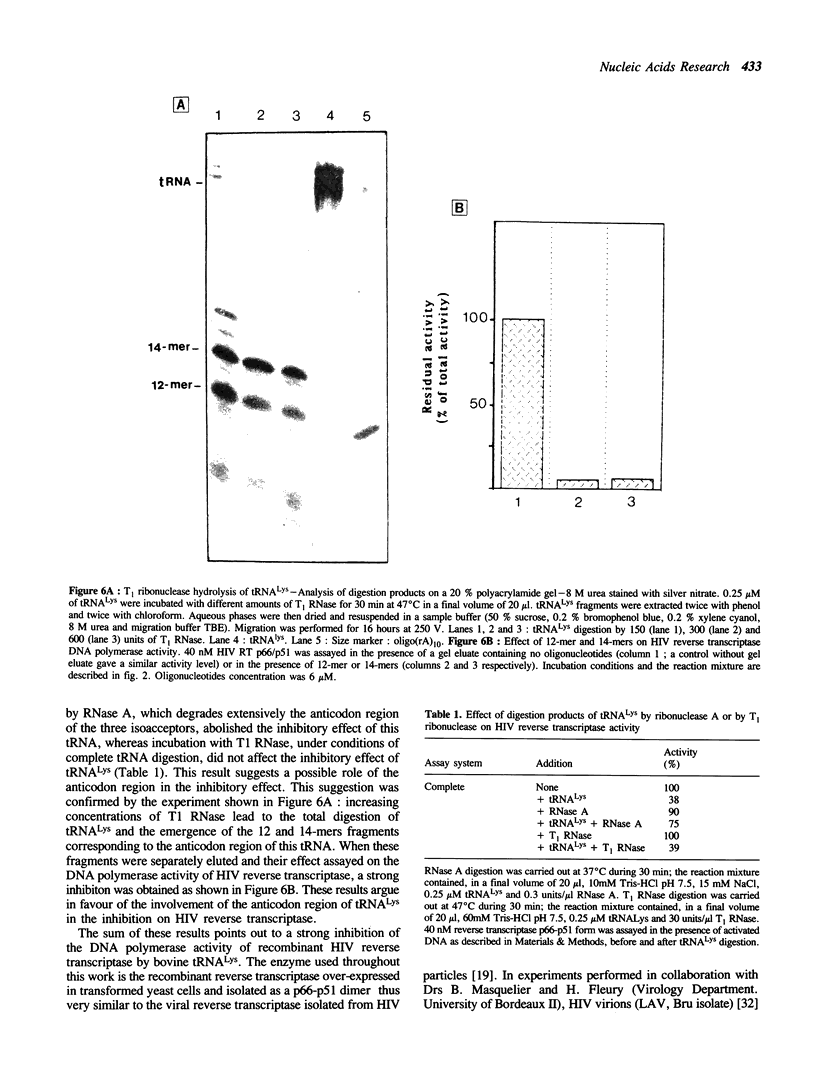
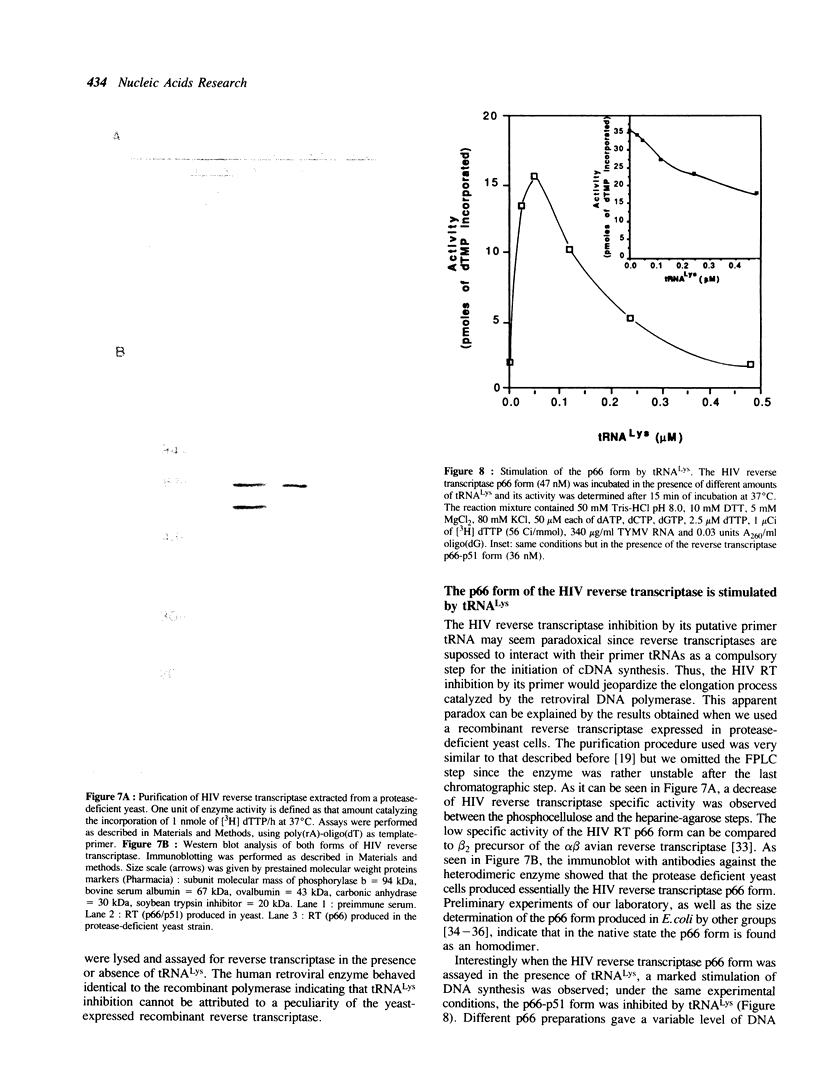
Abstract
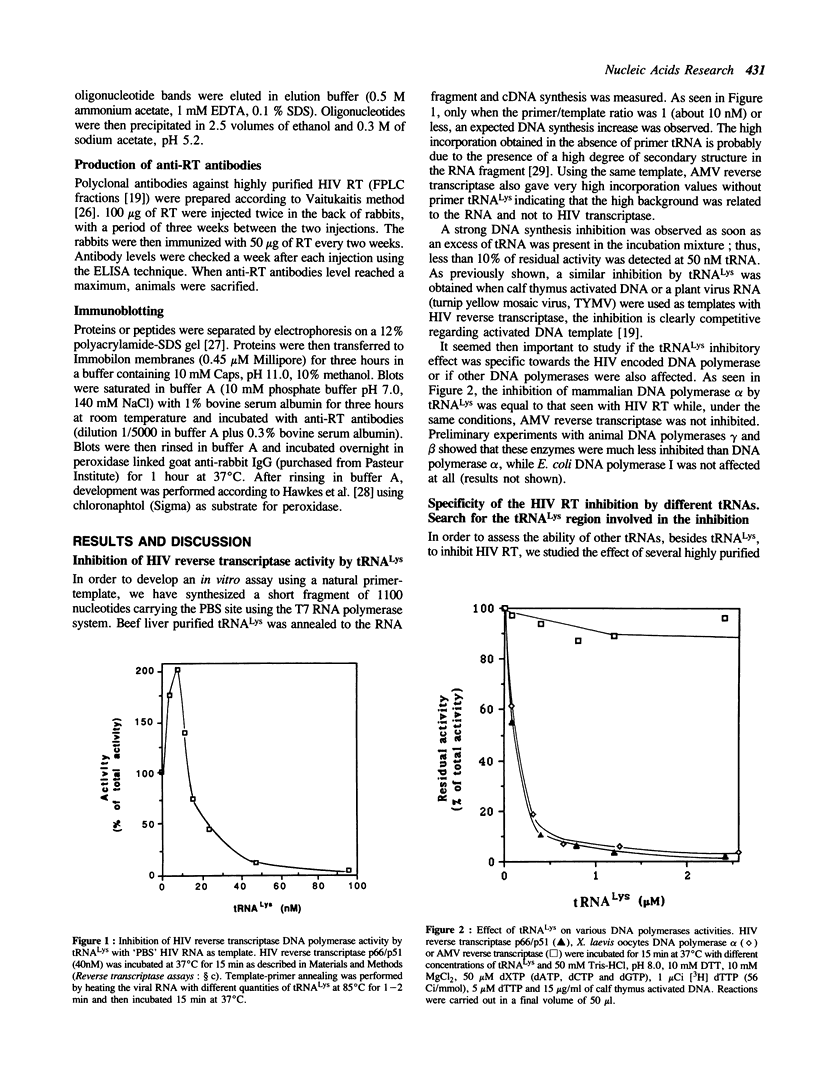
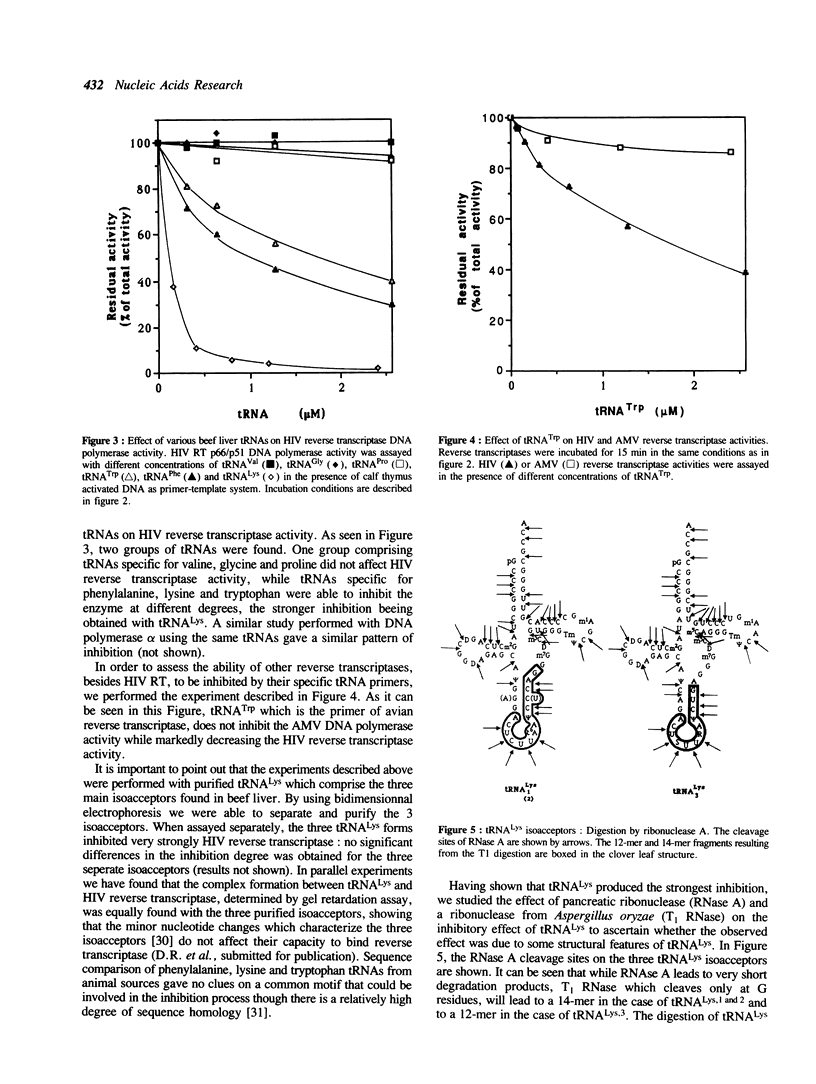
Human immunodeficiency virus (HIV) reverse transcriptase (RT) uses host tRNA(Lys) partially annealed to the primer binding site (PBS) as primer for the initiation of cDNA synthesis. When assaying cDNA synthesis with a template-primer complex formed by an RNA fragment carrying the PBS site and bovine tRNA(Lys) we noticed that an excess of primer tRNA inhibited strongly the DNA polymerase activity of a recombinant HIV RT (p66-p51 heterodimeric form) produced in transformed yeast cells. The same inhibitory effect was observed with animal DNA polymerase alpha, while avian retrovirus RT was neither affected by tRNA(Lys) nor by its specific primer tRNA(Trp). Although the strongest inhibition was observed with tRNA(Lys), other tRNas like tRNA(Phe) and tRNA(Trp) inhibited also the HIV RT, whereas tRNAs specific for valine, proline and glycine had no effect on enzyme activity. Digestion of tRNA(Lys) with pancreatic RNase abolished the inhibition; on the other hand T1 RNase digestion had no effect on the inhibition suggesting a role of the anticodon region in this effect. The 12- and 14-mers corresponding to the anticodon regions of the three bovine tRNA(Lys) isoacceptors inhibited RT activity, indicating that at least an important part of the inhibitory effect could be ascribed to this tRNA region. A strong stimulation of DNA polymerase activity was observed when the effect of tRNA(Lys) was assayed on a recombinant HIV reverse transcriptase produced in a protease deficient yeast strain, which leads to the production of an active p66 enzyme. The same tRNAs that inhibited strongly the heterodimeric form stimulated the p66 form of HIV reverse transcriptase. The results suggest that although both enzymatic forms are able to interact with tRNA(Lys) the topography, as well as the functional implications of the interaction between the precursor and the mature form of HIV reverse transcriptase with the tRNA(Lys) primer, are different.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barat C., Lullien V., Schatz O., Keith G., Nugeyre M. T., Grüninger-Leitch F., Barré-Sinoussi F., LeGrice S. F., Darlix J. L. HIV-1 reverse transcriptase specifically interacts with the anticodon domain of its cognate primer tRNA. EMBO J. 1989 Nov;8(11):3279–3285. doi: 10.1002/j.1460-2075.1989.tb08488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Farmerie W. G., Loeb D. D., Casavant N. C., Hutchison C. A., 3rd, Edgell M. H., Swanstrom R. Expression and processing of the AIDS virus reverse transcriptase in Escherichia coli. Science. 1987 Apr 17;236(4799):305–308. doi: 10.1126/science.2436298. [DOI] [PubMed] [Google Scholar]
- Fournier M., Dorizzi M., Sarger C., Labouresse J. Purification of tRNATrp, tRNAVal, and partial purification of tRNAIle and tRNAMfet from beef liver. Biochimie. 1976;58(10):1159–1165. doi: 10.1016/s0300-9084(76)80114-9. [DOI] [PubMed] [Google Scholar]
- Grandgenett D. P., Vora A. C., Faras A. J. Different states of avian myeloblastosis virus DNA polymerase and their binding capacity to primer rRNATrp. Virology. 1976 Nov;75(1):26–32. doi: 10.1016/0042-6822(76)90004-0. [DOI] [PubMed] [Google Scholar]
- Hansen J., Schulze T., Mellert W., Moelling K. Identification and characterization of HIV-specific RNase H by monoclonal antibody. EMBO J. 1988 Jan;7(1):239–243. doi: 10.1002/j.1460-2075.1988.tb02805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada F., Peters G. G., Dahlberg J. E. The primer tRNA for Moloney murine leukemia virus DNA synthesis. Nucleotide sequence and aminoacylation of tRNAPro. J Biol Chem. 1979 Nov 10;254(21):10979–10985. [PubMed] [Google Scholar]
- Harada F., Sawyer R. C., Dahlberg J. E. A primer ribonucleic acid for initiation of in vitro Rous sarcarcoma virus deoxyribonucleic acid synthesis. J Biol Chem. 1975 May 10;250(9):3487–3497. [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Igloi G. L. A silver stain for the detection of nanogram amounts of tRNA following two-dimensional electrophoresis. Anal Biochem. 1983 Oct 1;134(1):184–188. doi: 10.1016/0003-2697(83)90281-6. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y., Ando Y., Ichimura N., Noda A. Exoribonuclease activity of purified reverse transcriptase preparations from retroviruses. J Biochem. 1989 Jun;105(6):974–978. doi: 10.1093/oxfordjournals.jbchem.a122790. [DOI] [PubMed] [Google Scholar]
- Krug M. S., Berger S. L. Ribonuclease H activities associated with viral reverse transcriptases are endonucleases. Proc Natl Acad Sci U S A. 1989 May;86(10):3539–3543. doi: 10.1073/pnas.86.10.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le S. Y., Chen J. H., Braun M. J., Gonda M. A., Maizel J. V. Stability of RNA stem-loop structure and distribution of non-random structure in the human immunodeficiency virus (HIV-I). Nucleic Acids Res. 1988 Jun 10;16(11):5153–5168. doi: 10.1093/nar/16.11.5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightfoote M. M., Coligan J. E., Folks T. M., Fauci A. S., Martin M. A., Venkatesan S. Structural characterization of reverse transcriptase and endonuclease polypeptides of the acquired immunodeficiency syndrome retrovirus. J Virol. 1986 Nov;60(2):771–775. doi: 10.1128/jvi.60.2.771-775.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe D. M., Aitken A., Bradley C., Darby G. K., Larder B. A., Powell K. L., Purifoy D. J., Tisdale M., Stammers D. K. HIV-1 reverse transcriptase: crystallization and analysis of domain structure by limited proteolysis. Biochemistry. 1988 Dec 13;27(25):8884–8889. doi: 10.1021/bi00425a002. [DOI] [PubMed] [Google Scholar]
- Nishimura S., Weinstein I. B. Fractionation of rat liver transfer ribonucleic acid. Isolation of tyrosine, valine, serine, and phenylalanine transfer ribonucleic acids and their coding properties. Biochemistry. 1969 Mar;8(3):832–842. doi: 10.1021/bi00831a011. [DOI] [PubMed] [Google Scholar]
- Panet A., Haseltine W. A., Baltimore D., Peters G., Harada F., Dahlberg J. E. Specific binding of tryptophan transfer RNA to avian myeloblastosis virus RNA-dependent DNA polymerase (reverse transcriptase). Proc Natl Acad Sci U S A. 1975 Jul;72(7):2535–2539. doi: 10.1073/pnas.72.7.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prats A. C., Sarih L., Gabus C., Litvak S., Keith G., Darlix J. L. Small finger protein of avian and murine retroviruses has nucleic acid annealing activity and positions the replication primer tRNA onto genomic RNA. EMBO J. 1988 Jun;7(6):1777–1783. doi: 10.1002/j.1460-2075.1988.tb03008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raba M., Limburg K., Burghagen M., Katze J. R., Simsek M., Heckman J. E., Rajbhandary U. L., Gross H. J. Nucleotide sequence of three isoaccepting lysine tRNAs from rabbit liver and SV40-transformed mouse fibroblasts. Eur J Biochem. 1979 Jun;97(1):305–318. doi: 10.1111/j.1432-1033.1979.tb13115.x. [DOI] [PubMed] [Google Scholar]
- Sallafranque-Andreola M. L., Robert D., Barr P. J., Fournier M., Litvak S., Sarih-Cottin L., Tarrago-Litvak L. Human immunodeficiency virus reverse transcriptase expressed in transformed yeast cells. Biochemical properties and interactions with bovine tRNALys. Eur J Biochem. 1989 Sep 15;184(2):367–374. doi: 10.1111/j.1432-1033.1989.tb15028.x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pescador R., Power M. D., Barr P. J., Steimer K. S., Stempien M. M., Brown-Shimer S. L., Gee W. W., Renard A., Randolph A., Levy J. A. Nucleotide sequence and expression of an AIDS-associated retrovirus (ARV-2). Science. 1985 Feb 1;227(4686):484–492. doi: 10.1126/science.2578227. [DOI] [PubMed] [Google Scholar]
- Sarih L., Araya A., Litvak S. Characterization of the cDNA synthesized by avian retrovirus reverse transcriptase using 35 S avian myeloblastosis virus RNA and an exogenous bovine primer tRNA. FEBS Lett. 1988 Mar 28;230(1-2):61–66. doi: 10.1016/0014-5793(88)80642-2. [DOI] [PubMed] [Google Scholar]
- Starnes M. C., Cheng Y. C. Human immunodeficiency virus reverse transcriptase-associated RNase H activity. J Biol Chem. 1989 Apr 25;264(12):7073–7077. [PubMed] [Google Scholar]
- Tanese N., Sodroski J., Haseltine W. A., Goff S. P. Expression of reverse transcriptase activity of human T-lymphotropic virus type III (HTLV-III/LAV) in Escherichia coli. J Virol. 1986 Sep;59(3):743–745. doi: 10.1128/jvi.59.3.743-745.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaitukaitis J. L. Production of antisera with small doses of immunogen: multiple intradermal injections. Methods Enzymol. 1981;73(Pt B):46–52. doi: 10.1016/0076-6879(81)73055-6. [DOI] [PubMed] [Google Scholar]
- Varmus H. Retroviruses. Science. 1988 Jun 10;240(4858):1427–1435. doi: 10.1126/science.3287617. [DOI] [PubMed] [Google Scholar]
- Verma I. M. The reverse transcriptase. Biochim Biophys Acta. 1977 Mar 21;473(1):1–38. doi: 10.1016/0304-419x(77)90005-1. [DOI] [PubMed] [Google Scholar]
- Wain-Hobson S., Sonigo P., Danos O., Cole S., Alizon M. Nucleotide sequence of the AIDS virus, LAV. Cell. 1985 Jan;40(1):9–17. doi: 10.1016/0092-8674(85)90303-4. [DOI] [PubMed] [Google Scholar]