Abstract
Intestinal tissue engineering is an emerging field due to a growing demand for intestinal lengthening and replacement procedures secondary to massive resections of the bowel. Here, we demonstrate the potential use of a chitosan/collagen scaffold as a 3D matrix to support the bioengineered circular muscle constructs maintain their physiological functionality. We investigated the biocompatibility of chitosan by growing rabbit colonic circular smooth muscle cells (RCSMCs) on chitosan-coated plates. The cells maintained their spindle-like morphology and preserved their smooth muscle phenotypic markers. We manufactured tubular scaffolds with central openings composed of chitosan and collagen in a 1:1 ratio. Concentrically-aligned 3D circular muscle constructs were bioengineered using fibrin-based hydrogel seeded with RCSMCs. The constructs were placed around the scaffold for 2 weeks, after which they were taken off and tested for their physiological functionality. The muscle constructs contracted in response to Acetylcholine (Ach) and potassium chloride (KCl) and they relaxed in response to vasoactive intestinal peptide (VIP). These results demonstrate that chitosan is a biomaterial possibly suitable for intestinal tissue engineering applications.
Keywords: Rabbit Circular Smooth Muscle (RCSM) constructs, Chitosan, Force Generation, Scaffold, Concentric
1. Introduction
Damaged or diseased segments of the intestinal tract require surgical intervention and extensive resection. This can lead to malabsorption and malnutrition eventually resulting in high mortality and morbidity rates in children and in adults [1–3]. Intestinal transplantation is a common treatment but its limitation resides in the high incidence of rejection, availability of donor organs and the size of the donor graft [4–6]. Hence, there is a clinical demand for generating physiologically functional intestinal replacement. The challenge in tissue engineering functional intestinal replacement lies in the anatomic complexity of this tubular organ. The intestine is composed of two layers of smooth muscle cells that are arranged in distinct orientations; the inner muscle layer consisting of concentrically oriented circular smooth muscle cells, and the external muscle layer consisting of parallel longitudinal smooth muscle cells. These two smooth muscle layers, along with their innervations, perform propulsive peristaltic function.
Tissue engineering provides an approach to generate new tissues using a variety of biomaterials with different cells. In intestinal tissue engineering, it is essential to develop a physiologically functional intestinal segment that could serve as a replacement. To date, both synthetic and natural polymers are used to design 3-D scaffolds to serve as temporary matrices for cell seeding until mature regeneration of the intestine occurs. Synthetic biomaterials used in intestinal tissue engineering applications include polyglycolic acid (PGA) and polycaprolactone (PCL). Vacanti et. al have successfully seeded intestinal organoids isolated from rat guts onto PGA scaffolds and reported recovery after small bowel resection in rat models [7]. Another group has reported the biocompatibility of PCL as an alternative biomaterial for intestinal regeneration [8]. These polymers demonstrate adequate mechanical properties and are easily reproducible. Intestinal segments have been tissue engineered using collagen due to its excellent biocompatibility, cell-binding affinity and biodegradability [9, 10]. However, the mechanical properties of collagen are unsuitable for intestinal tissue engineering applications [11]. In vivo, collagen scaffolds display rapid degradation rate; therefore they do not allow enough time for tissue regeneration. This imposes limitations on the use of collagen alone as biomaterial in tissue engineering.
Chitosan is a partially or fully deacetylated form of chitin, which is the second most abundant natural polymer found in the shells of shrimps, lobsters and crabs. It is a natural polymer that is commonly used as a biomaterial in many tissue engineering applications. Chitosan is a linear polysaccharide linked by (1→4)-β-glycosidic bonds. [12–14]. It has a degradation rate that can be controlled by enzymatic reactions and its biocompatibility has shown promising results in vivo [15–17]. The hydroxyl and amino groups of chitosan allows this polymer to be derivatized under mild conditions [15]. Chitosan has the ability to modify the mechanical properties of collagen when mixed together [18]. Little is known about the use of chitosan in intestinal tissue engineering applications; however, it is thought to be well-tolerated by the digestive system as it has been used as a component of dietary supplements for a number of studies [19–21].
Our laboratory first reported the bioengineering of 3-D physiological model of rabbit internal anal sphincter (IAS) and colonic smooth muscle constructs using fibrin-based gels. These constructs showed cellular alignment similar to native tissue [22]. Yoshikawa and colleagues have implanted collagen sponges seeded with smooth muscle cells as a potential for intestinal regeneration [10]. The smooth muscle cells demonstrated an arrangement similar to the native circular muscle layer but functionality was not assessed. Also, the degradation time of the collagen sponges in vivo was very short which caused shrinkage of the graft sites.
In our current study, we have developed a composite scaffold composed of collagen as the bioactive component combined with chitosan to provide mechanical stability to the scaffold. The composite scaffold was then cross linked with heparan sulfate using carbodiimide cross linker. We propose to use the composite chitosan scaffold as a support for the bioengineered constructs. We first investigated the biocompatibility of chitosan with intestinal smooth muscle cells on 2D composite chitosan membranes by evaluating cellular attachment and smooth muscle phenotypic markers expression. Secondly, we bioengineered 3D smooth muscle tissue constructs and placed them around the composite chitosan tubular scaffold. The bioengineered muscle constructs were taken off the scaffold after a period of 2 weeks and their physiological functionality was compared to constructs that were not around the scaffold.
2. Materials and Methods
2.1. Reagents
All cell culture reagents including growth medium and supplements were purchased from Invitrogen (Carlsbad, CA). Growth medium consisted of Dulbecco’s modified Eagle medium (DMEM) (supplemented with 10% fetal bovine serum (FBS), 1.5% antibiotic-antimycotic, and 0.6% L-glutamine. Differentiation medium consisted of 73% DMEM, 20% medium 199, 7% heatinactivated horse serum, and 1% antibiotic-antimycotic. Collagenase type II was from Worthington (Lakewood, NJ).
Medium molecular weight chitosan (75-85% deacetylation), glycosaminoglycan heparan sulfate, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC), acetylcholine (ACh) and vasoactive intestinal peptide (VIP) were purchased from Sigma (St. Louis, MO). Sylgard [poly(dimethylsiloxane); PDMS] was from World Precision Instruments (Sarasota, FL). Type I collagen was purchased from BD Biosciences.
2.2. Isolation of Rabbit Colonic Circular Smooth Muscle cells
Rabbit colonic circular smooth muscle cells (RCSMCs) were isolated as described previously [23]. Briefly, rabbit sigmoid colon was cleaned of fecal content. The serosa, longitudinal smooth muscle and mucosa were removed. The circular smooth muscle was finely minced, digested twice in type II Collagenase (Worthington), and filtered through a 300 μm mesh to eliminate cellular debris. The filtrate was centrifuged at 600 g for 5 min, washed three times in Hank's buffered salt solution HBSS, and then plated in growth medium. Cells were grown to confluence before use in the experiments.
2.3. Fabrication of composite chitosan membranes and scaffolds
A 2% w/v chitosan solution was prepared by dissolution in 0.2 M acetic acid. 500 μl chitosan: type I collagen (1:1) solution was added to each well of a 12-well plate and the plate was left to air-dry overnight. The resulting dry membranes were cross linked with heparan sulfate using the carbodiimide cross linker (EDC) under moderate shaking at room temperature following the method used by Uygun et. al [24]. The membranes were neutralized with 0.2 M sodium hydroxide and washed several times with 1X PBS. Heparan sulfate (10mg/ml) solution was activated by EDC in a 1:10 ratio. The activated heparan sulfate was then applied to the dried chitosan. The plates were left under moderate shaking at room temperature overnight. This resulted in the production of composite chitosan 2D membranes, which were then sterilized with 70% ethanol and UV light. Membranes were washed several times with sterile PBS before use.
Composite chitosan scaffolds were prepared using the freezing and lyophilizing method described by Madihally et. al. [15]. Briefly, the 2 w/v % chitosan solution was mixed with type I collagen in a volume ratio of 1:1. The mixture was poured into a custom made mold. The mold consisted of a cylindrical tube with a central port. The inner lumen was created by inserting tubing in the center of the mold. The mold, containing the mixture, was frozen at −80°C for 3 hours and then lyophilized for 24 hours. The scaffolds were neutralized in 0.2 M NaOH and covalently crosslinked with heparan sulfate using EDC. The scaffolds were then washed several times with PBS and distilled water and then placed in 70% ethanol until time of use.
2.4. Microscopic Analysis
RCSMCs were seeded on 2D composite chitosan matrices at a density of 20,000 cells per well. Fresh growth medium was supplied every 2 days. Microscopic analysis was performed on days 2 and 7 to determine whether the smooth muscle cells had attached to the chitosan membranes and to record their morphology. Cells grown on chitosan were compared to control cell cultures, which consisted of RCSMC grown directly on tissue culture dishes (no membrane).
2.5. Immunofluorescence Analysis
RCSMCs were grown to confluence on microscope chamber slides and were supplied with growth medium. The cells were fixed, blocked and permeabilized. Primary antibodies directed against smooth muscle markers; α-smooth muscle actin (F3777; Sigma) and smooth muscle specific Caldesmon (c-4562; Sigma) were used. Fluorophore-conjugated secondary antibodies were used to detect immunofluorescence using a Nikon Ti-E fluorescence microscope.
2.6. Scanning Electron Microscopy
Lyophilized scaffolds were sectioned using a sharp scalpel. Cross sections were placed on a stub with double sticky tape and colloidal graphite and then sputter-coated with gold prior to examination under an AMRAY 1910 Field Emission Scanning Electron Microscope (FEG-SEM). Porosity and average pore sizes were determined by scanning 3 images of the scaffold and averaging the pore size using Sigmascan Pro 5 software (Systat software Inc, San Jose, CA).
2.7. Fabrication of three-dimensional tissue constructs using rabbit circular smooth muscle cells and placing them around a composite chitosan scaffold
The process of bioengineering three dimensional circular smooth muscle constructs is similar to the one previously described using internal anal sphincter smooth muscle cells [22]. Briefly, gels were fabricated by polymerizing 20 mg/ml of fibrinogen with 10 units of thrombin. RCSMC density was adjusted to 2×105 cells/ml and 2 ml of solution was added to fibrin gels. Plates were incubated in growth medium overnight. The next day, the growth medium was replaced with differentiation medium to promote smooth muscle differentiation. These fully formed, 3-dimensional bioengineered rabbit circular smooth muscle (RCSM) tissue constructs remained stable in culture for 7 days until used for the purpose of the experiment.
At day 7, RCSM tissue constructs were completely formed. Four of the constructs were placed around a tubular composite chitosan scaffold and left in culture for an additional two weeks. The four constructs were placed 1 mm apart.
2.8. Physiologic functionality measurement and testing protocol
The physiological functionality of the bioengineered rabbit colon tissue constructs was assessed in terms of real-time force generation. The force measurements of the constructs were conducted following the protocol previously described in human Internal Anal Sphincter (IAS) and implanted mouse IAS constructs [25]. All constructs were tested using an isometric force transducer with an attached vernier control (Harvard Apparatus, Holliston, MA). The force transducer set up consisted of a warm tissue bath keeping the tissue samples at conditions of 37°C ± 1°C. Noise and liquid measurements were evaluated by recording air tension and warm liquid force respectively. The bioengineered RCSM tissue constructs were taken off the scaffold at day 14 and real time force generation was measured on each one. RCSM constructs that were not placed around the scaffold were also tested as control.
One end of the tissue sample was hooked onto the measuring arm of the transducer and the other end was hooked onto a fixed reference pin. Tissue constructs were allowed to equilibrate in the tissue bath containing fresh medium. All reported values of force are active tension produced as a result of the tissue. The tissue constructs were immersed in the fluid throughout the testing period to avoid any air vibration. After establishment of baseline, the tissues were stretched by 10% – 15% using the micromanipulator. The same stretch was maintained during the testing. The stretch baseline established by the tissue samples was arbitrarily set to zero and the values represent change in force generation. Potassium chloride (KCl) was used for testing electromechanical coupling, Acetylcholine (Ach) for cholinergic stimulation and Vasoactive Intestinal Peptide (VIP) for relaxation. Before addition of any reagent, the baseline was established. At the end of each experiment, the tissues were washed with buffer and supplied with fresh basal DMEM.
2.9. Quantification of protein
Rabbit colonic circular smooth muscle cells grown on tissue culture plates were scraped using PBS. Aliquots of 200,000 cells were lysed in 100 μl of lysis buffer (Radioimmunoprecipitation assay buffer) by sonication. The lysates were centrifuged at the end of which the pellets were discarded. For protein quantification, 2 μl of the protein extract was removed and added to water. Coomassie blue dye was added to the solution and absorbance was measured at 490 nm. The results were plotted against a standard curve made with γ-globulin ranging from 0 to 11.5 μg/μL. Total protein was then calculated by extrapolation for the volume of the lysate. All reported forces are expressed in terms of Newtons per gram of protein content in the bioengineered constructs.
2.10. Data Analysis
Force generation data was acquired using LabScribe2 (iWorx, Hanover, NH). GraphPad Prism 5.01 for Windows (GraphPad Software, San Diego CA; www.graphpad.com) was used for data analysis. Data was exported at 100 samples/second. All values were normalized to millinewton per milligram of protein content in the bioengineered constructs. Second order Savitzky-Golay smoothing was applied to raw data. Expressed values represent mean and standard error of the mean. Student t-test was used to compare the means. A p-value less than 0.05 was considered significant.
3. Results
3.1. Microscopic analysis
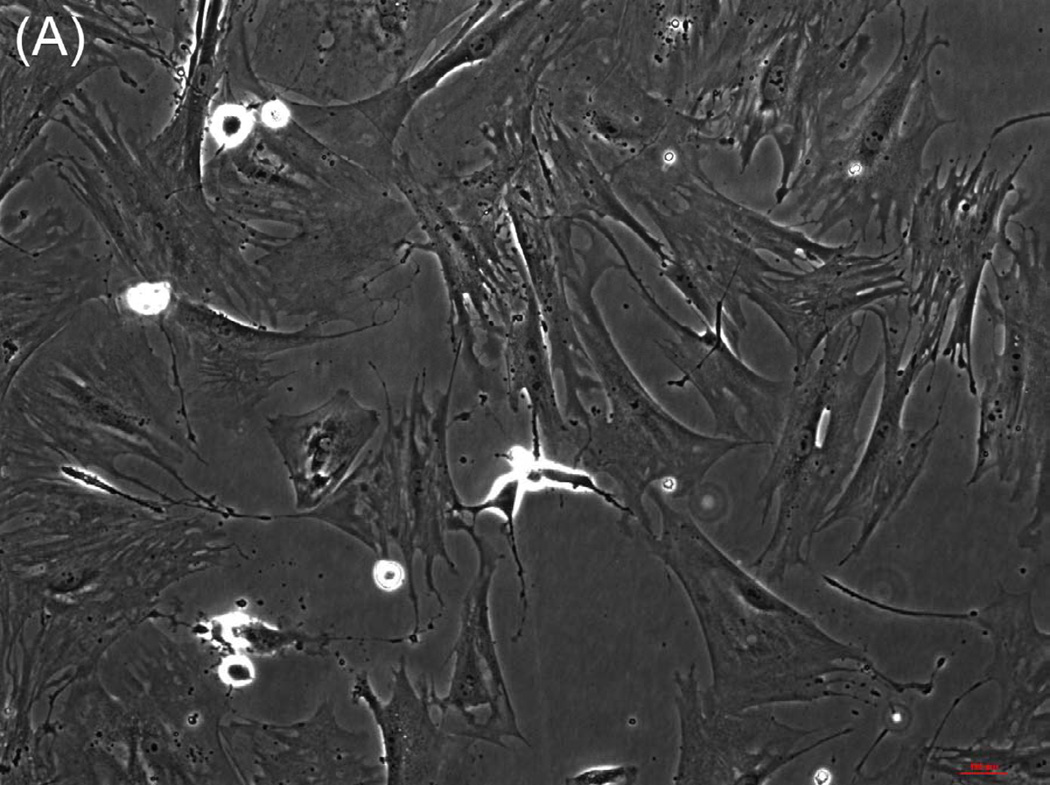
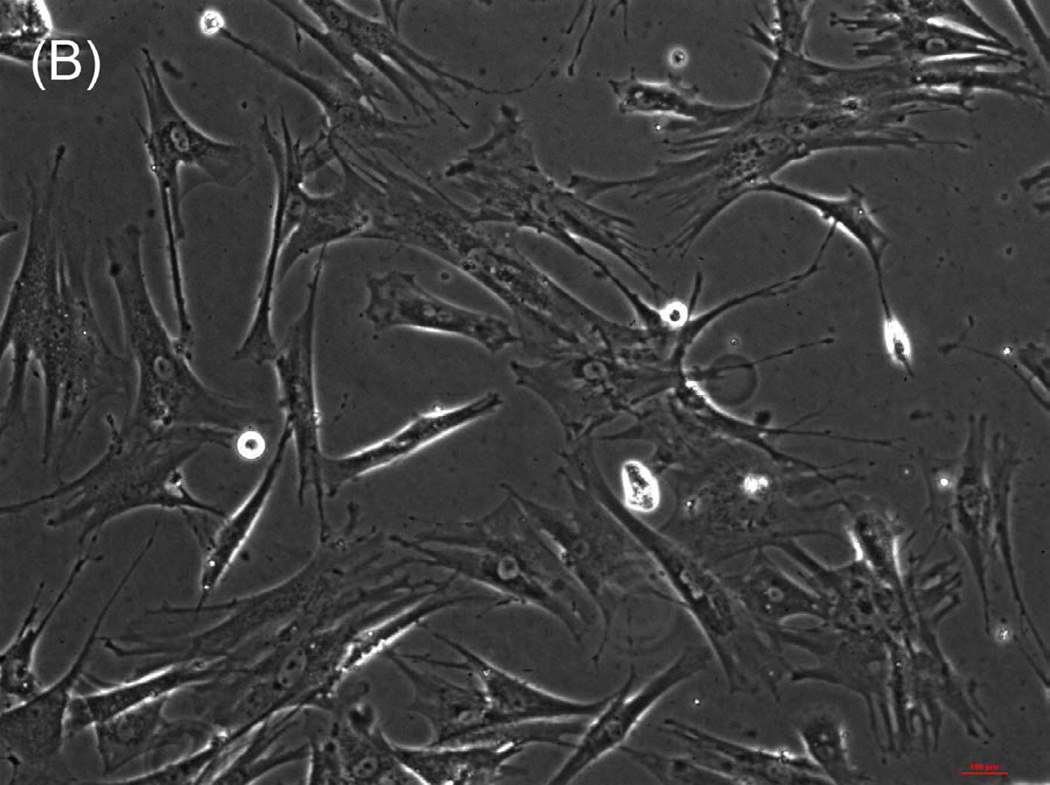
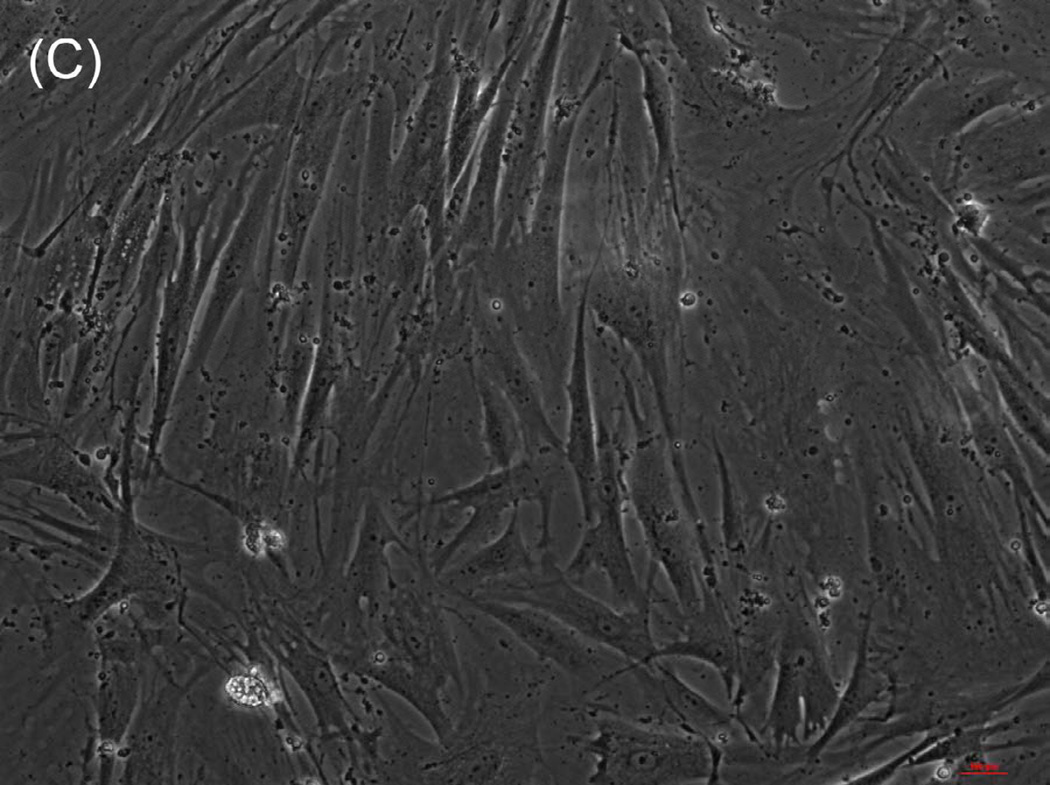
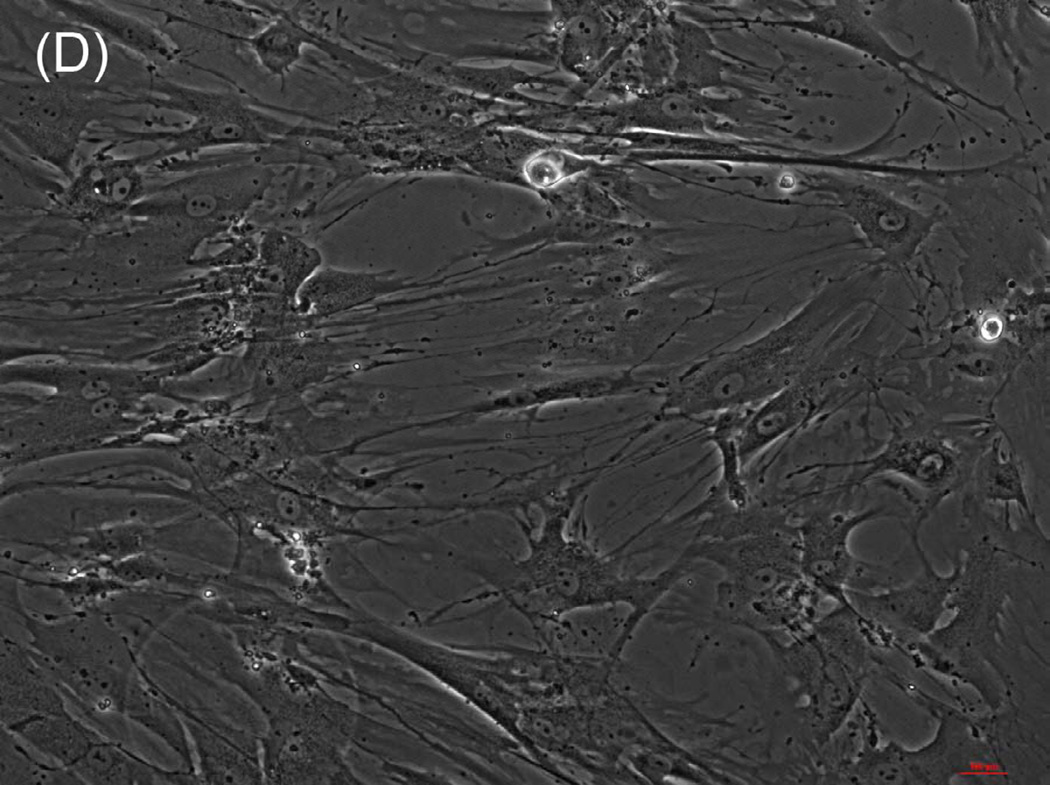
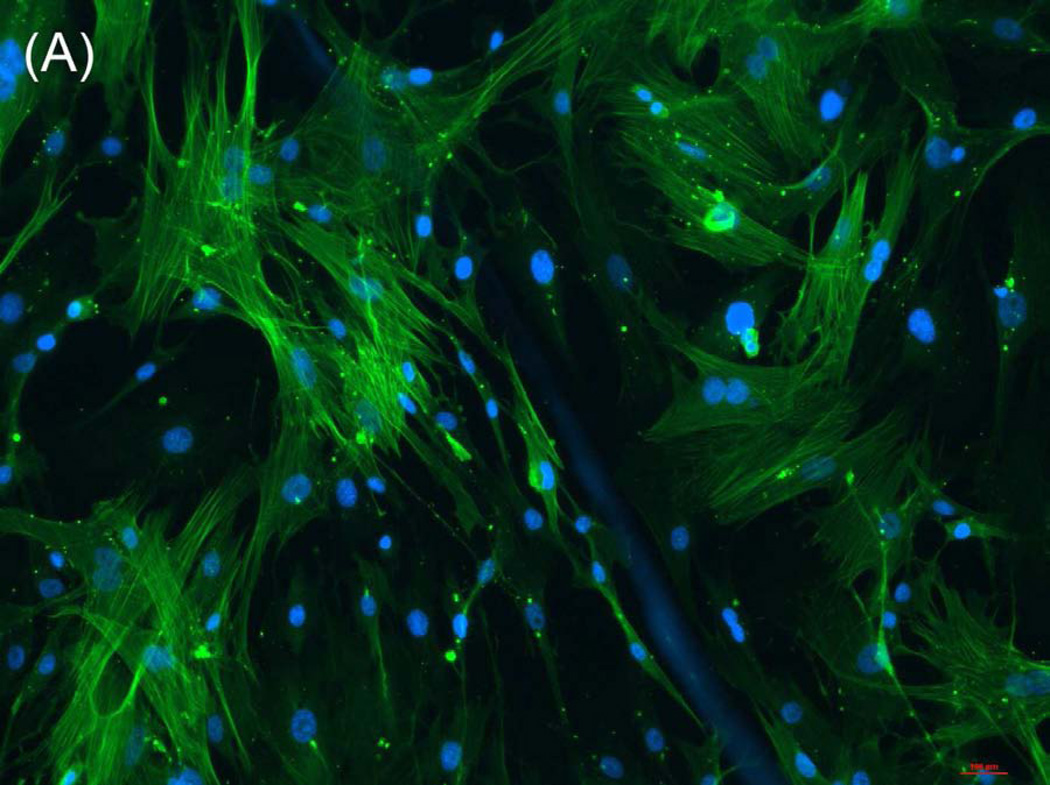
The ability of composite chitosan membranes to support attachment, viability and growth of circular smooth muscle cells was evaluated. Rabbit circular smooth muscle cells were seeded on composite-chitosan-coated wells and on non-coated wells of a 12 well plate for comparison. Growth medium was supplied every other day for a period of 7 days. Microscopic analysis showed good attachment of RCSMCs on composite chitosan membranes at day 2 (Fig.1-B). Cells on composite chitosan membranes displayed the normal spindle-like morphology of smooth muscle, when compared to cells on non-coated wells (Fig.1-A). The cells showed uniform distribution on the composite chitosan membrane. The smooth muscle cells maintained their spindle-like morphology (Fig.1-C, D) at day 7 on both coated and non-coated wells. Thus, chitosan did not change the normal morphology of the smooth muscle cells.
Figure 1.
Microscope pictures of RCSMCs on tissue culture plates (A, C) and composite chitosan membranes (B, D) at day 2 (A, B) and day 7 (C, D) after cell seeding. Pictures were taken at 10X magnification. Cells attached on composite chitosan membranes and they maintained spindle-like morphology, a characteristic of smooth muscle cells.
3.2. Immunofluorescence assay
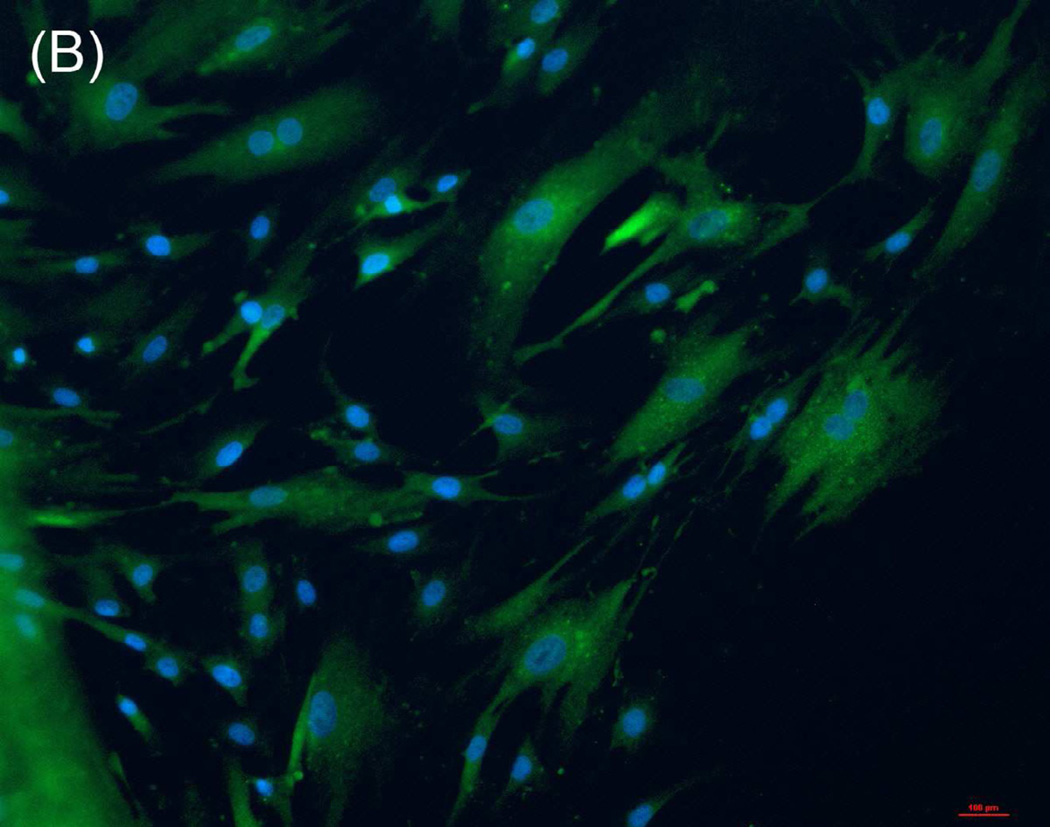
RCMCs were cultured on cover slips coated with composite chitosan for one week and growth medium was applied every 48 hours. The cells were analyzed by immunofluorescence using antibodies to smooth muscle markers α-smooth muscle actin (Fig.2-A) and smooth-muscle-specific heavy Caldesmon (Fig.2-B). All the cells cultured on composite chitosan membranes stained positive for both markers, indicating a highly enriched population of smooth muscle cells. The nuclei stained positive with DAPI.
Figure 2.
Immunofluorescence assay on RCSMCs grown on composite chitosan membranes for α-smooth muscle actin (A, green color) and smooth muscle specific heavy Caldesmon (B, green color). Nuclei stain 4′-6-diamidino-2-phenylindole (DAPI) appears in blue. Cells stained positive for α-SMA and smooth muscle specific heavy Caldesmon, indicating the maintenance of smooth muscle contractile phenotype on composite chitosan membranes.
3.3. Characterization of composite chitosan scaffolds
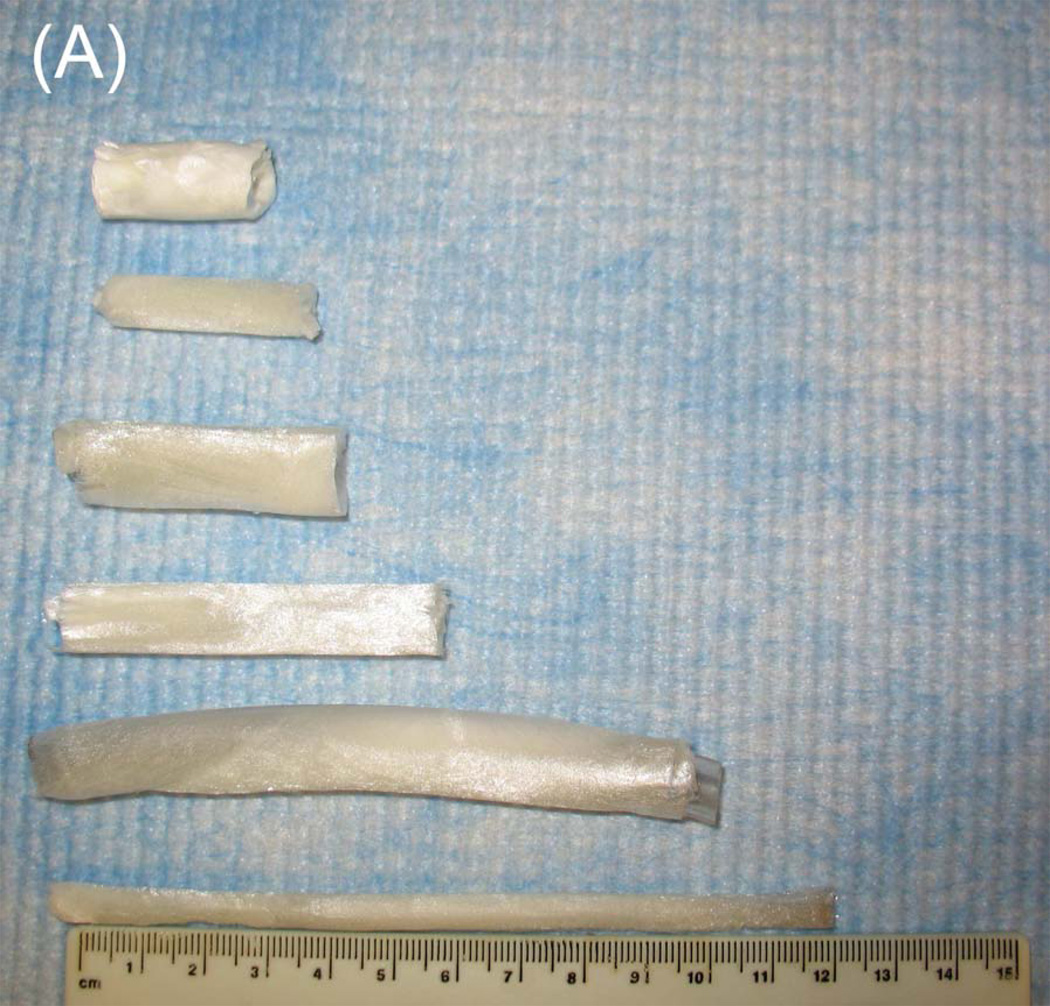
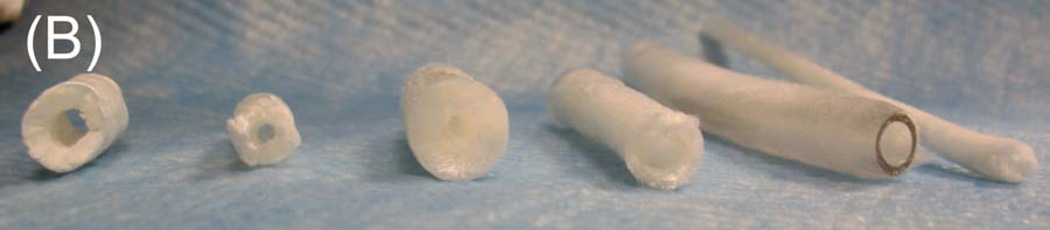
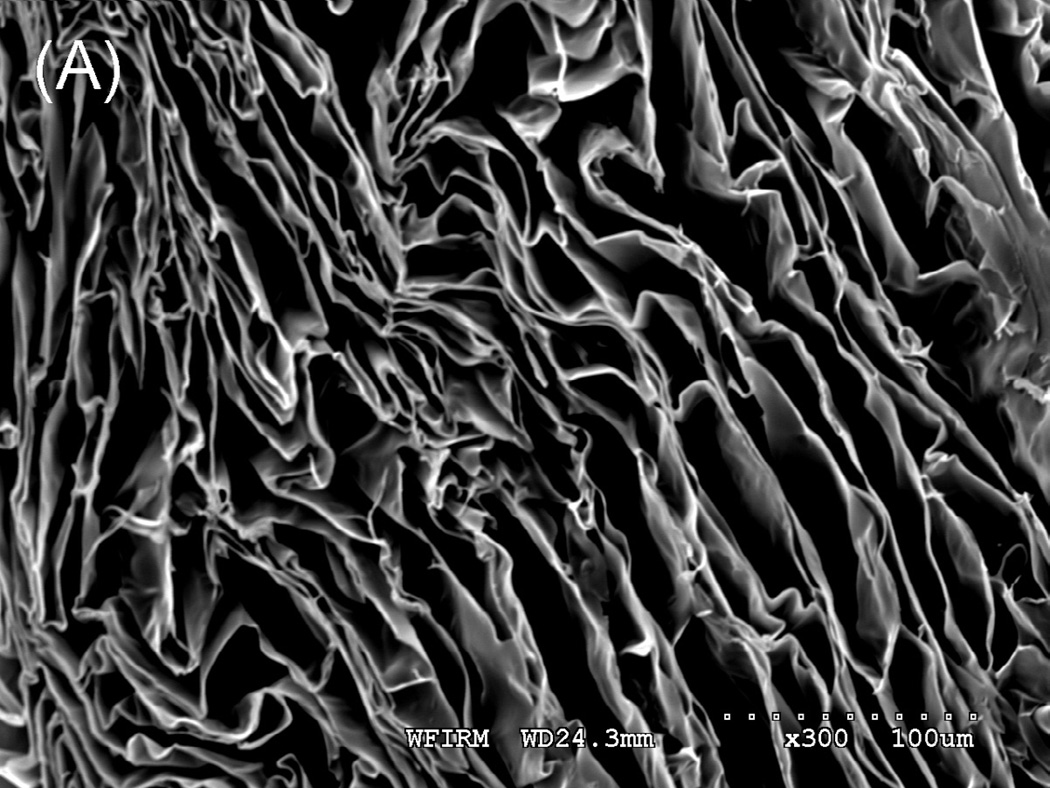
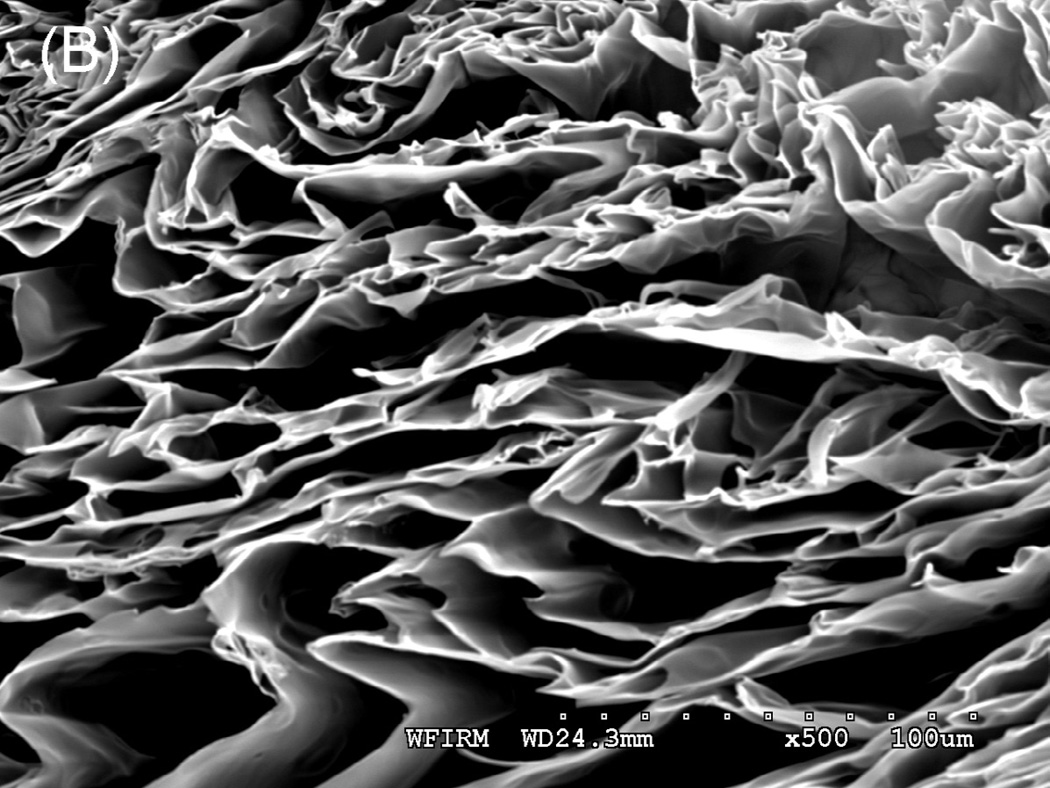
Tubular chitosan scaffolds were fabricated using the freezing and drying method [15]. The custom-made cylindrical molds consisted of cylindrical tubes with annular spaces. The mold containing the chitosan-collagen mixture was frozen for 3 hours at −80°C and then lyophilized for 24 hours. Figure 3A shows different tubular scaffolds fabricated with different lengths ranging from 3 cm up to 12 cm. The diameter of the inner lumen and the wall thickness of the mold were controlled during the manufacturing process by controlling the tube sizes. The fabricated scaffolds had different wall thicknesses and different diameter of central opening as seen in figure 3B. The porosity of the scaffolds was observed under low power using a scanning electron microscope. Pore sizes were measured from 3 samples and averaged using Sigmascan Pro 5 software. Figure 4 shows highly porous scaffolds with mean pore size of 170 µm. Additionally, the pores had a uniform and homogenous pattern.
Figure 3.
Composite chitosan tubular scaffolds with different lengths ranging from 3 cm up to 12 cm (A) and different lumen diameters and wall thicknesses (B).
Figure 4.
Scanning Electron Microscopy: A section of the scaffold was mounted on a stub and coated with gold using sputter coater. Porosity was characterized by SEM under low power. The image depicted a highly porous scaffold with uniform pore structure at 300X (A) and 500X (B). The mean pore size was found to be 170 μm.
3.4. Bioengineered RCSM constructs placed around the tubular scaffold
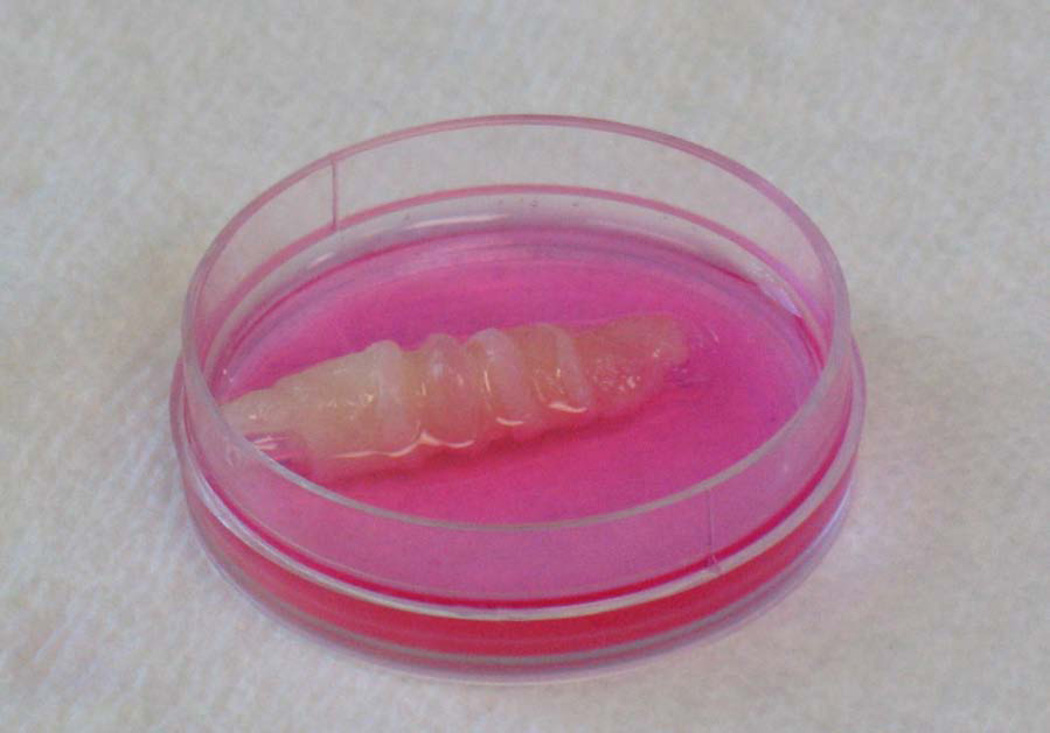
The RCSM constructs were formed using the fibrin-based method [22]. The circular smooth muscle cells were seeded on a fibrin gel. The cells were concentrically aligned around the post at the center of the plate. The constructs were fully formed by day 7 in culture and they were separated from the post using forceps. Figure 5 shows the RCSM construct with an inner diameter of 5 mm and a thickness of 2 mm. The tubular scaffold had the following dimensions: 2.5cm length, 5mm outer diameter and 3.25mm luminal diameter. The bioengineered RCSM tissue constructs were placed around the composite chitosan tubular scaffold and left in culture for 2 weeks (Fig. 6). The scaffold was easy to handle and there was no sign of disruption or disintegration of the RCSM constructs while sliding them around the scaffold. They maintained their integrity throughout the culture period of 14 days. Precaution was taken to ensure complete immersion of the scaffold along with the constructs in medium. These bioengineered constructs did not adhere to the tubular scaffold as they were easily taken off the scaffold at the end of the culture period. The bioengineered constructs didn’t appear to change the structure of the scaffold as it maintained its luminal patency.
Figure 5.
Day 7 - Three dimensional RCSM bioengineered construct with concentrically aligned smooth muscle cells. The internal lumen diameter is 5 mm and wall thickness is 2 mm.
Figure 6.
Four bioengineered constructs were mounted around the composite chitosan tubular scaffold and maintained in culture for 2 weeks. Tissue constructs were placed 1 mm apart.
3.5. Physiological testing of the bioengineered constructs
The muscle constructs were taken off the scaffold and immersed in a tissue bath for force measurements using the force transducer set up. The response of the bioengineered constructs taken off the scaffold was compared to that of the control constructs (that were not around the scaffold). Pharmacologic studies were carried out to evaluate the maximal contractile responses observed on the bioengineered constructs in response to depolarization with KCl as well as in response to acetylcholine. Relaxation was determined in response to VIP. All reported force values are expressed in Newtons per gram of protein content in the bioengineered constructs.
3.5.1. Contractile Response
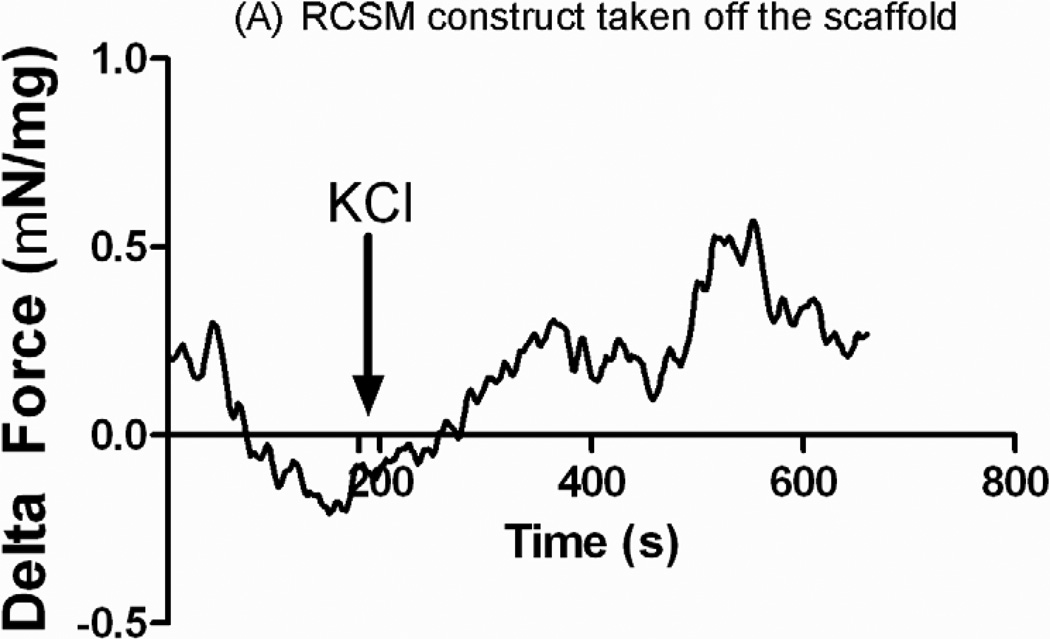
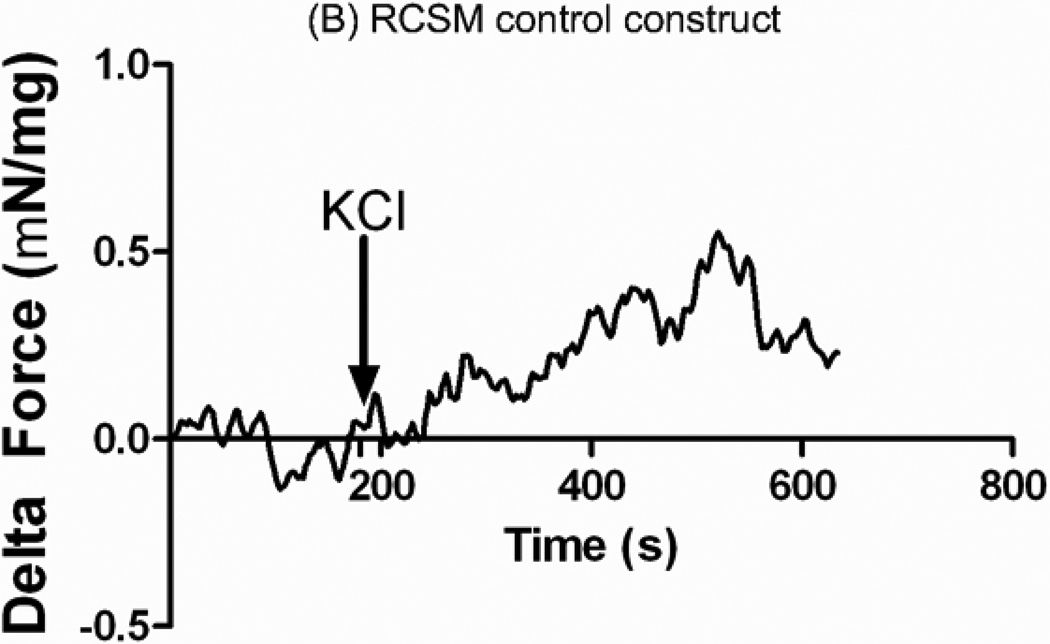
A final concentration of 60 mmol/L of KCl was added to the 4 ml tissue bath containing the RCSM constructs taken off the scaffold (Figure 7-A) or the control RCSM constructs (figure 7-B). The bioengineered constructs that were around the scaffold and the control constructs both produced an increase in force generation with a mean maximal contractile response of 0.48 ± 0.10 mN/mg of protein (n=3) and 0.48 ± 0.05 mN/mg of protein (n=4) respectively.
Figure 7.
Real time force generation was measured on the constructs taken off the scaffold at day 14 and on the control constructs using a force transducer. The graphs show the average force generated up on treatment with 60 mM potassium chloride (KCl) of an average of 0.48 ± 0.10 mN/mg of protein (n = 3) in the constructs that were around the scaffold (A) and 0.48 ± 0.05 mN/mg of protein (n = 4) in the control constructs (B). Addition of KCl is marked by the arrow at 180 seconds. Representative tracings for 3D bioengineered scaffold and control constructs have been chosen.
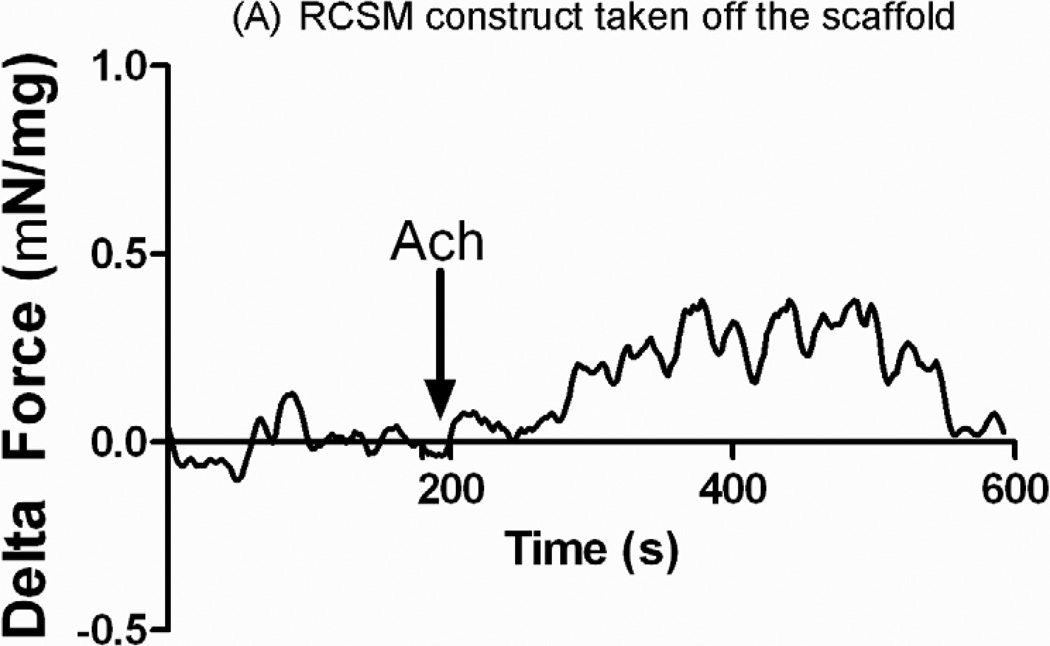
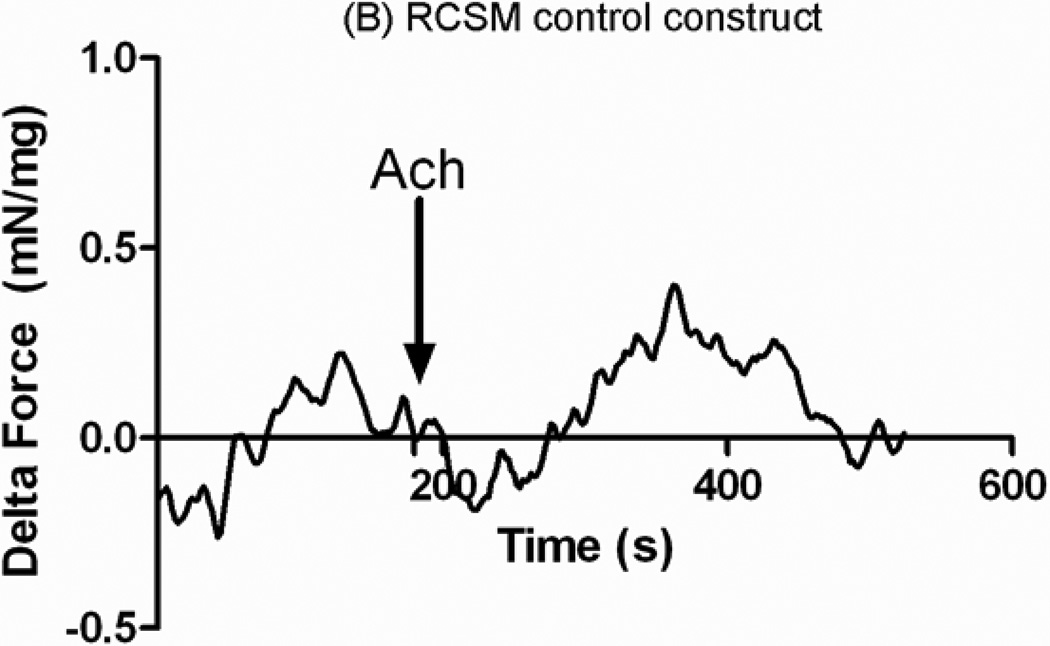
Acetylcholine was added after the bioengineered constructs established baseline. The arrow indicates the time of treatment with Ach. Addition of 1 μM Acetylcholine to the RCSM constructs that were around the scaffold for 14 days (Fig. 8-A) caused a mean contractile force of 0.55 ± 0.08 mN/mg of protein (n=4). Similarly, treatment of the control constructs with 1 μM Acetylcholine caused a contraction with an average of 0.5 ± 0.17 mN/mg of protein (n=4) (Fig. 8-B). Both groups of bioengineered RCSM constructs showed a sustained contraction in response to Ach. The bioengineered constructs that were around the scaffold preserved the integrity of their cellular receptors. Their maximal contractile response seen with KCl and Ach was similar to that observed for the corresponding responses in the control constructs that were not around the scaffold.
Figure 8.
Response in μN/μg of protein of the constructs that were around the scaffold (A) and control constructs (B) to Acetylcholine (Ach). Addition of 1μM of Ach is marked by the arrow. The constructs taken off the scaffold generated an average contraction of 0.55 ± 0.08 mN/mg of protein (n = 4) and the control constructs showed an average contraction of 0.5 ± 0.17 mN/mg of protein (n = 4). This graph is a representative figure.
3.5.2. Relaxation Response
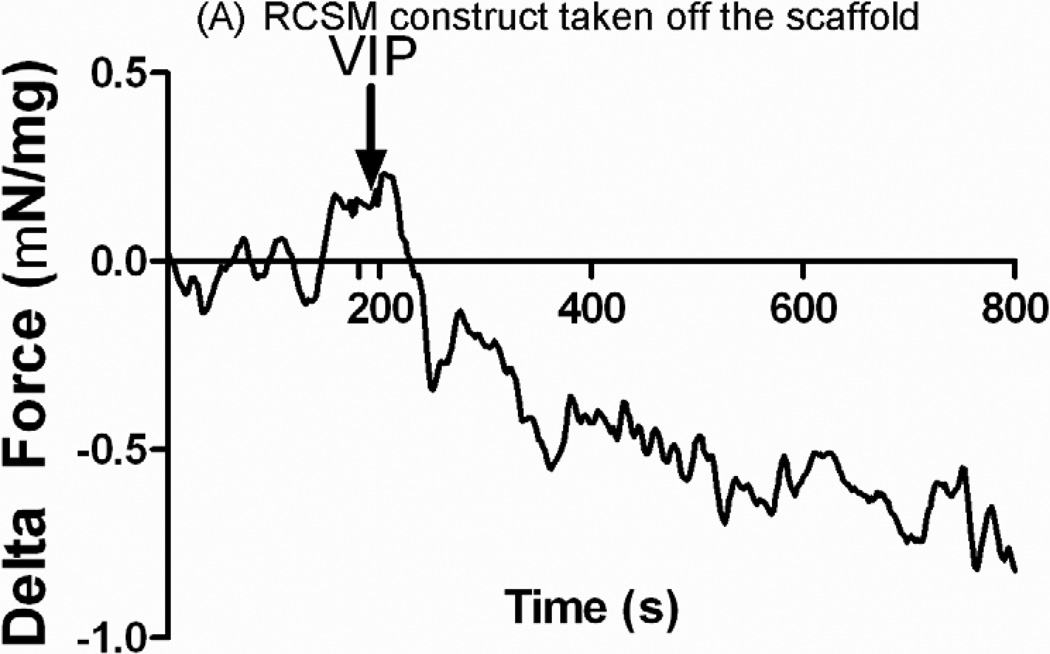
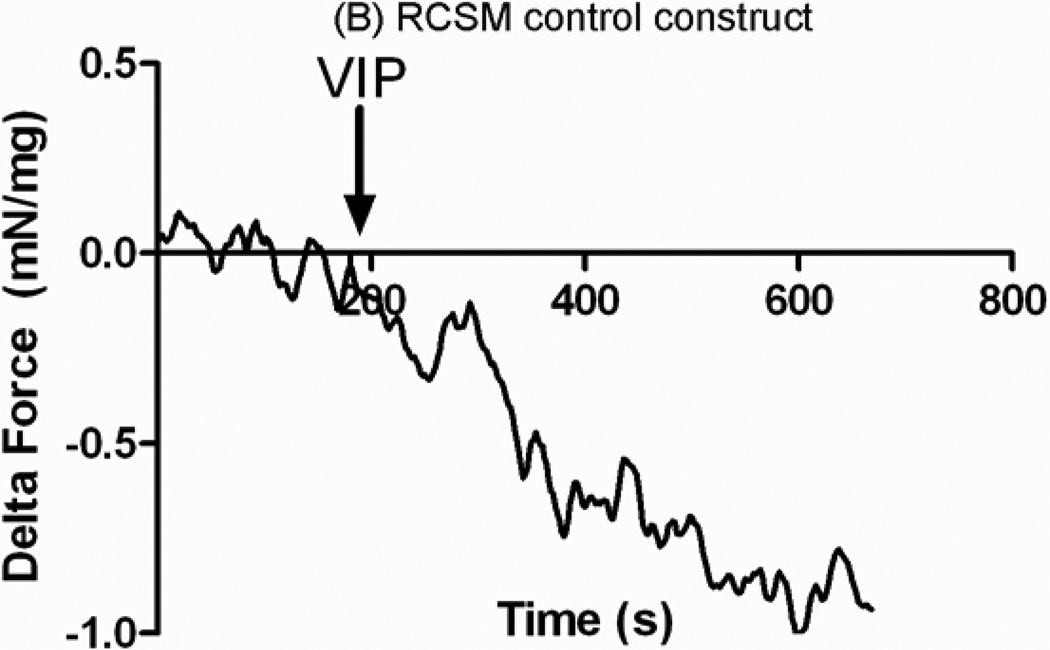
The ability of the RCSM constructs to relax in response to 1 μM VIP was investigated. The observed relaxation in both the constructs that were around the scaffold and the control constructs was rapid. An average relaxation of −0.79 ± 0.06 mN/mg of protein (n=3) was shown in the constructs that were around the scaffold following the addition of VIP (Fig. 9-A). The control constructs showed an average relaxation of −0.89 ± 0.18 mN/mg of protein (n=4) up on treatment with VIP (Fig. 9-B). There was no difference seen in relaxation in response to VIP between the constructs taken off the scaffold and the control constructs.
Figure 9.
RCSM constructs taken off the scaffold at day 14 (A) and control constructs (B) were tested for relaxation with Vasoactive Intestinal Peptide (VIP). VIP caused an average relaxation of −0.79 ± 0.06 mN/mg (n = 3) in the constructs that were around the scaffold and an average of −0.89 ± 0.18 mN/mg (n = 4) in the control constructs. This graph is a representative figure.
4. Discussion
Severe weight loss and malnutrition constitute a major threat to patients with extensive bowel resections. Patients with inflammatory bowel disease typically require surgical intervention. Surgical resection has shown improved conditions in patients but the chance of recurrence is still high [26]. Tissue engineered intestinal grafts are beneficial to create intestinal replacements or to lengthen the bowel. Smooth muscle is a crucial component of the gastrointestinal system, and the myoarchitecture of the intestinal wall is of paramount importance. The use of oriented smooth muscles to mimic the native tissue arrangement is more advantageous than randomly seeding smooth muscle cells onto a scaffold. In dysmotility, smooth muscle layer loses its ability to function normally. Thus, in intestinal tissue engineering it is necessary to duplicate the structure and function of the muscle layers in vitro. Dunn et. al. used fibroblast growth factor incorporated into a scaffold seeded with smooth muscle cells in order to induce vascularization and to help regenerating the smooth muscle layer of the intestine, which is a significant challenge in intestinal tissue engineering [27].
Several challenges to tissue-engineer intestinal grafts reside in mimicking in vivo arrangement of cells and extracellular components. Research is ongoing to bioengineer scaffolds with aligned structure to serve the purpose of their application. Our group has previously bioengineered longitudinally aligned colonic smooth muscle constructs [28]. The specific alignment of these constructs is thought to be responsible for the similarity in force generation between the bioengineered constructs and the native tissue. Other examples include the use of tissue scaffolds with longitudinal grooves structures to enhance the alignment of vascular smooth muscle cells [29]. A biodegradable polymer scaffold made out of aligned nanofibers improved the adhesion, proliferation and alignment of vascular smooth muscle cells while maintaining contractile phenotype [30]. Scaffolds with longitudinally oriented structures are shown to be required for the reconstruction of injured peripheral axons [31]. Graft replacements are usually developed using scaffolds and seeding them with cells, but in our method, we successfully developed concentrically oriented smooth muscle constructs to mimic the native architecture. The alignment of the cells was done using fibrin-based 3D culture. The potential of placing the muscle constructs around the tubular composite chitosan scaffold for 14 days with the ability to maintain physiological functionality in vitro is promising for intestinal regeneration. Our approach has the advantage of combining bioengineered muscle constructs aligned in an identical way to the native tissue with a biodegradable chitosan scaffold.
In this study, a 2 w/v % medium molecular weight chitosan solution was used to prepare the scaffolds by the freeze-drying method. Scanning electron microscopy was employed to characterize the porosity of the scaffold. Our results revealed a porous scaffold with an average pore size of 170 μm. Porosity is essential in intestinal tissue engineering in order to facilitate vascularization. Previous attempts to induce vascularization and to enhance cell survival have seeded smooth muscle cells in bFGF-releasing collagen scaffolds [27]. To mimic the physiological environment, we cross linked the scaffolds with heparan sulfate because of the abundance of this glycosaminoglycan in the intestinal extracellular matrix [32]. This is the first report that assesses the biocompatibility of chitosan using intestinal smooth muscle. Our data indicates that chitosan was not toxic to these cells as they maintained their spindle-like morphology. The rabbit intestinal smooth muscle cells preserved their contractile phenotype and their smooth muscle specificity as shown by the positive expression of differentiation markers (α-smooth muscle actin and smooth muscle specific heavy Caldesmon). Moreover, the above findings show that these smooth muscle cells were able to contract and relax in response to contractile and relaxant agonists, which are functions associated with high level of differentiation.
In recent years, acellular collagen scaffolds or collagen scaffolds seeded with mesenchymal stem cells have been developed. Results have shown lack of smooth muscle layer regeneration which is essential for the functionality of the tissue-engineered intestine [9, 33]. Other approaches have used collagen sponges seeded with smooth muscle cells as a potential for intestinal regeneration [10]. The main limitation is the lack of a functional smooth muscle layer. In our study, the three-dimensional circular smooth muscle tissue constructs were bioengineered by seeding circular smooth muscle cells isolated from rabbit colon in a thrombin polymerized fibrinogen hydrogel. The cells were self-organized concentrically around the central post of the plate and formed three-dimensional ring-like structures. We showed that 14 days post-placing the muscle constructs around the composite scaffold in culture, they displayed characteristics of colonic physiology, including contraction and relaxation mediated by physiologically relevant neurotransmitters like Acetylcholine and Vasoactive Intestinal Peptide. We were able to slide the constructs without causing rupture, and this shows the flexibility of the composite chitosan scaffold. During the culture period, the composite chitosan scaffold did not cause damage to the tissue constructs as they maintained their integrity and morphology. Also, there was no sign of damage to the scaffold throughout the culture period as it retained its luminal patency.
Some studies have seeded collagen scaffolds with autologous smooth muscle cells to regenerate the intestine. The smooth muscle cells formed a circular smooth muscle layer similar to in vivo arrangement, but there was no evidence for functionality [10]. The group of Vacanti has reported the use of intestinal organoids derived from animal guts to seed polymer scaffolds in order to regenerate the intestine [7, 34]. Histological studies have shown positive staining for the muscularis propria and electrophysiological studies have shown similar parameters to the native colon tissue [34]. In our case, the force generated by the constructs that were around the scaffold was compared to that of the control ones. Force was expressed in terms of Newton per microgram of protein content in the constructs. The RCSM constructs taken off the scaffold displayed a physiologic response to KCl in a way similar to the control constructs. This shows the maintenance of the electromechanical coupling and integrity of the cell membrane of the constructs after being placed around the composite chitosan scaffold for 14 days. Smooth muscle receptor-mediated contraction in response to Ach has been previously reported [35, 36]. Both the constructs that were around the scaffold and the control constructs demonstrated a similar sustained contraction with the addition of 1 μM Ach. This indicates that the smooth muscle cells in the RCSM constructs maintained their receptor integrity after being around the composite chitosan scaffold for 14 days. Smooth muscle relaxation has been documented to be dependent on protein kinase PKA [37, 38]. There was no difference in relaxation response between the constructs taken off the scaffold and the control constructs, which proves again that chitosan did not affect the receptors on the smooth muscle cells. Preincubation of our bioengineered constructs in a medium containing PKA inhibitor abolished the relaxation seen with VIP (data not shown), which means that the intracellular signaling pathway leading to relaxation was also preserved. Our constructs produced only a fraction of the contractile response (13%) compared to muscle strips from rabbit sigmoid native colon tissues (data not shown). The difference in response compared to native tissue was reported in previous studies using vascular smooth muscle cells [39, 40].
Alignment of smooth muscle cells is essential for muscle peristaltic function and therefore for the coordination of motility. To date, several scaffolds have been successfully seeded with smooth muscle cells and have shown to be capable of maintaining the smooth muscle characteristics. However, these structures lack the orientation of the native tissue, which is considered as the gold standard for treating colonic motility. Until today, no work has been done to successfully bioengineer concentrically oriented smooth muscle tissue constructs however our bioengineered constructs are expected to provide promising results in regenerating gut motility and functionality. Hori et. al. have used collagen sponges seeded with mesenchymal stem cells to reconstruct the intestine. Their results showed cells positively stained with α-SMA on the scaffold but there was no regeneration of the muscle layer [33]. Our model suggests the potential use of chitosan as an appealing biomaterial in the field of intestinal regeneration. Chitosan can easily form a porous tubular scaffold that can serve as a temporary support for physiologically functional smooth muscle constructs. In this study, we provide evidence that RCSM constructs exhibited fundamental functional characteristics of intestinal tissues. In the future, it will be necessary to include other cell types in the bioengineering of these muscle constructs in order to gain higher force.
5. Conclusion
Tissue engineering of the intestine imposes the challenge of regeneration the smooth muscle layer. This present study describes the use of chitosan as a biomaterial in intestinal tissue engineering applications. The use of aligned smooth muscle constructs is beneficial to the restoration of intestinal motility and propulsion of the intestinal content. The evidence of functionality of the smooth muscle constructs after being placed around the composite chitosan scaffold suggests the potential use of this biomaterial to produce a functional tissue. Ongoing work in our laboratory aims to create a continuous circular smooth muscle layer around the tubular scaffold and to characterize its physiological functionality and mechanical properties. Additional studies also intend to explore the ability of chitosan to support the regeneration of the intestine.
Impact Statement.
The work presented in this paper shows the potential use of bioengineered smooth muscle constructs around a chitosan-based scaffold for regenerating intestinal segments.
Acknowledgment
The authors would like to thank Sita Somara and Saranyaraajan Varadarajan for their assistance with this study and the colleagues at Wake Forest Institute for Regenerative Medicine for providing assistance with the microscopy. The authors gratefully acknowledge the funding support provided by National Institutes of Health RO1 DK-042876 and RO1 DK-071614.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cosnes J, Gendre JP, Le Quintrec Y. Role of the ileocecal valve and site of intestinal resection in malabsorption after extensive small bowel resection. Digestion. 1978;18:329–336. doi: 10.1159/000198220. [DOI] [PubMed] [Google Scholar]
- 2.Galea MH, Holliday H, Carachi R, Kapila L. Short-bowel syndrome: a collective review. J Pediatr Surg. 1992;27:592–596. doi: 10.1016/0022-3468(92)90455-g. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt T, Pfeiffer A, Hackelsberger N, Widmer R, Meisel C, Kaess H. Effect of intestinal resection on human small bowel motility. Gut. 1996;38:859–863. doi: 10.1136/gut.38.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kato T, Ruiz P, Thompson JF, Eskind LB, Weppler D, Khan FA, et al. Intestinal and multivisceral transplantation. World J Surg. 2002;26:226–237. doi: 10.1007/s00268-001-0210-5. [DOI] [PubMed] [Google Scholar]
- 5.Gillian B. Quality of life issues: parenteral nutrition to small bowel transplantation–a review. Nutrition. 1998;14:813–816. doi: 10.1016/s0899-9007(98)00091-4. [DOI] [PubMed] [Google Scholar]
- 6.Janson DD. Commentary on "Three years clinical experience with intestinal transplantation" and the nutritional implications. 1994. Nutr Clin Pract. 2002;17:361–364. doi: 10.1177/0115426502017006361. [DOI] [PubMed] [Google Scholar]
- 7.Grikscheit TC, Siddique A, Ochoa ER, Srinivasan A, Alsberg E, Hodin RA, et al. Tissue-engineered small intestine improves recovery after massive small bowel resection. Ann Surg. 2004;240:748–754. doi: 10.1097/01.sla.0000143246.07277.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta A, Vara DS, Punshon G, Sales KM, Winslet MC, Seifalian AM. In vitro small intestinal epithelial cell growth on a nanocomposite polycaprolactone scaffold. Biotechnol Appl Biochem. 2009;54:221–229. doi: 10.1042/BA20090214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hori Y, Nakamura T, Matsumoto K, Kurokawa Y, Satomi S, Shimizu Y. Tissue engineering of the small intestine by acellular collagen sponge scaffold grafting. Int J Artif Organs. 2001;24:50–54. [PubMed] [Google Scholar]
- 10.Nakase Y, Hagiwara A, Nakamura T, Kin S, Nakashima S, Yoshikawa T, et al. Tissue engineering of small intestinal tissue using collagen sponge scaffolds seeded with smooth muscle cells. Tissue Eng. 2006;12:403–412. doi: 10.1089/ten.2006.12.403. [DOI] [PubMed] [Google Scholar]
- 11.Sachlos E, Gotora D, Czernuszka JT. Collagen scaffolds reinforced with biomimetic composite nano-sized carbonate-substituted hydroxyapatite crystals and shaped by rapid prototyping to contain internal microchannels. Tissue Eng. 2006;12:2479–2487. doi: 10.1089/ten.2006.12.2479. [DOI] [PubMed] [Google Scholar]
- 12.Di Martino A, Sittinger M, Risbud MV. Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials. 2005;26:5983–5990. doi: 10.1016/j.biomaterials.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Francis Suh JK, Matthew HWT. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials. 2000;21:2589–2598. doi: 10.1016/s0142-9612(00)00126-5. [DOI] [PubMed] [Google Scholar]
- 14.Kim IY, Seo SJ, Moon HS, Yoo MK, Park IY, Kim BC, et al. Chitosan and its derivatives for tissue engineering applications. Biotechnol Adv. 2008;26:1–21. doi: 10.1016/j.biotechadv.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Madihally SV, Matthew HW. Porous chitosan scaffolds for tissue engineering. Biomaterials. 1999;20:1133–1142. doi: 10.1016/s0142-9612(99)00011-3. [DOI] [PubMed] [Google Scholar]
- 16.VandeVord PJ, Matthew HW, DeSilva SP, Mayton L, Wu B, Wooley PH. Evaluation of the biocompatibility of a chitosan scaffold in mice. J Biomed Mater Res. 2002;59:585–590. doi: 10.1002/jbm.1270. [DOI] [PubMed] [Google Scholar]
- 17.Ma L, Gao C, Mao Z, Zhou J, Shen J, Hu X, et al. Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials. 2003;24:4833–4841. doi: 10.1016/s0142-9612(03)00374-0. [DOI] [PubMed] [Google Scholar]
- 18.Taravel MN, Domard A. Collagen and its interactions with chitosan, III some biological and mechanical properties. Biomaterials. 1996;17:451–455. doi: 10.1016/0142-9612(96)89663-3. [DOI] [PubMed] [Google Scholar]
- 19.Mhurchu CN, Poppitt SD, McGill AT, Leahy FE, Bennett DA, Lin RB, et al. The effect of the dietary supplement, Chitosan, on body weight: a randomised controlled trial in 250 overweight and obese adults. Int J Obes Relat Metab Disord. 2004;28:1149–1156. doi: 10.1038/sj.ijo.0802693. [DOI] [PubMed] [Google Scholar]
- 20.Mhurchu CN, Dunshea-Mooij C, Bennett D, Rodgers A. Effect of chitosan on weight loss in overweight and obese individuals: a systematic review of randomized controlled trials. Obes Rev. 2005;6:35–42. doi: 10.1111/j.1467-789X.2005.00158.x. [DOI] [PubMed] [Google Scholar]
- 21.Muzzarelli RAA. Chitosan-based dietary foods. Carbohyd Polym. 1996;29:309–316. [Google Scholar]
- 22.Hecker L, Baar K, Dennis RG, Bitar KN. Development of a three-dimensional physiological model of the internal anal sphincter bioengineered in vitro from isolated smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G188–G196. doi: 10.1152/ajpgi.00335.2004. [DOI] [PubMed] [Google Scholar]
- 23.Somara S, Gilmont RR, Varadarajan S, Bitar KN. Phosphorylated HSP20 modulates the association of thin-filament binding proteins: caldesmon with tropomyosin in colonic smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1164–G1176. doi: 10.1152/ajpgi.00479.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uygun BE, Stojsih SE, Matthew HW. Effects of immobilized glycosaminoglycans on the proliferation and differentiation of mesenchymal stem cells. Tissue Eng Part A. 2009;15:3499–3512. doi: 10.1089/ten.tea.2008.0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Somara S, Gilmont RR, Dennis RG, Bitar KN. Bioengineered internal anal sphincter derived from isolated human internal anal sphincter smooth muscle cells. Gastroenterology. 2009;137:53–61. doi: 10.1053/j.gastro.2009.03.036. [DOI] [PubMed] [Google Scholar]
- 26.Krupnick AS, Morris JB. The long-term results of resection and multiple resections in Crohn's disease. Semin Gastrointest Dis. 2000;11:41–51. [PubMed] [Google Scholar]
- 27.Lee M, Wu BM, Stelzner M, Reichardt HM, Dunn JC. Intestinal smooth muscle cell maintenance by basic fibroblast growth factor. Tissue Eng Part A. 2008;14:1395–1402. doi: 10.1089/ten.tea.2007.0232. [DOI] [PubMed] [Google Scholar]
- 28.Raghavan S, Lam MT, Foster LL, Gilmont RR, Somara S, Takayama S, et al. Bioengineered three-dimensional physiological model of colonic longitudinal smooth muscle in vitro. Tissue Eng Part C Methods. 2010;16:999–1009. doi: 10.1089/ten.tec.2009.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarkar S, Dadhania M, Rourke P, Desai TA, Wong JY. Vascular tissue engineering: microtextured scaffold templates to control organization of vascular smooth muscle cells and extracellular matrix. Acta Biomater. 2005;1:93–100. doi: 10.1016/j.actbio.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Xu CY, Inai R, Kotaki M, Ramakrishna S. Aligned biodegradable nanofibrous structure: a potential scaffold for blood vessel engineering. Biomaterials. 2004;25:877–886. doi: 10.1016/s0142-9612(03)00593-3. [DOI] [PubMed] [Google Scholar]
- 31.Hu X, Huang J, Ye Z, Xia L, Li M, Lv B, et al. A novel scaffold with longitudinally oriented microchannels promotes peripheral nerve regeneration. Tissue Eng Part A. 2009;15:3297–3308. doi: 10.1089/ten.TEA.2009.0017. [DOI] [PubMed] [Google Scholar]
- 32.de Toledo OMS, Marquezini MV, Jia KB, Pinheiro MdC, Mora OA. Biochemical and Cytochemical Characterization of Extracellular Proteoglycans in the Inner Circular Smooth Muscle Layer of Dog Small Intestine. IUBMB Life. 2002;54:19–25. doi: 10.1080/15216540213824. [DOI] [PubMed] [Google Scholar]
- 33.Hori Y, Nakamura T, Kimura D, Kaino K, Kurokawa Y, Satomi S, et al. Experimental study on tissue engineering of the small intestine by mesenchymal stem cell seeding. J Surg Res. 2002;102:156–160. doi: 10.1006/jsre.2001.6294. [DOI] [PubMed] [Google Scholar]
- 34.Grikscheit TC, Ochoa ER, Ramsanahie A, Alsberg E, Mooney D, Whang EE, et al. Tissue-engineered large intestine resembles native colon with appropriate in vitro physiology and architecture. Ann Surg. 2003;238:35–41. doi: 10.1097/01.SLA.0000074964.77367.4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.An JY, Yun HS, Lee YP, Yang SJ, Shim JO, Jeong JH, et al. The intracellular pathway of the acetylcholine-induced contraction in cat detrusor muscle cells. Br J Pharmacol. 2002;137:1001–1010. doi: 10.1038/sj.bjp.0704954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hosey MM. Diversity of Structure, Signaling and Regulation within the Family of Muscarinic Cholinergic Receptors. Faseb J. 1992;6:845–852. [PubMed] [Google Scholar]
- 37.Van Geldre LA, Lefebvre RA. Interaction of NO and VIP in gastrointestinal smooth muscle relaxation. Curr Pharm Des. 2004;10:2483–2497. doi: 10.2174/1381612043383890. [DOI] [PubMed] [Google Scholar]
- 38.Murthy KS, Makhlouf GM. Interaction of cA-kinase and cG-kinase in mediating relaxation of dispersed smooth muscle cells. Am J Physiol. 1995;268:C171–C180. doi: 10.1152/ajpcell.1995.268.1.C171. [DOI] [PubMed] [Google Scholar]
- 39.Neff LP, Tillman BW, Yazdani SK, Machingal MA, Yoo JJ, Soker S, et al. Vascular smooth muscle enhances functionality of tissue-engineered blood vessels in vivo. J Vasc Surg. 2011;53:426–434. doi: 10.1016/j.jvs.2010.07.054. [DOI] [PubMed] [Google Scholar]
- 40.L'Heureux N, Stoclet JC, Auger FA, Lagaud GJ, Germain L, Andriantsitohaina R. A human tissue-engineered vascular media: a new model for pharmacological studies of contractile responses. Faseb J. 2001;15:515–524. doi: 10.1096/fj.00-0283com. [DOI] [PubMed] [Google Scholar]