Abstract
Age-related differences in thymic function influence the rapidity of T cell reconstitution following hematopoietic stem cell transplantation (HSCT). In adults, thymic reconstitution is delayed until after marrow engraftment is established, and is significantly improved by approaches that increase marrow chimerism, such as pre-transplant irradiation. In contrast, we show that neonatal mice undergo more rapid and efficient thymic reconstitution than adults, even when bone marrow engraftment is minimal and in the absence of pre-transplant radiation. We have previously shown that the neonatal thymus produces high levels of vascular endothelial growth factor (VEGF) that drives angiogenesis locally. In this report we show that inhibition of VEGF prior to HSCT prevents rapid thymic reconstitution in neonates, but has no effect on thymic reconstitution in adults. These data suggest that the early, radiation independent, thymic reconstitution unique to the neonatal host is mediated through VEGF, and reveals a novel pathway that might be targeted to improve immune reconstitution post-HSCT.
INTRODUCTION
Immune reconstitution following allogeneic hematopoietic stem cell transplantation (HSCT) is frequently slow and imperfect, with consequent morbidity from opportunistic infections1–5. Several factors contribute to immune dysfunction after allogeneic HSCT, including the presence of graft versus host disease, immune suppressive medications and damage to the thymic microenvironment from conditioning regimens and age-related involution 1–3.
Mature tolerant T cells are generated after HSCT through a complex stepwise process that begins with homing and engraftment in the marrow. Pre-transplant conditioning facilitates the initial stage of HSC engraftment and proliferation in the marrow, with subsequent differentiation into progeny that are recruited to the thymus to generate donor-derived thymocytes3. In murine studies, more than 2 weeks are required for donor derived CD4+CD8+ cells to first appear in the thymus after transplantation6 . In the clinical setting, severe T lymphopenia persists in patients for at least 100 days post transplant1–5.
The paradigm that assumes robust thymic reconstitution requires pre-transplant conditioning is challenged by observations made over several decades in the setting of HSCT for severe combined immune deficiency (SCID). When newborns and young infants with SCID undergo HSCT from HLA-matched siblings, T cell reconstitution can often be accomplished without pre-transplant conditioning7–10. In addition T cell reconstitution occurs rapidly and is more robust when HSCT for SCID is performed in the newborn period 3,11,12, suggesting that neonatal thymus is particularly permissive to reconstitution with donor cells.
Postnatal thymic growth in immune-competent humans and mice is most rapid during the neonatal period13–15. Our group has shown that during the first few days of murine postnatal life, the thymus is a site of vigorous thymocyte proliferation and VEGF-dependent angiogenesis13. Under the influence of locally produced VEGF, neonatal thymic vasculature is a dense network of capillaries. Beyond the first week of life as VEGF dependence is lost, the capillaries metamorphose to a mature hierarchic adult vasculature. Blocking VEGF during the neonatal period not only causes loss of vascular density and capillary pruning, but also reduces thymocyte numbers13. In the current studies, we show that the unique pattern of thymic reconstitution after bone marrow transplant (BMT) in the neonate is likely influenced by the same VEGF-mediated mechanisms that contribute to robust thymopoiesis during normal neonatal development.
MATERIALS AND METHODS
BMT and VEGF inhibition
In all experiments, unfractionated BM (1 × 107 cells/animal) from 6–8 week old C57Bl6/J (CD45.2) mice was transplanted intravenously into neonatal (one day old, via facial vein) or adult (six week old, via retro-orbital injection) NOD/SCID/IL-2Rγ−/− (NSG, CD45.1) mice (Jackson Research Laboratories, Bar Habor, Maine). Where indicated, mice were sub-lethally irradiated 2 hours pre-BMT with 150 cGy (neonates) or 270 cGy (adults) using a Cs-137 source13. For the timed transplant experiments in 1, 4, 7 and 10-day-old NSG mice, 1 × 107 unfractionated C57Bl6/J BM (CD45.2) cells were injected intra-hepatically without pre-transplant irradiation. In all indicated experiments, neonates and adults received either VEGF-Trap or hFc control (25 mg/kg) (Regeneron Pharmaceuticals, Tarrytown, NJ) 16 twenty-four hours prior to BMT (and 22 hours prior to irradiation). Mice were kept in specific pathogen-free facilities at Children’s Hospital Los Angeles (CHLA) and University of California, Los Angeles (UCLA) under approved protocols.
In vivo labeling of thymic vasculature
Mice were anesthetized and perfused with a mixture of biotinylated tomato lectin (10mg/kg; Vector Laboratories, Burlingame, CA) and streptavidin-Cy3 (3.1mg/kg; Jackson Immunoresearch, West Grove, PA) via facial vein (neonates) or cardiac injection (adults). Two minutes later, mice were perfused with PBS followed by 4% paraformaldehyde (PFA). Isolated thymi were fixed in 4% PFA13. Sections (200μm each) of 3% agarose–embedded thymi were examined for lectin labeling using a Leica TCS SP1 confocal microscope with a Plan Apo 10x/0.4NA objective and images acquired using Leica LCS software. Where indicated, mice received either 25mg/kg hFc or VEGF trap intraperitoneally twenty-four hours prior to the lectin staining. Due to the difficulty in identifying and dissecting thymi from non-transplanted adult NSG mice, thymi from NOD SCID β2m−/− were used to study vasculature of adult immune deficient mice.
Analysis
Mice were euthanized three or six weeks post-transplant for analysis of engraftment. Single cell suspensions were created from thymi and bone marrow of all animals. The cells were incubated with Mouse BD Fc Block (BD Biosciences, San Jose California) followed by incubation with rat anti-mouse CD45.2 (FITC) to identify donor cells and rat anti-mouse CD4 (APC) and CD8 (PE) antibodies (all BD Biosciences). Cells were acquired on a FACSCalibur or LSRII (BD Biosciences) and data was analyzed using FlowJo software (Tree Star, Ashland Oregon). For morphological analyses, 5 mm sections of paraffin-embedded thymi were stained with hematoxylin and eosin (H&E) and examined using an Olympus BX51 microscope fitted with a 4x/0.13 phl objective. Images were acquired with an Olympus DP 72 digital colour camera and DP2-BSW software.
Statistical Analysis
Data were analyzed using Wilcoxon rank-sum test or mixed model analysis of variance to account for inter-experiment variability. P-values < 0.05 were considered significant. All statistical analyses were carried out by statistical software SAS version 9.117.
RESULTS
Neonatal murine thymus is uniquely permissive to rapid and robust reconstitution after BMT, independent of pre-transplant conditioning
To examine if the impact of radiation on thymic and BM reconstitution is affected by recipient age, immune deficient neonatal and adult mice underwent allogeneic BMT with or without prior conditioning with sublethal doses of total body irradiation. Six weeks after transplantation, donor chimerism in the BM was significantly higher in all irradiated compared to non-irradiated mice, regardless of age at transplantation (Figure 1A, p < 0.001). Thus pre-transplant irradiation, even at sublethal doses, is required for efficient BM engraftment irrespective of the host age.
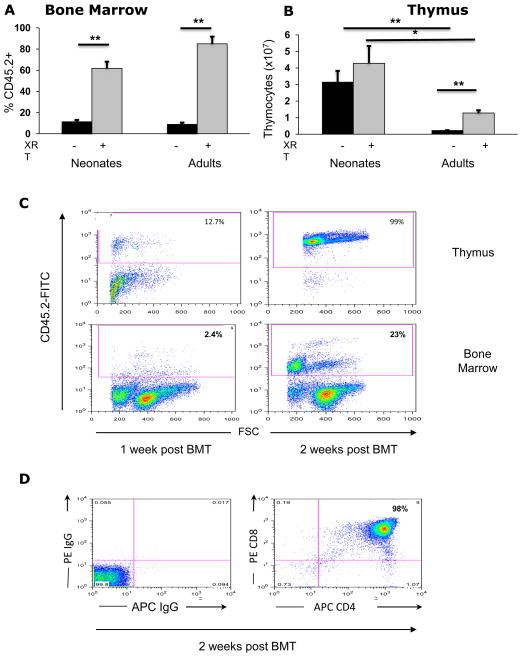
Figure 1. Neonatal mice show rapid and robust thymic reconstitution after HSCT.
(A) Frequency of CD45.2+ donor cells in the bone marrow of non-irradiated (-, black bars) or irradiated (+, grey bars) neonates or adult NSG mice analyzed six weeks post transplantation (neonates: no XRT n=22 vs. XRT n=16; adults: no XRT n=13 vs. XRT n=18). All numbers are mean ± standard error of the mean (SEM). (B) Thymocyte numbers, six weeks post transplantation (neonates: no XRT n=13 vs XRT n=14; adults: no XRT n=9 vs XRT n=8). (C) FACS plot comparing thymic and bone marrow reconstitution with donor (CD45.2+) cells at 1 and 2 weeks post transplant in NSG neonates. (D) FACS plots showing de novo generation of double positive (CD4+CD8+) thymocytes two weeks post transplantation in non-irradiated neonatal NSG mice (Right panel). Left panel: isotype control for CD4 and CD8. (*P<0.01, (**p < 0.001)
Despite similar patterns of BM chimerism in both age groups, a profound difference was seen in the pattern of thymic reconstitution between neonatal and adult recipients. In parallel with BM chimerism, reconstitution of the thymus in adults was almost undetectable in non-irradiated recipients, and was significantly increased with irradiation (Figure 1B, p < 0.001)
Thymic reconstitution was significantly greater in mice that received transplants as neonates when compared with adult recipients, irrespective of irradiation (Figure 1B, p<0.01 for irradiated, p <0.001 for non-irradiated cohorts). In addition, thymic reconstitution in neonates was rapid; even in the absence of irradiation, donor cells were detected in the thymus as early as 48 hours (data not shown) and efficiently reconstituted the thymus at one and two weeks post transplantation when compared to bone marrow reconstitution (Figure 1C). By 2 weeks most donor thymocytes had progressed to the DP (CD4+CD8+) stage (Figure 1D).
Thymic vasculature in immune-deficient and immune-competent mice is VEGF-dependent during the neonatal period
We have previously reported that the thymic vasculature in immune competent neonatal mice consists of a dense capillary network that is VEGF-dependent13. By adulthood, the vasculature has matured to a hierarchic pattern losing most of the capillaries and becoming VEGF-independent13. Although largely devoid of thymocytes, thymi of immune deficient neonatal mice have the same dense capillary network as immune competent neonatal mice, and this vascular phenotype is similarly lost by adulthood (Figure 2A). As with immune competent mice, inhibition of VEGF in the immune deficient neonatal thymus causes loss of dense capillaries and the appearance of an adult vascular pattern (Figure 2B).
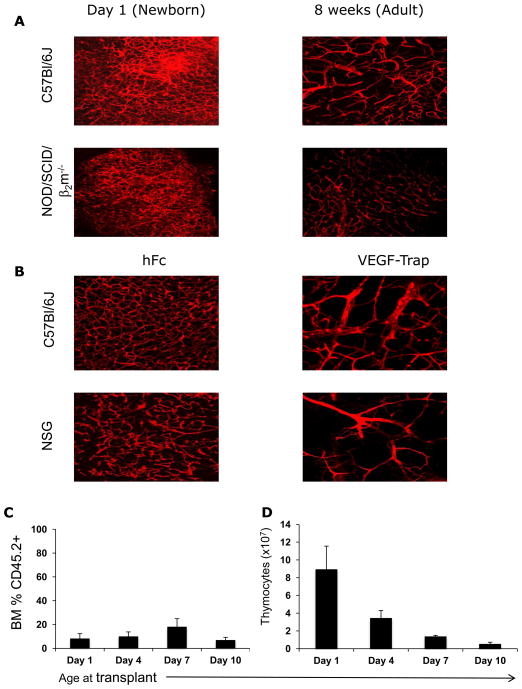
Figure 2. Immature vascular architecture and robust thymic reconstitution are lost during the first week of postnatal life.
(A) In vivo tomato lectin staining comparing vasculature between newborn (left panel) and adult (right panel) immune-competent (C57Bl/6) and immune- deficient (NOD/SCID/β2m−/−) mice. (B) VEGF-TRAP reduces vascular density of newborn thymi in immune-competent (C57Bl/6) and immune deficient (NSG) mice. Control mice were treated with hFC. (C, D) Effect of host age on bone marrow (C) and thymic reconstitution (D). NSG neonatal mice were transplanted with CD45.2+ BM without irradiation at Day 1, 4, 7 and 10 of life, (n = 4, 7, 7 and 9 mice respectively) and analyzed at 3 weeks post BMT. All numbers are mean ± standard error of the mean (SEM).
The window of efficient, radiation-independent thymic reconstitution closes during the first week of postnatal life
Our previous work showed that VEGF-dependent vasculature of the thymus transitions to a mature VEGF-independent vasculature during the first week of life (i.e. by the end of the murine neonatal period)13. We therefore evaluated if the permissive environment for thymic reconstitution post HSCT also changes during the first week of life. Animals were transplanted without prior irradiation at 1, 4, 7 or 10 days after birth, and thymocyte numbers were analyzed three weeks after transplantation. Age at time of transplant did not affect the level of donor chimerism in the bone marrow (Figure 2C). However, the ability of donor cells to reconstitute the thymus declined markedly during the first week of life (Figure 2D), a pattern coinciding with the previously reported temporal changes in vascular density and VEGF-dependence13.
VEGF inhibition significantly delays thymic reconstitution in neonatal NSG mice
In view of the parallel decline in thymic reconstitution and VEGF-dependence of thymic vasculature, we administered VEGF-Trap (a VEGFR1/2 fusion receptor18) twenty-four hours prior to transplantation to determine if thymic reconstitution was VEGF-dependent. VEGF inhibition significantly delayed thymic reconstitution in non-irradiated neonatal mice as shown by a significant reduction in donor thymopoiesis at 3 weeks post BMT (Figure 3A, p<0.01). A similar trend was seen three weeks after transplant of irradiated neonatal mice (Figure 3B). By six weeks post transplant, thymocyte numbers had increased to equivalent levels in control and VEGF-Trap treated neonates (Figure 3A, B), demonstrating that VEGF inhibition did not limit the ability of the thymus to recruit and support proliferation of precursors through the more typical mechanisms of reconstitution that occur after marrow engraftment is well-established. In contrast, thymic reconstitution of irradiated adults was not affected by pre-transplant VEGF inhibition (Figure 3C).
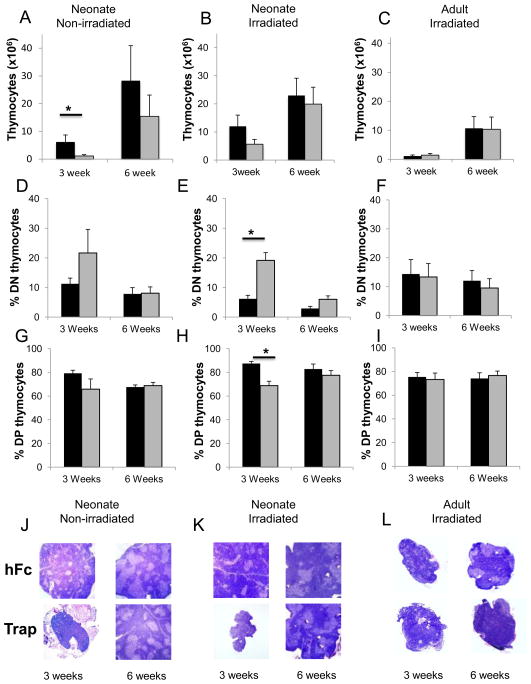
Figure 3. VEGF inhibition impairs early thymic engraftment of donor cells in mice transplanted as neonates, but not adults.
(A) Number of thymocytes in non-irradiated neonates harvested at three weeks [hFc (black bars) n=9; VEGF-Trap (grey bars) n=11, *p<0.01], and six weeks [hFc n=14; VEGF-Trap n=11; p = 0.3]. (B) Number of thymocytes in irradiated neonates harvested at three weeks (hFc n=6; VEGF-Trap n=5; p = 0.14) and 6 weeks (hFc n=5; VEGF-Trap n=4; p = 0.5) (C) Number of thymocytes in irradiated adults harvested at three weeks (hFc n=8; VEGF-Trap n=6; p = 0.48) or six weeks (hFc n=7, VEGF-Trap n=6; p = 0.84). (P values were unchanged when thymocyte numbers were normalized to body weight, to account for the smaller size of the VEGF-TRAP treated mice). (D-I) Percentage of CD4-CD8-; DN (D-F) and CD4+CD8+; DP (G-I) thymocytes from mice treated with hFc (black bars) and VEGF-Trap (grey bars) prior to transplant. (D, G) Non-irradiated neonates harvested 3 weeks (hFc n=9; VEGF-Trap n=5) and 6 weeks (hFc n=8; VEGF-Trap n=6) post transplant . (E,H) Irradiated neonates harvested 3 weeks (hFc n=5; VEGF-Trap n=3, *p < 0.01) and 6 weeks (hFc n=5; VEGF-Trap n=4,) post-transplant. (F, I) Irradiated adults harvested 3 weeks (hFc n=4; VEGF-Trap n=2) and 6 weeks (hFc n=6; VEGF-Trap n=6) post transplant. (J-L) Hematoxylin and eosin stains of thymi from NSG mice treated as described in Figures 3A-C, harvested at 3 and 6 weeks. All images shown are at original magnification x4 (J) Non-irradiated NSG mice transplanted as neonates. (K) Irradiated NSG mice transplanted as neonates. (L) Irradiated NSG mice transplanted as adults. In (L), the largest examples of tissue from the 3 week adult cohorts are shown as in most cases the thymus was not detected).
The age-specific effect of VEGF inhibition was also seen when examining the differentiation stages of donor-derived thymocytes. Treatment with VEGF-Trap resulted in a higher frequency of the immature CD4-CD8- double negative (DN) fraction in both non- irradiated (Figure 3D) and irradiated newborn mice (Figure 3E, p < 0.01) at 3 weeks post BMT. The frequency of CD4+CD8+ double positive (DP) thymocytes correspondingly dropped with VEGF inhibition in non-irradiated (Fig 3G) and irradiated (Fig3H, p <0.01) neonatal mice. However, by 6 weeks, VEGF inhibition had no effect on the frequencies of DN or DP thymocytes in the newborn mice (Fig 3D-H). VEGF inhibition had no effect on the frequency of DP or DN cells in adults (Fig 3F, I). Thus, VEGF inhibition in the neonate resulted in both lower numbers of total thymocytes and a more immature thymocyte profile at 3 weeks post BMT, consistent with a delay in thymic reconstitution. The frequencies of single positive CD4+ and CD8+ cells in both newborn and adult mice were not affected by VEGF inhibition (data not shown) suggesting that VEGF is unlikely to be involved in egress of mature thymocytes.
The effects of VEGF inhibition on thymic reconstitution were confirmed by analysis of thymic size and histology. Three weeks post transplant, thymi from VEGF-Trap treated neonates were smaller and lacked the medullary and cortical structures of control mice, regardless of whether the mice were irradiated prior to transplant. By six weeks, thymi from VEGF-Trap treated mice showed similar thymic architecture and size compared to control mice again demonstrating that VEGF inhibition did not permanently damage the thymus architecture or function (Figures 3J-K).
The size and histology of the thymi from irradiated adult NSG mice was not affected by VEGF inhibition (Figure 3L). Of note, at three weeks post BMT, thymi from mice transplanted as adults were significantly smaller and less cellular than those transplanted as neonates, in most cases being too small to clearly identify. By six weeks post BMT, even though thymi from adult recipients had developed distinct cortical and medullary structures (Figure 3L), they never achieved sizes equivalent to those from mice transplanted as neonates.
DISCUSSION
Our data demonstrate that the thymus of the newborn immune-deficient mouse provides a highly permissive environment for rapid thymic reconstitution after BMT, and that VEGF-mediated mechanisms have a role in producing this unique environment. The thymic microenvironment changes during the first seven days after birth, during which time the thymic vasculature matures and becomes VEGF-independent13. Neonatal thymic reconstitution is both rapid and robust, does not require pre-transplant conditioning, and does not require a high level of BM chimerism.
During steady state, ingress of progenitors into the thymus occurs in waves in response to the availability of empty intra-thymic niches19–21. These waves of recruitment and “settling” of marrow-derived progenitors in the adult murine thymus are thought to be mediated at least partly by interactions between the homing molecules P-selectin with PSGL-122, and the chemokine receptors CCR7 and CCR9 with their ligands23, 24. Zlotoff et al found that although long-term thymic reconstitution after bone marrow transplantation utilized the same mechanisms as seen in steady state, early (3 weeks post BMT) thymic reconstitution occurred independently of CCR7 and CCR925. It is interesting to note that in our own study with neonatal animals, dependence on VEGF was also different between the 3 week and 6 week phases of reconstitution, irrespective of irradiation. Although the data from Zlotoff et. al. was generated in a different transplant setting from our own (adult irradiated mice vs. neonatal mice), the two studies share the concept that early and late thymic reconstitution may be mediated by different mechanisms.
We propose that high local VEGF specifically affects the early phase of thymic seeding in neonates, as VEGF inhibition reduces thymocyte numbers 3 weeks after BMT. By six weeks after BMT, when the delivery of thymic precursors is dependent on previously described mechanisms of recruitment from the BM, the effect of VEGF inhibition on thymocyte numbers is not evident. This later production of marrow-derived thymocytes presumably overlaps and further adds to the thymopoiesis derived from the initial phase of thymic seeding, thereby accounting for higher levels of thymocyte production in neonates even at later time points.
Porritt et. al. found that at least 13 days (average >15 days) were required for cells entering the adult thymus to reach the outer cortex and begin to differentiate into DP cells, and even by 3 weeks after transplantation, most donor cells had not passed the DN3 stage of differentiation6. In our own studies, >95% of donor cells had reached the double positive stage of thymocyte differentiation by 2 weeks after transplantation of neonatal animals. VEGF inhibition in the newborn mice skewed the thymocyte differentiation towards a more immature phenotype with a higher frequency of DN and relatively fewer DP thymocytes. We postulate that in newborn mice, VEGF-dependent vasculature permits rapid ingress of thymic progenitors (possibly even by direct seeding prior to marrow engraftment) that then undergo proliferation in an empty niche resulting in rapid and robust reconstitution of the thymus. It is also possible that local VEGF mediates rapid reconstitution of the thymus by augmenting differentiation of DN to DP thymocytes, and subsequent proliferation through mechanisms that are yet to be elucidated.
It is intriguing to speculate whether the processes described here in the murine setting may also contribute to the robust thymopoiesis seen in clinical transplants of infants, particularly in those with SCID who, without pre-transplant conditioning, can achieve rapid T cell reconstitution with little or no donor contribution to the myeloid and B cell lineages26, 27. Our findings reveal previously unreported mechanisms that mediate efficient thymic reconstitution in neonates, and present novel pathways that might be targeted to improve post BMT immune reconstitution in the adult.
Acknowledgments
We would like to thank Gavin Thurston (Regeneron Pharmaceuticals, Tarrytown, NY) for generously providing hFc and VEGF-Trap; Lora Barsky and Ewa Zelinska (CHLA), and Jessica Scholes and Felicia Codrea (Broad Stem Cell Research Center flow cytometry core, UCLA) for assistance with flow cytometry; Chintan Parekh, Brile Chung, Erica Sloan and Katherine Monaghan for invaluable comments and feedback. This work was supported by a California Institute for Regenerative Medicine fellowship to A.R.C (CHLA), and grants from the National Institutes of Health (1P01AI072686) and California Institute of Regenerative Medicine grant (RM1-01707) to G.M.C.
Footnotes
Financial Disclosure: The authors have nothing to disclose
Author Contributions and Conflict of Interests
A.R.C., B.T.S. and G.M.C. designed research and wrote the paper. A.R.C., B.T.S., S.G., L.A.K., J.J. and J.A. performed research, collected, analyzed and interpreted data. X.W. performed statistical analysis. The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parkman R, Weinberg KI. Immunological reconstitution following bone marrow transplantation. Immunol Rev. 1997;157:73–8. doi: 10.1111/j.1600-065x.1997.tb00975.x. [DOI] [PubMed] [Google Scholar]
- 2.Storek J, Geddes M, Khan F, Huard B, Helg C, Chalandon Y, et al. Reconstitution of the immune system after hematopoietic stem cell transplantation in humans. Semin Immunopathol. 2008;30(4):425–37. doi: 10.1007/s00281-008-0132-5. [DOI] [PubMed] [Google Scholar]
- 3.Krenger W, Blazar BR, Hollander GA. Thymic T-cell development in allogeneic stem cell transplantation. Blood. 2011;117(25):6768–76. doi: 10.1182/blood-2011-02-334623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ciurea SO, Mulanovich V, Jiang Y, Bassett R, Rondon G, McMannis J, et al. Lymphocyte recovery predicts outcomes in cord blood and T cell-depleted haploidentical stem cell transplantation. Biol Blood Marrow Transplant. 2011;17(8):1169–75. doi: 10.1016/j.bbmt.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seggewiss R, Einsele H. Immune reconstitution after allogeneic transplantation and expanding options for immunomodulation: an update. Blood. 2010;115(19):3861–8. doi: 10.1182/blood-2009-12-234096. [DOI] [PubMed] [Google Scholar]
- 6.Porritt HE, Gordon K, Petrie HT. Kinetics of Steady-state Differentiation and Mapping of Intrathymic-signaling Environments by Stem Cell Transplantation in Nonirradiated Mice. Journal of Experimental Medicine. 2003;198(6):957–962. doi: 10.1084/jem.20030837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haynes BF, Markert ML, Sempowski GD, Patel DD, Hale LP. The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection. Annu Rev Immunol. 2000;18:529–60. doi: 10.1146/annurev.immunol.18.1.529. [DOI] [PubMed] [Google Scholar]
- 8.Mackall CL, Fleisher TA, Brown MR, Andrich MP, Chen CC, Feuerstein IM, et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med. 1995;332(3):143–9. doi: 10.1056/NEJM199501193320303. [DOI] [PubMed] [Google Scholar]
- 9.Hakim FT, Memon SA, Cepeda R, Jones EC, Chow CK, Kasten-Sportes C, et al. Age-dependent incidence, time course, and consequences of thymic renewal in adults. J Clin Invest. 2005;115(4):930–9. doi: 10.1172/JCI22492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eyrich M, Wollny G, Tzaribaschev N, Dietz K, Brugger D, Bader P, et al. Onset of thymic recovery and plateau of thymic output are differentially regulated after stem cell transplantation in children. Biol Blood Marrow Transplant. 2005;11(3):194–205. doi: 10.1016/j.bbmt.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Buckley RH. Molecular defects in human severe combined immunodeficiency and approaches to immune reconstitution. Annu Rev Immunol. 2004;22:625–55. doi: 10.1146/annurev.immunol.22.012703.104614. [DOI] [PubMed] [Google Scholar]
- 12.Myers LA. Hematopoietic stem cell transplantation for severe combined immunodeficiency in the neonatal period leads to superior thymic output and improved survival. Blood. 2002;99(3):872–878. doi: 10.1182/blood.v99.3.872. [DOI] [PubMed] [Google Scholar]
- 13.Cuddihy AR, Ge S, Zhu J, Jang J, Chidgey A, Thurston G, et al. VEGF-mediated crosstalk within the neonatal murine thymus. Blood. 2009;113(12):2723–31. doi: 10.1182/blood-2008-06-162040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinmann GG. Changes in the human thymus during aging. Curr Top Pathol. 1986;75:43–88. doi: 10.1007/978-3-642-82480-7_2. [DOI] [PubMed] [Google Scholar]
- 15.Haynes BF, Sempowski GD, Wells AF, Hale LP. The human thymus during aging. Immunol Res. 2000;22(2–3):253–61. doi: 10.1385/IR:22:2-3:253. [DOI] [PubMed] [Google Scholar]
- 16.Andrade J, Ge S, Symbatyan G, Rosol MS, Olch AJ, Crooks GM. Effects of sublethal irradiation on patterns of engraftment after murine bone marrow transplantation. Biol Blood Marrow Transplant. 2011;17(5):608–19. doi: 10.1016/j.bbmt.2010.12.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.SAS I. SAS/GeneticsTM 9.1.3 User’s Guide. SAS Institute Inc; Cary, NC: 2005. [Google Scholar]
- 18.Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, Russell M, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99(17):11393–8. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foss DL, Donskoy E, Goldschneider I. The importation of hematogenous precursors by the thymus is a gated phenomenon in normal adult mice. J Exp Med. 2001;193(3):365–74. doi: 10.1084/jem.193.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donskoy E, Foss D, Goldschneider I. Gated importation of prothymocytes by adult mouse thymus is coordinated with their periodic mobilization from bone marrow. J Immunol. 2003;171(7):3568–75. doi: 10.4049/jimmunol.171.7.3568. [DOI] [PubMed] [Google Scholar]
- 21.Cyster JG. Settling the thymus: immigration requirements. J Exp Med. 2009;206(4):731–734. doi: 10.1084/jem.20090458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossi FM, Corbel SY, Merzaban JS, Carlow DA, Gossens K, Duenas J, et al. Recruitment of adult thymic progenitors is regulated by P-selectin and its ligand PSGL-1. Nat Immunol. 2005;6(6):626–34. doi: 10.1038/ni1203. [DOI] [PubMed] [Google Scholar]
- 23.Ueno T, Saito F, Gray DH, Kuse S, Hieshima K, Nakano H, et al. CCR7 signals are essential for cortex-medulla migration of developing thymocytes. J Exp Med. 2004;200(4):493–505. doi: 10.1084/jem.20040643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krueger A, Willenzon S, Lyszkiewicz M, Kremmer E, Forster R. CC chemokine receptor 7 and 9 double-deficient hematopoietic progenitors are severely impaired in seeding the adult thymus. Blood. 2010;115(10):1906–12. doi: 10.1182/blood-2009-07-235721. [DOI] [PubMed] [Google Scholar]
- 25.Zlotoff DA, Zhang SL, De Obaldia ME, Hess PR, Todd SP, Logan TD, et al. Delivery of progenitors to the thymus limits T-lineage reconstitution after bone marrow transplantation. Blood. 2011;118(7):1962–70. doi: 10.1182/blood-2010-12-324954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buckley RH, Schiff SE, Schiff RI, Roberts JL, Markert ML, Peters W, et al. Haploidentical bone marrow stem cell transplantation in human severe combined immunodeficiency. Semin Hematol. 1993;30(4 Suppl 4):92–101. discussion 102–4. [PubMed] [Google Scholar]
- 27.Buckley RH, Schiff SE, Schiff RI, Markert L, Williams LW, Roberts JL, et al. Hematopoietic stem-cell transplantation for the treatment of severe combined immunodeficiency. N Engl J Med. 1999;340(7):508–16. doi: 10.1056/NEJM199902183400703. [DOI] [PubMed] [Google Scholar]