Abstract
Risk of recurrent CIN2+ (including cervical intraepithelial neoplasia grade 2 [CIN2], CIN3, carcinoma and in situ, adenocarcinoma in situ or cancer) remains elevated for years following treatment. The role of long-term post-treatment HPV presence on subsequent risk of CIN2+ was evaluated in the 10,049-women Guanacaste cohort. 681 women were referred to colposcopy because of high-grade cytology, positive cervicography and/or suspicion of cancer based on visual assessment; 486 were judged to require treatment. After excluding women with <12 months of follow-up (N=88), prior cancer or hysterectomy (n=37) or other reasons (N=14), 347 were included in the analysis. Infections were categorized as persistent if present at both pre- and post-treatment visits and new if detected only post-treatment. Median time between the treatment and post-treatment visits was 6.7 years (IQR 3.8 to 7.8). At the post-treatment visit, 8 (2.4%), 2 (0.6%), and 8 (2.4%) of the 347 treated women had persistent HPV16, HPV18, or other carcinogenic HPV, respectively. Two (0.8%), 3 (1.0%), and 13 (4.0%) had new HPV16, HPV18, and other carcinogenic HPV, respectively. Six CIN2+ cases were identified at the post-treatment visit, all with persistent infections (three HPV16, one HPV18, and two other carcinogenic HPV). No recurrent disease was observed among women with new HPV infections during the follow-up period. Thus, persistence of HPV infection a median of six years after treatment was uncommon but, when present, posed a substantial risk of subsequent CIN2+. Serial follow-up data from other studies would further strengthen these conclusions.
Introduction
Treatment for cervical precancer is highly effective and the majority of women require no further treatment. Nonetheless, approximately 10% of women develop cervical intraepithelial neoplasia (CIN; including grade 2 [CIN2], CIN3, carcinoma in situ, adenocarcinoma in situ or cancer) after treatment due to either residual or recurrent disease1- 4. Soutter et al conducted a meta-analysis of 26 cohorts and estimated that the rate of post-treatment invasive cervical cancer exceeds the expected rate by nearly three-fold, and that the rate remains elevated for up to 20 years following treatment (5). Most of this literature focused on follow-up after cold knife cone excision, whose advantages compared with loop electrosurgical excision procedure (LEEP) include better preservation of mucosal orientation, intact removal that permits better margin assessment, avoidance of cautery artifact, and the ability to remove a larger amount of tissue. LEEP is often the preferred treatment for cervical precancer because it is an outpatient procedure conducted with fewer side effects.
Until recently, the success of treatment was monitored by repeat cytology and colposcopy, both with inherent poor sensitivity and reproducibility given their subjective nature (6, 7). We and others have provided evidence that carcinogenic human papillomavirus (HPV), the causative agent of cervical cancer and its precursor lesions, can sometimes be detected shortly after treatment (8-16). This has motivated the use of HPV testing as a surveillance tool for identifying women at high risk of recurrence (17).
Most studies that have researched the prognostic meaning of HPV detection following treatment have had limited follow-up time post-treatment (<5 years): a recent systematic review of the literature evaluating the use of Hybrid Capture 2 for detection of post-treatment CIN2+ showed that the maximum mean follow-up time from the eight included studies was less than three years; most studies only had 1 to 2 years of follow-up (18). It therefore appears logical that longer follow-up and HPV typing are needed to understand the value of HPV testing to predict disease recurrence several years following treatment.
To extend previous findings, we had the opportunity to examine type-specific HPV detection and risk of recurrent CIN2+ several years following treatment predominantly by LEEP in the 10,049 women, population-based Costa Rica Natural History Study (19). We quantified risk of recurrent disease and evaluated whether recurrent CIN2+ was due to persistent or new HPV infections.
Materials and Methods
Study design and population
Women in this analysis were invited to attend a cervical cancer screening visit (“post-treatment visit”) between 2000 and 2001 if they previously had excisional cervical treatment during enrollment or follow-up in the Guanacaste Natural History Study, which has been described previously (19, 20). Briefly, between 1993 and 1994, a random sample of 10,049 women ≥ 18 years old living in Guanacaste, Costa Rica was recruited and later followed for 7 years at different screening intervals. The main objective of this population-based cohort was to study the natural history of HPV infection and cervical neoplasia. The overall participation rate for the main cohort study was 93.6% and for the post-treatment visit was 96.5% of those eligible. Women signed informed consent documents for each of the studies. The study protocols were reviewed by US National Cancer Institute and Costa Rican Institutional Review Boards.
Women were exited from the main study cohort at enrollment or during the 7 years of the study if they presented at a screening visit with an HSIL cytologic result or if the cervigram suggested CIN2, CIN3, or cancer, regardless of whether the final diagnosis was confirmed histologic CIN2+ (this will be referred to as the ‘final screening visit’). According to the colposcopy and treatment algorithms (see below), some women without biopsy confirmation of CIN2+ were treated. If invasive cancer was confirmed according to Costa Rican or US pathologists, the woman was treated following Costa Rican national protocols. If CIN2 or CIN3 was confirmed, the local practice when this study began in mid-1993 was to treat women by use of cold knife cone. In early 1994, the study colposcopist/ gynecologist introduced LEEP as the standard of care. Women were examined ~3 to 4 months after LEEP by repeat colposcopy and cytology; if these tests were within normal limits, the women were released, censored from the study, and referred to the Social Security system for follow-up and future cervical cancer screening.
Clinical Procedures
At all visits, a pelvic examination was performed on women reporting prior sexual activity, including collection of exfoliated cells with a Cervex brush (Unimar, CT, USA) for conventional and liquid based cytology (ThinPrep, Cytyc Corporation [now Hologic], Marlborough, MA, USA). Additional cells were collected with a Dacron swab and stored initially in ViraPap DNA transport medium and later in DNA standard transport medium (STM) (both from Digene Corporation [now Qiagen], Gaithersburg, MD, USA) for HPV DNA detection and typing. Finally, two magnified photographic images from the cervix were taken after rinsing with 5% acetic acid (cervigrams, National Testing Laboratories, MO, USA).
Women were asked for intervening relevant medical histories between the final screening and post-treatment visits. Upon report that they sought related medical care outside of the research infrastructure, the information was verified by requisitioning medical records from the administration that provides universal health care in Costa Rica.
HPV determination by PCR
As in the main study, specimens from the post-treatment visit were tested for HPV DNA, which was detected and genotyped from exfoliated cervical cell specimens using MY09/MY11 L1 degenerate primer PCR with AmpliTaq Gold polymerase, as detailed elsewhere (21, 22) In brief, after amplification, PCR products were analyzed by electrophoresis and hybridized with radiolabeled generic probes for HPV. Positive samples using the radiolabeled probe were typed by dot-blot hybridization with biotinylated type-specific oligonucleotide probes (2, 6, 11, 13, 16, 18, 26, 31-35, 39, 40, 42-45, 51-59, 61, 62, 64, 66-74, 81-85, and 89). We considered types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59 as carcinogenic (23, 24).
Cytology
In the main study, the cell samples obtained with the Cervex collection brooms were used to make conventional Pap smears and liquid-based ThinPrep preparations. The ThinPreps were interpreted twice, in the United States (US) and, for the last half of the 7 years, in Costa Rica as well (20). Thus, there were 2-3 cytologic interpretations at each examination; if any showed HSIL, the woman was referred to colposcopy. At the post-treatment visit, only liquid-based cytology was used; cytology specimens were interpreted in both Costa Rica and the US. All results were reported using the Bethesda System (25, 26).
Cervicography
The cervigrams taken at each pelvic examination were sent to National Testing Laboratories Worldwide for processing and interpretation by an expert evaluator (20,27). At enrollment in the main study, all women who had abnormal looking cervigram images (including P0, P1, P2 and P3 cervigrams) were referred to colposcopy. During the 7 years of main study follow-up and at the post-treatment visit, only an interpretation of P2 (consistent with CIN2 or CIN3) or P3 (consistent with cancer) led to colposcopic referral. In this analysis, it happens that no women were referred solely based on P0 or P1 cervigrams taken at enrollment.
Colposcopy
The colposcopic treatment algorithm during the follow-up phase of the main study (after LEEP was introduced) incorporated age, parity, colposcopic appearance, and screening results to determine whether punch biopsy, LEEP or no procedure was performed (20). For example, during the colposcopic exam, if the colposcopist saw a lesion consistent with CIN2+ or if the woman had two or more screening tests with HSIL, and fertility was no longer an issue due to age and parity, a diagnostic LEEP procedure was performed. Particularly because of the cytology component of the algorithm that called for women with two HSIL cytologic interpretations to be treated, many women who did not have CIN2+ received treatment. Women were referred to the social security administration if they required advanced treatment (e.g., surgery, radiotherapy or chemotherapy) according to Costa Rican national protocols (20).
Histology review and final diagnostic group
The Costa Rican pathologists’ diagnoses were used for clinical purposes. For study purposes, a masked re-review of histology slides was performed in the US by one or more of a group of expert pathologists (Mark Sherman, Diane Solomon, and/or Thomas Wright of Columbia University, New York). The majority opinion including the original Costa Rican diagnosis constituted the final diagnosis for study purposes. If all of the pathologists disagreed, a joint review in the US yielded the final diagnosis.
Statistical analysis
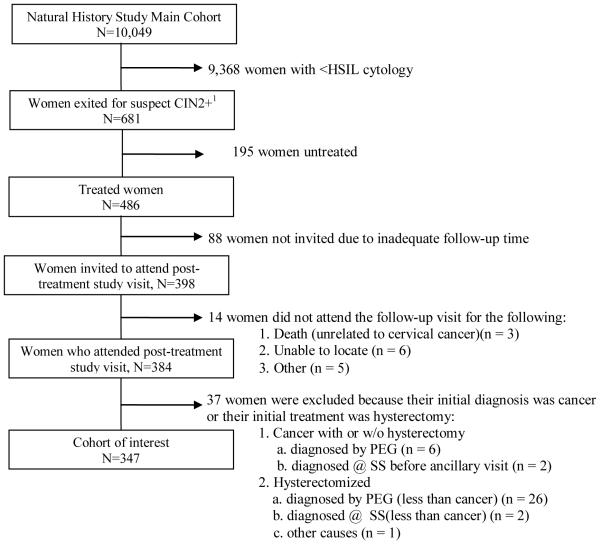
There were 10,049 women recruited into the main cohort (Figure 1) and 681 were exited from the main cohort due to the above-mentioned criteria; the majority of women were exited because of an HSIL cytology (78.7%). 486 women received treatment; 98.0% required only one treatment; data from only the first treatment was used, due to concerns that the later treatment might not be representative. Eighty-eight women were not invited for the post-treatment visit (for reasons described above) and 14 women did not attend the post-treatment visit (due to death unrelated to cervical cancer [n= 3], inability to locate them [n= 6], pregnancy [n= 2], and refusal [n= 3]). These women were excluded from this analysis, yet, a special search was conducted at the Costa Rican population-based cancer registry to determine if any of those women had ever been reported as having cervical cancer. Additional information about intervening diagnoses (including cancer) and treatments was available for all women and was included in the analysis.
Figure 1. Composition of study population.
1 Women were exited from the main cohort at enrolment or during the study follow-up if they presented with an HSIL cytologic result (which accounted for the majority of exit) or if the cervigram suggested CIN2, CIN3, or cancer, regardless of whether the final diagnosis was confirmed CIN2 or worse. Women were also exited if visual examination during a follow-up visit by the examining nurse suggested cancer, but only if the lesion was confirmed by the gynecologist’s colposcopy at referral; otherwise the visual inspection was considered non-specific and as such, no action was taken.
Abbreviations: PEG- Proyecto Epidemiológico Guanacaste, SS- Social Security system, HSIL- high-grade squamous intraepithelial lesion, CIN2- cervical intraepithelial neoplasia grade 2, CIN3- cervical intraepithelial neoplasia grade 3
Women were also excluded from this analysis if their initial diagnosis was cancer (n= 8, 6 of these women also had hysterectomy) or had hysterectomy due to reasons other than CIN or cervical cancer (n= 29)
347 women underwent initial treatment mainly by LEEP (implemented after 1994) or sometimes cold-knife cone (in the few months before LEEP was introduced). The majority of women (>90%) had HPV test results at the final screening and post-treatment visits. The total study size was 328 for the HPV-related analyses.
We describe the demographic characteristics, as well as HPV infection status and type measured in the cytology specimens (not the lesions) and disease recurrence rates. For multiple infections, HPV PCR results were used to categorize women hierarchically into one of five groups: (a) positive for HPV16; (b) else positive for HPV18; (c) else positive for other carcinogenic HPV types; (d) else positive only for noncarcinogenic HPV types; or (e) else HPV negative.
A persistent infection was defined as type-specific HPV infections present during the screening visits leading up to the final screening visit, and at the post-treatment visit as well. HPV results for all study visits leading up to the final screening visit were reviewed for women defined as having persistent HPV infections; in fact, all persistent infections appeared to be causal in our opinion in that they persisted in study visits including the visit immediately preceding treatment.
A new infection was defined as a type found at the post-treatment visit that was not present at any screening visit prior to the post-treatment visit. Therefore, only women who were always negative for a specific HPV type in the main cohort prior to treatment were considered at risk for a new infection by that type at post-treatment visit. Because new infections were only computed after removal from the analysis of the prevalent infections at the final screening visit, the denominators for each HPV type differ (HPV16, n=238, HPV18, n=306, and other carcinogenic types, n=328).
Following treatment, rates of persistent and new carcinogenic infections were investigated as were the rates of post-treatment CIN2+. We also examined the association between HPV infections (new and persistent) and resultant post-treatment CIN2+. To rule out bias in follow-up time, we calculated the median time from the final screening visit to the post-treatment visit among women who did and did not recur; this was stratified by new and persistent HPV infections. Further stratifications by either time (such as by year between the final screening and follow-up study visits) or by HPV genotype were not possible due to the small number of post-treatment HPV infections; as such, we choose to display the raw data for time instead of stratified summary measures.
Results
In this ancillary study we included 347 women who underwent at least one treatment by cone or LEEP during the main study; there was a median of 0.6 years (IQR 0.4 to 0.8 years) delay between the final screening visit and the treatment mainly because ThinPrep cytology was read in the US thereby prolonging the turnaround time for this component. At the final screening visit, treated women had a median age of 34 years and 26.8%, 5.5% and 36.9% had HPV16, 18, and other carcinogenic types, respectively. Of the treated women, 46.7% had histologically confirmed CIN2+ (Table 1). The median time between the actual treatment and the post-treatment visits was 6.7 years (IQR 3.8 to 7.8).
Table 1.
Descriptive characteristics of the 347 treated women at the pre-treatment final screening visit and the post-treatment visit.
| Pre-treatment screening visit |
Post-treatment visit |
|
|---|---|---|
| Median age (IQR), years | 34 (27-42) | 40 (34-48) |
| Reason for colposcopy referral | ||
| HSIL cytology | 263/347 (75.8%) | 53/347 (15.3%) |
| CIN2+ cervigram | 54/347 (15.6%) | 1/347 (0.3%) |
| Visual examination suggested cancer | 7/347 (2.0%) | 1/347 (0.3%) |
| Other | 23/347 (6.6%) | 0/347 (0%) |
| Not referred post-treatment | N/A | 292/347 (84.2%) |
| Histologic disease | ||
| CIN3+ | 88/347 (25.4%) | 6/347 (1.7%) |
| CIN2 | 74/347 (21.3%) | 0/347 (0%) |
| <CIN2 | 185/347 (53.3%) | 6/347 (1.7%) |
| No tissue taken post-treatment | N/A | 335/347 (96.5%) |
| HPV status1 | ||
| HPV16 | 93/347 (26.8%) | 10/347 (2.9%) |
| HPV18 | 19/347 (5.5%) | 5/347 (1.4%) |
| Other carcinogenic HPV | 128/347 (36.9%) | 18/347 (5.2%) |
| Non-carcinogenic HPV | 34/347 (9.8%) | 53/347 (15.3%) |
| HPV negative | 71/347 (20.5%) | 244/347 (70.3%) |
| Missing HPV results | 2/347 (0.6%) | 17/347 (4.9%) |
| Persistent infection2 | ||
| HPV16 | N/A | 8/328 (2.4%) |
| HPV18 | 2/328 (0.6%) | |
| Other Carcinogenic HPV | 8/328 (2.4%) | |
| New Infection.3 | ||
| HPV16 | N/A | 2/238 (0.8%) |
| HPV18 | 3/306 (1.0%) | |
| Other Carcinogenic HPV | 11/328 (3.4%) | |
IQR Interquartile range
For multiple infections, HPV PCR results were used to categorize women hierarchically into one of five groups: (a) positive for HPV16; (b) else positive for HPV18; (c) else positive for other carcinogenic HPVs; (d) else noncarcinogenic HPV positive; or (e) else HPV negative. This approach HPV16 infection was the most disease-relevant infection, followed by HPV18, etc.
Persistent infections were defined as type-specific HPV infections present during any of the main cohort visits and the follow-up visit; 328 women had type specific HPV results at both time points and therefore served as the denominators for the respective analyses.
Women were at risk for developing a new infection if the HPV type in question was not present at any visit prior to follow-up and was subsequently present at the follow-up visit. Therefore, only women who were negative for each specific HPV type were considered at risk for a new infection: denominators for women, HPV16, n=238, HPV18, n=306, and other carcinogenic types, n=328.
At the post-treatment visit, 2.9%, 1.4% and 5.2% had infections with HPV16, 18, and other carcinogenic types, respectively (Table 1). Most HPV16 infections were persistent (2.4%) while new HPV16 infections were less common (0.8%); 4.0 % of women had new non-16/18 carcinogenic HPV infection.
Based on study data alone, the observed risk of histologically confirmed CIN2+ following treatment was 1.7% (n=6; four cases of CIN3, one case of adenocarcinoma in situ, and one case of invasive cancer) (Table 1). Restricting to women who had CIN2+ (but less than cancer) as part of their original diagnosis, the post-treatment recurrence rate by subsetting to women was 6.0% (95% CI 2.9% to 10.7%). To be comprehensive, we included the five women (1 Normal, 2 CIN3, and 2 Cancer) who had hysterectomies through the social security system prior to attending the post-treatment visit and estimated the overall post-treatment recurrence rate: 2.8% (10 out of 352; 95% CI: 1.4% to 5.2%), with the important caveat that for women who never attended the post-treatment visit, their history could only be reviewed at the cancer registry; therefore, if they were ever diagnosed with cervical disease less than invasive cancer we had no opportunity to recover that information.
Eighteen new carcinogenic infections were detected at post-treatment visits, however, none of these infections resulted in CIN2+ (Table 2). Eighteen type-specific persistent carcinogenic HPV infections were detected at the post-treatment visit: eight HPV16 infections, two HPV18 infections, and eight other carcinogenic infections (Table 2). Of the eight persistent HPV16 infections, three (37.5%) were associated with post-treatment CIN2+ at six, six and eight years post-treatment. Thus, women with persistent HPV16 infection had 41-fold (95%CI 4.6 to 341; 3 of 8 women with HPV16 recurred compared to 3 of 330 without HPV16 infection) increased risk of having a recurrent CIN2+ at the post-treatment visit compared to all other treated women including those with other persistent HPV types. Three cases of post-treatment CIN2+ were diagnosed among the 10 other women with persistent carcinogenic infections other than HPV16: the persistent HPV types implicated in the post-treatment disease were HPV18, HPV52 and HPV31 (time to detection: three, five and eight years, respectively). The median time between treatment visit and diagnosis of recurrent CIN2+ was six years. For the persistent infections that did not result in post-treatment CIN2+, the median time to the post-treatment visit was four years. Again, after restricting to women who had CIN2+ (but less than cancer) as part of their original diagnosis and had persistent carcinogenic HPV infection, the estimated post-treatment recurrence rate was 46.0% (95% CI 19.2% to 74.9%).
Table 2.
Persistent and new HPV infections and resultant CIN2+ at the post-treatment study visit: time to detection of infection.
| # of infections |
% and 95%CI resulting in CIN2+ | Time between treatment and post-treatment visits (years) | ||
|---|---|---|---|---|
| Women with recurrent disease | Women with no recurrent disease | |||
| Persistent infections1 | ||||
| HPV16 | 8 | 37.5% (8.5% to 75.5%); (n=3) | 6, 6, 8 | 1, 3, 4, 4, 8 |
| HPV18 | 2 | 50.0% (1.3% to 98.7%); (n=1) | 3 | 8 |
| Other carcinogenic HPV | 8 | 25.0% (3.2% to 65.1%); (n=2) | 5, 8 | 1, 2, 3, 4, 7, 8 |
| New Infections2 | ||||
| HPV16 | 2 | 0% (n=0) | N/A | 7,8 |
| HPV18 | 3 | 0% (n=0) | N/A | 7, 8, 8 |
| Other carcinogenic HPV | 13 | 0% (n=0) | N/A | 4, 5, 5, 5, 5, 7, 7, 7, 7, 7, 8, 8, 8 |
Persistent infections were defined as type-specific HPV infections present during any of the pre-treatment study visits and at the post-treatment visit.
Women were at risk for developing a new infection if the HPV type in question was not present at any visit prior to follow-up was subsequently present at the post-treatment visit.
Besides persistent HPV infections, factors that elevated the risk of recurrence included having CIN3 (instead of CIN2; finding limited by small number, data not shown) and having positive margins on post-surgical histopathological review (N= 4 of the 10 women with recurrent disease, the denominator is composed of 6 women with recurrent disease at the posttreament visit and 4 women found to have CIN2+ diagnosis outside the study).
Discussion
On average, more than six years following treatment for CIN2+, the presence of carcinogenic HPV infection was rare, but when detected both before and after treatment, elevated the risk of CIN recurrence substantially. Acquisition of a new carcinogenic infection following treatment posed no risk for CIN2+ within the timeframe of our study. Although the efficacy of the intervention was generally high, one case of adenocarcinoma in situ and one case of invasive cancer were identified at the follow-up study visit, and two additional cases of cancer were identified through the registry match.
As in any study, it is important to consider the impact of the inclusion/exclusion criteria on the analysis. In this work, the necessary exclusion of women who did not attend the post-treatment visit (n= 14) or had an initial diagnosis and/or treatment of cancer/hysterectomy (n= 37) (as these women could not meet our definitions for HPV and clinical disease recurrence) could have been at higher risk for disease recurrence. For example, four women who were excluded from our final analytic cohort had an initial diagnosis of cervical precancer, were treated successfully by LEEP, but prior to our post-treatment visit, were diagnosed with CIN3 (N=2) or cancer (N=2) and sought treatment outside of the study through the social security system. These women technically could have contributed to our estimate of disease recurrence had they not been excluded, and therefore, our estimates of post-treatment disease may be underestimated. As such, we attempted to compensate for this by expanding our dataset to include information from the social security system: our estimate of the ‘true’ recurrence rate among treated women (2.8%) was higher than that reported in the initial analysis (1.7%). An additional limitation to this work is that we only had one study visit post-treatment (instead of serial measures) that happened an average 6 yrs post-treatment; therefore our study should not be viewed as a study of short-term prognosis. As some CIN2 lesions are transient, having less intense follow-up may have allowed for less disease detection. Although, lesions that were still present after seven years may yield a more serious threat in terms of cancer.
It has been previously shown, and again with these data, that women who are treated for CIN2+ remain at elevated risk for CIN and cervical cancer many years after treatment (5). Several explanations for this phenomenon seem possible, including treatment failure (incomplete excision at either the lesion and/or infection level) and/or inherent susceptibility of the woman to HPV persistence and disease progression, likely due to a sub-clinical immune impairment. Co-factors such as smoking, oral contraceptive use and parity could also impact risk of subsequent disease.
When creating guidelines for surveillance of women treated for CIN2+, the clinical assay for HPV detection and the testing interval are two important considerations. In the future, when type-specific HPV tests that can delineate persistent from new infections are available, clinicians might be able to further stratify risk for post-treatment CIN2+; in this study, all of the post-treatment CIN2+ was due to persistent HPV infections (i.e., the negative predictive value using type-specific HPV data could approach 100%). And, while this study does not directly test the monitoring interval, in our data, the minimum duration of persistent infection that resulted in a diagnosis of post-treatment CIN2+ was three years. Current USA consensus guidelines for the management of women treated for CIN recommend follow-up by either cervical cytology at 4 to 6-month intervals or HPV DNA testing at least 6 months after treatment (28). While our data suggest that it may be safe to extend the monitoring interval, it is important to note that persistent HPV16 that did not lead to recurrent CIN2+ had shorter follow up time (four years) compared to the seven years time between treatment and diagnosis of recurrent disease. We therefore cannot dismiss the possibility that additional disease would have developed should additional follow-up time have been available. In addition, questions remain about the management of women with persistent carcinogenic infection in the absence of cytologic abnormality.
In conclusion, while treated women were similarly likely to have new and persistent infections, all of the cases of CIN2+ following treatment were identified among women with persistent carcinogenic infections. For new infections, no cases of post-treatment disease were identified. This work confirms the low rate of disease post-treatment, and affirms that linking disease recurrence to HPV infection status at follow-up could improve the predictive value, thereby appropriately triaging lower risk women to less intensive follow-up. For clinical management, if the HPV type that caused the lesions is present following treatment, we recommend close colposcopic follow-up (i.e.: every 6 months). Lastly, it should be noted that the women in our study who had disease recurrence would not have benefited from the prophylactic HPV vaccine if applied after the initial treatment since all disease recurrence was due to infections present prior to treatment (29).
Acknowledgements
The authors thank the following individuals, without whom this work would not be possible: Fernando Cárdenas (Field Supervisor), Osman López (Field supervisor assistant), Marlen Jara (study nurse), María Gutiérrez (interviewer), Diego Guillen (pathologist), Jorge Morales (gynecologist), Mario Alfaro (cyto-pathologist), Diane Solomon (pathologist) and Greg Rydzak and the staff of Information Management Systems, Inc. (statistical analysis).
Source of the work: The Guanacaste cohort (design and conduct of the study, sample collection, management, analysis and interpretation of the data) for the enrollment and follow-up phases was supported by the National Cancer Institute (NCI), National Institutes of Health (NIH), Department of Health and Human Services of the United States of America. This ancillary study was sponsored and funded by NCI (contract N01-CP-11005) with support from the NIH Office for Research on Women’s Health. Dr. Burk was supported by National Cancer Institute grant CA78527 and used the facilities available through the Einstein Cancer Research Center and CFAR.
References
- 1).Mitchell MF, Tortolero-Luna G, Cook E, Whittaker L, Rhodes-Morris H, Silva E. A randomized clinical trial of cryotherapy, laser vaporization, and loop electrosurgical excision for treatment of squamous intraepithelial lesions of the cervix. Obstet Gynecol. 1998;92:737–44. [PubMed] [Google Scholar]
- 2).Alvarez RD, Helm CW, Edwards RP, Naumann RW, Partridge EE, Shingleton HM, McGee JA, Hall JB, Higgins RV, Malone JM., Jr. Prospective randomized trial of LLETZ versus laser ablation in patients with cervical intraepithelial neoplasia. Gynecol Oncol. 1994;52:175–9. doi: 10.1006/gyno.1994.1027. [DOI] [PubMed] [Google Scholar]
- 3).Arbyn M, Paraskevaidis E, Martin-Hirsch P, Prendiville W, Dillner J. Clinical utility of HPV-DNA detection: triage of minor cervical lesions, follow-up of women treated for high-grade CIN: an update of pooled evidence. Gynecol Oncol. 2005 Dec;99(3 Suppl 1):S7–11. doi: 10.1016/j.ygyno.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 4).Arbyn M, Sasieni P, Meijer CJ, Clavel C, Koliopoulos G, Dillner J. Chapter 9: Clinical applications of HPV testing: a summary of meta-analyses. Vaccine. 2006 Aug 31;24(Suppl 3):S3/78–89. doi: 10.1016/j.vaccine.2006.05.117. [DOI] [PubMed] [Google Scholar]
- 5).Soutter WP, Sasieni P, Panoskaltsis T. Long-term risk of invasive cervical cancer after treatment of squamous cervical intraepithelial neoplasia. Int J Cancer. 2006;118:2048–55. doi: 10.1002/ijc.21604. [DOI] [PubMed] [Google Scholar]
- 6).Massad LS, Jeronimo J, Katki HA, Schiffman M. The accuracy of colposcopic grading for detection of high-grade cervical intraepithelial neoplasia. J Low Genit Tract Dis. 2009;13:137–44. doi: 10.1097/LGT.0b013e31819308d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Stoler MH, Schiffman M. Interobserver reproducibility of cervical cytologic and histologic interpretations: realistic estimates from the ASCUS-LSIL Triage Study. JAMA. 2001;285:1500–5. doi: 10.1001/jama.285.11.1500. [DOI] [PubMed] [Google Scholar]
- 8).Kreimer AR, Guido RS, Solomon D, Schiffman S, Wacholder S, Jeronimo J, Wheeler CM, Castle PE. HPV testing following loop electrosurgical excision procedure (LEEP) identifies women at risk for post-treatment cervical intraepithelial neoplasia grade 2 or 3 disease. Cancer Epidemiol Biomarkers Prev. 2006;15:908–14. doi: 10.1158/1055-9965.EPI-05-0845. [DOI] [PubMed] [Google Scholar]
- 9).Nobbenhuis MA, Walboomers JM, Helmerhorst TJ, Rozendaal L, Remmink AJ, Risse EK, van der Linden HC, Voorhorst FJ, Kenemans P, Meijer CJ. Relation of human papilloma virus status to cervical lesions and consequences for cervical-cancer screening: a prospective study. The Lancet. 1999;354:20–5. doi: 10.1016/S0140-6736(98)12490-X. [DOI] [PubMed] [Google Scholar]
- 10).Acladious NN, Sutton C, Mandal D, Hopkins R, Zaklama M, Kitchener H. Persistent human papillomavirus infection and smoking increase risk of failure of treatment of cervical intraepithelial neoplasia (CIN) Int J Cancer. 2002;98:435–9. doi: 10.1002/ijc.10080. [DOI] [PubMed] [Google Scholar]
- 11).Debarge V Houfflin, Collinet P, Vinatier D, Ego A, Dewilde A, Boman F, Leroy JL. Value of human papillomavirus testing after conization by loop electrosurgical excision for high-grade squamous intraepithelial lesions. Gynecol Oncol. 2003;90:587–92. doi: 10.1016/s0090-8258(03)00372-x. [DOI] [PubMed] [Google Scholar]
- 12).Kjellberg L, Wadell G, Bergman F, Isaksson M, Angstrom T, Dillner J. Regular disappearance of the human papillomavirus genome after conization of cervical dysplasia by carbon dioxide laser. Am J Obstet Gynecol. 2000;183:1238–42. doi: 10.1067/mob.2000.107322. [DOI] [PubMed] [Google Scholar]
- 13).Elfgren K, Jacobs M, Walboomers JM, Meijer CJ, Dillner J. Rate of human papillomavirus clearance after treatment of cervical intraepithelial neoplasia. Obstet Gynecol. 2002;100:965–71. doi: 10.1016/s0029-7844(02)02280-9. [DOI] [PubMed] [Google Scholar]
- 14).Fen J, Yoshinouchi M, Nakamura K, Kodama J, Nasu Y, Yamato K, Hiramatsu Y. Eradication of HPV post-surgical treatments, its correlation with specific types, types of surgery and the physical status. Oncol Rep. 2004;12:375–9. [PubMed] [Google Scholar]
- 15).Verguts J, Bronselaer B, Donders G, Arbyn M, Van Eldere J, Drijkoningen M, Poppe W. Prediction of recurrence after treatment for high-grade cervical intraepithelial neoplasia: the role of human papillomavirus testing and age at conisation. BJOG. 2006;113:1303–7. doi: 10.1111/j.1471-0528.2006.01063.x. [DOI] [PubMed] [Google Scholar]
- 16).Jordan J, Martin-Hirsch P, Arbyn M, Schenck U, Baldauf JJ, Da Silva D, Anttila A, Nieminen P, Prendiville W. European guidelines for clinical management of abnormal cervical cytology, part 2. Cytopathology. 2009 Feb;20(1):5–16. doi: 10.1111/j.1365-2303.2008.00636.x. [DOI] [PubMed] [Google Scholar]
- 17).Bais AG, Eijkemans MJ, Rebolj M, Snijders PJ, Verheijen RH, van Ballegooijen M, Meijer CJ, Helmerhorst TJ. Post-treatment CIN: randomised clinical trial using hrHPV testing for prediction of residual/recurrent disease. Int J Cancer. 2009;124(4):889–95. doi: 10.1002/ijc.23824. [DOI] [PubMed] [Google Scholar]
- 18).Chan BK, Melnikow J, Slee CA, Arellanes R, Sawaya GF. Posttreatment human papillomavirus testing for recurrent cervical intraepithelial neoplasia: a systematic review. Am J Obstet Gynecol. 2009;200(4):422. doi: 10.1016/j.ajog.2008.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Herrero R, Schiffman MH, Bratti C, Hildesheim A, Balmaceda I, Sherman ME, Greenberg M, Cárdenas F, Gómez V, Helgesen K, Morales J, Hutchinson M, et al. Design and methods of a population-based natural history study of cervical neoplasia in a rural province of Costa Rica: the Guanacaste Project. Rev Panam Salud Publica. 1997;1:362–75. doi: 10.1590/s1020-49891997000500005. [DOI] [PubMed] [Google Scholar]
- 20).Bratti MC, Rodriguez AC, Schiffman M, Hildesheim A, Morales J, Alfaro M, Guillen D, Hutchinson M, Sherman ME, Eklund C, Schussler J, Buckland J, et al. Description of a seven-year prospective study of human papillomavirus infection and cervical neoplasia among 10000 women in Guanacaste, Costa Rica. Rev Panam Salud Publica. 2004;15:75–89. doi: 10.1590/s1020-49892004000200002. [DOI] [PubMed] [Google Scholar]
- 21).Herrero R, Castle PE, Schiffman M, Bratti MC, Hildesheim A, Morales J, Alfaro M, Sherman ME, Wacholder S, Chen S, Rodriguez AC, Burk RD. Epidemiologic profile of type-specific human papillomavirus infection and cervical neoplasia in Guanacaste, Costa Rica. J Infect Dis. 2005;191:1796–807. doi: 10.1086/428850. [DOI] [PubMed] [Google Scholar]
- 22).Castle PE, Schiffman M, Gravitt PE, Kendall H, Fishman S, Dong H, Hildesheim A, Herrero R, Bratti MC, Sherman ME, Lorincz A, Schussler JE, et al. Comparisons of HPV DNA detection by MY09/11 PCR methods. J Med Virol. 2002;68:417–23. doi: 10.1002/jmv.10220. [DOI] [PubMed] [Google Scholar]
- 23).Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, Snijders PJ, Meijer CJ. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 24).Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V, WHO International Agency for Research on Cancer Monograph Working Group A review of human carcinogens--Part B: biological agents. Lancet Oncol. 2009;10:321–2. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 25).The 1988 Bethesda System for reporting cervical/vaginal cytological diagnoses. National Cancer Institute Workshop. JAMA. 1989;262:931–4. [PubMed] [Google Scholar]
- 26).Hutchinson ML, Zahniser DJ, Sherman ME, Herrero R, Alfaro M, Bratti MC, Hildesheim A, Lorincz AT, Greenberg MD, Morales J, Schiffman M. Utility of liquid-based cytology for cervical carcinoma screening: results of a population-based study conducted in a region of Costa Rica with a high incidence of cervical carcinoma. Cancer. 1999;87:48–55. doi: 10.1002/(sici)1097-0142(19990425)87:2<48::aid-cncr2>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 27).Schneider DLS, Herrero R, Bratti C, Greenberg MD, Hildesheim A, Sherman ME, Morales J, Hutchinson ML, Sedlacek TV, Lorincz A, Mango L, Wacholder S, et al. Cervicography screening for cervical cancer among 8460 women in a high-risk population. Am J Obstet Gynecol. 1999;180:290–8. doi: 10.1016/s0002-9378(99)70202-4. [DOI] [PubMed] [Google Scholar]
- 28).Ferenczy A, Choukroun D. Loop electrosurgical procedure for squamous intraepithelial lesions of the cervix: advantages and potential pitfalls. Obstet Gynecol. 1996;87(3) doi: 10.1016/0029-7844(95)00453-x. [DOI] [PubMed] [Google Scholar]
- 29).Hildesheim A, Herrero R, Wacholder S, Rodriguez AC, Solomon D, Bratti MC, Schiller JT, Gonzalez P, Dubin G, Porras C, Jimenez SE, Lowy DR, et al. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. JAMA. 2007;298(7):743–53. doi: 10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]