Abstract
Innate immunity is highly conserved and relies on pattern recognition receptors (PRRs) such as Toll-like receptors (identified through their homology to Drosophila Toll) for pathogen recognition. While Drosophila Toll is vital for immune recognition and defense, roles for the other eight Drosophila Tolls in immunity have remained elusive. Here we have shown that Toll-7 is a PRR both in vitro and in adult flies; loss of Toll-7 led to increased Vesicular Stomatitis virus (VSV) replication and mortality. Toll-7, along with additional uncharacterized Drosophila Tolls, were transcriptionally induced by VSV infection. Furthermore, Toll-7 interacted with VSV at the plasma membrane and induced antiviral autophagy independently of the canonical Toll signaling pathway. These data uncover an evolutionarily conserved role for a second Drosophila Toll receptor that links viral recognition to autophagy and defense, and suggest that other Drosophila Tolls may restrict specific as yet untested pathogens, perhaps via non-canonical signaling pathways.
INTRODUCTION
Detection and clearance of viruses by the innate immune system involves several distinct and essential pathways that are evolutionarily conserved (Janeway and Medzhitov, 2002). These pathways rely on pattern recognition receptors (PRRs) to recognize pathogen-associated molecular patterns (PAMPs), molecular signatures shared by wide classes of invading organisms, and induce an appropriate effector response to clear the infection. One important class of PRRs are the Toll-like receptors (TLRs), which were first identified in Drosophila through their homology to Toll, and are now recognized as the canonical pathogen recognition system in all metazoans (Uematsu and Akira, 2006).
Drosophila encodes nine Toll receptors (Bilak et al., 2003). The first to be identified, Toll, is the upstream receptor for the Toll pathway, which is the main defense against Gram-positive bacterial and fungal infections and is conserved in many insects (Cerenius et al., 2010; Lemaitre and Hoffmann, 2007; Lemaitre et al., 1996). These microbes are sensed by a variety of recognition molecules that activate a proteolytic cascade converging on the activation of spätzle, a cytokine that binds to Toll thereby inducing an NF-kB-dependent transcriptional program for antimicrobial defense. Surprisingly, a role for the additional eight Drosophila Toll homologues in innate immune defense has yet to be established. Toll-2 (18-wheeler) may have a minor role in the antibacterial response (Ligoxygakis et al., 2002; Williams et al., 1997), and Toll-5 (Tehao) and Toll-9 can activate the expression of the antifungal gene Drosomycin (Bilak et al., 2003; Luo et al., 2001; Ooi et al., 2002; Tauszig et al., 2000). However, these receptors have not been implicated as essential components of the immune response or in the recognition of any pathogen (Narbonne-Reveau et al., 2011; Yagi et al., 2010).
In contrast to Drosophila, studies have quickly identified a role for the 10 human TLRs in immunity. Mutants in the TLRs are more susceptible to infection, and the PAMPs recognized by TLRs have been well-characterized. Viral nucleic acids are recognized via endolysosomal TLRs (TLRs 3,7,8,9) while viral glycoproteins can be recognized by TLRs present on the cell surface (e.g. TLR4) (Akira et al., 2006; Kawai and Akira, 2006). Unlike the indirect recognition of microbes by Toll, the mammalian TLRs generally bind microbial PAMPs directly to activate innate immune effectors (Jin and Lee, 2008).
One such effector pathway is autophagy, which can be induced by TLR signaling, although its in vivo significance is unknown (Delgado et al., 2009; Xu and Eissa, 2010). Autophagy is an ancient and conserved pathway that degrades intracellular components and can restrict a variety of intracellular pathogens, including viruses (Deretic and Levine, 2009; Lee et al., 2007; Levine et al., 2011; McPhee and Baehrecke, 2009). In Drosophila, autophagy is triggered upon recognition of the Vesicular Stomatitis virus (VSV) glycoprotein, VSV-G, and this pathway is essential for antiviral defense in adult flies (Shelly et al., 2009). The response can be activated by viral recognition independently of viral replication, and thus we hypothesized that VSV might be recognized by a Drosophila PRR controlling antiviral autophagy. As the TLRs are known PRRs and VSV-G was previously shown to induce TLR4 signaling in mammalian cells (Georgel et al., 2007), we reasoned that one of the nine Drosophila Tolls could be the PRR linking viral recognition to this innate immune response. By screening mutants in the nine Drosophila Tolls both in cells and adult flies, we found that VSV was recognized by Toll-7, which restricted viral replication and thereby protected flies from an otherwise lethal infection. Toll-7 interacted with VSV virions at the plasma membrane, and this recognition was required for the induction of antiviral autophagy. Together, these data demonstrate that pathogen recognition by Drosophila Tolls may be more similar than previously assumed to the mammalian systems and that there may be unknown roles for the additional Tolls in antiviral defense.
RESULTS
Toll-7 restricts VSV infection in cultured cells
To determine whether any of the Drosophila Tolls are involved in antiviral defense against VSV, we generated double-stranded RNA (dsRNA) against each of the nine Toll receptors and depleted them in Drosophila S2 cells using RNA interference (RNAi). Efficient silencing for each Toll receptor was confirmed by reverse transcriptase-polymerase chain reaction (RT-PCR) (Supplementary Figure 1). Next, we challenged RNAi-treated cells with VSV-GFP and subsequently analyzed the infection using fluorescence microscopy and automated image analysis. We observed an increase in the percentage of infected cells upon silencing of Toll-7 and Toll-2 but not other Tolls (Figure 1A, B). This increase was similar to that observed upon silencing of Atg8, an essential autophagy protein. Immunoblot analysis further confirmed that there was an elevation in the amount of GFP production in cells depleted of Toll-7 or Toll-2 but not other Toll receptors (Figure 1C, not shown). Interestingly, Toll-7 and Toll-2 are highly similar, showing 61% identity and 74% similarity, and are located in close chromosomal proximity (250kb apart). Taken together, our data suggest that Toll-7 and Toll-2 might represent a gene duplication and play a similar antiviral role in vivo (Yagi et al., 2010).
Figure 1. Drosophila Toll-7 and Toll-2 are antiviral in cells.
A. Drosophila cells pretreated with dsRNAs against the indicated genes were infected with VSV (MOI=0.1) for 20 hr and processed for immunofluorescence. Infected cells express GFP, and the percent infection is calculated with automated image analysis (MetaXpress) from three wells, with three sites per well (virus, green; nuclei, blue). B. Percent infection for three experiments is shown; Mean±SE, *p<0.01, Student’s t-test. C. Cells pretreated with the indicated dsRNAs were infected (MOI=0.1) and processed for immunoblot 20 hr.p.i; a representative experiment of three is depicted.
Toll-7 is essential for antiviral defense in adult flies
As Drosophila Toll-7 and Toll-2 were antiviral in vitro, we next investigated whether these receptors or any of the other Tolls play similar innate antiviral roles in the adult organism. Using in vivo RNAi, we screened these genes to determine whether loss of any of these factors had an effect on VSV replication. Toll receptor-depleted flies were generated by driving expression of transgenes bearing long hairpin double-stranded RNA constructs targeting each Toll gene. For Toll (Tl) and Toll-4 through Toll-9, we crossed control and transgenic flies to a strong ubiquitous driver, Actin-GAL4, to constitutively express the transgene. Because the Toll-2 (18w) and Toll-3 (Mstprox) transgenes were lethal when driven ubiquitously during development, we crossed them to Heat-shock-GAL4 to allow for inducible transgene expression. Once again, silencing of each Toll was confirmed, although we were unable to detect Toll-3 and Toll-4 expression (Supplementary Figure 2A). Silenced flies along with their sibling controls were challenged with VSV and monitored for changes in viral infection at day 6 post infection. Only the loss of Toll-7 had a significant effect on VSV infection and led to an increase in viral RNA production (Figure 2A). Furthermore, increased viral replication upon Toll-7 depletion was also observed at day 9 post infection (Figure 2B). To validate the Toll-7 phenotype, we challenged a second independent transgenic RNAi line and similarly found that silencing of Toll-7 resulted in increased VSV replication as measured by Northern blot at day 6, as well as at later time points (Supplementary Figure 2B, C). Finally, adult flies expressing heat shock-driven Toll-7 dsRNA exhibited increased viral replication, suggesting that the susceptibility of Toll-7 depleted flies to VSV infection is not due to developmental defects (Supplementary Figure 2D).
Figure 2. Toll-7 is antiviral in adult flies.
A. Adult flies expressing dsRNA against the indicated Toll receptors and their sibling controls were challenged with VSV and monitored for viral replication at 6 d.p.i. by RNA blot and quantified relative to a cellular control mRNA (Actin). Fold change of the Mean±SD compared to sibling controls for three experiments is shown; *p<0.05, Student’s t-test. B. Toll-7 depleted flies (Actin-Gal4> UAS-Toll-7 IR) or sibling controls (+> UAS-Toll-7 IR) were challenged with VSV and viral RNA was monitored by RNA blot at the indicated time points p.i. A representative blot is depicted; results were repeated in at least three experiments. C. Adult flies expressing dsRNA against Toll-7 (Actin-Gal4> UAS-Toll-7 IR) or sibling controls (+> UAS-Toll-7 IR) were challenged with vehicle (PBS) or VSV, and morbidity was monitored as a function of time after infection. Mean±SE is shown for three experiments (Log-rank test, p<0.02). D. Toll-7 mutant (Df(2R)BSC22/Toll-7g1–5) or control flies (+/Toll-7g1–5) were infected with VSV and viral replication was monitored by Northern blot. E. Average fold change of viral RNA in Toll-7 mutants compared to controls normalized to RpS6 expression at 6 d.p.i; *p<0.05, Student’s t-test. F. Survival of VSV-challenged Toll-7 mutant (Df(2R)BSC22/Toll-7g1–5) or sibling control flies (Df(2R)BSC22/+ and +/Toll-7g1–5) for three experiments (Mean±SE; Log-rank test, p<0.001).
Because RNAi-mediated silencing is incomplete and Toll-2 was antiviral in cell culture (Figure 1), we tested whether previously characterized Toll-2 mutant flies (18wΔ7–35/Df(2R)017) were more susceptible to VSV infection (Ligoxygakis et al., 2002). In contrast to our in vitro results, Toll-2 was dispensable for defense against VSV in adult flies (Supplementary Figure 2E). Taken together, these data suggest that Toll-7 but not Toll-2 is an essential component of the antiviral arsenal in both cells and adult flies.
Next, we evaluated whether Toll-7 depletion alters the susceptibility of flies to VSV infection. Depletion of Toll-7 had no effect on the lifespan of adult flies (Figure 2C). We challenged control (+> UAS-Toll-7 IR) or Toll-7 depleted flies (Actin-GAL4> UAS-Toll-7 IR) with VSV and found that while the control flies were viable, the Toll-7 depleted flies succumbed to infection (Figure 2C). Thus, Toll-7 depletion in adult flies promotes increased viral replication, leading to mortality from an otherwise non-lethal infection.
Although silenced flies exhibited decreased Toll-7 mRNA expression, RNAi carries potential caveats such as driver overexpression and off-target silencing. To address these concerns, we obtained a recently reported Toll-7 mutant fly line harboring a deletion in the Toll-7 coding region (Toll-7g1–5) (Yagi et al., 2010). These flies were crossed to a deficiency strain to generate flies lacking Toll-7 expression, and we confirmed the deletion at the DNA level by genotyping and the RNA level by RT-PCR (Supplementary Figure 2F–H). Toll-7 mutants and control flies were infected with VSV, and consistent with the in vivo RNAi results, the Toll-7 mutants demonstrated significantly elevated viral replication (Figure 2D, E; Supplementary Figure 2I). This increased viral RNA load correlated with decreased survival of the Toll-7 mutants after infection (Figure 2F). Collectively, these data further verify Toll-7 as a critical antiviral factor against VSV in vivo.
VSV infection induces Toll-7 expression but not other canonical signaling pathways
Drosophila has evolved multiple pathways to defend against invading pathogens, among which are the Toll, IMD and Jak-Stat pathways (Lemaitre and Hoffmann, 2007; Sabin et al., 2010). Each of these pathways responds to different invading pathogens and ultimately leads to the induction of specific antimicrobial peptides (AMPs) (Lemaitre and Hoffmann, 2007). Since all the Drosophila Tolls have a conserved Toll and Interleukin-1 receptor (TIR) domain (Imler and Zheng, 2004), we explored whether Toll-7 signals via the canonical Toll signaling pathway. The Toll-dependent AMP gene Drosomycin is potently activated after fungal infection, but it was only modestly induced by VSV infection in cultured cells (~2-fold, Figure 3A). To examine whether this induction reflects a requirement for the Toll signaling pathway in restricting VSV infection in vivo, we challenged flies mutant for canonical pathway components including the TIR adapter MyD88 and NF-kB member Dif, which are both essential for fungal and Gram-positive bacterial immunity in adult flies (Bilak et al., 2003; Ip et al., 1993; Tauszig-Delamasure et al., 2002). Loss of these critical Toll pathway components had no impact on VSV replication in vivo, suggesting that Toll-7 signals through a distinct pathway (Figure 3B).
Figure 3. Toll-7, but not the canonical innate immune signaling pathways, is transcriptionally induced by VSV infection.
A. Drosophila cells were either uninfected or infected with VSV for four hours and processed for RT-qPCR of the Toll pathway AMP Drosomycin, the Imd pathway AMP Diptericin and the Jak-Stat pathway readout vir-1. These were normalized to a control gene (Rp49) and fold change of Mean±SD for three experiments is shown; *p<0.01, Student’s t-test. B. Adult flies either heterozygous or homozygous mutant for the Toll pathway components MyD88 and the NF-kB transcription factor Dif were challenged with VSV and viral replication was monitored by RNA blot at 6 d.p.i. and quantified relative to a cellular control (Actin). Fold change compared to heterozygous control of the Mean±SD for three experiments is shown. C. Expression of the Drosophila Tolls from VSV-infected cells 4 hr.p.i. compared to uninfected cells was analyzed by RT-qPCR; Mean±SD, *p<0.01, Student’s t-test.
The IMD pathway is also activated by a PRR and converges on alternative NF-kB transcription factors that induce a different spectrum of AMPs including Diptericin (Lemaitre and Hoffmann, 2007). We also explored this pathway to see if Toll-7 might be signaling through downstream members and found that VSV infection did not affect Diptericin expression in cell culture (Figure 3A).
Lastly, we examined the Jak-Stat signaling pathway, which has been shown to play antiviral roles both in flies and mammals (Dostert et al., 2005; Garcia-Sastre and Biron, 2006). Upon VSV infection of cells, we found that expression of vir-1, a virus-specific Stat-dependent gene in Drosophila, was unperturbed (Figure 3B). These data suggest that Toll-7 mediates its antiviral effects through a signaling cascade distinct from the canonical Toll, IMD or Jak-Stat pathways.
As many genes with roles in immunity are regulated by infection, we examined the expression of Toll-7 (and the other Toll receptors) after VSV infection. Cells were challenged with VSV, and Toll-7 along with Toll, Toll-2, Toll-4 and Toll-8 were transcriptionally induced, indicating a potential role for these genes in immunity (Figure 3C).
Toll-7 is a surface receptor that interacts with VSV
TLRs can reside either at the plasma membrane or within endosomal compartments where they interact directly or indirectly with pathogens. Therefore, we characterized the subcellular localization of Toll-7. For these studies we generated an antibody that recognizes endogenous Toll-7 and found that RNAi against Toll-7 efficiently depleted the protein in both cells and flies (Figure 4A). Toll-7 protein was also undetectable in the Toll-7 mutant flies (Figure 4A), and transgenic flies expressing Toll-7 under control of Heat-shock-GAL4 exhibited increased Toll-7 protein, further validating the antibody’s activity (Supplementary Figure 3). To test whether Toll-7 is a plasma membrane resident protein, we surface biotinylated Drosophila cells with a cell impermeable form of biotin and precipitated the biotinylated proteins with avidin. Similar to the known surface resident protein Toll, Toll-7 was precipitated by avidin while tubulin, an intracellular protein, was not found in the precipitate (Figure 4B).
Figure 4. Toll-7 is a membrane-bound receptor that interacts with VSV.
A. Immunoblot of Drosophila cells treated with control or Toll-7 dsRNA (left), adult flies expressing dsRNA against Toll-7 (Actin-Gal4> UAS-Toll-7 IR) or sibling controls (+> UAS-Toll-7 IR) (center) and Toll-7 mutant (Df(2R)BSC22/Toll-7g1–5) or control flies (+/Toll-7g1–5) (right) probed for Toll-7 and control (tubulin). A representative experiment is shown; similar findings were made in at least three experiments. B. Cells at 4°C were left untreated or biotinylated for one hour, and lysates were probed with the indicated antibodies (input, left; precipitate, right). A representative experiment is shown; similar findings were made in at least three experiments. C. Drosophila cells treated with the indicated dsRNA were left untreated or incubated with biotinylated IgG or biotinylated VSV for one hour at 4°C. Lysates were precipitated with streptavidin beads and immunoblotted for Toll-7 or VSV-G. Coomassie staining is shown as a loading control. A representative experiment is shown; similar findings were made in at least three experiments. D. Cells were left untreated or incubated with biotinylated VSV for one hour at 4°C. Lysates were precipitated with streptavidin beads and immunoblotted with the indicated antibodies. A representative experiment is shown; similar findings were made in at least three experiments.
In general, mammalian TLRs bind directly to their PAMPs, while recognition by Drosophila Toll is indirect. Toll is instead activated by the cytokine spätzle, which is the product of a proteolytic cascade induced upon upstream recognition of bacterial and fungal PAMPs (Akira et al., 2006; Ferrandon et al., 2004; Lemaitre et al., 1996). Therefore, we tested whether VSV interacted with Toll-7 at the cell surface. Cells were pre-bound with purified biotinylated infectious VSV at 4°C to allow for surface binding. After one hour, unbound virus was removed and cell lysates were applied to avidin beads. Precipitation of proteins bound to VSV revealed that VSV-G was efficiently precipitated, as we were unable to detect the low amount in the input (Figure 4C). We found that VSV interacted with endogenous Toll-7 at the plasma membrane, and that this interaction was lost upon RNAi depletion of Toll-7 (Figure 4C). Moreover, the interaction between Toll-7 and VSV was specific, as Toll-7 did not bind biotinylated IgG (Figure 4C, D). Lastly, while Toll-7 precipitated with VSV, the plasma membrane protein Toll and the intracellular protein tubulin did not precipitate, suggesting that Toll-7 is a specific and bona fide PRR for VSV (Figure 4D).
VSV-induced autophagy is dependent on Toll-7 in cultured cells
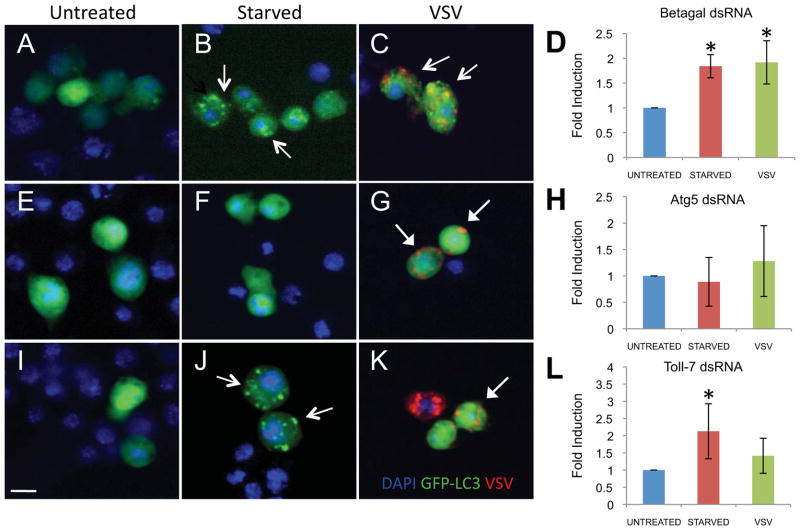
Since both Toll-7 and autophagy show similar antiviral activity against VSV, we tested whether Toll-7 is the PRR upstream of autophagy. In order to examine autophagy, we implemented a commonly used assay dependent upon the change in localization of an expressed GFP-tagged Light Chain 3 (GFP-LC3) in Drosophila cells (Juhasz and Neufeld, 2008; McPhee et al., 2010; Rusten et al., 2004; Shelly et al., 2009). Under normal conditions, LC3 shows diffuse cytoplasmic staining; however, it is translocated to autophagosomes when autophagy is induced, appearing as bright puncta within the cell (Klionsky et al., 2008; Mizushima et al., 2010). Upon VSV infection or starvation, we observed a significant increase in the number of LC3 puncta per cell compared to control cells (Figure 5A–C; quantified in D and Supplementary Figure 4). This induction was dependent on canonical autophagy proteins, as depletion of Atg5, a core component of this pathway, blocked the puncta formation induced by either VSV infection or starvation (Figure 5E–G; quantified in H and Supplementary Figure 4). In contrast, upon silencing of Toll-7, VSV-induced puncta were lost while starvation-induced puncta were unaffected (Figure 5I–K; quantified in L and Supplementary Figure 4). Taken together, these results indicate that Toll-7 is specifically required for antiviral autophagy but dispensable for starvation-induced autophagy.
Figure 5. Toll-7 is required for antiviral autophagy in cell culture.
A–L. Cells transfected with a GFP-LC3 reporter (green) were treated with dsRNA against a negative control (Betagal), canonical autophagy component Atg5 or Toll-7. Cells were left uninfected (A, E, I), starved (B, F, J), or infected with VSV for 22 hr (MOI=10) (C, G, K). Representative images are shown (nuclei, blue; GFP-LC3, green; VSV-G, red). Open arrows indicate GFP-LC3+ puncta while closed arrows indicate VSV+ cells devoid of GFP-LC3 puncta. Scale bar: 10μm. D, H, L. Quantification of the fold change in puncta per cell for triplicate experiments; Mean±SD is shown; *p<0.02, Student’s t-test.
Toll-7 mediates the antiviral autophagy response in adult flies
Next, we evaluated whether Toll-7 is required for VSV-induced autophagy in vivo. To examine autophagy in adult flies, we used a well-characterized assay that takes advantage of Lysotracker, a marker of acidified compartments, to observe the induction of late-stage autophagosomes in the fat body, which lacks an acidic pH under normal conditions (Arsham and Neufeld, 2009; Bilen and Bonini, 2007; Chen et al., 2008; McPhee et al., 2010; Rusten et al., 2004; Shelly et al., 2009). Toll-7 silenced flies or sibling controls were infected with VSV-GFP and dissected three days after infection, at which time the fat body was removed and stained with Lysotracker. While control flies showed significant Lysotracker staining in VSV-infected fat body cells, Toll-7 depleted flies exhibited minimal Lysotracker staining despite extensive viral infection, as monitored by GFP expression (Figure 6A, quantified in B). Uninfected Toll-7 silenced flies or sibling controls had little Lysotracker staining of fat body cells (data not shown).
Figure 6. Toll-7 is required for antiviral autophagy in adult flies.
A. Control flies (+> UAS-Toll-7 IR) or Toll-7 depleted flies (Actin-Gal4> UAS-Toll-7 IR) were challenged with VSV-GFP for 3 days. The flies were monitored for infection (GFP+) and autophagy (Lysotracker+). Representative images of fat body demonstrate that autophagy is induced in infected wild type cells but not in the infected Toll-7 depleted cells. Scale bar: 100μm. B. The percentage of virally infected cells (GFP+) with puncta (Lysotracker+) was quantified. Mean±SD shown for three experiments; *p<0.0001, Student’s t-test. C. Immunoblot of control flies (+> UAS-Toll-7IR) or Toll-7 depleted flies (Actin-Gal4> UAS-Toll-7 IR) challenged with VSV for 2 days. Autophagy was monitored by size shift of Atg8 (Atg8-II accumulation) and samples were normalized to the control protein tubulin. These data show representative experiments; similar findings were made in at least three experiments. D. Immunoblot of Atg8 expression from VSV-challenged Toll-7 mutant (Df(2R)BSC22/Toll-7g1–5) or control flies (+/Toll-7g1–5) day 3 post infection. A representative image of three experiments is presented.
To further verify that Toll-7 is required for the induction of autophagy downstream of VSV infection in adult flies, we implemented an immunoblot assay. During autophagy, cytosolic LC3 (LC3-I or Atg8-I) is conjugated with phosphatidylethanolamine, forming a lipidated form of LC3 (LC3-II or Atg8-II) that decorates the autophagic membrane and results in a size shift by immunoblot (Shelly et al., 2009). Control flies exhibited a strong induction of autophagy after VSV infection as monitored by increased Atg-II amounts; however, VSV-activated autophagy was severely abrogated in Toll-7 depleted flies (Figure 6C). Consistent with these results, Toll-7 mutant flies demonstrated a reduction in Atg8-II production after VSV challenge compared to the controls (Figure 6D). Autophagy was induced independently of canonical Toll signaling as Myd88 mutant flies showed substantial Atg8-II accumulation after VSV infection (Supplementary Figure 5). Together, our results confirm that Toll-7 is required for VSV-induced antiviral autophagy both in vitro and in vivo.
DISCUSSION
The essential role for Drosophila Toll in antimicrobial defense is firmly established; however, whether other Toll receptors serve important immune functions has been poorly understood. We have identified a role for a second Drosophila Toll receptor, Toll-7, in antiviral defense both in cells and animals. Toll-7 depleted cells exhibited increased VSV infectivity, and Toll-7 deficient flies demonstrated significantly elevated viral replication and mortality after VSV challenge. Furthermore, Toll-7 acted as a PRR by interacting with VSV at the plasma membrane to induce an effector program that converged on antiviral autophagy. The function of Toll-7 appears to be specific to antiviral immunity, as Toll-7 deficient flies mount appropriate AMP responses to septic injury (Yagi et al., 2010).
Multiple innate immune pathways in Drosophila rely on the activation of the transcription factor NF-kB; however, the Toll-7 dependent autophagy response is likely elicited via an NF-kB-independent mechanism. Unlike Toll-7 deficient flies, flies lacking core Toll pathway components did not demonstrate increased susceptibility to VSV. Moreover, the IMD pathway was not activated by viral infection. In agreement with these data, MyD88 was also not required for the induction of antiviral autophagy. This NF-kB independence is consistent with previous studies that found that the NFkB-dependent AMPs Diptericin and Drosomycin are not induced in Drosophila cells when stimulated with a hyperactive form of Toll-7 (Tauszig et al., 2000) and that Toll-7 is dispensable for immunity to NF-kB-dependent bacterial challenges (Yagi et al., 2010). Hence, while Toll-7 likely activates non-canonical signaling pathways, the exact pathways downstream of Toll-7 remain to be determined.
Recent studies in mammals found that TLR activation can lead to the induction of autophagy in a variety of cultured cells (Delgado et al., 2008; Sanjuan et al., 2007; Shin et al., 2010; Xu et al., 2007). However, the mechanism by which TLR stimulation converges on autophagy is unclear. Moreover, the dependence on specific signaling molecules is controversial and whether TLR-induced autophagy is important in restricting infection in vivo is unknown (Delgado et al., 2009; Xu and Eissa, 2010). Our data, together with the findings that Listeria recognition via a peptidoglycan recognition protein induces autophagy (Yano et al., 2008), suggest that multiple classes of PRRs are involved in the induction of antimicrobial autophagy, which plays an important role in the control of a diverse set of pathogens.
While the discovery of Toll as an innate immune receptor led to the identification of TLRs as a large family of PRRs, studies demonstrating a role for the additional eight Toll receptors in immunity have lagged behind. This discrepancy may be in part due to the lack of studies probing the role of the additional eight Toll receptors in antiviral defense. Perhaps the lack of classical cytoplasmic sensors (RIG-I and MDA5) has required Drosophila to be more heavily dependent on the Tolls for viral recognition, opening up the possibility that additional Drosophila Toll receptors play roles in antiviral immunity. This hypothesis is further supported by our finding that a number of uncharacterized Tolls are induced by viral infection similar to the two major antiviral TLRs, TLR3 and TLR7, which are transcriptionally induced by viral infection in mammalian systems (Siren et al., 2005; Takeda et al., 2003). Importantly, Toll-7 is conserved in vector mosquitoes, suggesting that Toll-7 and other Toll receptors may be involved in the recognition and restriction of human arboviruses (Waterhouse et al., 2007).
TLRs are generally thought to directly bind their PAMPS, whereas Drosophila Toll functions indirectly by recognizing a host cytokine. Our findings that Toll-7 interacts with VSV virions suggest that Toll-7 might act directly as a pattern recognition receptor more similar to mammalian TLRs, a previously unknown mechanism for an insect Toll receptor. Although VSV is an arbovirus, the natural vectors have been proposed to be biting insects such as sand flies and blackflies (Comer et al., 1990; Mead et al., 2004); nevertheless, for several reasons we believe that VSV is a bona fide ligand for Drosophila Toll-7. First, Toll-7 is highly conserved between insect species that have been sequenced (66% identity and 77% homology to Aedes aegypti Toll-7), indeed, more so than many other Toll receptors. Second, while nucleic acids have been well-characterized as viral PAMPs, emerging evidence suggests that viral proteins including glycoproteins can also activate TLRs (Barbalat et al., 2009; Barton, 2007). Importantly, there are several examples of murine TLRs that recognize PAMPs from viruses that naturally do not infect mice. Humans are the natural host of measles virus, yet the viral hemagglutinin still activates mouse TLR2 (Bieback et al., 2002). Likewise, Tlr2−/− murine macrophages have reduced cytokine responses to hepatitis C virus core and NS3, as well as to human cytomegalovirus, despite the fact that both viruses are human viruses (Chang et al., 2007; Compton et al., 2003). Moreover, in mouse macrophages and myeloid dendritic cells, VSV-G activates an antiviral response dependent on TLR4, even though VSV does not normally infect mice in the wild (Georgel et al., 2007). These results are consistent with the idea that PAMPs are molecular signatures often conserved across wide groups of pathogens and not necessarily restricted to a single microbe. It is therefore not unexpected that TLRs (as well as Tolls) can recognize these structures even if they have not yet encountered that particular pathogen. Third, while the Rhabdovirus VSV does not normally infect fruit flies, the closely related Rhabdovirus sigma virus is a natural Drosophila pathogen (Fleuriet, 1988). The Drosophila sigmaviruses phylogenetically cluster more closely to the vesiculoviruses than other groups of Rhabdoviruses (Longdon et al., 2010). Furthermore, while autophagy has not formally been shown to restrict sigma virus, flies deficient in Drosophila p62 (ref(2)p), which serves as an autophagy cargo receptor implicated in the clearance of Sindbis virus capsids and other pathogens, are more susceptible to infection (Contamine et al., 1989; Dru et al., 1993; Orvedahl et al., 2010). Given the relatedness of sigma virus to VSV, we posit that the Toll-7 ligand on VSV may be similar to that of a natural Drosophila pathogen.
Intriguingly, the interaction between Toll-7 and VSV suggests that other Toll receptors may recognize presently undefined ligands, including pathogen-derived molecules. Taken together with studies on Toll in microbial defense, our data suggest that Toll receptors likely evolved to recognize foreign microbes and elicit antimicrobial effector mechanisms, therefore uncovering an evolutionarily conserved intrinsic antiviral program that links pathogen recognition to autophagy, which may be amenable to therapeutic intervention.
METHODS
Cells and viruses
Drosophila S2 cells and BHK cells were grown and maintained as described (Shelly et al., 2009). VSV or VSV-eGFP was grown as described (Ramsburg et al., 2005).
RNAi and infections
dsRNAs for RNAi were generated and used for RNAi as described (Cherry et al., 2005). Amplicons used are described at http://flyrnai.org. Three days after dsRNA bathing, cells were infected with the indicated viral innoculum and assayed at the indicated time point post infection.
Immunofluorescence
Cells were processed for immunofluorescence as previously described (Shelly et al., 2009) and imaged using an automated microscope (ImageXpress Micro). Three wells per treatment with three sites per well were collected and quantified (MetaXpress). S2* cells were transfected with pMT-Gal4 and UAS-GFP-LC3 and infected with VSV as previously described (Shelly et al., 2009). Greater than 150 cells per treatment were counted for three independent experiments.
Immunoblotting, Northern blots, qPCR and Titers
Cells or flies were collected at the indicated time points and lysed in radioimmunoprecipitation assay (RIPA) buffer supplemented with a protease inhibitor cocktail (Boehringer) and blotted as previously described for immunoblots (Shelly et al., 2009). Cells or purified virus were biotinylated using Sulfo-NHS-LC-Biotin following the manufacturer’s protocol at 4°C (Thermo). For immunoprecipitations, samples were lysed in lysis buffer (20mM Tris at pH 7.6, 150 mM NaCl, 2mM EDTA, 10% glycerol, 1% Triton X-100, 1mM DTT, and protease inhibitors) (Aggarwal et al., 2008). Protein lysates were precipitated with streptavidin-agarose and immunoblotted. For Northern blot, total RNA was purified by Trizol and analyzed as previously described (Shelly et al., 2009). qPCR was performed on DNAse-treated total RNA that had been reverse transcribed using random primers.
Adult infections
4–7 day old adults of the stated genotypes were inoculated with vehicle or VSV-GFP as previously described (Shelly et al., 2009). Flies were processed at the indicated time point post infection. For autophagy studies, flies were dissected in complete Schneider’s media with Lysotracker red (Invitrogen), incubated for 10 min, rinsed in media and mounted live for imaging (Leica) (Shelly et al., 2009).
Supplementary Material
Highlights.
Toll-7 is required for antiviral defense in cells and animals
Toll-7 interacts with VSV at the plasma membrane
Toll-7 is required for autophagy in response to VSV
Toll-7 along with additional Tolls is transcriptionally induced by VSV infection
Acknowledgments
We thank S. Ross, R. Doms and M. Tudor for critical reading of the manuscript; the VDRC for Toll snapback transgenic flies (Dietzl et al., 2007); the TRiP at Harvard Medical School (NIH/NIGMS R01-GM084947) for providing transgenic RNAi fly stocks; Y. Yagi for Toll-7 mutants; The Bloomington Stock center for other fly stocks; J. Rose for VSV-GFP; R. Doms for anti-VSV-G (I1) antibody. This work was supported NIH grants T32AI007324 to RHM, and R01AI074951 and U54AI057168 to SC. SC is a recipient of the Burroughs Wellcome Investigators in the Pathogenesis of Infectious Disease Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal K, Rus F, Vriesema-Magnuson C, Erturk-Hasdemir D, Paquette N, Silverman N. Rudra interrupts receptor signaling complexes to negatively regulate the IMD pathway. PLoS Pathog. 2008;4:e1000120. doi: 10.1371/journal.ppat.1000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Arsham AM, Neufeld TP. A genetic screen in Drosophila reveals novel cytoprotective functions of the autophagy-lysosome pathway. PLoS One. 2009;4:e6068. doi: 10.1371/journal.pone.0006068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbalat R, Lau L, Locksley RM, Barton GM. Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nature immunology. 2009;10:1200–1207. doi: 10.1038/ni.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton GM. Viral recognition by Toll-like receptors. Seminars in immunology. 2007;19:33–40. doi: 10.1016/j.smim.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Bieback K, Lien E, Klagge IM, Avota E, Schneider-Schaulies J, Duprex WP, Wagner H, Kirschning CJ, Ter Meulen V, Schneider-Schaulies S. Hemagglutinin protein of wild-type measles virus activates toll-like receptor 2 signaling. Journal of virology. 2002;76:8729–8736. doi: 10.1128/JVI.76.17.8729-8736.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilak H, Tauszig-Delamasure S, Imler JL. Toll and Toll-like receptors in Drosophila. Biochem Soc Trans. 2003;31:648–651. doi: 10.1042/bst0310648. [DOI] [PubMed] [Google Scholar]
- Bilen J, Bonini NM. Genome-wide screen for modifiers of ataxin-3 neurodegeneration in Drosophila. PLoS Genet. 2007;3:1950–1964. doi: 10.1371/journal.pgen.0030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerenius L, Kawabata S, Lee BL, Nonaka M, Soderhall K. Proteolytic cascades and their involvement in invertebrate immunity. Trends Biochem Sci. 2010;35:575–583. doi: 10.1016/j.tibs.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Chang S, Dolganiuc A, Szabo G. Toll-like receptors 1 and 6 are involved in TLR2-mediated macrophage activation by hepatitis C virus core and NS3 proteins. Journal of leukocyte biology. 2007;82:479–487. doi: 10.1189/jlb.0207128. [DOI] [PubMed] [Google Scholar]
- Chen GC, Lee JY, Tang HW, Debnath J, Thomas SM, Settleman J. Genetic interactions between Drosophila melanogaster Atg1 and paxillin reveal a role for paxillin in autophagosome formation. Autophagy. 2008;4:37–45. doi: 10.4161/auto.5141. [DOI] [PubMed] [Google Scholar]
- Cherry S, Doukas T, Armknecht S, Whelan S, Wang H, Sarnow P, Perrimon N. Genome-wide RNAi screen reveals a specific sensitivity of IRES-containing RNA viruses to host translation inhibition. Genes Dev. 2005;19:445–452. doi: 10.1101/gad.1267905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer JA, Tesh RB, Modi GB, Corn JL, Nettles VF. Vesicular stomatitis virus, New Jersey serotype: replication in and transmission by Lutzomyia shannoni (Diptera: Psychodidae) Am J Trop Med Hyg. 1990;42:483–490. doi: 10.4269/ajtmh.1990.42.483. [DOI] [PubMed] [Google Scholar]
- Compton T, Kurt-Jones EA, Boehme KW, Belko J, Latz E, Golenbock DT, Finberg RW. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. Journal of virology. 2003;77:4588–4596. doi: 10.1128/JVI.77.8.4588-4596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contamine D, Petitjean AM, Ashburner M. Genetic resistance to viral infection: the molecular cloning of a Drosophila gene that restricts infection by the rhabdovirus sigma. Genetics. 1989;123:525–533. doi: 10.1093/genetics/123.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M, Singh S, De Haro S, Master S, Ponpuak M, Dinkins C, Ornatowski W, Vergne I, Deretic V. Autophagy and pattern recognition receptors in innate immunity. Immunol Rev. 2009;227:189–202. doi: 10.1111/j.1600-065X.2008.00725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MA, Elmaoued RA, Davis AS, Kyei G, Deretic V. Toll-like receptors control autophagy. EMBO J. 2008;27:1110–1121. doi: 10.1038/emboj.2008.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V, Levine B. Autophagy, immunity, and microbial adaptations. Cell Host Microbe. 2009;5:527–549. doi: 10.1016/j.chom.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- Dostert C, Jouanguy E, Irving P, Troxler L, Galiana-Arnoux D, Hetru C, Hoffmann JA, Imler JL. The Jak-STAT signaling pathway is required but not sufficient for the antiviral response of drosophila. Nat Immunol. 2005;6:946–953. doi: 10.1038/ni1237. [DOI] [PubMed] [Google Scholar]
- Dru P, Bras F, Dezelee S, Gay P, Petitjean AM, Pierre-Deneubourg A, Teninges D, Contamine D. Unusual variability of the Drosophila melanogaster ref(2)P protein which controls the multiplication of sigma rhabdovirus. Genetics. 1993;133:943–954. doi: 10.1093/genetics/133.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandon D, Imler JL, Hoffmann JA. Sensing infection in Drosophila: Toll and beyond. Semin Immunol. 2004;16:43–53. doi: 10.1016/j.smim.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Fleuriet A. Maintenance of a hereditary virus, the sigma virus, in populations of its host, D. melanogaster. In: HMK, editor. Evolutionary Biology. New York: Plenum Press; 1988. [Google Scholar]
- Garcia-Sastre A, Biron CA. Type 1 interferons and the virus-host relationship: a lesson in detente. Science. 2006;312:879–882. doi: 10.1126/science.1125676. [DOI] [PubMed] [Google Scholar]
- Georgel P, Jiang Z, Kunz S, Janssen E, Mols J, Hoebe K, Bahram S, Oldstone MB, Beutler B. Vesicular stomatitis virus glycoprotein G activates a specific antiviral Toll-like receptor 4-dependent pathway. Virology. 2007;362:304–313. doi: 10.1016/j.virol.2006.12.032. [DOI] [PubMed] [Google Scholar]
- Imler JL, Zheng L. Biology of Toll receptors: lessons from insects and mammals. J Leukoc Biol. 2004;75:18–26. doi: 10.1189/jlb.0403160. [DOI] [PubMed] [Google Scholar]
- Ip YT, Reach M, Engstrom Y, Kadalayil L, Cai H, Gonzalez-Crespo S, Tatei K, Levine M. Dif, a dorsal-related gene that mediates an immune response in Drosophila. Cell. 1993;75:753–763. doi: 10.1016/0092-8674(93)90495-c. [DOI] [PubMed] [Google Scholar]
- Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- Jin MS, Lee JO. Structures of the toll-like receptor family and its ligand complexes. Immunity. 2008;29:182–191. doi: 10.1016/j.immuni.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Juhasz G, Neufeld TP. Experimental control and characterization of autophagy in Drosophila. Methods Mol Biol. 2008;445:125–133. doi: 10.1007/978-1-59745-157-4_8. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA, Ballabio A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science. 2007;315:1398–1401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligoxygakis P, Bulet P, Reichhart JM. Critical evaluation of the role of the Toll-like receptor 18-Wheeler in the host defense of Drosophila. EMBO Rep. 2002;3:666–673. doi: 10.1093/embo-reports/kvf130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longdon B, Obbard DJ, Jiggins FM. Sigma viruses from three species of Drosophila form a major new clade in the rhabdovirus phylogeny. Proc Biol Sci. 2010;277:35–44. doi: 10.1098/rspb.2009.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C, Shen B, Manley JL, Zheng L. Tehao functions in the Toll pathway in Drosophila melanogaster: possible roles in development and innate immunity. Insect Mol Biol. 2001;10:457–464. doi: 10.1046/j.0962-1075.2001.00284.x. [DOI] [PubMed] [Google Scholar]
- McPhee CK, Baehrecke EH. Autophagy in Drosophila melanogaster. Biochim Biophys Acta. 2009;1793:1452–1460. doi: 10.1016/j.bbamcr.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhee CK, Logan MA, Freeman MR, Baehrecke EH. Activation of autophagy during cell death requires the engulfment receptor Draper. Nature. 2010;465:1093–1096. doi: 10.1038/nature09127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead DG, Howerth EW, Murphy MD, Gray EW, Noblet R, Stallknecht DE. Black fly involvement in the epidemic transmission of vesicular stomatitis New Jersey virus (Rhabdoviridae: Vesiculovirus) Vector Borne Zoonotic Dis. 2004;4:351–359. doi: 10.1089/vbz.2004.4.351. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narbonne-Reveau K, Charroux B, Royet J. Lack of an antibacterial response defect in Drosophila Toll-9 mutant. PLoS One. 2011;6:e17470. doi: 10.1371/journal.pone.0017470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi JY, Yagi Y, Hu X, Ip YT. The Drosophila Toll-9 activates a constitutive antimicrobial defense. EMBO Rep. 2002;3:82–87. doi: 10.1093/embo-reports/kvf004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvedahl A, MacPherson S, Sumpter R, Jr, Talloczy Z, Zou Z, Levine B. Autophagy protects against Sindbis virus infection of the central nervous system. Cell host & microbe. 2010;7:115–127. doi: 10.1016/j.chom.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsburg E, Publicover J, Buonocore L, Poholek A, Robek M, Palin A, Rose JK. A vesicular stomatitis virus recombinant expressing granulocyte-macrophage colony-stimulating factor induces enhanced T-cell responses and is highly attenuated for replication in animals. J Virol. 2005;79:15043–15053. doi: 10.1128/JVI.79.24.15043-15053.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusten TE, Lindmo K, Juhasz G, Sass M, Seglen PO, Brech A, Stenmark H. Programmed autophagy in the Drosophila fat body is induced by ecdysone through regulation of the PI3K pathway. Dev Cell. 2004;7:179–192. doi: 10.1016/j.devcel.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Sabin LR, Hanna SL, Cherry S. Innate antiviral immunity in Drosophila. Curr Opin Immunol. 2010;22:4–9. doi: 10.1016/j.coi.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjuan MA, Dillon CP, Tait SW, Moshiach S, Dorsey F, Connell S, Komatsu M, Tanaka K, Cleveland JL, Withoff S, et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253–1257. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- Shelly S, Lukinova N, Bambina S, Berman A, Cherry S. Autophagy is an essential component of Drosophila immunity against vesicular stomatitis virus. Immunity. 2009;30:588–598. doi: 10.1016/j.immuni.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DM, Yuk JM, Lee HM, Lee SH, Son JW, Harding CV, Kim JM, Modlin RL, Jo EK. Mycobacterial lipoprotein activates autophagy via TLR2/1/CD14 and a functional vitamin D receptor signalling. Cell Microbiol. 2010;12:1648–1665. doi: 10.1111/j.1462-5822.2010.01497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siren J, Pirhonen J, Julkunen I, Matikainen S. IFN-alpha regulates TLR-dependent gene expression of IFN-alpha, IFN-beta, IL-28, and IL-29. J Immunol. 2005;174:1932–1937. doi: 10.4049/jimmunol.174.4.1932. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Tauszig-Delamasure S, Bilak H, Capovilla M, Hoffmann JA, Imler JL. Drosophila MyD88 is required for the response to fungal and Gram-positive bacterial infections. Nat Immunol. 2002;3:91–97. doi: 10.1038/ni747. [DOI] [PubMed] [Google Scholar]
- Tauszig S, Jouanguy E, Hoffmann JA, Imler JL. Toll-related receptors and the control of antimicrobial peptide expression in Drosophila. Proc Natl Acad Sci U S A. 2000;97:10520–10525. doi: 10.1073/pnas.180130797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu S, Akira S. Toll-like receptors and innate immunity. J Mol Med. 2006 doi: 10.1007/s00109-006-0084-y. [DOI] [PubMed] [Google Scholar]
- Waterhouse RM, Kriventseva EV, Meister S, Xi Z, Alvarez KS, Bartholomay LC, Barillas-Mury C, Bian G, Blandin S, Christensen BM, et al. Evolutionary dynamics of immune-related genes and pathways in disease-vector mosquitoes. Science. 2007;316:1738–1743. doi: 10.1126/science.1139862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MJ, Rodriguez A, Kimbrell DA, Eldon ED. The 18-wheeler mutation reveals complex antibacterial gene regulation in Drosophila host defense. EMBO J. 1997;16:6120–6130. doi: 10.1093/emboj/16.20.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Eissa NT. Autophagy in innate and adaptive immunity. Proc Am Thorac Soc. 2010;7:22–28. doi: 10.1513/pats.200909-103JS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Jagannath C, Liu XD, Sharafkhaneh A, Kolodziejska KE, Eissa NT. Toll-like receptor 4 is a sensor for autophagy associated with innate immunity. Immunity. 2007;27:135–144. doi: 10.1016/j.immuni.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi Y, Nishida Y, Ip YT. Functional analysis of Toll-related genes in Drosophila. Dev Growth Differ. 2010;52:771–783. doi: 10.1111/j.1440-169X.2010.01213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano T, Mita S, Ohmori H, Oshima Y, Fujimoto Y, Ueda R, Takada H, Goldman WE, Fukase K, Silverman N, et al. Autophagic control of listeria through intracellular innate immune recognition in drosophila. Nat Immunol. 2008;9:908–916. doi: 10.1038/ni.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.