Abstract
Biomineral formation is widespread in Nature and occurs in bacteria, single-celled protists, plants, invertebrates and vertebrates. Minerals formed in the biological environment often show unusual physical properties (e.g., strength, degree of hydration) and often have structures that exhibit order on many length scales. Biosilica, found in single cell organisms through to higher plants and primitive animals (sponges) is formed from an environment that is undersaturated with respect to silicon and under conditions of around neutral pH and low temperature ca. 4–40 °C. Formation of the mineral may occur intra- or extra-cellularly and specific biochemical locations for mineral deposition that include lipids, proteins and carbohydrates are known. In most cases the formation of the mineral phase is linked to cellular processes, understanding of which could lead to the design of new materials for biomedical, optical and other applications.
In this contribution we describe the aqueous chemistry of silica, from uncondensed monomer through to colloidal particles and three dimensional structures, relevant to the environment from which the biomineral forms. We then describe the chemistry of silica formation from alkoxides such as tetraethoxysilane as this and other silanes have been used to study the chemistry of silica formation using silicatein and such precursors are often used in the preparation of silicas for technological applications. The focus of this article is on the methods, experimental and computational by which the process of silica formation can be studied with emphasis on speciation.
Keywords: silica, silicic acid, condensation, speciation
Silicic acid condensation in aqueous media- the fundamentals
The simplest soluble form of silica, orthosilicic acid ‘Si(OH)4’, is a weakly acidic molecule (pKa 9.8) with silicon tetrahedrally coordinated to four hydroxyl groups [1, 2]. It is found universally (sea water, fresh water, soil water) at low concentrations (a few ppm) and it is most likely that it is in this form that all organisms are able take up ‘silicon’. Evidence for transport of the element by biochemical transporters is available for three classes of well studied silicified organisms (diatoms, sponges and higher plants) [3-6]. How the silicic acid reaches its final site of deposition is still largely unknown and remains an active area of study across the globe.
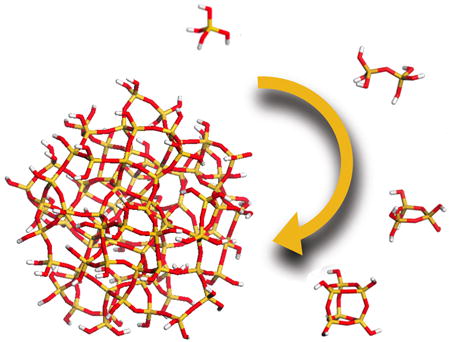
Orthosilicic acid is stable in water at room temperature as long as its concentration remains below the solubility limit of the amorphous phase (typically around 100 ppm, ca. 1 mM). Above this concentration, however, it will undergo autopolycondensation to lower the concentration of orthosilicic acid in solution with each condensation reaction between two orthosilicic acid molecules additionally generating one molecule of water. In addition, even with a pKa of 9.8, a small number of orthosilicic acid molecules will be ionized at neutral pH (ca. 0.18%) and these will very readily react with neutral orthosilicic acid molecules to generate oligomers, Scheme 1. The condensation process is spontaneous with a range of small oligomers being formed initially, Figures 1 and 2. These serve as nuclei for the formation of stable particles that eventually aggregate/ coalesce to form a gel or aggregate network, Figures 1 and 2.
Scheme 1.
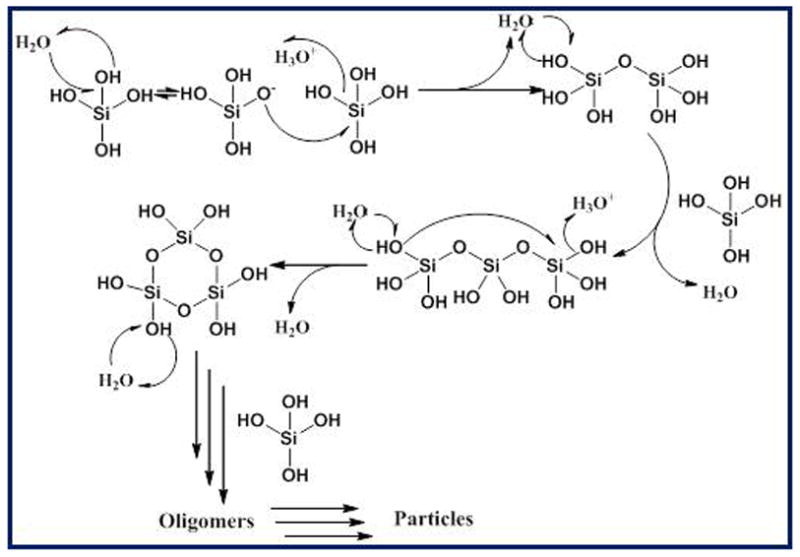
Mechanism of condensation of silicic acid species to generate linear and cyclic structures.
Figure 1.
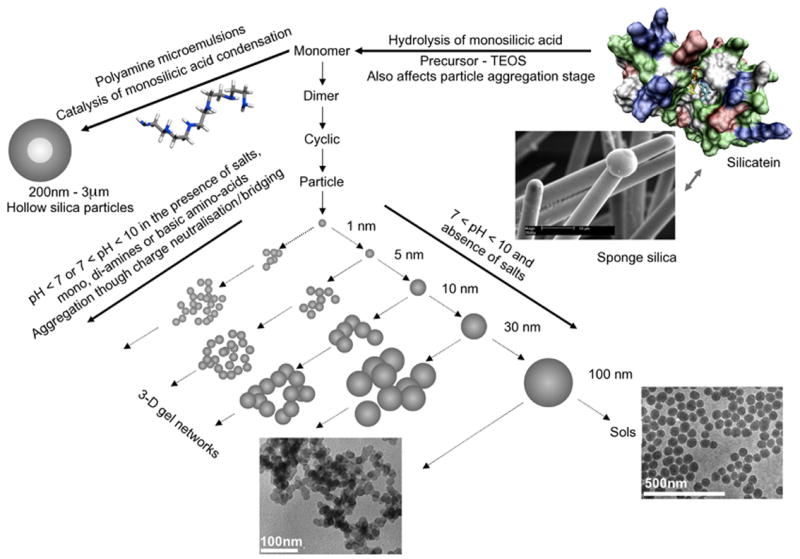
Schematic showing the generally accepted pathways to silica formation from orthosilicic acid through oligomers to particles and aggregated structures. Annotations to show where in the process the sponge protein silicatein and a few other selected biomolecules act are also shown.
Figure 2.
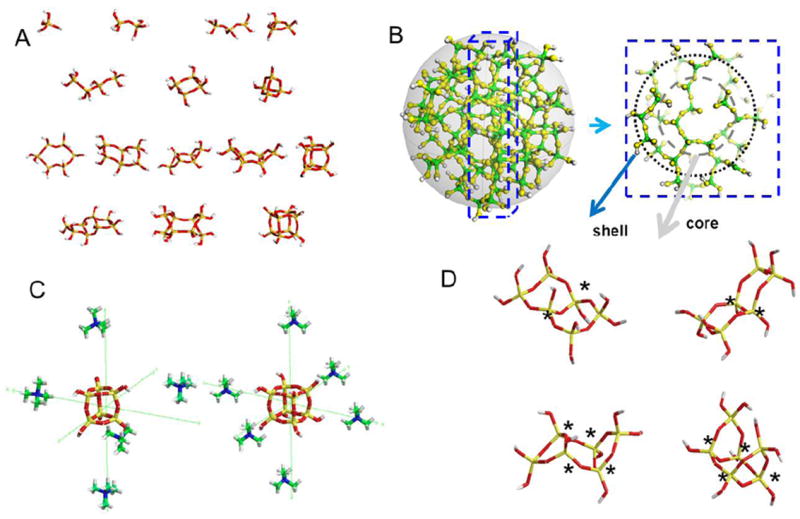
A: Energy minimised (MM2 followed by application of the AM1 programme) structures of exemplar silicic acid structures from momomers through to octamers. B: Schematic of an amorphous silica particle obtained from the thermal annealing of a quartz structure, correction of defects (3 and 5 coordinate Si and 3 coordinate O), extraction of 2nm particle followed by protonation of dangling Si-O bonds and additional condensation on the surface to eliminate Q1 species. The core contains predominantly Q4 species and the shell a mixture of Qn species. C: TMA+ stabilised 6:1 cubic octamer and 5:1 prismatic hexamer. D: Exemplars of energy minimised stereoisomers, LHS bicyclic hexamers, trans and cis form and RHS tricyclic hexamers chair and boat forms. In all cases potential chiral centres are marked with a *.
A perhaps unusual feature of silicic acid chemistry is the fact that although the silanol groups in the monomer are mildly basic, the pKa’s of small oligomers have been reported to vary between 9.5-10.7, and the pKa of silanol groups on the outside of oligomers 1nm in diameter (essentially a small particle) is 6.8. This means that as silica particles grow to the size of building blocks present in biological silicas, essentially a few nm in diameter, they will carry a surface negative charge and it is these particles that will interact with the biochemical environment in which the silica is deposited. As a rule, silicic acid molecules condense in such a way as to maximise the number of Si-O-Si bonds with cyclic species being formed early in the condensation process, Scheme 1. We will come back to this later when silicate speciation is considered in more detail. Almost all silicas contain high proportions of cyclic species unless they have been formed under conditions of extreme acidity. Furthermore, once the cyclic species dominate, monomers and dimers and other small oligomers react preferentially with these as the oligomers have a higher density of ionized silanol groups. As the levels of orthosilicic acid above the solubility limit decrease, smaller, more soluble particles dissolve releasing silicic acid that re-deposits onto the larger particles present, a process known as Ostwald ripening, Scheme 2. At circumneutral pH (6.0 – 8.0 – typically ~ 7), particles can continue growing as isolated particles until the levels of soluble silica reach the solubility of amorphous silica as the particles carry negative charge and repel one another, Figure 1. If salts or other charge species are present (inevitable in a biological environment) then the surface charge that causes repulsion between individual particles is reduced or cancelled and the particles aggregate to form dense three-dimensional networks, Figure 1. Note that in the case of silica found in sponges, the smallest silica particles that have been measured by electron microscopy are of the order of 2-3 nm in size with little space between the particles. These particles are then arranged into larger scale hybrid structures with biomolecules that include silicatein, silicase, galectin, sponge collagen and other proteins (see associated review). The possible role(s) that proteins, small molecules and ions can play in the generation of a functional condensed silica structure in relation to different stages of silica formation are indicated in Figure 1.
Scheme 2.
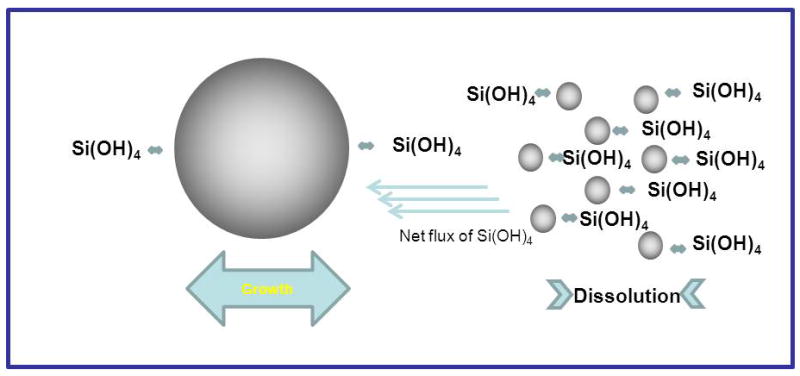
Pictorial representation of Ostwald ripening
Many factors affect the process of silica condensation from the molecular level upwards including concentration, temperature, pressure, pH and the presence of other ions, small molecules and polymers [1 and many subsequent reviews, including 2, 7] but in all cases the materials that form are amorphous on the 1nm length scale and are built up from SiO4 tetrahedra with variable Si-O-Si bond angles and Si-O bond distances. The materials contain hydroxyl groups (some will be present as Si-O-) and according to the reaction environment in which the mineral forms, silica from different organisms/ precipitation conditions can vary greatly with regard to density, hardness, solubility and composition [8].
Condensation from alkoxysilanes
Alkoxysilanes such as tetramethoxysilane and tetraethoxysilane are common silicon containing precursors used to study silica formation and generate materials for technological applications [9]. They have been extensively used by scientists exploring silica formation in the presence of silicatein [e.g. 10,11] although there are issues of water miscibility in the case of tetraethoxysilane until the compound is hydrolysed. In these compounds the central silicon atom is attached covalently via ether linkages to organic groups, although silanes with organic groups covalently attached to the silicon atom are also used so as to tune the condensation/aggregation mechanisms and also the properties of the materials formed. Alkoxy groups are usually hydrolysed when the compounds are placed in an aqueous environment, particularly in the presence of acid or basic catalysts, to generate silanol groups, Scheme 3. Once these are present condensation can occur with generation of alcohol or water as a side product of the reaction depending on whether condensation occurs between one silanol group and an alkoxide group or between two silanol groups respectively, Scheme 3. In water, there is a single building block from which silica structures are generated (orthosilicic acid) but for condensation from alkoxides a range of monomers with various functionalities can coexist, Scheme 3. The hydrolysis and condensation of silanes is affected by the identity of the precursor, solvent and catalyst, temperature and pH amongst other simple operating parameters including order of addition of individual reagents [9]. The catalyst used can dramatically modify the structure of the material produced, acid hydrolysis leading to largely linear structures, whereas base catalysis leads to branched structures. In addition, the length of the alkyl chain present in the precursor also has an effect on hydrolysis and condensation, as the use of tetramethoxysilane or tetraethoxysilane leads to structures built up from a preponderance of ring structures containing respectively three or four silicon atoms. Recent studies using UV-Raman and 29Si NMR spectroscopy coupled with molecular modelling [12] have further investigated the difference between the two precursors. When the amount of water is well below the stoichiometric value (e.g 10x below), 3-silicon rings are only formed from TMOS late in the oligomerization process, while starting from TEOS under similar reaction conditions, four silicon rings are formed early in the process by cyclodimerization. Even slight modifications in the alkoxide to water ratio (0.9 to 1.3) leads to significant changes in the condensed structures detected, including a great decrease in the concentration of ring structures, and the presence of partially condensed silicon species.
Scheme 3.
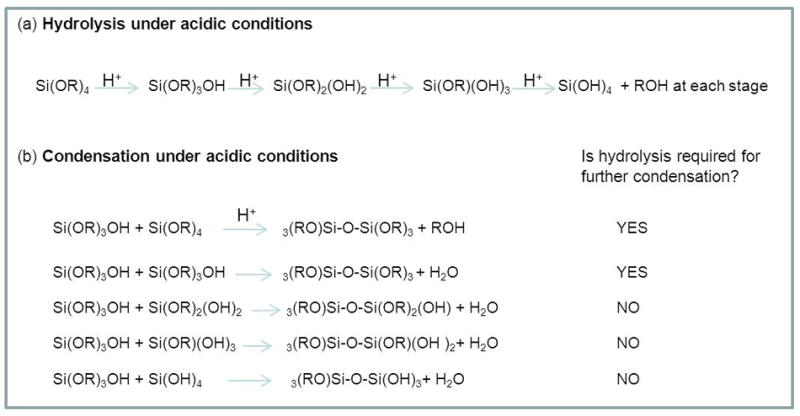
For the hydrolysis and condensation of an alkoxysilane (R = organic group) under acidic conditions, (a) species present on hydrolysis, (b) species present on condensation
It should be noted that studies of silica formation in the presence of biochemical isolates from silicifying organisms either proceed by prehydrolysis of the alkoxides (typically 10-15 minutes in the presence of an acid) followed by a transfer to the buffered medium containing the biomolecule, or by direct addition of the un-hydrolysed siloxane to the buffered medium. The presence of buffer ions including phosphate will modify the activity of the precursors, change the condensation pathway and can be a decisive factor in the formation of a gel or particulate structure.
Characterisation of the siliceous materials formed
The mineralised structures built up from nanosized particles intermixed with organic material arise from a complex interplay between inorganic chemistry (silica formation) and cellular biology (biomolecule structure and location). A wide range of techniques are being used to probe these complex materials at different levels, from the micrometer to the nanometre length scale, and down to their molecular structures[7]. Some examples of the types of information that can be gained from a subset of the techniques described below is presented in Figure 3.
Figure 3.
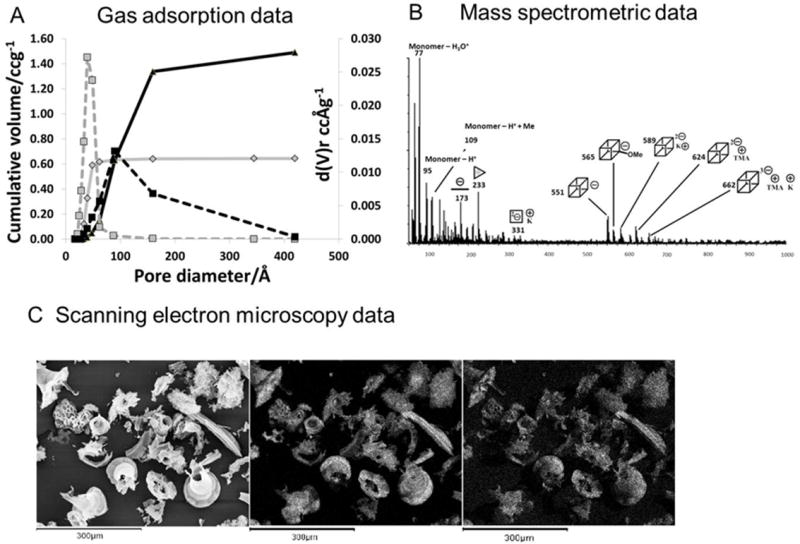
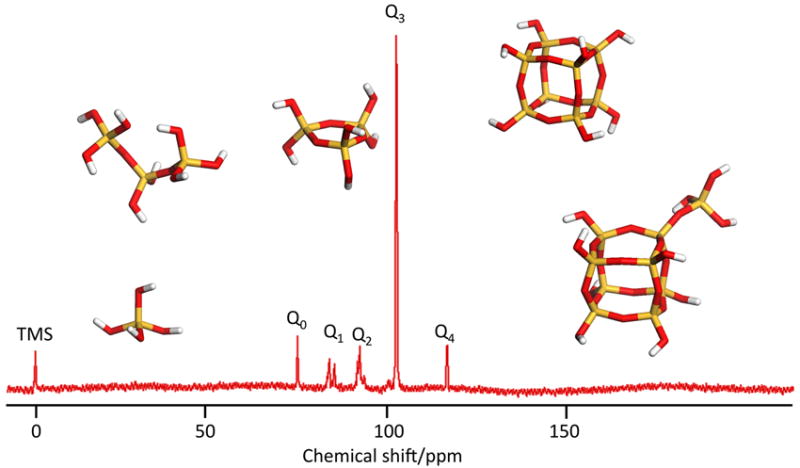
Methods used for characterisation of silica species in solution and in the solid state. A: nitrogen gas adsorption analysis of a fumed silica(small particles treated at high temperature); grey lines and a silicalite; black lines. B: ESI/MS mass spectrometric data for a solution of silica (30 mM dm-3) dissolved in a 12.5% wgt/vol solution of tetramethylammonium hydroxide after heating to 60°C for 8 hours to aid solubility. C:scanning electron microscopy images of silica extracts from Curcurbita (marrow) leaves. D: solid state 29Si nmr of silicon species in Q° to Q4 coordination environments.
Electron microscopy (scanning and transmission modes) is used to identify the sizes of fundamental particles and their interconnection to one another. This technique is often used in conjunction with energy dispersive X-ray analysis (EDXa), to locate the mineral within an organism as well as locate co-localized inorganic species. An example shown in Figure 3 is the siliceous structures isolated from leaves of a member of the Cucurbita (marrow) family.
Small angle, ultra small angle and wide angle X-ray diffraction (SAXS, USAXS and WAXS) can detect order at the nm level with USAXS being used successfully to identify structures at different length scales including pore structures [13]. Scanning probe microscopies constitute an arsenal of techniques which can be used to characterise not only the topological features of the materials formed, but also their physical and chemical properties [14]; Confocal microscopy coupled with the use of fluorescently labelled biomolecules such as polyamines has been used to explore the co-localisation of the organic matrix and mineral phase [15] and further experimentation in this direction may provide more detailed time dependent information on the process of biological silicification. Another technique which gives information on bulk samples is nitrogen (or other gas) adsorption analysis and this can be used to measure porosity, including the distribution of pore sizes, Figure 3.
At the molecular level, solid state 29Si NMR allows for the observation of connectivity and degree of condensation in solid siliceous materials by comparing the intensities of the Q0,Q1, Q2, Q3 and Q4 signals, Figure 3. Infra red and Raman spectroscopies have also been used to observe siliceous materials in solution, colloidal and solid state. Comparison of surface and bulk properties may be considered with these by the use of reflection techniques such as attenuated total reflection (ATR) and diffuse reflection infrared Fourier transform spectroscopy (DRIFTS) which only penetrate the first few molecular layers of the material whilst the bulk properties can be observed by transmission techniques. The use of these instruments in ‘mapping’ mode can be used to identify any co-localization of the mineral with specific molecule types (protein, carbohydrate etc.) that can be of great use in planning further biochemical investigations.
Study of the condensation process
For information on solution species, a range of chemical and instrumental techniques are available. Complexation with molybdic acid reagent produces silicomolybdic acid, a strongly absorbing yellow complex which only forms in combination with monosilicic acid. This complex can be reduced to the blue silicomolybdous acid complex to give a highly sensitive method for monitoring monomeric and dimeric solution concentrations, for example for kinetic studies. The yellow complex may also be monitored directly to gain information about the species present in solution, the rate of complexation being directly related to the rate of dissociation of oligomeric species into the monomer [16-18] (Figure 4). Silicate species have also been successfully monitored by gas chromatography/mass spectrometry (GC/MS) tandem techniques. The silylation of the oligomers with alkylchlorosilanes [19,20] prevents further condensation reactions and also facilitates their transfer to the gas phase for chromatographic separation. To analyse directly stable aqueous solution molecules, electrospray ionisation mass spectrometry (ESI/MS) has been employed [21,22], Figure 3. The implementation of this technique to the study of dynamic changes such as during silica condensation, the solution speciation needs to be “frozen” during the evaporative stage in the sample ionisation step and this possibility is currently under investigation. 29Si NMR has been used extensively to observe silicate structures in stable solutions. It has been successfully applied using both 1D and 2D pulsed techniques such as COSY (Correlation SpectroscopY), INEPT (Insensitive Nuclei Enhanced by Polarization Transfer) and INADEQUATE (Incredible Natural Abundance DoublE QUAntum Transfer Experiment) in the identification of in excess of 30 solution species and more are being discovered [23,24] The abundance of the active isotope 29Si is only 4.7% of all Si species, and relaxation times, even with the use of relaxation agents such as chromium acetylacetone (Cr(acac)3), are in the order of 20-30 seconds. Because of these limitations the use of 29Si NMR in dilute and dynamic systems is limited. 29Si enrichment has been used but the cost and availability of the active isotope are currently prohibitive for extensive studies. An additional method for quantifying silicon in solution or colloidal state is the atomic emission method based on inductively coupled plasma analysis (ICP) which analyses the total ‘Si’ content but does not give any information on speciation.
Figure 4.
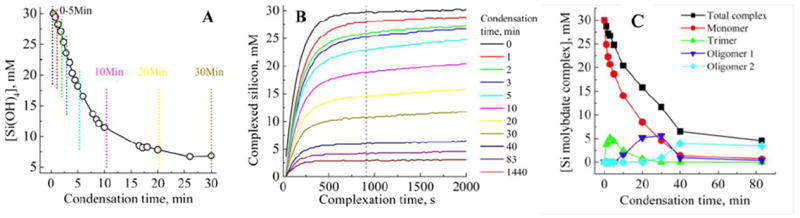
A: The decrease in silicomolybdous acid (molybdenum blue) complex with time following pH reduction for 30 mM initial [Si(OH)4] at pH 6.8, B: the formation of silicomolybdic acid yellow complex after increasing condensation times. The vertical lines in A: represent a selection of the sampling times used to obtain the molybdenum yellow data presented in B:. The vertical line on B: denotes the time at which the blue silicomolybdic acid complex is formed from species available in solution. C: Silicate speciation during the condensation process for a 4 species fit.
Speciation of aqueous silicate solutions
Silicate speciation in aqueous media has been studied during silica dissolution, condensation and in stabilised solutions. Speciation studies during dissolution have included natural systems using both amorphous and crystalline (mineral) silicas and observations of natural waters with a range of characteristics. During the dissolution of amorphous silica under neutral pH conditions an increase in the ratio of dimer to monomer is observed as saturation is approached [25]. Silica of a wide range of origins (mineral, quartz, opal, volcanic, silica gel and amorphous) have all been shown to produce solutions of monomer at equilibrium but during the early stages of dissolution the presence of polymeric species was indicated by their relative rates of complexation with molybdic acid [26]. These polymeric species were found to convert to monomer over time and were thought to probably represent the rapidly dissolving species found at high energy sites on the surface of irregular and angular materials. Natural waters contain usually from 0.03 to 1 mM dissolved silica together with silica particles due to erosive pressures. Irrespective of the silicate concentration all of these sources only contain monomeric silicic acid associated with various cations, however only Na+ concentrations are consistently correlated to the soluble silica concentrations [27]. Under conditions of elevated temperature and pressure the ratio of dimer to monomer increases and under more extreme hydrothermal conditions (>850 °C and >12 bar) a contribution from dissolved higher oligomers has been detected by Raman spectroscopy [28].
During the condensation of silica from meta-stable orthosilicic acid solutions a number of oligomeric species have been either identified (by spectroscopic methods) or inferred (by kinetic methods and molybdic acid complexation rates). These studies have shown that the early stages of condensation proceed by the formation of dimers and short linear oligomers which rapidly cyclise [12, 16, 18, 29, 30].
Cyclisation does not universally occur end to end within a chain but can also take place along the chain, resulting in a number of cyclic siliceous species with linear appendages [31]. Under neutral pH conditions, the pKa of the oligomers decreases with connectivity. These oligomers become increasingly anionic and favourable for condensation with the remaining monomers and preformed oligomers. Hence the condensing system becomes rapidly dominated by monomer and particulate silica. Under strongly alkaline conditions, these oligomers are stabilised and may be monitored by techniques which are unavailable to condensing systems due to the fast processes involved. In dilute solutions, small oligomers (monomer and dimers) are stabilised, but structures as large as the cubic octamer and prismatic decamer are found in more concentrated systems [21, 22, 24, 32-35], Figure 2, 3.
Depending on the base used for solution preparation, silicon speciation can be modified and stable solutions of different oligomers can be obtained. In sodium and potassium hydroxide solutions, many of the small and large oligomers coexist, whereas in tetraalkylammonium hydroxide stabilised solutions, various cage oligomers dominate according to the alkyl chain present. In tetramethylammonium hydroxide solution the cubic octamer is the major species, Figure 3, in tetraethylammonium hydroxide the prismatic hexamer is preferentially stabilised and in tetrapropylammonium hydroxide the cubic octamer and its dimer occur [36]. Solutions containing specific oligomers can be formed by varying the ratio of the alkylammonium hydroxide to silicate precursor with tetramethylammonium hydroxide being able to select for the cubic octamer at a 6:1 TMAOH to oligomer ratio and the prismatic hexamer at a 5:1 ratio. An explanation for this specific stabilisation of oligomers has been obtained from molecular modelling. These studies demonstrated that the stabilisation is due to a lowering of the difference in entropy caused by the disruption of the hydrogen bonding network of bulk water rather than by a templating effect [37]. Figure 2c shows the spatial location of TMA relative to the oligomerfaces.
If we now consider a potentially ‘simpler’ system initially containing silicate anions and sodium ions produced from the dissolution of amorphous silica in sodium hydroxide, 1-D and 2-D NMR studies using 29Si enriched sodium silicate solutions, with comparison to simulated COSY spectra have identified oligomers with multiple stereoisomers, including conformers and/ or diastereomers, which were not possible from the 1D spectra alone [12]. The 1-D methods are effective only where the spectra of the oligomers present give rise to well-resolved and minimally overlapped features. Oligomers that have been identified from the correlation experiments contain a small number of chemically inequivalent silicon sites, attributable to small, symmetric structures which show a multiplicity of stereoisomers when the potential chirality of Q3 sites is considered. An example of such structures is shown in Figure 2D. The presence of such structures has consequences for the way in which a silicate network can develop both in solution and ultimately in the solid state, and may well have a bearing on the way we think about biomineral development in Nature.
Computational studies of silica formation
Computational methods have been applied to the study of silica formation, including the interaction between silicic acid species and biomolecules since the mid 1990’s [38]. In recent years, due to the improvement of processing power, computer modelling has become a valuable tool in determining mechanisms and routes of silica oligomerization and the effect of external parameters on the process. Earlier work usually used non-explicit water models such as the COSMO (conductor like screening model) solvation model with the solvent being treated as a continuum rather than containing individual solvent molecules. These studies showed that the anionic route to condensation was favoured in agreement with experimental observations, [39-41], and that the activation energy for cyclisation was higher than that for linear oligomerisation, with the rate determining step being the removal of water. However, when explicit water molecules were included in molecular dynamic simulations, the Si-O-Si bond cyclisation activation energies were found to be much reduced, making the models come closer to the experimental observation where tricyclic oligomers are often favoured over linear and higher cyclic oligomers [42]. Moreover, the simulations demonstrated that the linear growth mechanism dominates under circumneutral pH conditions, with cyclisation dominating at higher pH [42]. Furthermore, a kinetic Monte Carlo simulation study using parameters calculated by density functional theory found ring closure and linear growth to be the rate limiting steps respectively at circumneutral and basic pH [43]. Although the semi empirical PM3 force field gives a good approximation to molecular dynamic models [44] it does not fully explain the role of entropy in the formation of cyclical structures.
Other modelling has focussed on explaining the stabilisation of the larger cage like oligomers by tetraalkylammonium cations. By calculating the mean force for silicate polyion and tetramethyl ammonium cation ion pair interactions and entropic effects studies have shown the preferred ratio and therefore orientations of the ionic species to be 6:1 TMA+ to oligomer for the cubic octamer stabilisation and 5:1 for that of the prismatic hexamer with the cation oriented towards each face of their respective cages as is found experimentally. [45], Figure 2c.
Conclusions
Fundamental studies of the early stages of silica condensation are continuing to reveal much about the wide range of species present in solution and the mechanisms by which these species form. However, the majority of the experimental information has been obtained from condensing systems where the reaction can effectively be ‘quenched’ either because of the pH at which it is run or by other chemical means. We still know much less concerning condensation under physiologically relevant conditions. Increases in computing power are now enabling realistic calculations of species present, their relative energies and likely condensation pathways. However we are still a long way from being able to manipulate the chemistry of silica, either in silico or in the laboratory to produce materials with the exquisite form and function that occur in the natural world (see associated review on sponge proteins). The relevance of solution species to final structure is still unclear and methods which can monitor the process in real time need to be developed in order for us to be able to study and thereafter understand and fully appreciate the contribution of all species in the reaction medium to mineral formation. We should remember that silica formation in the natural environment occurs in the presence of a very wide range of ions, small molecules and larger biomolecules; polyamines and a range of proteins being associated with silica produced by sponges and the presence of all of these will affect in some way the condensation process from monomer through stable nuclei to the development of macroscopic three dimensional siliceous structures.
Acknowledgments
We gratefully acknowledge support of the European Union through a Marie-Curie Network for aspects of this work and funding from the American Airforce Office of Scientific Research (AFOSR FA 9550-06-1-0154) and the National Institutes of Health (USA) award (5RO1DE017207-05).
Glossary
The terminology which is used relevant for the study of silica formation in an aqueous and non-aqueous environment is as follows:
- Si
the chemical symbol for the element and the generic term used when the nature of the specific silicon compound is not known.
- Si(OH)4
orthosilicic acid, the fundamental building block used in the formation of silicas.
- SiO2•nH2O or SiO2−x(OH)2x•2H2O
amorphous, hydrated, polymerized material.
- Oligomerization
the formation of dimers and small oligomers from orthosilicic acid by removal of water. e.g., 2Si(OH)4 ↔ (HO)3Si–O–Si(OH)3 + H2O
- Polymerization
the mutual condensation of silicic acid to give molecularly coherent units of increasing size.
- Q0, Q1, Q2, Q3, Q4
notation used in 29Si NMR spectroscopy for Si with four oxygens attached, the number 0-4 denotes the number of ‘Si’ units attached through the oxygen to an individual silicon atom.
- Silane
a compound have silicon atom(s) and organic chemical groups often connected through an oxygen linkage; e.g. tetraethoxy or tetramethoxysilane. Silanes may also contain Si-organic groups with direct connection of organic groupings to the silicon atom.
- TMOS, TEOS
Alkoxy silanes; tetramethoxysilane and tetraethoxysilane. In the context of the work described herein, used as precursors for supersaturated orthosilicic acid solutions for condensation experiments.
- Silanol
hydroxyl group bonded to silicon atom
- Silicate
a chemically specific ion having negative charge (e.g., SiO32−), term also used to describe salts (e.g., sodium silicate Na2SiO3)
- Opal
the term used to describe the gem-stone and often used to describe the type of amorphous silica produced by biological organisms. The two are similar in structure at the molecular level (disordered or amorphous) but at higher levels of structural organization are distinct from one another.
References
- 1.Iler RK. The chemistry of silica. John Wiley & Sons; New York: 1979. [Google Scholar]
- 2.Perry CC. Chemical Studies of Biogenic silica. In: Mann S, Webb J, Williams RJP, editors. Biomineralisation, chemical and biological perspectives. VCH; Weinheim: 1989. pp. 233–256. [Google Scholar]
- 3.Hildebrand M, Dahlin K, Volcani BE. Characterization of a silicon transporter gene family in Cylindrotheca fusiformis: sequences, expression analysis, and identification of homologs in other diatoms. Molec Gen Genetics. 1998;260:480–486. doi: 10.1007/s004380050920. [DOI] [PubMed] [Google Scholar]
- 4.Hildebrand M, Volcani BE, Gassmann W, Schroeder JI. A gene family of silicon transporters. Nature. 1997;385:688–689. doi: 10.1038/385688b0. [DOI] [PubMed] [Google Scholar]
- 5.Schröder HC, Perović-Ottstadt S, Rothenberger M, Wiens M, Schwertner H, Batel R, Korzhev M, Müller IM, Müller WEG. Silicon transport in the demosponge Suberites domuncula: fluorescence emission analysis using the PDMPO probe and cloning of a potential transporter. Biochem J. 2004;381:665–673. doi: 10.1042/BJ20040463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma J-F, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, Yano M. A silicon transporter in rice. Nature. 2006;440:688–69. doi: 10.1038/nature04590. [DOI] [PubMed] [Google Scholar]
- 7.Perry CC. Silicification: The processes by which Organisms Capture and Mineralize Silica. In: Dove PM, De Yoreo JJ, Weiner S, editors. Rev Miner and Geochem. Vol. 54. 2003. pp. 291–327. [Google Scholar]
- 8.Perry CC, Keeling-Tucker T. Biosilicification: The role of the organic matrix in structure control. J Biol Inorg Chem. 2000;5:537–550. doi: 10.1007/s007750000130. [DOI] [PubMed] [Google Scholar]
- 9.Brinker CJ, Scherrer GW. Sol-Gel Science- the physics and chemistry of sol-gel processing. Academic Press; London: 1990. [Google Scholar]
- 10.Tahir MN, Theato P, Müller WEG, Schröder HC, Janshoff A, Zhang J, Huth J, Tremel W. Monitoring the formation of biosilica catalysed by histidine-tagged silicatein. Chem Commun. 2004;24:2848–2849. doi: 10.1039/b410283e. [DOI] [PubMed] [Google Scholar]
- 11.Rai A, Perry CC. Facile Fabrication of uniform silica films with tunable physical properties using silicatein protein from sponges. Langmuir. 2010;26(6):4152–4159. doi: 10.1021/la903366a. [DOI] [PubMed] [Google Scholar]
- 12.Depla A, Verheyen E, Veyfeykan A, Van Houteghem M, Houthoofd K, Van Speybroeck V, Waroquier M, Kirshhock CEA, Martens J. UV-Raman and 29Si NMR spectroscopy investigation of the nature of silicate oligomers formed by acid catalysed hydrolysis and polycondensation of tetramethylorthosilicate. J Phys Chem C. 2011;115:11077–11088. [Google Scholar]
- 13.Vrieling EG, Beelen TPM, van Santen RA, Gieskes WWC. Mesophases of (bio)polymer-silica particles inspire a model for silica biomineralization in diatoms. Angew Chem Int Edn. 2002;41:1543–1546. doi: 10.1002/1521-3773(20020503)41:9<1543::aid-anie1543>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 14.Wetherbee R, Crawford S, Mulvaney P. The nanostructure and development of diatom biosilica. In: Bauerlein E, editor. Biomineralization, from Biology to Biotechnology and Medical Applications. Wiley VCH; Weinheim, Germany: 2000. pp. 189–206. [Google Scholar]
- 15.Annenkov VV, Danilovtseva EN, Zelinskiy SN, Basharina TN, Dafonova TA, Korneva ES, Likhoshway YV, Grachev MA. Novel Fluorescent dyes based on oligopropylamines for the in vivo staining of eukaryotic unicellular algae. Anal Biochem. 2010;407(1):44–51. doi: 10.1016/j.ab.2010.07.032. [DOI] [PubMed] [Google Scholar]
- 16.Iler RK. Isolation and characterization of particle nuclei during the polymerization of silicic acid to colloidal silica. J Coll Int Sci. 1980;75(1):138–148. [Google Scholar]
- 17.Belton DJ, Patwardhan SV, Annenkov VV, Danilovtseva EN, Perry CC. From biosilicification to tailored materials: Optimizing hydrophobic domains and resistance to Protonation of polyamines. Proc Natl Acad Sci USA. 2008;105(16):5063–5068. doi: 10.1073/pnas.0710809105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belton DJ, Deschaume O, Patwardhan SV, Perry CC. A Solution Study of Silica Condensation and Speciation with Relevance to in Vitro investigations of biosilicification. J Phys Chem B. 2010;114:9947–9955. doi: 10.1021/jp101347q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lentz CW. Silicate minerals as sources of trimethylsilyl silicates and silicate structure analysis of sodium silicate solutions. Inorg Chem. 1964;3:574–579. [Google Scholar]
- 20.Glasser LSD, Sharma SK. Experiences with the method of trimethylsilylation for the study of soluble silicates. Br Polym J. 1974;6(6):283–291. [Google Scholar]
- 21.Bussian P, Sobott F, Brutschy B, Schrader W, Schüth F. Speciation in solution: Silicate oligomers in aqueous solutions detected by mass spectrometry. Angew Chem Int Ed. 2000;39(21):3901–3905. doi: 10.1002/1521-3773(20001103)39:21<3901::AID-ANIE3901>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 22.Eggers K, Eichner T, Woenckhaus J. Mass spectrometric investigation of small polyhedra in solution. International Journal of Mass Spectrometry. 2005;244:72–75. [Google Scholar]
- 23.Delak KM, Farrar TC, Sahai N. 29Si NMR sensitivity enhancement methods for the quantitative study of organosilicate hydrolysis and condensation. J Non Cryst Sol. 2005;351:2244–2250. [Google Scholar]
- 24.Haouas M, Taulelle F. Revisiting the identification of structural units in aqueous silicate solutions by two-dimensional silicon-29 INADEQUATE. J Phys Chem B. 2006;110(7):3007–3014. doi: 10.1021/jp0557823. [DOI] [PubMed] [Google Scholar]
- 25.Applin KR. The diffusion of dissolved silica in dilute aqueous solutions. Geochim Cosmochim Acta. 1987;51:2147–2152. [Google Scholar]
- 26.Dietzel M. Dissolution of silicates and the stability of polysilicic acid. Geochim Cosmochim Acta. 2000;64(19):3275–3281. [Google Scholar]
- 27.Tanaka M, Takahashi K, Sahoo YV. Speciation of dissolved silicates in natural waters containing alkaline and alkaline-earth ions. Anal Bioanal Chem. 2004;378:789–797. doi: 10.1007/s00216-003-2337-8. [DOI] [PubMed] [Google Scholar]
- 28.Zotov N, Keppler H. Silica speciation in aqueous fluids at high pressures and high temperatures. Chemical Geology. 2002;184:71–82. [Google Scholar]
- 29.Icopini GA, Brantley SL, Heaney PJ. Kinetics of silica oligomerization and nanocolloid formation as a function of pH and ionic strength at 25°C. Geochimica et Cosmochimica Acta. 2005;69(2):293–303. [Google Scholar]
- 30.Harris RK, Newman RH. 29Si NMR studies of aqueous silicate solutions. J Chem Soc Faraday transactions 2. 1977;73:1204–1215. [Google Scholar]
- 31.Rankin SE, Macosko CW, McCormick AV. Importance of cyclization during the condensation of hydrolysed alkoxysilanes. Chem Mater. 1998;10(8):2037–2040. [Google Scholar]
- 32.Cary LW, De Jong BHWS. A 29Si NMR study of silica species in dilute aqueous solution. Geochimica et Cosmochimica Acta. 1982;46:1317–1320. [Google Scholar]
- 33.Cho H, Felmy AR, Craciun R, Keenum JP, Shah N, Dixon DA. Solution state structure determination of silicate oligomers by 29Si NMR spectroscopy and molecular modelling. J Am Chem Soc. 2006;128:2324–2335. doi: 10.1021/ja0559202. [DOI] [PubMed] [Google Scholar]
- 34.Caremans TP, Loppinet B, Follens LRA, van Erp TS, Vermant J, Goderis B, Kirschhock CEA, Martens JA, Aerts A. Investigation of nanoparticles occurring in the colloidal silicalite-1 zeolite crystallization process using dissolution experiments. Chem Mater. 2010;22:3619–3629. [Google Scholar]
- 35.Tognonvi MT, Massiot D, Lecomte A, Rossignol S, Bonnet J-P. Identification of solvated species present in concentrated and dilute sodium silicate solutions by combined 29Si NMR and SAXS studies. J Coll Int Sci. 2010;352:309–315. doi: 10.1016/j.jcis.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 36.Kinrade SD, Donovan JCH, Schach AS, Knight CTG. Two substituted cubic octameric silicate cages in aqueous solution. J Chem Soc Dalton Trans. 2002:1250–1252. [Google Scholar]
- 37.Caratzoulas S, Vlachos DG, Tsapatsis M. Potential mean force for tetramethylammonium binding to cagelike oligosilicates in aqueous solution. J Am Chem Soc. 2006;128:16138–16147. doi: 10.1021/ja064597f. [DOI] [PubMed] [Google Scholar]
- 38.Lobel KD, West JK, Hench LL. Computational model for protein mediated biomineralization of the diatom frustule. Marine Biol. 1996;126(3):353–360. [Google Scholar]
- 39.Mora-Fonz MJ, Catlow CRA, Lewis DW. Oligomerisation and cyclisation processes in the nucleation of microporous silicas. Angew Chemie Int Ed. 2005;44:3082–3086. doi: 10.1002/anie.200462524. [DOI] [PubMed] [Google Scholar]
- 40.Mora-Fonz MJ, Catlow CRA, Lewis DW. Modelling of aqueous silica chemistry in aqueous media. J Phys Chem C. 2007;111(49):18155–18158. [Google Scholar]
- 41.Trinh TT, Jansen APJ, van Santen RA. Mechanism of oligomerisation reactions of silica. J Phys Chem B. 2006;110:23099–23106. doi: 10.1021/jp063670l. [DOI] [PubMed] [Google Scholar]
- 42.Trinh TT, Jansen APJ, van Santen RA, Meijer EJ. Role of water in silica oligomerisation. Physical Chemistry Letters C. 2009;113:2647–2652. [Google Scholar]
- 43.Zhang Z-Q, Trinh TT, van Santen RA, Jansen APJ. Mechanism of the initial stages of silicate oligomerisation. JACS. 2011;133:6613–6625. doi: 10.1021/ja110357k. [DOI] [PubMed] [Google Scholar]
- 44.Nedelec JM, Hench LL. Ab initio molecular orbital calculations on silica rings. Journal of Non-Crystalline Solids. 1999;255:163–170. [Google Scholar]
- 45.Caratazoulas S, Vlachos DG, Tsapatsis M. Potential of mean force for the tetramethylammounium binding to cagelike oligosilicates in aqueous solution. JACS. 2006;128:16138–161. doi: 10.1021/ja064597f. [DOI] [PubMed] [Google Scholar]


