Abstract
Objective
Both subclinical hypothyroidism and the metabolic syndrome have been associated with increased risk of coronary heart disease events. It is unknown if the prevalence and incidence of metabolic syndrome is higher as TSH levels increase, or in individuals with subclinical hypothyroidism. We sought to determine the association between thyroid function and the prevalence and incidence of the metabolic syndrome in a cohort of older adults.
Design
Data was analyzed from the Health, Aging, and Body Composition Study, a prospective cohort of 3,075 community-dwelling US adults.
Participants
2,119 participants with measured TSH and data on metabolic syndrome components were included in the analysis.
Measurements
TSH was measured by immunoassay. Metabolic syndrome was defined per revised ATP III criteria.
Results
At baseline, 684 participants met criteria for metabolic syndrome. At 6yr follow-up, incident metabolic syndrome developed in 239 individuals. In fully adjusted models, each unit increase in TSH was associated with a 3% increase in the odds of prevalent metabolic syndrome (OR 1.03, 95% CI 1.01–1.06, p=0.02), and the association was stronger for TSH within the normal range (OR 1.16, 95% CI 1.03–1.30, p=0.02). Subclinical hypothyroidism with a TSH>10mIU/L was significantly associated with increased odds of prevalent metabolic syndrome (OR 2.3, 95% CI 1.0–5.0, p=0.04); the odds of incident MetS was similar (OR 2.2), but the confidence interval was wide (0.6–7.5).
Conclusions
Higher TSH levels and subclinical hypothyroidism with a TSH>10 mIU/L are associated with increased odds of prevalent but not incident metabolic syndrome.
Keywords: Thyroid Function, Metabolic Syndrome, Subclinical Hypothyroidism
Introduction
The association between overt hypothyroidism and atherosclerotic cardiovascular disease is well-described1 and increasing evidence suggests an association exists with milder degrees of thyroid dysfunction such as subclinical hypothyroidism.2 Several observational studies have examined the relationship between subclinical hypothyroidism and cardiovascular disease and mortality with conflicting results;3–6 however, a recent individual participant data meta-analysis of 11 prospective studies reported a positive association.2 In studies where an association has been found, the observed relationship persists after adjustment for traditional risk factors, suggesting an alternative mechanism by which subclinical hypothyroidism increases cardiovascular risk.2, 5, 6
The metabolic syndrome (MetS), which has been linked to cardiovascular disease and mortality in the general population,7, 8 is one such potential mechanism. MetS is characterized by atherogenic dyslipidemia, insulin resistance and hypertension,9 all of which may be increased in hypothyroid or subclinical hypothyroid states. It is presently unknown if higher TSH within the euthyroid range or subclinical hypothyroidism is associated with an increased prevalence and incidence of MetS. Several recent cross-sectional studies have sought to determine this relationship with conflicting findings.10–14 To date, there have been no prospective studies on the association between thyroid function and MetS.
Although most individuals at risk for cardiovascular disease are euthyroid, older adults have a higher prevalence of subclinical hypothyroidism15 and therefore a potential relationship between elevated TSH and the risk factors characterizing the metabolic syndrome may be more important to identify in this group. To characterize the association between thyroid function and the prevalence and incidence of the metabolic syndrome in older adults, we examined 6 years of follow-up data from the Health, Aging, and Body Composition (Health ABC) cohort study.
Participants and Methods
Study Population
Data were analyzed from the Health ABC study, a prospective cohort of 3,075 White and Black community-dwelling persons aged 70–79 at enrollment designed to study body composition and physical function. Participants were recruited from a random sample of White Medicare beneficiaries and all age-eligible Black community residents in Pittsburgh, PA and Memphis, TN. Eligibility criteria included the ability to walk ¼ mile, up 10 stairs without rest and perform basic activities of daily living independently.16 The institutional review board at each of the two sites approved of the protocol and all subjects provided written informed consent.
2799 participants of the cohort had TSH measured from fasting blood samples at the second year visit. Free thyroxine (FT4) levels were reflexively measured on any participant with a TSH value of less than 0.1 mIU/L or greater than 7.0 mIU/L.17
We excluded participants with diabetes at baseline (n=632), defined as a self-reported diagnosis of diabetes, use of diabetes medications, fasting glucose of 7.0 mmol/L or higher, or 2-h glucose of 11.1 mmol/L or higher after a 75-g oral glucose load, because they already exhibit a more complete phenotype of insulin resistance and dyslipidemia than individuals with the MetS. We further excluded participants on amiodarone (n=5), lithium (n=2), and antithyroid medications (n=1) due to their variable effects on thyroid function. Individuals with overt hyperthyroidism (n=4) were excluded as this represented a small number of participants, and 36 persons were excluded for missing data on the MetS. The final sample for this analysis was 2,119 participants.
Measurements
Thyroid Hormones
TSH was measured by a central lab at the University of Vermont by immunoassay (ACS; Chiron Diagnostics Corp, Emeryville, Calif). According to the manufacturer, the coefficient of variation for TSH was 4.71% at a level of 0.30 mIU/L and 3.64% at a level of 15.85 mIU/L. The normal range for TSH in this assay is 0.35–5.5 mIU/L. FT4 was measured by competitive immunoassay (ACS; Chiron Diagnostics). The normal range for FT4 in this assay is 10.3–23.2 pmol/L (0.8–1.8 ng/dL). Categories of thyroid function were defined as subclinical hyperthyroid (TSH <0.35 mIU/L), euthyroid (TSH 0.35–4.5 mIU/L), mild subclinical hypothyroid (TSH 4.5–9.9 mIU/L, normal FT4), marked subclinical hypothyroid (TSH 10–20 mIU/L, normal FT4), and overt hypothyroid (TSH>4.5 mIU/L and FT4 < 10.3 pmol/L).
Metabolic Syndrome
Metabolic syndrome was defined per revised NCEP/ATP III criteria as any 3 of 5 criteria: waist circumference ≥102cm for men or ≥88cm for women, elevated triglycerides (TG) ≥1.7 mmol/L or on drug treatment for high triglycerides, reduced HDL cholesterol <1.03 mmol/L in men or <1.29 mmol/L in women or on drug treatment for reduced HDL-C, elevated blood pressure ≥130 mmHg systolic BP or ≥85 mmHg diastolic BP or on antihypertensive drug treatment for elevated BP, and elevated fasting glucose ≥5.6 mmol/L.9 Blood glucose, total cholesterol, LDL, HDL, triglycerides were measured from fasting blood samples (Vitros, Johnson & Johnson, Rochester, NY), and blood pressure was measured in the entire cohort annually. Waist circumference was measured at baseline and at 6-yr follow-up.
Insulin Resistance
Insulin resistance was assessed at baseline using the homeostasis model of insulin resistance (HOMA-IR), calculated as fasting insulin (uU/mL) (Abbott Laboratories Diagnostics Division, South Pasadena, CA) × fasting glucose (mmol/L)/22.5.18
Covariates
BMI was calculated as weight (kilograms)/height squared (meters2). Participants self-reported smoking status (current, past, or never) and alcohol intake (categorized as 0–1, 2–7, or >7 drinks per week).
Medication use
Participants were asked to bring any medications taken over the preceding 2 weeks to all study visits. Medications were classified using a hierarchical drug dictionary based upon the Iowa Drug Information System.19
Statistical Analysis
Differences in baseline characteristics by MetS status were assessed using x2-testfor categorical variables and t-test for continuous variables. For continuous variables which did not follow a normal distribution, the appropriate non-parametric test was employed.
The relationship between continuous TSH as a predictor of prevalent MetS at baseline and incident MetS at 6-yr follow-up was determined using logistic regression. Potential confounders such as age, sex, race, BMI, and HOMA-IR were chosen based on biological plausibility and the published literature. We screened for additional confounders based on univariate association with MetS using a cutoff of p<0.1, which resulted in the inclusion of smoking status as a covariate. Self-reported alcohol intake was a proposed covariate but was excluded from our model as it was not independently associated with either thyroid function (p=0.5) or MetS (p=0.5).
Univariate models were performed first and subsequently adjusted for age, sex and race. Final multivariable models included age, sex, race, smoking status, BMI and HOMA-IR as covariates. We then assessed for interaction with sex, race, and smoking status.
We used restricted cubic splines to flexibly model the association between TSH and MetS at baseline, adjusted for age, sex, race, BMI, and HOMA-IR. The spline curve for the probability of MetS vs. TSH level was plotted with 95% confidence intervals.
We determined the association between the categories of thyroid function (subclinical hyperthyroid, euthyroid, subclinical hypothyroid, and overt hypothyroid) and the prevalence of MetS at baseline and the incidence of MetS at 6-yr follow-up using the same covariates described above. Subclinical hypothyroid participants were stratified by TSH level into two categories: mild (TSH 4.5–9.9) and marked (TSH 10–20).
In addition to MetS as a composite endpoint, we also evaluated the association between continuous TSH and thyroid function category with each individual component of MetS using both logistic and linear regression. Triglycerides and HDL were natural-log transformed for linear regression due to significant departure from the normal distribution.
Finally, in order to address the possibility of misclassification of thyroid function category due to incomplete FT4 measurements in this cohort (FT4 was measured only in those with TSH<0.1 or >7mIU/L), we performed a sensitivity analysis excluding any participants with abnormal TSH who did not have measured FT4 (n= 216).
Data were analyzed using STATA 11 software (StataCorp, College Station, TX). All analyses were confirmed by an independent analyst at the San Francisco Coordinating Center (S.H.).
Results
Baseline Participants Characteristics
Characteristics of the participants are described in Table 1. The mean age of this cohort was 73.6 years (±2.9), and 53% were women.
Table 1.
Baseline Characteristics of the Cohort
| MetS+ (N= 684) | MetS − (N= 1,435) | P-value | |
|---|---|---|---|
| Age, years | 73.4 (2.8) | 73.7 (2.9) | 0.03 |
| Sex (n) | <0.01 | ||
| Male | 280 (40.9) | 712 (49.6) | |
| Female | 404 (59.1) | 723 (50.4) | |
| Race (n) | |||
| White | 477 (69.7) | 871 (60.7) | <0.01 |
| Black | 207 (30.3) | 564 (39.3) | |
| Weight, kg | 79.6 (14.6) | 71.6 (14.0) | <0.01 |
| BMI, kg/m2 | 29.0 (4.6) | 25.9 (4.3) | <0.01 |
| Smoking (n) | <0.01 | ||
| Current | 49 (7.2) | 165 (11.5) | |
| Never/Past | 634 (92.7) | 1,268 (88.4) | |
| Alcohol Intake (n) | 0.26 | ||
| 0–1 drink/wk | 328 (48.0) | 652 (45.4) | |
| 2–7 drink/wk | 348 (50.9) | 759 (52.9) | |
| >7 drink/wk | 8 (1.2) | 22 (1.5) | |
| Weekly exercise (n) | 22 (3.2) | 37 (2.6) | 0.40 |
| SBP, mmHg | 139.1 (19.9) | 132.8 (20.8) | <0.01 |
| DBP, mmHg | 72.4 (11.3) | 71.1 (11.7) | 0.02 |
| Abdominal | 104.4 (11.3) | 95.2 (12.4) | <0.01 |
| Circumference, cm | |||
| TSH, mIU/L | 2.4 (2.1)* | 2.1 (1.8)* | <0.01 |
| Total Cholesterol, mmol/L | 5.38 (1.03) | 5.21 (0.96) | <0.01 |
| LDL Cholesterol, mmol/L | 3.26 (0.93) | 3.13 (0.86) | <0.01 |
| HDL Cholesterol, mmol/L | 1.1 (0.36)* | 1.48 (0.52)* | <0.01 |
| Triglycerides, mmol/L | 1.90 (1.05)* | 1.14 (0.55)* | <0.01 |
| Fasting Glucose, mmol/L | 5.40 (0.57) | 5.0 (0.44) | <0.01 |
| HOMA-IR | 2.1 (1.6)* | 1.3 (0.9)* | <0.01 |
Data are given as mean (SD) or n(percent) unless otherwise noted.
For multilevel categorical variables, the P-value for test of trend is reported.
Median (interquartile range)
Thyroid Function
Most (84%) of the individuals in our study were euthyroid. As previously reported, 2% (n=50) had subclinical hyperthyroidism, less than 1% (n=21) had overt hypothyroidism and nearly 13% (n=268) of individuals had subclinical hypothyroidism.17 TSH was significantly associated with race, with higher mean TSH in White participants (mean TSH 3.2 vs. 2.2 mIU/L, p<0.01). Thyroid function was not associated with age, sex, weight or BMI (data not shown).
Metabolic Syndrome
At baseline, 32% (n=684) of the cohort met criteria for MetS. Participants with prevalent MetS were more likely to be female, White, and non-smokers. As expected, those with MetS had higher mean blood pressure, abdominal circumference, triglycerides, fasting glucose, HOMA-IR and lower HDL cholesterol. Individuals with MetS also had higher mean TSH, LDL and total cholesterol levels. Prevalent MetS was not associated with self-reported alcohol intake or physical activity.
At 6-yr follow-up, among the 1032 individuals eligible for analysis there were 239 incident cases of MetS. MetS developed in 20% (n=100) of men vs. 26% (n=139) of women, and in 24% (n=87) of Black vs. 23% (n=152) of White participants.
TSH and Metabolic Syndrome
TSH as a continuous variable was significantly associated with an increased odds of prevalent MetS in an age, sex and race-adjusted model, with each one unit increase in TSH predicting a 3% increase in the odds of MetS (OR 1.03, 95% CI 1.01–1.06, p=0.017). The multivariable-adjusted odds ratio was similar (OR 1.03, 95% CI 1.01–1.06, p=0.018) (Table 2). Figure 1 shows the restricted cubic spline model of the association between TSH and prevalent MetS, adjusted for age, sex, and race.
Table 2.
Odds Ratio of Prevalent MetS by TSH
| TSH (per 1 unit increase) | Age, Sex, & Race Adjusted OR (95% CI) | Multivariable Adjusted OR* (95% CI) |
|---|---|---|
| Entire Cohort | ||
| All TSH values (n=2119) | 1.03 (1.01–1.06) | 1.03 (1.01–106) |
| Euthyroid range only (n=1779) | 1.18 (1.06–1.32) | 1.16 (1.03–1.30) |
| Excluding Thyroid Hormone Users | ||
| All TSH values (n=1915) | 1.03 (1.00–1.06) | 1.03 (1.00–1.06) |
| Euthyroid range only (n=1641) | 1.21 (1.08–1.35) | 1.14 (1.00–1.30) |
Adjusted for age, sex, race, smoking status, BMI, and HOMA-IR
Figure 1.
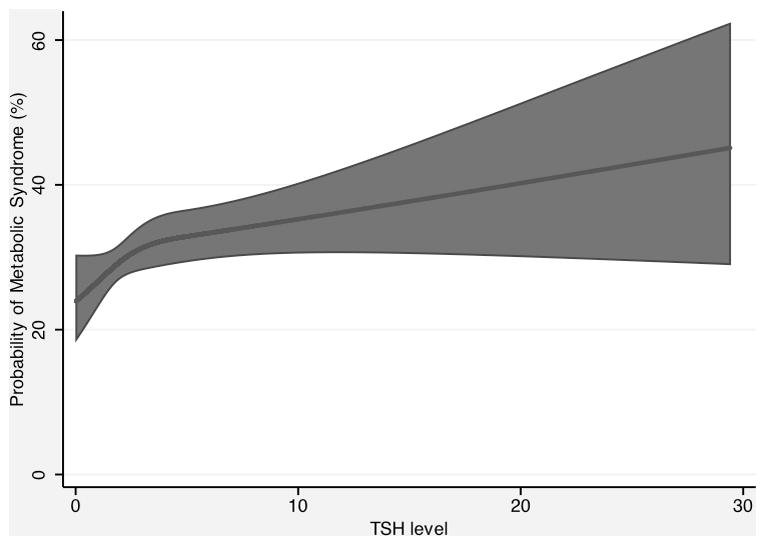
Fitted prevalence of metabolic syndrome as a function of TSH levels, with 95% confidence interval, holding age, sex, race, BMI, smoking status, and HOMA-IR constant at their sample means
In order to evaluate the relationship between TSH and prevalent MetS within the euthyroid range, we then restricted our analysis to include only normal TSH values (n=1,779). Among euthyroid individuals, each one unit increase in TSH predicted an 18% increase in the odds of MetS (OR 1.18, 1.06–1.32, p=0.002).
The association between continuous TSH and incident MetS was not significant (OR 1.00, 95% CI 0.96–1.04, p=0.92).
Thyroid Function Category and Metabolic Syndrome
Overall, there was no significant difference in the odds of prevalent MetS in subclinical hypothyroid compared with euthyroid participants (OR 1.2, 95% CI 0.9–1.5, p=0.3) in unadjusted or adjusted models. When levels of subclinical hypothyroidism were examined, we found no evidence of association between mild subclinical hypothyroidism and the odds of prevalent MetS. However, marked subclinical hypothyroidism (TSH 10–20) was significantly associated with increased odds of prevalent MetS (OR 2.2, 95% CI 1.1–4.4, p=0.02) after adjustment for age, sex, and race (Table 3). Multivariable analysis adjusted for age, sex, race, smoking status, BMI, and HOMA-IR resulted in similarly increased odds of prevalent MetS in participants with TSH 10–20 (OR 2.3, 95% CI 1.0–5.0, p=0.04).
Table 3.
Odds of Prevalent MetS at Baseline by Thyroid Function Category
| Thyroid Function Category | Participants with MetS at Baseline, n (%) | OR (95% CI) | |
|---|---|---|---|
| Age, Sex, & Race Adjusted | Multivariable Adjusted** | ||
| Subclinical Hyperthyroid | 16/50 (32) | 0.9 (0.5–1.7) | 0.8 (0.4–1.6) |
| Euthyroid | 561/1779 (32) | Referent | Referent |
| Mild Subclinical Hypothyroid* | 80/234 (34) | 1.0 (0.8–1.4) | 1.0 (0.7–1.5) |
| Marked Subclinical Hypothyroid* | 18/34 (53) | 2.2 (1.1–4.4) | 2.3 (1.0–5.0) |
| Overt Hypothyroid | 8/21 (38) | 1.1 (0.5–2.8) | 1.0 (0.4–2.6) |
Mild Subclinical Hypo = TSH 4.5–9.9 mIU/mL, Marked Subclinical Hypo = TSH 10–20 mIU/mL
Adjusted for age, sex, race, smoking status, BMI, and HOMA-IR
Neither subclinical hyperthyroidism nor overt hypothyroidism was associated with prevalent MetS.
In individuals with marked subclinical hypothyroidism (n=12), the odds of developing MetS at 6-yr follow-up adjusted for age, sex, and race was similar to the odds of prevalent MetS (OR 2.4, 95% CI 0.7–7.6, p=0.15) but did not achieve statistical significance (Table 4). The confidence interval for this association was wide likely due to a small number of cases of incident MetS among participants in this thyroid function category (n=5). Multivariable-adjusted results were similar.
Table 4.
Odds of Incident MetS at Year 6 by Thyroid function Category
| Thyroid Function Category | Participants with MetS at 6yr Follow-Up, n (%) | OR (95% CI) | |
|---|---|---|---|
| Age, Sex, & Race Adjusted | Multivariable Adjusted** | ||
| Subclinical Hyperthyroid | 5/21 (24) | 1.0 (0.3–2.7) | 0.8 (0.2–2.5) |
| Euthyroid | 200/871 (23) | reference | |
| Mild Subclinical Hypothyroid* | 27/117 (23) | 1.0 (0.6–1.6) | 1.2 (0.7–1.9) |
| Marked Subclinical Hypothyroid* | 5/12 (42) | 2.4 (0.7–7.6) | 2.2 (0.6–7.5) |
| Overt Hypothyroid | 2/11 (18) | 0.6 (0.1–3.0) | 0.6 (0.1–2.8) |
Mild Subclinical Hypo = TSH 4.5–9.9 mIU/L, Marked Subclinical Hypo = TSH 10–20 mIU/L
Adjusted for age, sex, race, smoking status, BMI, and HOMA-IR
Thyroid Function Category and Individual Components of the Metabolic Syndrome
In order to determine which components of the metabolic syndrome were most strongly associated with thyroid function, we evaluated the association of TSH and thyroid function categories with each of the five MetS criteria at baseline (Table 5).
Table 5.
Odds of Meeting Individual MetS Criteria at Baseline by Thyroid Function Category
| Thyroid Function Category (n) | Multivariable-Adjusted** OR (95% CI) | ||||
|---|---|---|---|---|---|
| Fasting Blood Glucose | Elevated Blood Pressure | Elevated Triglycerides | Low HDL Cholesterol | Increased Waist Circumference | |
| Subclinical Hyperthyroid (50) | 0.81 (0.36–1.79) | 0.48 (0.27–0.85) | 0.99 (0.52–1.90) | 1.80 (0.98–3.31) | 0.79 (0.36–1.75) |
| Euthyroid (1,779) | Referent | ||||
| Mild Subclinical Hypothyroid* (234) | 0.88 (0.59–1.30)) | 0.84 (0.63–1.14) | 1.19 (0.87–1.64) | 1.28 (0.93–1.76) | 0.88 (0.60–1.31) |
| Marked Subclinical Hypothyroid* (34) | 0.81 (0.30–2.19) | 1.31 (0.58–2.98) | 2.37 (1.13–4.96) | 2.08 (1.00–4.31) | 1.13 (0.41–3.09) |
| Overt Hypothyroid (21) | 1.27 (0.41–3.97) | 3.01 (0.87–10.47) | 1.34 (0.52–3.44) | 1.53 (0.61–3.85) | 0.35 (0.10–1.22) |
Mild Subclinical Hypo = TSH 4.5–9.9 mIU/L, Marked Subclinical Hypo = TSH 10–20 mIU/L
Adjusted for age, sex, race, smoking status, BMI, and HOMA-IR
In an age, sex, and race-adjusted model, continuous TSH was significantly associated with the odds of meeting the high triglyceride (OR 1.03, 95% CI 1.01–1.06, p=0.014) and low HDL (OR 1.03, 95% CI 1.00–1.05, p=0.043) components of MetS. One unit increase in TSH was associated with an approximately 1% increase in triglyceride levels (β=0.009, 95% CI 0.004–0.014, p=0.001). TSH was also marginally associated with decreasing HDL, with each unit increase in TSH predicting a 0.3% decrease in HDL levels (β= −0.003, 95% CI = −0.006–0.00, p=0.055). Although continuous TSH was associated with the odds of meeting the elevated blood pressure criteria of the MetS (OR 1.04, 95% CI 1.00–1.07, p=0.032), each unit increase in TSH did not predict a statistically significant increase in either systolic or diastolic blood pressure. Neither continuous TSH nor categories of thyroid function were associated with meeting the fasting glucose or waist circumference criteria of the MetS.
Subgroup analyses
There was no evidence of a differential effect of TSH on MetS between men and women (p=0.48 for interaction), or between race groups (p= 0.36). There was also no evidence for an interaction between TSH and smoking status (p=0.37). In addition, given the potential relationship between serum TSH and obesity, we repeated our analyses separately in obese participants (BMI>30 kg/m2). There was no significant difference in the association between thyroid function and prevalent or incident MetS in participants with BMI ≤ or > 30 kg/m2 and testing for interaction by obesity status was not significant (p=0.37).
Additional Analyses
Several additional analyses were performed in order to determine the sensitivity of our results to study inclusion and exclusion criteria. Since thyroid hormone users represented nearly 10% of our included cohort (n=204) we repeated our analyses excluding these individuals, although univariate models did not show an association between thyroid hormone use and prevalent or incident MetS (p=0.2 and p=0.4, respectively). Among participants who did not reported thyroid hormone use at baseline (n=1,915), we found that continuous TSH was still associated with increased odds of prevalent MetS (OR 1.02, 95% CI 1.00–1.06, p=0.057) but the association was no longer significant, likely due to loss of power. Analysis restricted to the normal range of TSH values remained significant with each unit increase in TSH predicting a 21% increase in the odds of MetS (OR 1.21, 95% CI 1.08–1.35, p<0.01) (Table 2).
Excluding thyroid hormone users from the analysis of the association between thyroid function categories and the odds of MetS at baseline substantially changed our results. Marked subclinical hypothyroidism was no longer significantly associated with prevalent MetS (OR 1.58, 95% CI 0.68–3.66, p=0.28). We also found that a greater proportion with marked subclinical hypothyroidism were thyroid hormone users compared with all other TSH categories except for subclinical hyperthyroidism. Thyroid hormone use was present in 8% of euthyroid participants, 9% with mild and 32% with marked subclinical hypothyroidism, and 24% with overt hypothyroidism. Participants with subclinical hyperthyroidism had the highest prevalence of thyroid hormone use (49%).
Exclusion of 182 participants with TSH 4.5–6.9 and 34 participants with TSH 0.1–0.35 who were potentially misclassified due to lack of FT4 measurements did not substantially change results. In analysis restricted to participants with complete thyroid function testing (n=1,903), the odds of prevalent MetS (95% CI) for mild subclinical hyperthyroidism was 0.58 (0.15–2.16), for mild subclinical hypothyroid OR 0.93 (0.46–1.85), for marked subclinical hypothyroid OR 2.34 (1.06–5.13), and for overt hypothyroid OR 0.97 (0.35–2.63).
In our analyses of incident MetS, we initially included participants who developed incident diabetes by year 6 (n=55). Analyses were repeated after excluding these individuals (remaining cohort, n=977) and results were similar. Primary analyses also excluded individuals with overt hyperthyroidism (n=4) as this represented a small number of participants who were likely to have variable thyroid function over time, however results were unchanged with their inclusion.
Because BMI and HOMA-IR are potentially on the causal pathway between thyroid function and MetS, their inclusion as covariates in multivariable models may have attenuated our results. We therefore repeated analyses removing BMI and HOMA-IR from our models and found similar results (for continuous TSH OR 1.03, 95% CI 1.00–1.06; for marked subclinical hypothyroidism OR 2.24, 95% CI 1.13–4.46), suggesting the association was not mediated by these factors.
Finally, we repeated our analyses using different cutpoints for categories of thyroid function. Using the manufacturer’s assay (0.35–5.5mIU/L) or literature based (0.45–4.5mIU/L) normal ranges 20 did not change our results (Data not shown).
Discussion
In a large population-based study of older adults, we found that increasing TSH levels were associated with greater odds of prevalent metabolic syndrome. We also observed a significant association between continuous TSH and prevalent MetS among euthyroid participants. This suggests that even within the normal range, there may be a higher prevalence of metabolic abnormalities as TSH levels increase. In addition, we found a significant increase in the odds of prevalent MetS in participants with marked subclinical hypothyroidism (TSH 10–20 mIU/L). This is the same category of individuals who, in a recent meta-analysis by Rodondi et al, were found to have increased incidence of CHD events and mortality, suggesting that cardiometabolic risk may be determined in part by degree of TSH elevation.2
Although results for the association between continuous TSH and prevalent MetS were unchanged after excluding thyroid hormone users, the relationship between marked subclinical hypothyroidism and prevalent MetS was no longer statistically significant. This suggests that our power to detect an association within this group may have been decreased by excluding 11 of 34 participants (32%). Another possibility is that the observed association among this group was due to the inclusion of under-treated overt hypothyroid participants; therefore excluding these individuals attenuated the effect.
Previous cross-sectional studies have inconsistently found an association between MetS and higher TSH levels or subclinical hypothyroidism.10, 11 This may be due to differences in how thyroid function was categorized, the outcome assessed, and the age of participants under study. In a Mexican cohort of over 3000 participants, Garduño-Garcia et al. reported a relationship between FT4 and HDL, insulin and HOMA-IR levels, however there was no difference in the prevalence of the MetS between euthyroid and subclinical hypothyroid individuals.11 Of note, participants in this study with TSH values ≥10 mIU/L were classified as overt hypothyroid regardless of FT4 level. This may have obscured an association between subclinical hypothyroidism and MetS since we found a significant relationship only in those with a TSH >10 mIU/L. In a cohort of 2700 euthyroid participants, Roos et al. found an association between FT4 and 4 of the 5 MetS criteria, however this study did not use MetS as a composite endpoint.10 In both of these studies, the average age of participants (mean age 40’s) was much younger than the Health ABC, suggesting that age might play a role in modifying the effect of TSH on the MetS and its components.
Two recent cross-sectional studies showed a positive association between TSH levels and prevalent MetS. One study of a euthyroid German cohort (mean age 52) found that TSH in the upper normal range (2.5–4.5 mIU/L) was associated with a 1.7-fold increased risk of MetS compared with a low-normal TSH (0.3–2.5 mIU/L).12 Another study of nearly 1000 euthyroid post-menopausal women in Korea (mean age 59) reported an odds ratio for MetS of 1.9 in participants with the highest vs. lowest quartiles of TSH.13 In both studies only the triglyceride component of MetS was independently associated with TSH. These finding are consistent with our results, although we also found HDL levels to be weakly associated.
Current evidence suggests that many of the individual components of the metabolic syndrome may be more prominent as TSH levels increase. Both the HUNT and Tromsø studies reported a linear positive association between TSH levels and blood pressure among euthyroid individuals which may be mediated by increased arterial stiffness and impaired vascular smooth muscle relaxation.21–23 The HUNT study also found higher TSH to be associated with higher triglycerides and lower HDL; furthermore, the relationship was stronger among overweight individuals (BMI>25 kg/m2).24 Thyroid hormone may suppress liver triglycerides synthesis, and there is increasing evidence that thyroid hormone increases reverse cholesterol transport, thereby promoting HDL cholesterol activity.25 Increased insulin resistance has also been documented in both overt and subclinical hypothyroidism.26, 27
Insulin resistance is generally thought to be the underlying cause of the metabolic syndrome.9 However, we did not find an association between thyroid function and insulin resistance as measured by HOMA-IR or fasting glucose. The only individual components of the metabolic syndrome independently associated with thyroid function were triglycerides and HDL cholesterol. HDL cholesterol is an independent predictor of cardiovascular disease even in individuals who are adequately treated for elevated LDL cholesterol.28, 29 Therefore the association between thyroid function and HDL observed in our study might represent one of the mechanisms by which subclinical hypothyroidism increases cardiovascular risk in previous studies that adjusted for traditional CVD risk factors.
Several limitations of this study should be noted. First, TSH values were measured only once despite previous data reporting spontaneous normalization of abnormal TSH in a significant proportion of people.30, 31 This would likely result in non-differential misclassification and attenuate our observations. In addition, FT4 levels were only measured in participants with TSH values <0.1 or greater than 7.0 mIU/L which may have resulted in misclassification of individuals with overt hyper- or hypothyroidism as having subclinical disease, though studies of outpatient populations suggest that overt thyroid disease is rare with mild TSH abnormalities.32 Furthermore, given the overall low prevalence of overt hyper- or hypothyroidism in Health ABC participants with markedly high or low TSH values (<1% for each condition),17 it is likely that few individuals were misclassified due to a lack of complete FT4 measurements and a sensitivity analysis excluding these potentially misclassified participants yielded similar results. Secondly, because only a small proportion of the cohort had FT4 measurements (n=129), we were unable to evaluate the association between FT4 and MetS.
The lack of significant association among individuals with overt hypothyroidism represents an inconsistent finding of this study. Although the association between continuous TSH and prevalent MetS was found to be linear, the odds ratios for prevalent disease in individuals with overt hypothyroidism were not elevated. This may be due to the small number of participants with overt hypothyroidism (n=21) and consequent lack of power.
In addition, there is some evidence that obesity itself may be responsible for elevated TSH levels which are not indicative of thyroid dysfunction, but rather a consequence of greater fat mass.33, 34 In obese individuals, increased adipose tissue may secrete greater amounts of leptin, which in turn may stimulate the hypothalamic-pituitary-thyroid axis to secrete TSH. Because of this bidirectional relationship, it is possible that in some participants obesity and MetS preceded the measurement of elevated TSH. However, we observed no correlation between TSH and measures of obesity (BMI or waist circumference) and TSH values were not significantly different between obese and non-obese participants (p=0.62). Furthermore, results remained unchanged after adjustment for BMI, and analyses stratified by BMI≤ or >30 kg/m2 were not significantly different. These results are in contrast to those reported by Rotondi et al, who found significantly higher TSH values in euthyroid morbidly obese (BMI>40) and obese (BMI>30) individuals versus normo-weight controls.35
Finally, our results showed an association between thyroid function and prevalent, but not incident MetS, and causal relationships cannot be established from cross-sectional data. Although our results for incident disease were not statistically significant, the point estimates for the odds of incident MetS were similar to those for prevalent disease. However, we were underpowered in this analysis due to the very small number or participants with marked subclinical hypothyroidism (n=12).
In conclusion, we describe an association between higher TSH levels and increased prevalence of the metabolic syndrome not only in those with abnormal thyroid function but also within the euthyroid range. In addition, individuals with subclinical hypothyroidism and TSH >10mIU/L may be at greater risk for MetS and its associated complications. The relationship between thyroid function and MetS appears to be driven primarily by higher triglycerides and lower HDL. Further prospective studies are needed to determine whether treatment of elevated TSH can attenuate these metabolic abnormalities.
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging. This research was supported by National Institute on Aging (NIA) Contracts N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050, and NINR grant R01-NR012459.
Footnotes
Conflicts of Interest and Financial Disclosures: The authors have nothing to declare.
References
- 1.Cappola AR, Ladenson PW. Hypothyroidism and atherosclerosis. J Clin Endocrinol Metab. 2003;88:2438–2444. doi: 10.1210/jc.2003-030398. [DOI] [PubMed] [Google Scholar]
- 2.Rodondi N, den Elzen WP, Bauer DC, Cappola AR, Razvi S, Walsh JP, Asvold BO, Iervasi G, Imaizumi M, Collet TH, Bremner A, Maisonneuve P, Sgarbi JA, Khaw KT, Vanderpump MP, Newman AB, Cornuz J, Franklyn JA, Westendorp RG, Vittinghoff E, Gussekloo J. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 304:1365–1374. doi: 10.1001/jama.2010.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cappola AR, Fried LP, Arnold AM, Danese MD, Kuller LH, Burke GL, Tracy RP, Ladenson PW. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA. 2006;295:1033–1041. doi: 10.1001/jama.295.9.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodondi N, Newman AB, Vittinghoff E, de Rekeneire N, Satterfield S, Harris TB, Bauer DC. Subclinical hypothyroidism and the risk of heart failure, other cardiovascular events, and death. Arch Intern Med. 2005;165:2460–2466. doi: 10.1001/archinte.165.21.2460. [DOI] [PubMed] [Google Scholar]
- 5.Walsh JP, Bremner AP, Bulsara MK, O’Leary P, Leedman PJ, Feddema P, Michelangeli V. Subclinical thyroid dysfunction as a risk factor for cardiovascular disease. Arch Intern Med. 2005;165:2467–2472. doi: 10.1001/archinte.165.21.2467. [DOI] [PubMed] [Google Scholar]
- 6.Imaizumi M, Akahoshi M, Ichimaru S, Nakashima E, Hida A, Soda M, Usa T, Ashizawa K, Yokoyama N, Maeda R, Nagataki S, Eguchi K. Risk for ischemic heart disease and all-cause mortality in subclinical hypothyroidism. J Clin Endocrinol Metab. 2004;89:3365–3370. doi: 10.1210/jc.2003-031089. [DOI] [PubMed] [Google Scholar]
- 7.Malik S, Wong ND, Franklin SS, Kamath TV, L’Italien GJ, Pio JR, Williams GR. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110:1245–1250. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 8.Butler J, Rodondi N, Zhu Y, Figaro K, Fazio S, Vaughan DE, Satterfield S, Newman AB, Goodpaster B, Bauer DC, Holvoet P, Harris TB, de Rekeneire N, Rubin S, Ding J, Kritchevsky SB. Metabolic syndrome and the risk of cardiovascular disease in older adults. J Am Coll Cardiol. 2006;47:1595–1602. doi: 10.1016/j.jacc.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 9.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 10.Roos A, Bakker SJ, Links TP, Gans RO, Wolffenbuttel BH. Thyroid function is associated with components of the metabolic syndrome in euthyroid subjects. J Clin Endocrinol Metab. 2007;92:491–496. doi: 10.1210/jc.2006-1718. [DOI] [PubMed] [Google Scholar]
- 11.Garduno-Garcia Jde J, Alvirde-Garcia U, Lopez-Carrasco G, Padilla Mendoza ME, Mehta R, Arellano-Campos O, Choza R, Sauque L, Garay-Sevilla ME, Malacara JM, Gomez-Perez FJ, Aguilar-Salinas CA. TSH and free thyroxine concentrations are associated with differing metabolic markers in euthyroid subjects. Eur J Endocrinol. 163:273–278. doi: 10.1530/EJE-10-0312. [DOI] [PubMed] [Google Scholar]
- 12.Kim BJ, Kim TY, Koh JM, Kim HK, Park JY, Lee KU, Shong YK, Kim WB. Relationship between serum free T4 (FT4) levels and metabolic syndrome (MS) and its components in healthy euthyroid subjects. Clin Endocrinol (Oxf) 2009;70:152–160. doi: 10.1111/j.1365-2265.2008.03304.x. [DOI] [PubMed] [Google Scholar]
- 13.Park HT, Cho GJ, Ahn KH, Shin JH, Hong SC, Kim T, Hur JY, Kim YT, Lee KW, Kim SH. Thyroid stimulating hormone is associated with metabolic syndrome in euthyroid postmenopausal women. Maturitas. 2009;62:301–305. doi: 10.1016/j.maturitas.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Ruhla S, Weickert MO, Arafat AM, Osterhoff M, Isken F, Spranger J, Schofl C, Pfeiffer AF, Mohlig M. A high normal TSH is associated with the metabolic syndrome. Clin Endocrinol (Oxf) 72:696–701. doi: 10.1111/j.1365-2265.2009.03698.x. [DOI] [PubMed] [Google Scholar]
- 15.Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab. 2007;92:4575–4582. doi: 10.1210/jc.2007-1499. [DOI] [PubMed] [Google Scholar]
- 16.de Rekeneire N, Rooks RN, Simonsick EM, Shorr RI, Kuller LH, Schwartz AV, Harris TB. Racial differences in glycemic control in a well-functioning older diabetic population: findings from the Health, Aging and Body Composition Study. Diabetes Care. 2003;26:1986–1992. doi: 10.2337/diacare.26.7.1986. [DOI] [PubMed] [Google Scholar]
- 17.Kanaya AM, Harris F, Volpato S, Perez-Stable EJ, Harris T, Bauer DC. Association between thyroid dysfunction and total cholesterol level in an older biracial population: the health, aging and body composition study. Arch Intern Med. 2002;162:773–779. doi: 10.1001/archinte.162.7.773. [DOI] [PubMed] [Google Scholar]
- 18.Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, Monauni T, Muggeo M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 19.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–411. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 20.Surks MI, Ortiz E, Daniels GH, Sawin CT, Col NF, Cobin RH, Franklyn JA, Hershman JM, Burman KD, Denke MA, Gorman C, Cooper RS, Weissman NJ. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA. 2004;291:228–238. doi: 10.1001/jama.291.2.228. [DOI] [PubMed] [Google Scholar]
- 21.Iqbal A, Figenschau Y, Jorde R. Blood pressure in relation to serum thyrotropin: The Tromso study. J Hum Hypertens. 2006;20:932–936. doi: 10.1038/sj.jhh.1002091. [DOI] [PubMed] [Google Scholar]
- 22.Asvold BO, Bjoro T, Nilsen TI, Vatten LJ. Association between blood pressure and serum thyroid-stimulating hormone concentration within the reference range: a population-based study. J Clin Endocrinol Metab. 2007;92:841–845. doi: 10.1210/jc.2006-2208. [DOI] [PubMed] [Google Scholar]
- 23.Owen PJ, Rajiv C, Vinereanu D, Mathew T, Fraser AG, Lazarus JH. Subclinical hypothyroidism, arterial stiffness, and myocardial reserve. J Clin Endocrinol Metab. 2006;91:2126–2132. doi: 10.1210/jc.2005-2108. [DOI] [PubMed] [Google Scholar]
- 24.Asvold BO, Vatten LJ, Nilsen TI, Bjoro T. The association between TSH within the reference range and serum lipid concentrations in a population-based study. The HUNT Study. Eur J Endocrinol. 2007;156:181–186. doi: 10.1530/eje.1.02333. [DOI] [PubMed] [Google Scholar]
- 25.Angelin B, Rudling M. Lipid lowering with thyroid hormone and thyromimetics. Curr Opin Lipidol. 2010;21:499–506. doi: 10.1097/MOL.0b013e3283402e9c. [DOI] [PubMed] [Google Scholar]
- 26.Maratou E, Hadjidakis DJ, Kollias A, Tsegka K, Peppa M, Alevizaki M, Mitrou P, Lambadiari V, Boutati E, Nikzas D, Tountas N, Economopoulos T, Raptis SA, Dimitriadis G. Studies of insulin resistance in patients with clinical and subclinical hypothyroidism. Eur J Endocrinol. 2009;160:785–790. doi: 10.1530/EJE-08-0797. [DOI] [PubMed] [Google Scholar]
- 27.Dimitriadis G, Mitrou P, Lambadiari V, Boutati E, Maratou E, Panagiotakos DB, Koukkou E, Tzanela M, Thalassinos N, Raptis SA. Insulin action in adipose tissue and muscle in hypothyroidism. J Clin Endocrinol Metab. 2006;91:4930–4937. doi: 10.1210/jc.2006-0478. [DOI] [PubMed] [Google Scholar]
- 28.Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, Jacobs DR, Jr, Bangdiwala S, Tyroler HA. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79:8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- 29.Barter P, Gotto AM, LaRosa JC, Maroni J, Szarek M, Grundy SM, Kastelein JJ, Bittner V, Fruchart JC. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007;357:1301–1310. doi: 10.1056/NEJMoa064278. [DOI] [PubMed] [Google Scholar]
- 30.Huber G, Staub JJ, Meier C, Mitrache C, Guglielmetti M, Huber P, Braverman LE. Prospective study of the spontaneous course of subclinical hypothyroidism: prognostic value of thyrotropin, thyroid reserve, and thyroid antibodies. J Clin Endocrinol Metab. 2002;87:3221–3226. doi: 10.1210/jcem.87.7.8678. [DOI] [PubMed] [Google Scholar]
- 31.Diez JJ, Iglesias P. Spontaneous subclinical hypothyroidism in patients older than 55 years: an analysis of natural course and risk factors for the development of overt thyroid failure. J Clin Endocrinol Metab. 2004;89:4890–4897. doi: 10.1210/jc.2003-032061. [DOI] [PubMed] [Google Scholar]
- 32.Bauer DC, Brown AN. Sensitive thyrotropin and free thyroxine testing in outpatients. Are both necessary? Arch Intern Med. 1996;156:2333–2337. [PubMed] [Google Scholar]
- 33.Rotondi M, Magri F, Chiovato L. Thyroid and obesity: not a one-way interaction. J Clin Endocrinol Metab. 2011;96:344–346. doi: 10.1210/jc.2010-2515. [DOI] [PubMed] [Google Scholar]
- 34.Biondi B. Thyroid and obesity: an intriguing relationship. J Clin Endocrinol Metab. 2010;95:3614–3617. doi: 10.1210/jc.2010-1245. [DOI] [PubMed] [Google Scholar]
- 35.Rotondi M, Leporati P, La Manna A, Pirali B, Mondello T, Fonte R, Magri F, Chiovato L. Raised serum TSH levels in patients with morbid obesity: is it enough to diagnose subclinical hypothyroidism? Eur J Endocrinol. 2009;160:403–408. doi: 10.1530/EJE-08-0734. [DOI] [PubMed] [Google Scholar]


