Abstract
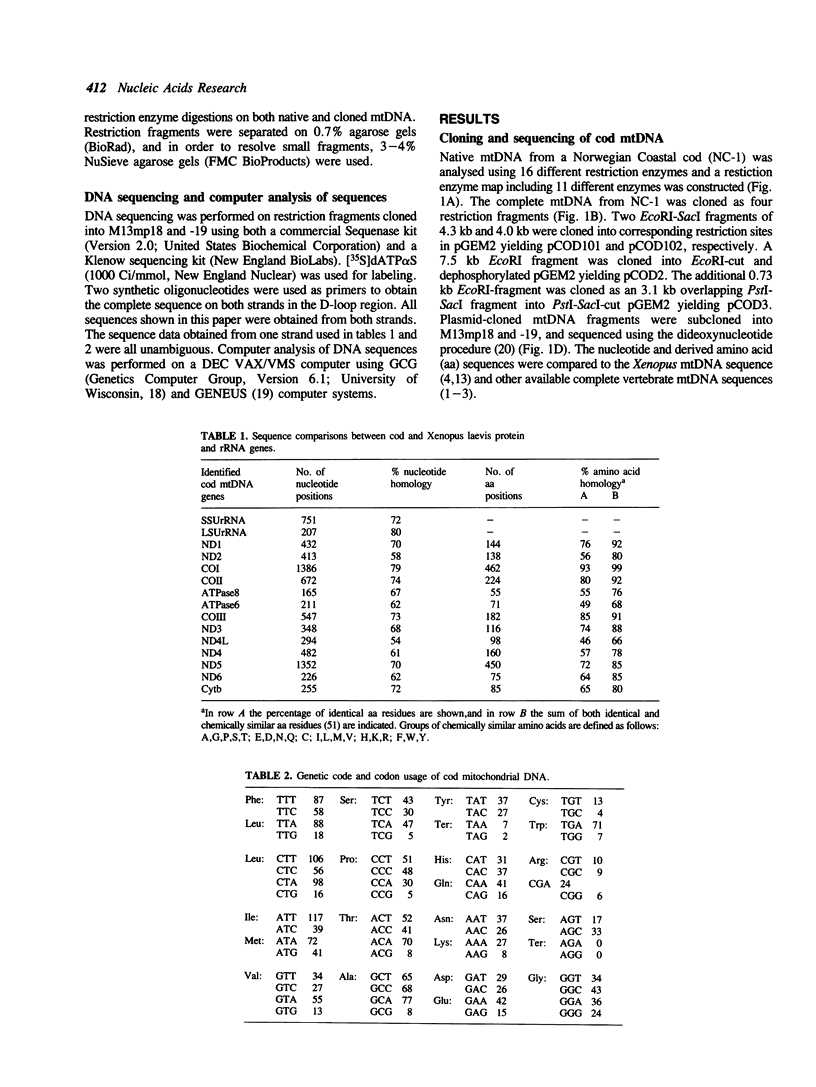
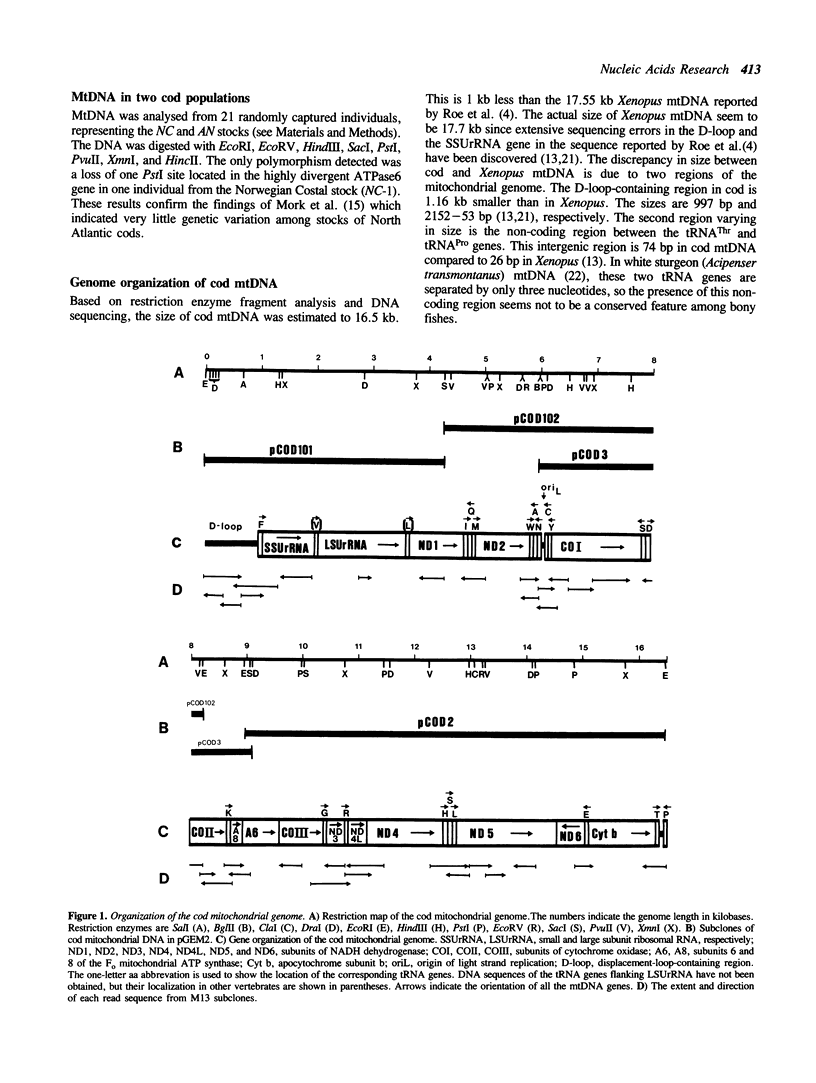
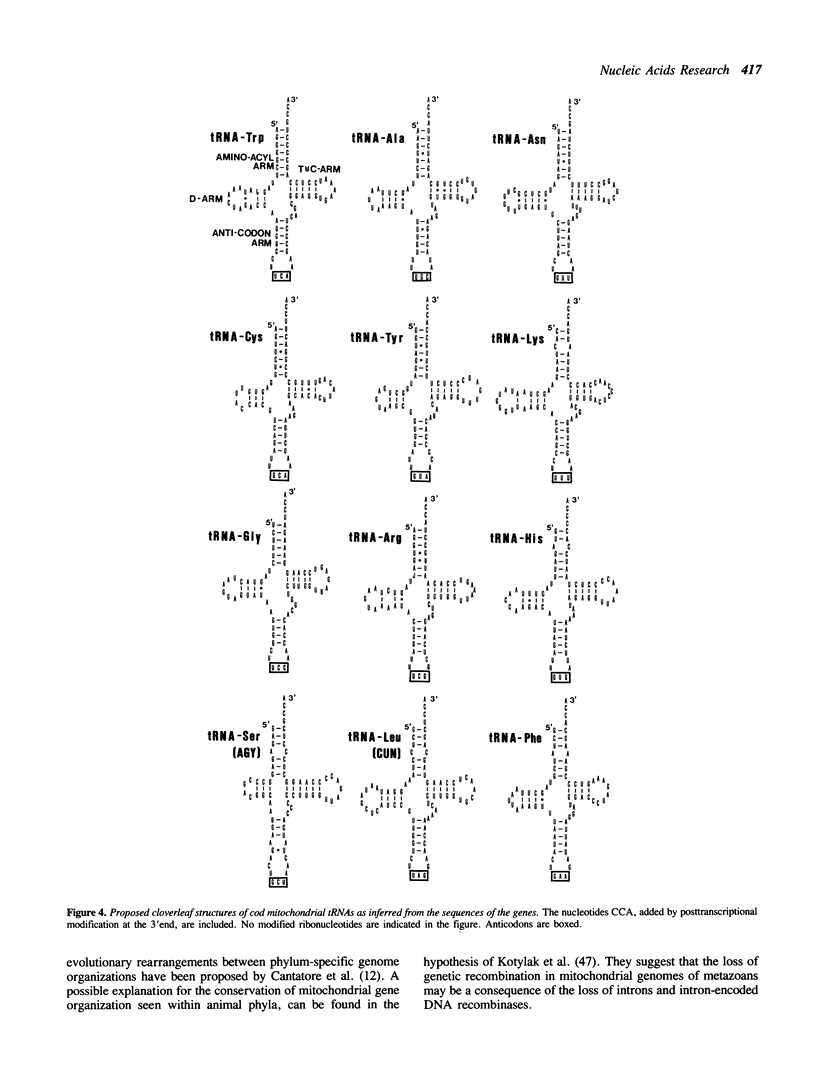
The mitochondrial DNA (mtDNA) from the Atlantic cod, Gadus morhua, was mapped using 11 different restriction enzymes and cloned into plasmid vectors. Sequence data obtained from more than 10 kilobases of cod mtDNA show that the genome organization, genetic code, and the overall codon usage have been conserved throughout the evolution of vertebrates. Comparison of the derived amino acid sequences of proteins encoded by cod mtDNA to the ones encoded by Xenopus laevis mtDNA revealed that the amino acid identity range from 46% to 93% for the different proteins. ND4L is most divergent while COI is most conserved. GUG was found as the translation initiation codon of the COI gene, indicating a dual coding function for this codon. The sequences of the 997 base pair displacement-loop (D-loop)-containing region and the origin of L-strand replication (oriL), are presented. Only few of the primary and secondary structure features found to be conserved among mammalian mitochondrial D-loops, can be identified in cod. Presence of CSB-2 in the D-loop-containing region and the conserved hairpin structure at oriL, indicates that replication of bony fish mtDNA may follow the same general scheme as described for higher vertebrates.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Anderson S., de Bruijn M. H., Coulson A. R., Eperon I. C., Sanger F., Young I. G. Complete sequence of bovine mitochondrial DNA. Conserved features of the mammalian mitochondrial genome. J Mol Biol. 1982 Apr 25;156(4):683–717. doi: 10.1016/0022-2836(82)90137-1. [DOI] [PubMed] [Google Scholar]
- Batuecas B., Garesse R., Calleja M., Valverde J. R., Marco R. Genome organization of Artemia mitochondrial DNA. Nucleic Acids Res. 1988 Jul 25;16(14A):6515–6529. doi: 10.1093/nar/16.14.6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzen P., Leggett W. C., Brown G. G. Length and restriction site heteroplasmy in the mitochondrial DNA of american shad (alosa sapidissima). Genetics. 1988 Mar;118(3):509–518. doi: 10.1093/genetics/118.3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham E., Lamb T., Avise J. C. Size polymorphism and heteroplasmy in the mitochondrial DNA of lower vertebrates. J Hered. 1986 Jul-Aug;77(4):249–252. doi: 10.1093/oxfordjournals.jhered.a110230. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Bogenhagen D. F., Yoza B. K. Accurate in vitro transcription of Xenopus laevis mitochondrial DNA from two bidirectional promoters. Mol Cell Biol. 1986 Jul;6(7):2543–2550. doi: 10.1128/mcb.6.7.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. G., Gadaleta G., Pepe G., Saccone C., Sbisà E. Structural conservation and variation in the D-loop-containing region of vertebrate mitochondrial DNA. J Mol Biol. 1986 Dec 5;192(3):503–511. doi: 10.1016/0022-2836(86)90272-x. [DOI] [PubMed] [Google Scholar]
- Cairns S. S., Bogenhagen D. F. Mapping of the displacement loop within the nucleotide sequence of Xenopus laevis mitochondrial DNA. J Biol Chem. 1986 Jun 25;261(18):8481–8487. [PubMed] [Google Scholar]
- Cantatore P., Gadaleta M. N., Roberti M., Saccone C., Wilson A. C. Duplication and remoulding of tRNA genes during the evolutionary rearrangement of mitochondrial genomes. 1987 Oct 29-Nov 4Nature. 329(6142):853–855. doi: 10.1038/329853a0. [DOI] [PubMed] [Google Scholar]
- Cantatore P., Roberti M., Rainaldi G., Gadaleta M. N., Saccone C. The complete nucleotide sequence, gene organization, and genetic code of the mitochondrial genome of Paracentrotus lividus. J Biol Chem. 1989 Jul 5;264(19):10965–10975. [PubMed] [Google Scholar]
- Cantatore P., Saccone C. Organization, structure, and evolution of mammalian mitochondrial genes. Int Rev Cytol. 1987;108:149–208. doi: 10.1016/s0074-7696(08)61438-2. [DOI] [PubMed] [Google Scholar]
- Chang D. D., Fisher R. P., Clayton D. A. Roles for a promoter and RNA processing in the synthesis of mitochondrial displacement-loop strands. Biochim Biophys Acta. 1987 Jul 14;909(2):85–91. doi: 10.1016/0167-4781(87)90029-7. [DOI] [PubMed] [Google Scholar]
- Clary D. O., Wolstenholme D. R. The mitochondrial DNA molecular of Drosophila yakuba: nucleotide sequence, gene organization, and genetic code. J Mol Evol. 1985;22(3):252–271. doi: 10.1007/BF02099755. [DOI] [PubMed] [Google Scholar]
- Clayton D. A. Replication of animal mitochondrial DNA. Cell. 1982 Apr;28(4):693–705. doi: 10.1016/0092-8674(82)90049-6. [DOI] [PubMed] [Google Scholar]
- Densmore L. D., Wright J. W., Brown W. M. Length variation and heteroplasmy are frequent in mitochondrial DNA from parthenogenetic and bisexual lizards (genus Cnemidophorus). Genetics. 1985 Aug;110(4):689–707. doi: 10.1093/genetics/110.4.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunon-Bluteau D. C., Brun G. M. Mapping at the nucleotide level of Xenopus laevis mitochondrial D-loop H strand: structural features of the 3' region. Biochem Int. 1987 Apr;14(4):643–657. [PubMed] [Google Scholar]
- Dunon-Bluteau D., Volovitch M., Brun G. Nucleotide sequence of a Xenopus laevis mitochondrial DNA fragment containing the D-loop, flanking tRNA genes and the apocytochrome b gene. Gene. 1985;36(1-2):65–78. doi: 10.1016/0378-1119(85)90070-8. [DOI] [PubMed] [Google Scholar]
- Fearnley I. M., Walker J. E. Initiation codons in mammalian mitochondria: differences in genetic code in the organelle. Biochemistry. 1987 Dec 15;26(25):8247–8251. doi: 10.1021/bi00399a034. [DOI] [PubMed] [Google Scholar]
- Foran D. R., Hixson J. E., Brown W. M. Comparisons of ape and human sequences that regulate mitochondrial DNA transcription and D-loop DNA synthesis. Nucleic Acids Res. 1988 Jul 11;16(13):5841–5861. doi: 10.1093/nar/16.13.5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadaleta G., Pepe G., De Candia G., Quagliariello C., Sbisà E., Saccone C. Nucleotide sequence of rat mitochondrial NADH dehydrogenase subunit 1. GTG, a new initiator codon in vertebrate mitochondrial genome. Nucleic Acids Res. 1988 Jul 11;16(13):6233–6233. doi: 10.1093/nar/16.13.6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garesse R. Drosophila melanogaster mitochondrial DNA: gene organization and evolutionary considerations. Genetics. 1988 Apr;118(4):649–663. doi: 10.1093/genetics/118.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert T. L., Brown J. R., O'Hara P. J., Buroker N. E., Beckenbach A. T., Smith M. J. Sequence of tRNA(Thr) and tRNA(Pro) from white sturgeon (Acipenser transmontanus) mitochondria. Nucleic Acids Res. 1988 Dec 23;16(24):11825–11825. doi: 10.1093/nar/16.24.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harr R., Fällman P., Häggström M., Wahlström L., Gustafsson P. GENEUS, a computer system for DNA and protein sequence analysis containing an information retrieval system for the EMBL data library. Nucleic Acids Res. 1986 Jan 10;14(1):273–284. doi: 10.1093/nar/14.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haucke H. R., Gellissen G. Different mitochondrial gene orders among insects: exchanged tRNA gene positions in the COII/COIII region between an orthopteran and a dipteran species. Curr Genet. 1988 Nov;14(5):471–476. doi: 10.1007/BF00521271. [DOI] [PubMed] [Google Scholar]
- Himeno H., Masaki H., Kawai T., Ohta T., Kumagai I., Miura K., Watanabe K. Unusual genetic codes and a novel gene structure for tRNA(AGYSer) in starfish mitochondrial DNA. Gene. 1987;56(2-3):219–230. doi: 10.1016/0378-1119(87)90139-9. [DOI] [PubMed] [Google Scholar]
- Hixson J. E., Wong T. W., Clayton D. A. Both the conserved stem-loop and divergent 5'-flanking sequences are required for initiation at the human mitochondrial origin of light-strand DNA replication. J Biol Chem. 1986 Feb 15;261(5):2384–2390. [PubMed] [Google Scholar]
- Jacobs H. T., Elliott D. J., Math V. B., Farquharson A. Nucleotide sequence and gene organization of sea urchin mitochondrial DNA. J Mol Biol. 1988 Jul 20;202(2):185–217. doi: 10.1016/0022-2836(88)90452-4. [DOI] [PubMed] [Google Scholar]
- Kocher T. D., Thomas W. K., Meyer A., Edwards S. V., Päbo S., Villablanca F. X., Wilson A. C. Dynamics of mitochondrial DNA evolution in animals: amplification and sequencing with conserved primers. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6196–6200. doi: 10.1073/pnas.86.16.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignotte B., Barat M., Mounolou J. C. Characterization of a mitochondrial protein binding to single-stranded DNA. Nucleic Acids Res. 1985 Mar 11;13(5):1703–1716. doi: 10.1093/nar/13.5.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignotte B., Dunon-Bluteau D., Reiss C., Mounolou J. C. Sequence deduced physical properties in the D-loop region common to five vertebrate mitochondrial DNAs. J Theor Biol. 1987 Jan 7;124(1):57–69. doi: 10.1016/s0022-5193(87)80252-7. [DOI] [PubMed] [Google Scholar]
- Monnerot M., Mounolou J. C., Solignac M. Intra-individual length heterogeneity of Rana esculenta mitochondrial DNA. Biol Cell. 1984;52(3):213–218. doi: 10.1111/j.1768-322x.1985.tb00339.x. [DOI] [PubMed] [Google Scholar]
- Nagley P. Eukaryote membrane genetics: the Fo sector of mitochondrial ATP synthase. Trends Genet. 1988 Feb;4(2):46–51. doi: 10.1016/0168-9525(88)90066-2. [DOI] [PubMed] [Google Scholar]
- Roe B. A., Ma D. P., Wilson R. K., Wong J. F. The complete nucleotide sequence of the Xenopus laevis mitochondrial genome. J Biol Chem. 1985 Aug 15;260(17):9759–9774. [PubMed] [Google Scholar]
- Saccone C., Attimonelli M., Sbisà E. Structural elements highly preserved during the evolution of the D-loop-containing region in vertebrate mitochondrial DNA. J Mol Evol. 1987;26(3):205–211. doi: 10.1007/BF02099853. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. J., Banfield D. K., Doteval K., Gorski S., Kowbel D. J. Gene arrangement in sea star mitochondrial DNA demonstrates a major inversion event during echinoderm evolution. Gene. 1989 Mar 15;76(1):181–185. doi: 10.1016/0378-1119(89)90022-x. [DOI] [PubMed] [Google Scholar]
- Thomas W. K., Beckenbach A. T. Variation in salmonid mitochondrial DNA: evolutionary constraints and mechanisms of substitution. J Mol Evol. 1989 Sep;29(3):233–245. doi: 10.1007/BF02100207. [DOI] [PubMed] [Google Scholar]
- Walberg M. W., Clayton D. A. Sequence and properties of the human KB cell and mouse L cell D-loop regions of mitochondrial DNA. Nucleic Acids Res. 1981 Oct 24;9(20):5411–5421. doi: 10.1093/nar/9.20.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welter C., Dooley S., Zang K. D., Blin N. DNA curvature in front of the human mitochondrial L-strand replication origin with specific protein binding. Nucleic Acids Res. 1989 Aug 11;17(15):6077–6086. doi: 10.1093/nar/17.15.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme D. R., Macfarlane J. L., Okimoto R., Clary D. O., Wahleithner J. A. Bizarre tRNAs inferred from DNA sequences of mitochondrial genomes of nematode worms. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1324–1328. doi: 10.1073/pnas.84.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T. W., Clayton D. A. In vitro replication of human mitochondrial DNA: accurate initiation at the origin of light-strand synthesis. Cell. 1985 Oct;42(3):951–958. doi: 10.1016/0092-8674(85)90291-0. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yoneyama Y. [The nucleotide sequences of the heavy and light strand replication origins of the Rana catesbeiana mitochondrial genome]. Nihon Ika Daigaku Zasshi. 1987 Aug;54(4):429–440. doi: 10.1272/jnms1923.54.429. [DOI] [PubMed] [Google Scholar]



