Abstract
Background
Cyanide (CN) toxicity is a serious clinical problem and can occur with sodium nitroprusside (SNP), accidental smoke inhalation, industrial mishaps and bio-terrorism. In this study we induced severe CN toxicity independently with SNP or sodium cyanide (NaCN) in a juvenile pig model to demonstrate reversal of severe CN toxicity with a new antidote, sulfanegen sodium, a prodrug of 3-mercaptopyruvate.
Methods
SNP study: A pilot study in eleven anesthetized, mechanically ventilated juvenile pigs allowed us to determine the dose of SNP to induce CN toxicity. Blood CN, serum lactates and blood gases were monitored. CN toxicity was defined as the occurrence of severe lactic acidosis accompanied by significant elevation in blood CN levels. Based on this pilot study, eight anesthetized pigs received a high-dose IV infusion of SNP (100mg/hr) for 2 hours to induce CN toxicity. They were then randomized to receive either sulfanegen sodium or placebo. Four pigs received 3 doses of sulfanegen sodium (2.5g IV) every hour after induction of severe CN toxicity, while 4 pigs received placebo.
NaCN study
A pilot study was conducted in four spontaneously ventilating pigs sedated with propofol plus ketamine to demonstrate hemodynamic and metabolic stability for several hours. After this, 6 pigs were similarly sedated and given NaCN in bolus aliquots to produce CN toxicity ultimately resulting in death. Hemodynamics and metabolic variables were followed to define peak CN toxicity. In another group of six pigs, severe CN toxicity was induced by this method, and at peak toxicity, the animals were given sulfanegen sodium (2.5 g IV) followed by a repeat dose 60 minutes later in surviving animals.
Results
SNP study: The pilot study demonstrated the occurrence of a significant increase in blood CN levels (p<0.05) accompanied by severe lactic acidemia (p<0.05) in all pigs receiving a high dose of SNP. Administration of the sulfanegen antidote resulted in progressive significant reduction in blood lactate and CN levels with 100% survival (p<0.05), whereas the placebo-treated pigs deteriorated and did not survive (p<0.05).
NaCN study
NaCN injection resulted in CN toxicity accompanied by severe lactic acidosis and mortality in all the pigs. Sulfanegen sodium reversed this toxicity and prevented mortality in all the pigs treated with this antidote.
Conclusions
CN toxicity can be successfully induced in a juvenile pig model with SNP or NaCN. The prodrug, sulfanegen sodium, is effective in reversing CN toxicity induced by SNP or NaCN.
Introduction
Cyanide (CN) inhibits the enzyme cytochrome C oxidase in the electron transport system of mitochondria and disrupts the aerobic production of adenosine triphosphate.1,2 CN toxicity is well-described and commonly occurs after smoke inhalation, industrial exposure and dietary ingestion.3 CN has also been used intentionally to induce toxicity in homicide, suicide, chemical warfare and bio-terrorism. Clinically, CN toxicity has been reported after the use of sodium nitroprusside (SNP) in intensive care units and perioperatively for blood pressure (BP) control.4 In this study we present a juvenile porcine model of CN toxicity induced by the injection of SNP and by the direct injection of sodium cyanide (NaCN), and its reversal with a new antidote, sulfanegen sodium, a prodrug of 3-mercaptopyruvate (3-MP).5 The enzyme 3-mercaptopyruvate sulfurtransferase (3-MST) is present in the soluble and mitochondrial cell compartments of most organs including the brain, heart, liver and kidneys.6 This enzyme uses the endogenous substrate 3-MP, available from the transamination of cysteine, to provide the needed sulfur to detoxify CN to thiocyanate (SCN). However, only limited amounts of cysteine, and therefore of 3-MP, are available in the heart and brain, thus limiting the ability of 3-MST to be effective in acute CN poisoning. Because 3-MP is rapidly metabolized and is ineffective when given IV,7 the administration of a prodrug of 3-MP, namely, sulfanegen sodium, will allow sustainable availability of 3-MP for 3-MST. Accordingly, we evaluated the effect of sulfanegen sodium in reversing severe CN toxicity induced by continuous IV SNP and by bolus IV injections of NaCN in juvenile Yorkshire pigs.
Background information about sulfanegen sodium
The structure of the prodrug sulfanegen sodium is displayed in Figure 1. This compound was prepared essentially as previously described5. Sulfanegen sodiumis a dimer, which dissociates nonenzymatically in physiological systems at pH 7.4 to two equivalents of the monomer 3-MP. In vivo, 3-MP is rapidly metabolized by the enzyme 3-MST to release a reactive sulfane sulfur intermediate that captures a CN anion and converts it to SCN, a nontoxic product that is excreted in the urine. This prodrug has been shown to be highly effective in reversing CN toxicity in a sublethal murine and rabbit model.5,8,9
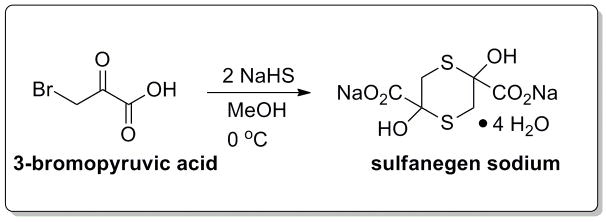
Figure 1.
Sulfanegen sodium (2,5-dihydroxy-1,4-dithiane-2,5-dicarboxylic acid disodium salt) is formulated from 3-bromopyruvic acid with the addition of sodium hydrogen sulphide and methyl alcohol5.
Methods
The study was conducted in healthy juvenile Yorkshire pigs weighing 20–30 kg. The Institutional Animal Care and Use Committee approved all the experimental protocols, and all procedures were conducted in accordance with the guidelines set by the Association for Assessment and Accreditation of Laboratory Animal Care.
Pilot studies
We conducted a pilot study in 11 pigs to demonstrate stability of our animal preparation and define the SNP dose and metabolic variable that would serve as a marker for fatal CN toxicity. All animals were sedated witha Telazol® (10 mg/kg i.m.), endotracheally intubated, their lungs mechanically ventilated after vecuronium administration and maintained under general anesthesia with isoflurane (FIO2=0.7). Normothermia was ensured with a forced-air warming device. Using sterile techniques, a femoral artery catheter was placed percutaneously for continuous BP monitoring and for obtaining arterial blood samples. A multilumen pulmonary artery catheter was also aseptically placed percutaneously in the femoral or jugular vein for obtaining mixed venous blood and continuously monitor central venous pressure (CVP) and pulmonary artery pressure (PAP). Electrocardiography, SPO2, end-tidal CO2 and inhaled anesthetic concentration were also monitored. After obtaining baseline hemodynamic and metabolic measurements (blood gases, lactates and CN levels), the animals remained anesthetized for 4 hours. Three animals served as controls during which time they received only placebo (saline) infusion. Six animals received a continuous infusion of SNP (2 animals were given ≈ 2.5 mg/kg/hr and the other 4 received ≈ 5 mg/kg/hr) to induce and evaluate the severity of CN toxicity. During SNP infusion, BP was supported at values within 30% of baseline with the infusion of phenylephrine (≈ 1–10 mg/hr). Two other pigs served as phenylephrine controls and received only phenylephrine (≈ 10 mg/hr). The pilot studies demonstrated the occurrence of significant and marked elevation in blood CN levels in the high dose SNP group (mean± SD peak CN levels achieved were 13.9± 0.018 mg/L) and this was accompanied by concomitant significant elevations in serum lactate levels (mean± SD peak lactate levels achieved were 14.1±0.9 mg/dL) and mortality. The low dose SNP group, as well as the phenyephrine and control groups survived the experimental procedures without significant lactic acidemia. They were killed 7 – 14 days later.
Effect of sulfanegen sodium on sodium nitroprusside toxicity
After completion of the pilot studies, we evaluated the effect of sulfanegen sodum on reversing CN toxicity. We studied 8 juvenile pigs that were induced into CN toxicity by the infusion of a high dose of SNP (≈ 5 mg/kg/hr) as in the pilot studies. As in the pilot study, the systolic BPs were supported within 30% of the control values with phenylephrine infusion (1–10 mg/hr). After two hours, the SNP infusion was stopped and the animals were administered either a placebo (4 animals) or the experimental prodrug sulfanegen sodium (4 animals). The dose of sulfanegen sodium extrapolated from a previous mouse study5,8 consisted of 2.5 g IV bolus and repeated hourly for a total of 3 doses in surviving pigs.
Effect of sulfanegen sodium on NaCN toxicity
For this experiment, the pigs were prepared similarly as in the previous pigs with the following changes. After sedation with Telazol®, the pigs were given atropine (0.4 mg IV) as an anti-sialagogue and sedated with a continuous infusion of propofol + ketamine (to 10 ml of propofol, 1% was added 400 mg ketamine). This was titrated at 6–15 ml/hour for continuous sedation with preservation of spontaneous breathing. Oxygen was delivered at 2 liters per minute via a nasal canula. Total IV anesthesia with spontaneous ventilation was used for this portion of the experiment to determine the effect of CN on ventilation and better mimic CN toxicity caused by smoke inhalation. We observed 4 pigs for 4 hours to demonstrate hemodynamic and metabolic stability.
Induction of CN toxicity: We induced CN toxicity in 6 pigs by bolus IV administration of NaCN given in aliquots of 0.55 mg/kg. This was repeated at 15-minute intervals until the occurrence of apnea. At this time, respiratory assistance was provided by oral endotracheal intubation, ambu-bag assistance and the transient administration of 100% oxygen. NaCN was continued at the same frequency until the occurrence of significant hemodynamic instability (severe bradycardia, arrhythmias and eventually cardiac arrest) and lactic acidemia leading to death. Blood chemistries, lactates and CN levels were monitored every 15 minutes. For the next 6 pigs, we administered NaCN in a similar manner until severe CN toxicity was observed, as confirmed by severe lactic acidemia and the occurrence of apnea requiring airway assistance. Sulfanegen sodium (2.5 g) was then given as a bolus IV dose and repeated 60 minutes later in surviving pigs.
Data are reported as mean ± SD. Significance (P<0.05) was determined by repeated ANOVA followed by the Tukey’s test or Fisher’s exact test as appropriate.
Results
SNP studies
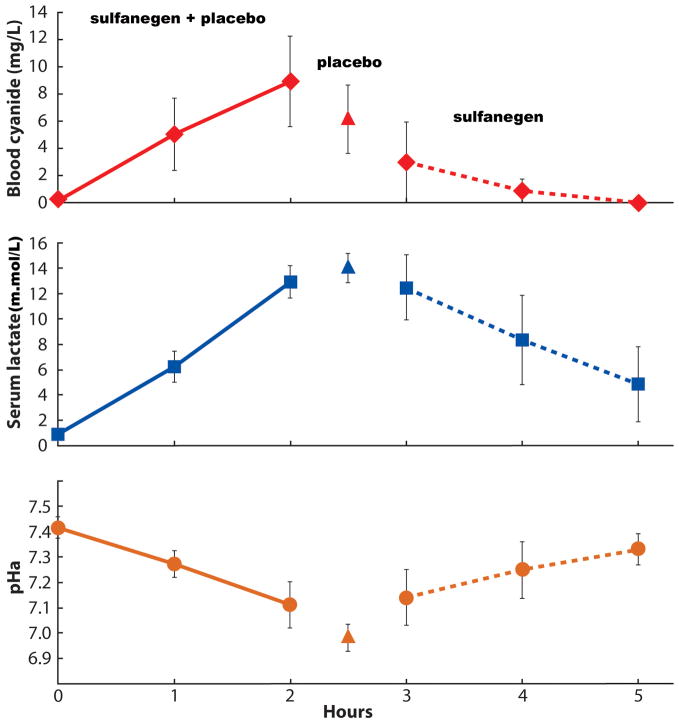
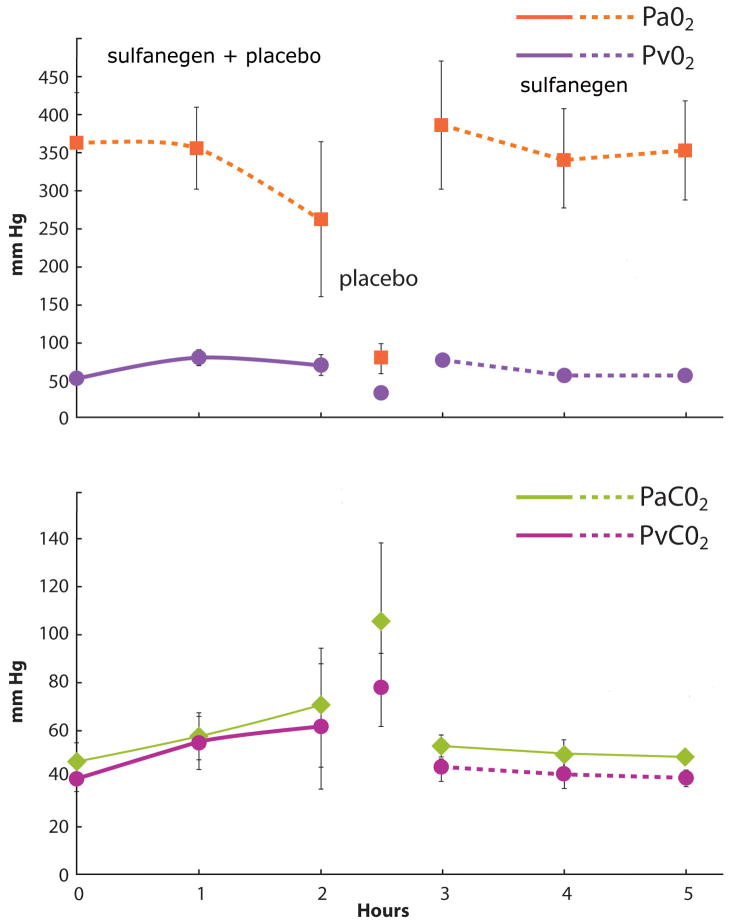
Table 1 summarizes the hemodynamics observed during high-dose SNP infusion, followed by the administration of either placebo or sulfanegen sodium during peak CN toxicity. The mean BP decreased significantly accompanied by an increase in heart rate. The mean PAP increased significantly without a significant change in mean CVP, except for a transient significant increase observed after the first dose of sulfanegen sodium. In the surviving pigs, these values returned to baseline levels. In the pigs that received placebo, there was deterioration in vital signs leading to mortality. SNP administration resulted in lactic acidosis and significant cyanidemia accompanied by a significant reduction in arterial pH in all 8 pigs (Figure 2). The changes observed in PaO2, PVO2, PaCO2 and PvCO2 are displayed in Figure 3. Unlike placebo, administration of sulfanegen sodium resulted in survival (p = 0.0286 placebo vs sulfanegen sodium) and recovery of these variables (Figure 2). All the surviving animals awakened successfully and were able to ambulate and feed within a few hours after tracheal extubation. No gross neurological deficits were evident.
Table 1.
Results of hemodynamic changes observed during SNP infusion, followed either by placebo or sulfanegen sodium given 2 hours after SNP infusion (all values are mean±SD).
| Group | Mean arterial blood pressure (mm Hg) | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | 1 hr | 2 hr | 30 min after placebo(last value before death) or sulfanegen Rx | 1 hr after placebo or sulfanegen Rx | 2 hr after placebo or sulfanegen Rx | 3 hr after placebo or sulfanegen Rx | |
| Placebo | 88±7 | *67±5 | *66±21 | *26±5 | Pigs died | ||
| Sulfanegen | 94±14 | *77±10 | *81±15 | *69±7 | 88±14 | 93±18 | 90±13 |
| Heart Rate (bpm) | |||||||
| Placebo | 104±19 | *149±13 | *155±24 | *60±19 | Pigs died | ||
| Sulfanegen | 116±28 | *140±10 | *148±23 | *178±26 | *165±29 | 121±14 | 119±11 |
| Mean pulmonary artery pressure (mm Hg) | |||||||
| Placebo | 12±3 | *22±4 | *26±6 | *21±5 | Pigs died | ||
| Sulfanegen | 16±3 | *22±4 | *29±2 | *21±5 | *28±5 | *22±5 | 15±2 |
| Mean central venous pressure (mm Hg) | |||||||
| Placebo | 5±2 | 6±4 | 7±3 | 7±2 | Pigs died | ||
| Sulfanegen | 4±1 | 7±4 | 7±1 | 5±2 | *9±1 | 7±3 | 5±1 |
SNP = sodium nitroprusside
p < 0.05 vs baseline
Figure 2.
Note the significant changes (p < 0.05 at 2 hours vs control value at time 0) in blood cyanide (upper graph), serum lactate (middle graph) and arterial pH (lower graph) in all 8 pigs during sodium nitroprusside infusion (sulfanegen and placebo group). At the end of the two hour infusion, 4 pigs were treated with sulfanegen sodium (2.5 g IV) and the other 4 were given a similar volume of normal saline placebo. None of the placebo-treated pigs survived (placebo group). All the antidote-treated pigs survived with significant improvements (p < 0.05 at 5 hours vs control) in pH, cyanide and lactate levels (sulfanegen group). The surviving pigs received 2 additional doses of sulfanegen sodium (2.5 g IV) at hours 3 and 4.
Figure 3.
Changes in arterial and mixed venous oxygen (upper graph; arterial oxygen tension is depicted by the square symbol and mixed venous oxygen tension by the circles) and carbon dioxide tensions (lower graph; arterial carbon dioxide tension is depicted by the diamond symbol and mixed venous carbon dioxide tension by circles) during sodium nitroprusside (SNP) infusion in all 8 pigs (duration of SNP infusion and times for placebo and sulfanegen injection are similar to Figure 1). The venous oxygen tension increased significantly as did the carbon dioxide tensions. There was also a significant decline in arterial oxygen tension (p < 0.05 at 2 hours vs control value at time 0). After SNP infusion, 4 pigs given saline placebo showed a marked decline in oxygen tensions associated with an increase in carbon dioxide levels just before death. The 4 pigs given sulfanegen sodium survived and showed recovery of arterial oxygen tension with normalization after two hours, as did the carbon dioxide levels (p > 0.05 at 5 hours vs control), pH, cyanide and lactate levels (C). The surviving pigs received 2 additional doses of sulfanegen sodium (2.5 g IV) at hours 3 and 4.
NaCN studies
The control pigs (n=4) were stable (Table 2). Table 3 summarizes the hemodynamic changes observed during NaCN infusion and the effect of sulfanegen sodium in the treated group. Administration of NaCN resulted in significant lactic acidosis and increases in blood CN levels (Figures 4 and 5). At peak CN toxicity, the nontreated pigs deteriorated and died (Table 4, Figure 4). The to-be treated pigs were tachycardic and demonstrated significant increases in CVP and PAP before they were given the antidote (Table 3). The administration of sulfanegen sodium resulted in survival, reversal of lactic acidosis and blood CN levels (Figure 5, Tables 3 and 4). All surviving animals awakened successfully, ambulated and began feeding a few hours after tracheal extubation. No gross neurological deficits were evident.
Table 2.
The control pigs of the sodium cyanide study did not have significant changes in arterial pH, blood cyanide and lactate levels.
| Item | Baseline | End of 4 hour period |
|---|---|---|
| pH (arterial) | 7.40 ± 0.07 | 7.44 ± 0.00 |
| Blood cyanide (mg/L) | 0.08 ± 0.07 | 0.08 ± 0.06 |
| Serum lactates (mmol/L) | 0.8 ± 0.24 | 1.3 ± 0.98 |
Table 3.
Hemodynamic changes observed during cyanide (CN) infusion. (all values are mean±SD)
| Mean Arterial Blood Pressure | ||||||
|---|---|---|---|---|---|---|
| Group | Baseline | peak Cyanide toxicity value | Last value before death (no antidote group); 15 minutes after sulfanegen (sulfanegen group) | 60 minutes after sulfanegen | 120 minutes after sulfanegen | 180 minutes after sulfanegen |
| No antidote | 92±11 | 117±23 | *22±10 | N.A. | N.A. | N.A. |
| Sulfanegen Group | 91±10 | *122±18 | *71±25 | *75±16 | 81±13 | 87±9 |
| Heart Rate (beats/minute) | ||||||
| No antidote | 111+20 | *182±36 | *43±20 | N.A. | N.A. | N.A. |
| Sulfanegen Group | 118±31 | *149±38 | *155±29 | *152±13 | *154±32 | 143±29 |
| Mean Pulmonary Artery Pressure (mmHg) | ||||||
| No antidote | 15±0 | *19±1 | 7±2 | N.A. | N.A. | N.A. |
| Sulfanegen Group | 16±3 | *27±9 | 16±4 | 15±3 | 15±2 | 14±1 |
| CVP (mm Hg) | ||||||
| No antidote | 6±2 | *18±8 | 9±5 | N.A. | N.A. | N.A. |
| Sulfanegen Group | 4±1 | *12±7 | 6±5 | 6±5 | 5±2 | 6±3 |
p < 0.05 vs baseline
(The results shown are those obtained at baseline and during peak CN toxicity. The group of pigs that did not receive the antidote all died. The other group of pigs received the antidote sulfanegen sodium at peak CN toxicity. A second dose of sulfanegen sodium was administered after 60 minutes to surviving pigs in this group).
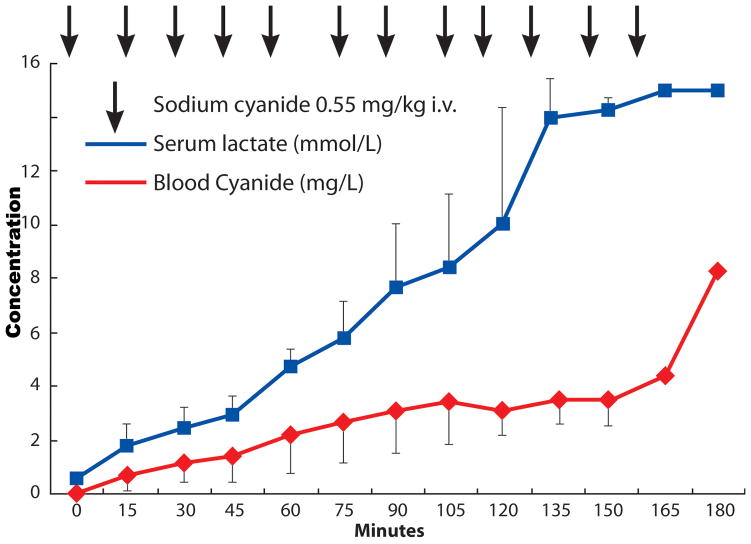
Figure 4.
Changes in blood cyanide (depicted by the diamond symbols) and serum lactate levels (depicted by the square symbols) with the bolus injections of sodium cyanide, followed every 15 minutes until the occurrence of cardiac arrest (all values are mean ± SD).
Figure 5.
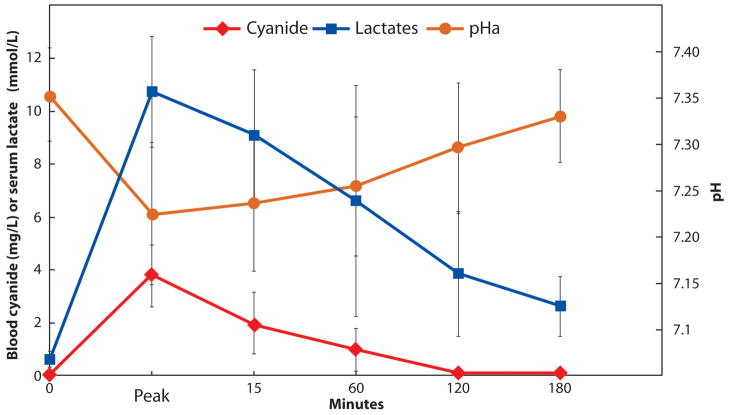
Note the significant increase (p < 0.05 peak vs control) in blood cyanide (depicted by the diamond symbols) and serum lactate (depicted by the square symbols) similar to those shown in Figure 4, along with a reduction in arterial pH (depicted by the circles) in this group of pigs given sodium cyanide. At peak toxicity during severe lactic acidemia, the pigs received the first dose of sulfanegen sodium (2.5 g IV) followed by a second dose 60 minutes later. All the pigs survived with significant (p > 0.05 control vs 180 minutes) improvements in serum lactate and arterial pH. The blood cyanide levels at 180 minutes were also significantly lower when compared to peak levels (p < 0.05).
Table 4.
Sulfanegen sodium was effective in reversing cyanide (CN) toxicity in both groups of pigs administered either sodium nitroprusside (SNP) or sodium cyanide (NaCN)
| Group | Intervention | Number | Survived | Died |
|---|---|---|---|---|
| High Dose SNP | Placebo | 4 | 0 | 4 |
| High Dose SNP | sulfanegen sodium | 4 | *4 | 0 |
| Sodium cyanide | None | 6 | 0 | 6 |
| Sodium cyanide | sulfanegen sodium | 6 | †6 | 0 |
p < 0.05 (Fisher’s exact test) vs high dose SNP placebo
p < 0.05 (Fisher’s exact test) vs sodium cyanide no intervention
Discussion
Lactic acidosis has been reported as the mechanism of CN toxicity.10 This is due to inactivation of cytochrome c oxidase that results in decreased use of pyruvate for adenosine triphospate production. The excess pyruvate is metabolized to lactate in the cells.6 During CN poisoning, CN is metabolized to the less toxic SCN accomplished via transsulfuration.6 This reaction requires the enzymes rhodanese and 3-MST. Both of these enzymes are widely distributed in tissues, with rhodanese being compartmentalized in mitochondria while 3-MST, being present in both cytoplasm and mitochondria, is more widely distributed than rhodanese. Whereas rhodanase is more abundant in liver and kidney, 3-MST is more universal and facilitates sulfuration of CN initially in the cytoplasm and then in the mitochondria6.
The currently available CN antidote, sodium thiosulfate, is a substrate for both rhodanese and 3-MST and functions by converting CN to the water soluble and less toxic SCN. However, kinetic limitations prevent its ability to reverse severe CN toxicity.7 Sodium nitrite is usually administered with sodium thiosulphate during CN poisoning. Sodium nitrite oxidizes hemoglobin to methhemoglobin. Methemoglobin has a high affinity for CN, forms cyanomethhemoglobin freeing up cytochrome oxidase.11 Sodium nitrite acts slowly and during mass casualty emergencies may be contraindicated because of high carboxyhemoglobin levels due to simultaneous carbon monoxide inhalation.5 The newer agent hydroxycobalamin (MW 1355), although poorly water soluble is an effective and safe antidote,12 requires a large dose (one mole of antidote is needed per mole of CN (MW 27); hence, may not be as effective in severe CN poisoning.5,13 Using both hydroxycobalamin and sodium thiosulphate was more effective for acute CN toxicity in a pig model.14
In our juvenile pig model, we were able to induce severe CN toxicity by SNP and NaCN infusion as demonstrated by the occurrence of extreme lactic acidosis. The placebo-treated pigs did not survive, whereas sulfanegen sodium administration prevented mortality (Table 4) by reversing lactic acidosis and CN levels. The pigs awakened without detectable deficits. This is because the prodrug of 3-MP, viz., sulfanegen sodium, was used and made available the sulfur needed for the more universally available 3-MST in key organs (vide supra). 3-MP although a natural endogenous product of cysteine metabolism cannot be used as a drug because of its chemical instability.15
Conclusions
We have demonstrated the efficacy of a new prodrug, sulfanegen sodium, in reversing acute CN toxicity induced by the infusion of SNP or NaCN in a juvenile pig model. This compound holds considerable promise for the treatment of severe CN toxicity from direct exposure, smoke inhalation or nitroprusside administration. Further studies are being conducted to evaluate the safety of sulfanegen sodium in other animal models, to be followed by its assessment in humans.
Acknowledgments
Funding: NIH Grant No. NIH NINDS/5U01-NS058087
The authors thank David JW Goon, Ph.D., Jonathan F. Cohen, Ph.D. and Aleixandre Monteil, Ph,D. for the preparation of research quantities of sulfanegen sodium. The authors also acknowledge the assistance of T Reihsen, H Pham, MD, D Weiland, MD for help during the studies. The authors thank Keogh Medical for providing the use of the Penlon Anesthesia Gas Machine.
Footnotes
Reprints will not be available from the authors.
This report was previously presented, in part, at the American Society of Anesthesiologists and The International Anesthesia Research Society.
Telazol® is a combination of equal parts by weight of tiletamine (dissociative drug) and zolazepam (benzodiazepine), and is available in sterile vials containing 50 mg of each.
This manuscript was handled by: Marcel E Durieux, MD PhD
DISCLOSURES:
Name: Kumar G. Belani, MBBS, MS
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript
Attestation: Kumar G. Belani has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files
Conflicts of Interest: Kumar G. Belani received honoraria from NONIN Inc, consulted for NONIN Inc, received research funding from NONIN Inc, received research funding from Augustine Temperature Management, LLC, reported a conflict of interest with Oakstone Publishing, consulted for Cadence Pharmaceuticals, and reported a conflict of interest with Cadence Pharmaceuticals. He is coordinating editor for Oakstone Publishing. He is a speaker for Cadence Pharmaceuticals. His authorship in this article will add to his academic productivity and may have an indirect influence on his compensation from University of Minnesota.
Name: Harpreet Singh, MBBS
Contribution: This author helped conduct the study and analyze the data
Attestation: Harpreet Singh has seen the original study data, reviewed the analysis of the data, and approved the final manuscript
Conflicts of Interest: This author has no conflicts of interest to declare, however, his authorship in this article will add to his academic productivity and may have an indirect influence on his compensation from University of Minnesota.
Name: David S. Beebe, MD
Contribution: This author helped conduct the study, analyze the data, and write the manuscript
Attestation: David S. Beebe has seen the original study data, reviewed the analysis of the data, and approved the final manuscript
Conflicts of Interest: David S. Beebe reported a conflict of interest with Oakstone Publishing. He is a contributing editor to Oakstone Publishing. His authorship in this article will add to his academic productivity and may have an indirect influence on his compensation from University of Minnesota.
Name: Preeta George, MBBS, MD
Contribution: This author helped conduct the study
Attestation: Preeta George has seen the original study data and approved the final manuscript
Conflicts of Interest: This author has no conflicts of interest to declare.
Name: Steven E. Patterson, PhD
Contribution: This author helped write the manuscript and provided the test drug sulfanegen and is the PI on the NIH grant for this study
Attestation: Steven E. Patterson has seen the original study data, reviewed the analysis of the data, and approved the final manuscript
Conflicts of Interest: Steven E. Patterson has a proprietary interest in sulfanegen. His authorship in this article will add to his academic productivity and may have an indirect influence on his compensation from University of Minnesota.
Name: Herbert T. Nagasawa, PhD
Contribution: This author helped write the manuscript
Attestation: Herbert T. Nagasawa has seen the original study data and approved the final manuscript
Conflicts of Interest: Herbert T. Nagasawa is the inventor of sulfanegen and thus has proprietary interest
Name: Robert Vince, PhD
Contribution: This author helped write the manuscript
Attestation: Robert Vince has seen the original study data and approved the final manuscript
Conflicts of Interest: Robert Vince has proprietary interest in sulfanegen. His authorship in this article will add to his academic productivity and may have an indirect influence on his compensation from University of Minnesota.
Contributor Information
Kumar G. Belani, Department of Anesthesiology, University of Minnesota, Minneapolis, Minnesota.
Harpreet Singh, Department of Anesthesiology, University of Minnesota, Minneapolis, Minnesota.
David S. Beebe, Department of Anesthesiology, University of Minnesota, Minneapolis, Minnesota.
Preeta George, Department of Anesthesiology, University of Minnesota, Minneapolis, Minnesota.
Steven E. Patterson, Center for Drug Design, University of Minnesota, Minneapolis, Minnesota.
Herbert T. Nagasawa, Medicinal Chem Pharmaconosy, University of Minnesota, Minneapolis, Minnesota.
Robert Vince, Center for Drug Design, University of Minnesota, Minneapolis, Minnesota.
References
- 1.Way JL, Leung P, Cannon E, Morgan R, Tamulinas C, Leong-Way J, Baxter L, Nagi A, Chui C. The mechanism of cyanide intoxication and its antagonism. Ciba Found Symp. 1988;140:232–43. doi: 10.1002/9780470513712.ch14. [DOI] [PubMed] [Google Scholar]
- 2.Vogel SN, Sultan TR, Ten Eyck RP. Cyanide poisoning. Clin Toxicol. 1981;18:367–83. doi: 10.3109/15563658108990043. [DOI] [PubMed] [Google Scholar]
- 3.Bronstein AC, Spyker DA, Cantilena LR, Jr, Green JL, Rumack BH, Heard SE. 2007 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 25th Annual Report. Clin Toxicol (Phila) 2008;46:927–1057. doi: 10.1080/15563650802559632. [DOI] [PubMed] [Google Scholar]
- 4.Rindone JP, Sloane EP. Cyanide toxicity from sodium nitroprusside: risks and management. Ann Pharmacother. 1992;26:515–9. doi: 10.1177/106002809202600413. [DOI] [PubMed] [Google Scholar]
- 5.Nagasawa HT, Goon DJ, Crankshaw DL, Vince R, Patterson SE. Novel, orally effective cyanide antidotes. J Med Chem. 2007;50:6462–4. doi: 10.1021/jm7011497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagahara N, Ito T, Minami M. Mercaptopyruvate sulfurtransferase as a defense against cyanide toxication: molecular properties and mode of detoxification. Histol Histopathol. 1999;14:1277–86. doi: 10.14670/HH-14.1277. [DOI] [PubMed] [Google Scholar]
- 7.Nagahara N, Li Q, Sawada N. Do antidotes for acute cyanide poisoning act on mercaptopyruvate sulfurtransferase to facilitate detoxification? Curr Drug Targets Immune Endocr Metabol Disord. 2003;3:198–204. doi: 10.2174/1568008033340162. [DOI] [PubMed] [Google Scholar]
- 8.Crankshaw DL, Goon DJ, Briggs JE, DeLong D, Kuskowski M, Patterson SE, Nagasawa HT. A novel paradigm for assessing efficacies of potential antidotes against neurotoxins in mice. Toxicol Lett. 2007;175:111–7. doi: 10.1016/j.toxlet.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenner M, Kim JG, Lee J, Mahon SB, Lemor D, Ahdout R, Boss GR, Blackledge W, Jann L, Nagasawa HT, Patterson SE. Sulfanegen sodium treatment in a rabbit model of sub-lethal cyanide toxicity. Toxicol Appl Pharmacol. 248(3):269–76. doi: 10.1016/j.taap.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baud FJ, Borron SW, Megarbane B, Trout H, Lapostolle F, Vicaut E, Debray M, Bismuth C. Value of lactic acidosis in the assessment of the severity of acute cyanide poisoning. Crit Care Med. 2002;30:2044–50. doi: 10.1097/00003246-200209000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Baskin SI, Horowitz AM, Nealley EW. The antidotal action of sodium nitrite and sodium thiosulfate against cyanide poisoning. J Clin Pharmacol. 1992;32:368–75. doi: 10.1002/j.1552-4604.1992.tb03849.x. [DOI] [PubMed] [Google Scholar]
- 12.Borron SW, Baud FJ, Megarbane B, Bismuth C. Hydroxocobalamin for severe acute cyanide poisoning by ingestion or inhalation. Am J Emerg Med. 2007;25:551–8. doi: 10.1016/j.ajem.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Megarbane B, Delahaye A, Goldgran-Toledano D, Baud FJ. Antidotal treatment of cyanide poisoning. J Chin Med Assoc. 2003;66:193–203. [PubMed] [Google Scholar]
- 14.Bebarta VS, Tanen DA, Lairet J, Dixon PS, Valtier S, Bush A. Hydroxocobalamin and sodium thiosulfate versus sodium nitrite and sodium thiosulfate in the treatment of acute cyanide toxicity in a swine (Sus scrofa) model. Ann Emerg Med. 2010;55:345–51. doi: 10.1016/j.annemergmed.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 15.Westley J. Methods of Enzymology. San Diego: Academic Press, Inc; 1981. [Google Scholar]