neurogenesis is ablated genetically at the time of injury, injured animals lose the ability to learn spatial memory tasks (Blaiss et al., 2011). These results indicate that TBI-induced newborn neurons can be connected to their targets and may contribute to spontaneous functional recovery after TBI.
The adult subgranular zone of the DG is one of two largest neurogenic areas of the adult mammalian brain (Laplagne et al., 2006; Toni et al., 2008; Zhao et al., 2008). The adult DG contains NSCs/NPCs that generate adult new neurons throughout life. NPCs are very sensitive to Ara-C treatment, however, NSCs are resistant to Ara-C treatment and can replenish NPCs (Doetsch et al., 1999; Zhang et al., 2004a). In a stroke study, the number of neuroblasts significantly increases in the subventricular zone (SVZ), and Ara-C treatment eliminates the actively proliferating cell population in the SVZ, which entirely repopulates at 7 days after termination of Ara-C infusion in stroke rats (Zhang et al., 2004a). In the present study, the spatial learning test started at Day 31 post injury, 17 days after termination of Ara-C infusion. We speculate that the NPCs may entirely repopulate on Day 31. Dynamic study of cell proliferation in the DG is needed to clarify this issue. However, previous studies indicate that new neurons do not show electrophysiological features of maturity until the third week after their birth (Ambrogini et al., 2004; Esposito et al., 2005). Anatomically, the axon, dendrites, and their spines reach maturity around weeks 3 to 4 after neuron birth (Esposito et al., 2005; Hastings and Gould, 1999; Sandoval et al., 2011; Zhao et al., 2006). Newborn DG neurons begin to receive afferent excitation at 2 weeks, but they can only generate action potentials in response to an excitatory drive at 24–28 days (Mongiat et al., 2009; Mongiat and Schinder, 2011). It is likely that newborn mature neurons other than immature neurons after TBI play a critical role in spatial learning after EPO treatment. However, a recent study indicates that NSCs/NPCs produce trophic factors including brain-derived neurotrophic factor (BDNF), which has been shown to provide trophic support for hippocampal neuron survival after cortical stroke (Li et al., 2010). Our previous studies show that EPO treatment increases brain BDNF level after TBI and stroke (Mahmood et al., 2007b; Wang et al., 2004). Further study is warranted to clarify whether Ara-C infusion affects brain BDNF level in TBI rats after EPO treatment.
There are several limitations to this study. First, Ara-C can inhibit proliferation of entirely different cells in the brain. We only examined its inhibitory effect on neurogenesis, astrocytes and microglia/macrophages. Second, we focused on the DG. Although the DG is one of the neurogenic regions in the adult brain and may play an important role in spatial learning, other brain regions are also involved in learning and memory (Spiers and Maguire, 2007). Third, we cannot exclude other effects of EPO including its effects on other brain regions as well as other cells including endothelial cells and oligodendrocyte precursor cells (Meng et al., 2011; Zhang et al., 2010), which may participate in brain repair after TBI.
Conclusions
The injured brain has a limited capacity for self repair, suggesting that functional recovery following TBI is likely to require cellular transplantation of exogenous cells to replace those lost to trauma (Schouten et al., 2004). Cellular transplantation has been evaluated in several models of experimental TBI, with promising results (Chen et al., 2007; Chopp and Li, 2008; Lu et al., 2001; Mahmood et al., 2004a; Richardson et al., 2010; Xiong et al., 2009); however, cell therapy is linked to limitations such as relatively low rate of direct differentiation to neurons, tumorigenicity, and questions regarding optimal dose and the time and routes of administration it should begin (Richardson et al., 2010). The findings in the present study suggest that EPO-amplified neurogenesis may, at least in part, contribute to spatial learning following TBI. The results from us and others provide strong evidence that neuronal replacement strategies through enhancing adult endogenous neurogenesis may hold potential for treatment of TBI and other neurodegenerative disorders.
Acknowledgements
The authors would like to thank Susan MacPhee-Gray for editorial assistance. This work was supported by NIH grants RO1 NS62002 (yx) and PO1 NS023393 (mc).
References
- Ambrogini P, Lattanzi D, Ciuffoli S, Agostini D, Bertini L, Stocchi V, Santi S, Cuppini R. Morpho-functional characterization of neuronal cells at different stages of maturation in granule cell layer of adult rat dentate gyrus. Brain Res. 2004;1017:21–31. doi: 10.1016/j.brainres.2004.05.039. [DOI] [PubMed] [Google Scholar]
- Blaiss CA, Yu TS, Zhang G, Chen J, Dimchev G, Parada LF, Powell CM, Kernie SG. Temporally specified genetic ablation of neurogenesis impairs cognitive recovery after traumatic brain injury. J Neurosci. 2011;31:4906–4916. doi: 10.1523/JNEUROSCI.5265-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, Itri LM, Cerami A. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci U S A. 2000;97:10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilla-Ortega E, Pedraza C, Estivill-Torrus G, Santin LJ. When is adult hippocampal neurogenesis necessary for learning? evidence from animal research. Rev Neurosci. 2011;22:267–283. doi: 10.1515/RNS.2011.027. [DOI] [PubMed] [Google Scholar]
- Celik M, Gokmen N, Erbayraktar S, Akhisaroglu M, Konakc S, Ulukus C, Genc S, Genc K, Sagiroglu E, Cerami A, Brines M. Erythropoietin prevents motor neuron apoptosis and neurologic disability in experimental spinal cord ischemic injury. Proc Natl Acad Sci U S A. 2002;99:2258–2263. doi: 10.1073/pnas.042693799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerami A. Beyond erythropoiesis: novel applications for recombinant human erythropoietin. Semin Hematol. 2001;38:33–39. doi: 10.1016/s0037-1963(01)90128-3. [DOI] [PubMed] [Google Scholar]
- Chen HI, Bakshi A, Royo NC, Magge SN, Watson DJ. Neural stem cells as biological minipumps: a faster route to cell therapy for the CNS? Curr Stem Cell Res Ther. 2007;2:13–22. doi: 10.2174/157488807779317044. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, Katakowski M, Lu M, Chopp M. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25:281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Woodlee MT, Hong JJ, Schallert T. A simple modification of the water maze test to enhance daily detection of spatial memory in rats and mice. J Neurosci Methods. 2006;156:182–193. doi: 10.1016/j.jneumeth.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Chopp M, Li Y. Treatment of stroke and intracerebral hemorrhage with cellular and pharmacological restorative therapies. Acta Neurochir Suppl. 2008;105:79–83. doi: 10.1007/978-3-211-09469-3_16. [DOI] [PubMed] [Google Scholar]
- Clausen F, Lewen A, Marklund N, Olsson Y, McArthur DL, Hillered L. Correlation of hippocampal morphological changes and morris water maze performance after cortical contusion injury in rats. Neurosurgery. 2005;57:154–163. doi: 10.1227/01.neu.0000163412.07546.57. discussion 154–163. [DOI] [PubMed] [Google Scholar]
- Cotena S, Piazza O, Tufano R. The use of erythtropoietin in cerebral diseases. Panminerva Med. 2008;50:185–192. [PubMed] [Google Scholar]
- Dempsey RJ, Raghavendra Rao VL. Cytidinediphosphocholine treatment to decrease traumatic brain injury-induced hippocampal neuronal death, cortical contusion volume, and neurological dysfunction in rats. J Neurosurg. 2003;98:867–873. doi: 10.3171/jns.2003.98.4.0867. [DOI] [PubMed] [Google Scholar]
- Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- Dixon CE, Kochanek PM, Yan HQ, Schiding JK, Griffith RG, Baum E, Marion DW, DeKosky ST. One-year study of spatial memory performance, brain morphology, and cholinergic markers after moderate controlled cortical impact in rats. J Neurotrauma. 1999;16:109–122. doi: 10.1089/neu.1999.16.109. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci U S A. 1999;96:11619–11624. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery DL, Fulp CT, Saatman KE, Schutz C, Neugebauer E, McIntosh TK. Newly born granule cells in the dentate gyrus rapidly extend axons into the hippocampal CA3 region following experimental brain injury. J Neurotrauma. 2005;22:978–988. doi: 10.1089/neu.2005.22.978. [DOI] [PubMed] [Google Scholar]
- Esposito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, Schinder AF. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci. 2005;25:10074–10086. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox GB, Fan L, Levasseur RA, Faden AI. Sustained sensory/motor and cognitive deficits with neuronal apoptosis following controlled cortical impact brain injury in the mouse. J Neurotrauma. 1998;15:599–614. doi: 10.1089/neu.1998.15.599. [DOI] [PubMed] [Google Scholar]
- Gage FH. Molecular and cellular mechanisms contributing to the regulation, proliferation and differentiation of neural stem cells in the adult dentate gyrus. Keio J Med. 2010;59:79–83. doi: 10.2302/kjm.59.79. [DOI] [PubMed] [Google Scholar]
- Gean AD, Fischbein NJ. Head trauma. Neuroimaging Clin N Am. 2010;20:527–556. doi: 10.1016/j.nic.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Gonzalez FF, McQuillen P, Mu D, Chang Y, Wendland M, Vexler Z, Ferriero DM. Erythropoietin enhances long-term neuroprotection and neurogenesis in neonatal stroke. Dev Neurosci. 2007;29:321–330. doi: 10.1159/000105473. [DOI] [PubMed] [Google Scholar]
- Grasso G, Sfacteria A, Erbayraktar S, Passalacqua M, Meli F, Gokmen N, Yilmaz O, La Torre D, Buemi M, Iacopino DG, Coleman T, Cerami A, Brines M, Tomasello F. Amelioration of spinal cord compressive injury by pharmacological preconditioning with erythropoietin and a nonerythropoietic erythropoietin derivative. J Neurosurg Spine. 2006;4:310–318. doi: 10.3171/spi.2006.4.4.310. [DOI] [PubMed] [Google Scholar]
- Hastings NB, Gould E. Rapid extension of axons into the CA3 region by adult-generated granule cells. J Comp Neurol. 1999;413:146–154. doi: 10.1002/(sici)1096-9861(19991011)413:1<146::aid-cne10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Im SH, Yu JH, Park ES, Lee JE, Kim HO, Park KI, Kim GW, Park CI, Cho SR. Induction of striatal neurogenesis enhances functional recovery in an adult animal model of neonatal hypoxic-ischemic brain injury. Neuroscience. 2010;169:259–268. doi: 10.1016/j.neuroscience.2010.04.038. [DOI] [PubMed] [Google Scholar]
- Jain KK. Neuroprotection in traumatic brain injury. Drug Discov Today. 2008;13:1082–1089. doi: 10.1016/j.drudis.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Koros C, Papalexi E, Anastasopoulos D, Kittas C, Kitraki E. Effects of AraC treatment on motor coordination and cerebellar cytoarchitecture in the adult rat. A possible protective role of NAC. Neurotoxicology. 2007;28:83–92. doi: 10.1016/j.neuro.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Laplagne DA, Esposito MS, Piatti VC, Morgenstern NA, Zhao C, van Praag H, Gage FH, Schinder AF. Functional convergence of neurons generated in the developing and adult hippocampus. PLoS Biol. 2006;4:e409. doi: 10.1371/journal.pbio.0040409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leker RR, Soldner F, Velasco I, Gavin DK, Androutsellis-Theotokis A, McKay RD. Long-lasting regeneration after ischemia in the cerebral cortex. Stroke. 2007;38:153–161. doi: 10.1161/01.STR.0000252156.65953.a9. [DOI] [PubMed] [Google Scholar]
- Li B, Piao CS, Liu XY, Guo WP, Xue YQ, Duan WM, Gonzalez-Toledo ME, Zhao LR. Brain self-protection: the role of endogenous neural progenitor cells in adult brain after cerebral cortical ischemia. Brain Res. 2010;1327:91–102. doi: 10.1016/j.brainres.2010.02.030. [DOI] [PubMed] [Google Scholar]
- Li CQ, Liu D, Huang L, Wang H, Zhang JY, Luo XG. Cytosine arabinoside treatment impairs the remote spatial memory function and induces dendritic retraction in the anterior cingulate cortex of rats. Brain Res Bull. 2008;77:237–240. doi: 10.1016/j.brainresbull.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Lu D, Li Y, Wang L, Chen J, Mahmood A, Chopp M. Intraarterial administration of marrow stromal cells in a rat model of traumatic brain injury. J Neurotrauma. 2001;18:813–819. doi: 10.1089/089771501316919175. [DOI] [PubMed] [Google Scholar]
- Lu D, Mahmood A, Qu C, Goussev A, Schallert T, Chopp M. Erythropoietin enhances neurogenesis and restores spatial memory in rats after traumatic brain injury. J Neurotrauma. 2005;22:1011–1017. doi: 10.1089/neu.2005.22.1011. [DOI] [PubMed] [Google Scholar]
- Lu D, Qu C, Goussev A, Jiang H, Lu C, Schallert T, Mahmood A, Chen J, Li Y, Chopp M. Statins increase neurogenesis in the dentate gyrus, reduce delayed neuronal death in the hippocampal CA3 region, and improve spatial learning in rat after traumatic brain injury. J Neurotrauma. 2007;24:1132–1146. doi: 10.1089/neu.2007.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood A, Lu D, Chopp M. Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury. J Neurotrauma. 2004a;21:33–39. doi: 10.1089/089771504772695922. [DOI] [PubMed] [Google Scholar]
- Mahmood A, Lu D, Chopp M. Marrow stromal cell transplantation after traumatic brain injury promotes cellular proliferation within the brain. Neurosurgery. 2004b;55:1185–1193. doi: 10.1227/01.neu.0000141042.14476.3c. [DOI] [PubMed] [Google Scholar]
- Mahmood A, Lu D, Qu C, Goussev A, Chopp M. Treatment of traumatic brain injury with a combination therapy of marrow stromal cells and atorvastatin in rats. Neurosurgery. 2007a;60:546–553. doi: 10.1227/01.NEU.0000255346.25959.99. discussion 553–544. [DOI] [PubMed] [Google Scholar]
- Mahmood A, Lu D, Qu C, Goussev A, Zhang ZG, Lu C, Chopp M. Treatment of traumatic brain injury in rats with erythropoietin and carbamylated erythropoietin. J Neurosurg. 2007b;107:392–397. doi: 10.3171/JNS-07/08/0392. [DOI] [PubMed] [Google Scholar]
- Marin-Burgin A, Schinder AF. Requirement of adult-born neurons for hippocampus-dependent learning. Behav Brain Res. 2012;227:391–399. doi: 10.1016/j.bbr.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Marklund N, Hillered L. Animal Modeling of Traumatic Brain Injury in Pre-clinical Drug Development - Where do we go from here? Br J Pharmacol. 2010 doi: 10.1111/j.1476-5381.2010.01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y, Xiong Y, Mahmood A, Zhang Y, Qu C, Chopp M. Dose-dependent neurorestorative effects of delayed treatment of traumatic brain injury with recombinant human erythropoietin in rats. J Neurosurg. 2011;115:550–560. doi: 10.3171/2011.3.JNS101721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongiat LA, Esposito MS, Lombardi G, Schinder AF. Reliable activation of immature neurons in the adult hippocampus. PLoS One. 2009;4:e5320. doi: 10.1371/journal.pone.0005320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongiat LA, Schinder AF. Adult neurogenesis and the plasticity of the dentate gyrus network. Eur J Neurosci. 2011;33:1055–1061. doi: 10.1111/j.1460-9568.2011.07603.x. [DOI] [PubMed] [Google Scholar]
- Ning R, Xiong Y, Mahmood A, Zhang Y, Meng Y, Qu C, Chopp M. Erythropoietin promotes neurovascular remodeling and long-term functional recovery in rats following traumatic brain injury. Brain Res. 2011;1384:140–150. doi: 10.1016/j.brainres.2011.01.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi CT, Wang L, Rogers HM, Teng R, Jia Y. Survival and proliferative roles of erythropoietin beyond the erythroid lineage. Expert Rev Mol Med. 2008;10:e36. doi: 10.1017/S1462399408000860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RM, Singh A, Sun D, Fillmore HL, Dietrich DW, 3rd, Bullock MR. Stem cell biology in traumatic brain injury: effects of injury and strategies for repair. J Neurosurg. 2010;112:1125–1138. doi: 10.3171/2009.4.JNS081087. [DOI] [PubMed] [Google Scholar]
- Richardson RM, Sun D, Bullock MR. Neurogenesis after traumatic brain injury. Neurosurg Clin N Am. 2007;18:169–181. doi: 10.1016/j.nec.2006.10.007. xi. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, Wen TC, Matsuda S, Masuda S, Morishita E, Nagao M, Sasaki R. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci U S A. 1998;95:4635–4640. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval CJ, Martinez-Claros M, Bello-Medina PC, Perez O, Ramirez-Amaya V. When are new hippocampal neurons, born in the adult brain, integrated into the network that processes spatial information? PLoS One. 2011;6:e17689. doi: 10.1371/journal.pone.0017689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallert T. Behavioral tests for preclinical intervention assessment. NeuroRx. 2006;3:497–504. doi: 10.1016/j.nurx.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouten JW, Fulp CT, Royo NC, Saatman KE, Watson DJ, Snyder EY, Trojanowski JQ, Prockop DJ, Maas AI, McIntosh TK. A review and rationale for the use of cellular transplantation as a therapeutic strategy for traumatic brain injury. J Neurotrauma. 2004;21:1501–1538. doi: 10.1089/neu.2004.21.1501. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, Maguire EA. The neuroscience of remote spatial memory: a tale of two cities. Neuroscience. 2007;149:7–27. doi: 10.1016/j.neuroscience.2007.06.056. [DOI] [PubMed] [Google Scholar]
- Stein SC, Georgoff P, Meghan S, Mizra K, Sonnad SS. 150 years of treating severe traumatic brain injury: a systematic review of progress in mortality. J Neurotrauma. 2010;27:1343–1353. doi: 10.1089/neu.2009.1206. [DOI] [PubMed] [Google Scholar]
- Stoica BA, Faden AI. Cell death mechanisms and modulation in traumatic brain injury. Neurotherapeutics. 2010;7:3–12. doi: 10.1016/j.nurt.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, McGinn MJ, Zhou Z, Harvey HB, Bullock MR, Colello RJ. Anatomical integration of newly generated dentate granule neurons following traumatic brain injury in adult rats and its association to cognitive recovery. Exp Neurol. 2007;204:264–272. doi: 10.1016/j.expneurol.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, Schinder AF. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008;11:901–907. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velly L, Pellegrini L, Guillet B, Bruder N, Pisano P. Erythropoietin 2nd cerebral protection after acute injuries: a double-edged sword? Pharmacol Ther. 2010;128:445–459. doi: 10.1016/j.pharmthera.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Villa P, Bigini P, Mennini T, Agnello D, Laragione T, Cagnotto A, Viviani B, Marinovich M, Cerami A, Coleman TR, Brines M, Ghezzi P. Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J Exp Med. 2003;198:971–975. doi: 10.1084/jem.20021067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Chopp M. Emerging treatments for traumatic brain injury. Expert Opin Emerg Drugs. 2009;14:67–84. doi: 10.1517/14728210902769601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Meng Y, Zhang Y, Zhang ZG, Morris DC, Chopp M. Treatment of traumatic brain injury with thymosin beta in rats. J Neurosurg. 2011a;114:102–115. doi: 10.3171/2010.4.JNS10118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Zhang Y, Meng Y, Zhang ZG, Qu C, Sager TN, Chopp M. Effects of posttraumatic carbamylated erythropoietin therapy on reducing lesion volume and hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome in rats following traumatic brain injury. J Neurosurg. 2011b;114:549–559. doi: 10.3171/2010.10.JNS10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsiv I, Grigoriadis N, Simeonidou C, Stahel PF, Schmidt OI, Alexandrovitch AG, Tsenter J, Shohami E. Erythropoietin is neuroprotective, improves functional recovery, and reduces neuronal apoptosis and inflammation in a rodent model of experimental closed head injury. FASEB J. 2005;19:1701–1703. doi: 10.1096/fj.05-3907fje. [DOI] [PubMed] [Google Scholar]
- Yau SY, Lau BW, Tong JB, Wong R, Ching YP, Qiu G, Tang SW, Lee TM, So KF. Hippocampal neurogenesis and dendritic plasticity support running-improved spatial learning and depression-like behaviour in stressed rats. PLoS One. 2011;6:e24263. doi: 10.1371/journal.pone.0024263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa G, Momiyama T, Oya S, Takai K, Tanaka J, Higashiyama S, Saito N, Kirino T, Kawahara N. Induction of striatal neurogenesis and generation of region-specific functional mature neurons after ischemia by growth factors. Laboratory investigation. J Neurosurg. 2010;113:835–850. doi: 10.3171/2010.2.JNS09989. [DOI] [PubMed] [Google Scholar]
- Zhang L, Chopp M, Zhang RL, Wang L, Zhang J, Wang Y, Toh Y, Santra M, Lu M, Zhang ZG. Erythropoietin amplifies stroke-induced oligodendrogenesis in the rat. PLoS One. 2010;5:e11016. doi: 10.1371/journal.pone.0011016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Wang Y, Zhang L, Zhang Z, Tsang W, Lu M, Chopp M. Sildenafil (Viagra) induces neurogenesis and promotes functional recovery after stroke in rats. Stroke. 2002;33:2675–2680. doi: 10.1161/01.str.0000034399.95249.59. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zhang Z, Wang L, Wang Y, Gousev A, Zhang L, Ho KL, Morshead C, Chopp M. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab. 2004a;24:441–448. doi: 10.1097/00004647-200404000-00009. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zhang Z, Zhang C, Zhang L, Robin A, Wang Y, Lu M, Chopp M. Stroke transiently increases subventricular zone cell division from asymmetric to symmetric and increases neuronal differentiation in the adult rat. J Neurosci. 2004b;24:5810–5815. doi: 10.1523/JNEUROSCI.1109-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Jr., Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Zhuge Q, Zhong M, Chen G, Shao B, Wang H, Mao X, Xie L, Jin K. Neurogenesis in Adult Human Brain after Traumatic Brain Injury. J Neurotrauma. 2011 doi: 10.1089/neu.2010.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]