Abstract
Maternal smoking during pregnancy can impair performance of the exposed offspring in tasks that require auditory stimulus processing and perception; however, the tobacco component(s) responsible for these effects and the underlying neurobiological mechanisms remain uncertain. In this study, we show that administration of nicotine during mouse perinatal development can impair performance in an auditory discrimination paradigm when the exposed animals are mature. This suggests that nicotine disrupts auditory pathways via nicotinic acetylcholine receptors (nAChRs) that are expressed at an early stage of development. We have also determined that mice which lack nAChRs containing the β2 subunit (β2* nAChRs) exhibit similarly compromised performance in this task, suggesting that β2* nAChRs are necessary for normal auditory discrimination or that β2* nAChRs play a critical role in development of the circuitry required for task performance. In contrast, no effect of perinatal nicotine exposure or β2 subunit knockout was found on the acquisition and performance of a differential reinforcement of low rate task. This suggests that the auditory discrimination impairments are not a consequence of a general deficit in learning and memory, but may be the result of compromised auditory stimulus processing in the nicotine-exposed and knockout animals.
Keywords: nicotine, nicotinic acetylcholine receptor, development, mouse, auditory
1. Introduction
Approximately 15% of pregnant women in the United States smoke tobacco [1]. In addition to deleterious effects on maternal health, smoking during pregnancy increases the risk of morbidity, mortality, and impaired growth in the offspring, both pre- and post-natally [2]. The offspring of pregnant smokers are also at elevated risk of diagnosis for various psychiatric disorders during childhood, including attention deficit hyperactivity disorder (ADHD) [3–7]. This suggests that tobacco exposure during gestation can alter processes during neurodevelopment and have persistent consequences for the offspring.
A number of studies have also shown that children exposed to tobacco smoke during gestation exhibit non-clinical deficits in various cognitive domains [3, 8–10]. It has been suggested that compromised processing of sensory stimuli may be a prominent contributor to these deficits [3, 11]. In particular, central processing of auditory stimuli appears to be highly sensitive to disruption by gestational tobacco exposure. The Ottawa Prenatal Prospective Study has reported alterations in the performance of various tasks dependent on auditory processing in gestational tobacco-exposed individuals at multiple ages, observing reduced orientation and habituation to sounds in infancy [12], increased errors of commission in an auditory task in childhood [13, 14], and difficulties with speech perception and language in adolescence [15–17]. Jacobsen and colleagues [18] also demonstrated that performance in auditory and visual attention tasks was impaired in adolescents exposed to tobacco prenatally. Enhanced hemodynamic signals in brain areas associated with auditory processing were also observed, suggesting reduced efficiency in the neural circuits involved in stimulus processing in these individuals.
Among the numerous components in tobacco, nicotine is a likely component that could disrupt neurodevelopment and induce these persistent changes in sensory processing through its actions at nicotinic acetylcholine receptors (nAChRs), which are expressed early in central nervous system development [19–23]. Notably, it has been reported that the α7 nAChR subtype has a critical role in the normal refinement of the thalamocortical terminals which relay sensory stimulus representations from primary thalamic sensory nuclei to layer IV of primary sensory cortex [11, 24–26]. It has also been suggested that the α4β2α5 nAChR subtype may have a role in the refinement of the layer VI corticothalamic terminals that permit cortical feedback modulation of thalamic activity [27, 28]. It is therefore likely that gestational nicotine exposure could have a profound effect on the maturation of thalamo-cortico-thalamic circuits and thereby alter the relay, and consequently the processing, of sensory stimuli. This is supported by evidence of impaired performance in an auditory-cued active avoidance task by adult rats exposed to nicotine during a developmental period approximately equivalent to the human third trimester of pregnancy, in which the ability of both exogenous nicotine and endogenous acetylcholine to enhance the sensitivity of the mature auditory cortex to sound stimuli was also blunted [29]. A small number of independent studies have also evaluated the persistent effects of various routes of developmental nicotine exposure on auditory-cued active avoidance performance [30, 31]. However, all of these experiments assessed the persistent effects of perinatal nicotine exposure on auditory processing in an aversive context. One recent study in rats examined the effects of perinatal nicotine exposure on performance in an appetitive task [32]. In this case, nicotine exposure delayed sensorimotor development and impaired performance in the 5-choice serial reaction time task when the visual stimulus was brief [32], suggesting either compromised visual stimulus processing or impaired attention.
In the current study, we examined whether compromised auditory stimulus processing is a consequence of developmental nicotine exposure that can be generalized beyond the aversive context by characterizing the performance of exposed mice in an appetitive auditory discrimination paradigm. We have shown previously that developmental nicotine exposure alters the processing of somatosensory stimuli in adult mice via α4β2α5 nAChRs expressed in corticothalamic projections [27]. We therefore extended the current study to explore the potential role for β2 subunit-containing nAChRs in this task by assessing β2 nAChR subunit knockout mice [33] in this paradigm. To dissociate impaired auditory discrimination learning from a general learning impairment or altered response rates, we tested both perinatally-exposed and β2 knockout mice in a differential reinforcement of low rate (DRL) task, a paradigm that is commonly used to assess differences in impulsivity and hyperactivity [34–36].
2. Methods
2.1. Perinatal nicotine exposure paradigm
All procedures involving animals in this study were approved by the Yale University Institutional Animal Care and Use Committee in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) were housed in a colony room at 22°C with ad libitum access to standard laboratory chow and normal drinking water on a 12 hour light-dark cycle (lights on: 7AM). Following acclimation to the animal facility (at least 7 days), females were mated in pairs or harems and immediately given ad libitum access to drinking water containing 200 μg/ml nicotine (hydrogen tartrate salt calculated with respect to the free base) with 2% saccharin (w/v) or pH-matched 2% saccharin (w/v) with 0.2% (v/v) tartaric acid (Sigma Aldrich, St. Louis, MO) as their sole water supply. Drinking solutions were changed twice weekly. The 200 μg/ml nicotine dose was selected as it has been shown previously to have both behavioral and biochemical efficacy in the adult mouse [37–40], to induce alterations in neurodevelopment [41], and to have minimal effects on pregnancy in the C57BL/6J strain [41]. It has also been shown that perinatal exposure using this dose and method of administration has minimal impact on birth weight or early postnatal weight gain or on measures of maternal care behavior in this strain [42] while affecting sensory processing in adulthood [27].
Dams were maintained on nicotine- or vehicle-containing water throughout pregnancy and after delivery until offspring were weaned at postnatal day (PND) 21. Developmentally-treated offspring received no further nicotine treatment before testing in adulthood and were not handled except for routine husbandry. Thirty-one male offspring perinatally exposed to nicotine and thirty-four male saccharin-exposed control mice were trained in the auditory discrimination task. Of these mice, twelve nicotine- and twelve saccharin-treated mice were trained and tested in a DRL procedure.
2.2. β2 nAChR subunit knockout mice
β2 nAChR subunit knockout mice and their wild-type siblings were generated as described previously [33] and were backcrossed onto the C57BL/6J background for at least twenty generations. Heterozygous breeding pairs were mated to yield the wild-type and homozygous knockout mice used in this study and were housed in the same breeding facility as described for developmentally treated mice. Mice were genotyped shortly after weaning from DNA obtained by tail biopsy. Eight β2 knockout and six wild-type male mice were trained in the auditory discrimination task. Of these mice, six β2 knockout and five wild-type mice were trained and tested in a DRL procedure.
2.3. Auditory discrimination procedure
2.3.1. Behavioral apparatus for auditory discrimination procedure
All behavioral training was carried out in Med-Associates (St. Albans, VT) operant arenas (ENV-307A) fitted with Med-Associates components and situated within sound-attenuating outer chambers (ENV-022V). For auditory discrimination, each arena contained a pellet magazine (ENV-303M) located in the center panel of one wall with a nosepoke response port (ENV-313M) located to either the left or right of the pellet magazine (counterbalanced across boxes; see Figure 1). Pellets (20 mg Dustless Precision Pellets; Bio-Serv, Frenchtown, NJ) were delivered to the magazine by a food hopper mounted on a pedestal external to the operant arena (ENV-203-20). Entries into the magazine and nosepoke ports were each detected by the interruption of an infrared photobeam.
Figure 1. Auditory discrimination task.
(A) Mice were trained to make head entries into the nosepoke port to initiate a discrimination trial (top). Nosepoke entries after the end of the ITI resulted in presentation of either the Rewarded or Unrewarded Tone (center). A response in the magazine following presentation of the Rewarded Tone was scored as a Hit, and triggered reward delivery. A magazine entry after the Unrewarded Tone was scored as a False Alarm, and had no programmed consequence except for the initiation of a new ITI. Lack of responding in the magazine also had no consequence for the mice. (B) Typical training and testing schedule. Behavioral training was carried out during single daily sessions. Mice were first trained to approach the magazine for food (`Magazine'). Next, mice were rewarded only for magazine entries during delivery of the Rewarded Tone (`Tone'). During the `Nosepoke' stage of training, mice learned to make nosepoke entries to trigger the Rewarded Tone. A fixed or random 20 sec interval schedule was next introduced (`Interval'). Finally, the ability of mice to discriminate between Rewarded and Unrewarded Tones was tested (`Auditory Discrimination Testing') over seven sessions.
For auditory discrimination, two speakers (ENV-224BM) were mounted within plastic casings above the operant arena and were connected to pure tone generators (ENV-230) set to either 12 kHz or 15 kHz and calibrated with a digital sound pressure meter (Model 732; B&K Precision, Yorba Linda, CA) to ~60dBA. Tone frequencies were selected based on audiograms measured in adult mice and are both well within the optimal hearing range [43]. The locations of speakers assigned to each tone were counterbalanced across boxes, either proximal or distal to the nosepoke ports and food magazine. A house light (ENV-315M) provided general illumination throughout behavioral sessions. The nosepoke port- and food magazine-associated lights (ENV-313M and ENV-321M, respectively) were also illuminated during behavioral testing. Stimuli were controlled and behavioral events were captured by a computer running MedPC IV (MedAssociates) software.
2.3.2. Auditory discrimination training
Mice were trained in an auditory discrimination procedure adapted from methods previously used in rats [44, 45]. A schematic of the behavioral arena and a timeline of events are shown in Figure 1. Behavioral training began after mice were at least 2 months of age. As there is a well-characterized loss of hearing around 7 months of age in the C57BL/6J strain [46–50], mice were tested at no older than 6 months of age with stimuli that would still be audible even with some diminution of hearing [49]. There was no statistical difference between the ages of saccharin- (4.05±0.18 months, mean±SEM) and nicotine-treated (4.54±0.23 months) mice at the beginning of auditory discrimination training (two-tailed t-test of independent samples: t(43)=1.73, p=0.09). The ages of wild-type (4.47±0.27 months) and β2 nAChR knockout (4.84±0.37 months) mice were also not statistically different (two-tailed t-test of independent samples: t(9)=0.78, p=0.46).
Mice were transferred from the breeding facility to a non-breeding room near the behavioral testing space with the same light-dark cycle, and were allowed to acclimate to the new environment for at least 7 days prior to training. Mice were then handled and weighed daily to establish baseline weight. If weights were stable over 3 days, mice were placed on a restricted diet and maintained at 85% of baseline weight. To habituate mice to food pellet rewards, their daily restricted food allowance was supplemented for at least two days with 25 pellets in the home cage. During training, mice received chow in the home cage at least 30 min after the end of the behavioral session. Water was available ad libitum in the home cage.
In single daily sessions, mice were initially trained to approach the food magazine by delivery of a pellet every 30 sec over a 15 min session. Next, mice had to make magazine entries only in the presence of a 3 sec pure tone stimulus (12 kHz or 15 kHz, counterbalanced across subjects) in order to receive a food pellet. In the third phase of training, the nosepoke port was revealed, and mice had to make an entry into the nosepoke port to trigger the 3 sec rewarded tone. Entries into the magazine in the presence of the reward-associated tone resulted in pellet delivery. Mice were then assigned to either a fixed or random 20 sec interval schedule in a counter-balanced fashion. Only nosepoke entries after the completion of an interval triggered 2 sec tone delivery, while other nosepoke or magazine entries had no consequence.
Different interval schedules were used to understand the nature of any responding during the intertrial interval (ITI). In the fixed interval schedule, ITI responding might indicate impulsive behavior or hyperactivity, or it could signify anticipation of the reward. To examine these possibilities, the random interval schedule used ITIs generated from an exponential distribution (mean of 20 sec) to reduce temporal predictability. A main effect of treatment group or genotype on ITI responding, without any difference across schedules, would imply hyperactivity or impulsivity, but would rule out impaired timing mechanisms.
Once mice successfully earned 50 pellets within an interval training session, they progressed to auditory discrimination training. Mice retained their assigned interval schedule and reward-associated tone (`Rewarded Tone'), but a second `Unrewarded Tone' was introduced. As before, a nosepoke entry after the end of an interval triggered tone delivery (1 sec); however, only magazine responses to the Rewarded Tone resulted in reward delivery, whereas responses to the Unrewarded Tone had no consequence. Mice were tested for seven consecutive days in the auditory discrimination task to assess task acquisition (i.e., the number of days it took for perinatal nicotine-treated mice to achieve a Discrimination Ratio that was statistically equivalent to that of their saccharin control group; see Results).
2.3.3. Behavioral measures for auditory discrimination performance
A head entry into the nosepoke port resulted in one of three possible scenarios: 1) delivery of the Rewarded Tone, 2) delivery of the Unrewarded Tone, or 3) no tone delivery (i.e., the response was made during the ITI). The mouse could then make a magazine entry in anticipation of reward. A magazine entry in response to the Rewarded Tone was scored as a `Hit'. A magazine entry in response to the Unrewarded Tone was scored as a `False Alarm'. Magazine entries in the absence of tone delivery were captured in order to explore potential differences in impulsive responding. Latencies between nosepoke entries and magazine entries were also calculated for each tone condition. The rate of nosepoking during the ITI was also determined to examine general activity. A Discrimination Ratio was calculated to explore differences in responding to the two tones (Discrimination Ratio = (P(Hits) − P(False Alarms) ) / (P(Hits) + P(False Alarms)) and was the primary measure of discrimination performance.
2.3.4. Statistical analyses for auditory discrimination performance
Behavioral data were stored for each subject and session by MedPC as .mpc files and were compiled for further analysis using custom written Matlab (R2009a, Version 7.8.0.347, MathWorks, Natick, MA) scripts. Outliers were determined by excluding animals with Discrimination Ratios outside of 1.5 x the interquartile range [51] on any single day of testing. While this effectively reduced the number of subjects in the study, it eliminated mice that had an exceptionally good or bad testing session, which could skew the data and was preferable to choosing data from particular days to exclude for individual animals. This normalized and homogenized the Discrimination Ratio data without the need for further transformation. All other variables were then subjected to tests of normality and homogeneity (Levene's Test),and were transformed as appropriate prior to statistical comparisons [51]. The probabilities (arcsine-transformed: and latencies (log-transformed) of Hits, False Alarms, and magazine entries with no stimulus, Discrimination Ratios (untransformed), and ITI nosepoke rates (log-transformed) were compared across treatment groups or genotypes using mixed factorial repeated measures analysis of variance (rmANOVA) over sessions in PASW Statistics 18 (Release 18.0.0, IBM, Armonk, NY). Huynh-Feldt corrections were applied when data failed Mauchly's test of sphericity. Stimulus and interval assignments were included as additional between-subjects factors. To assess the timing (i.e., session effects) and direction of differences between groups, we examined pair-wise comparisons of the estimated marginal means using Fisher's Least Significant Difference (LSD) [51]. For all analyses, significance was set at p<0.05.
2.4. DRL Procedure
2.4.1. Behavioral apparatus for DRL procedure
Behavioral training in the DRL procedure was carried out in Med-Associates chambers as described above. For the differential reinforcement of low rate (DRL) task, both left and right nosepoke ports were located in the wall opposite a liquid reward magazine cup (ENV-303LP). Plastic tubing attached the magazine to a syringe filled with liquid reward (50% Vanilla Ensure solution, Abbott); the syringe plunger was advanced using a Med-Associates pump (PHM-100; 3.33 rpm, 11.8μl/sec).
2.4.2. DRL training
See Figure 4 for a diagram of the DRL task and training regimen. To characterize the nature of differences in learning rates and in ITI nosepoke responding between groups observed in our initial cohorts of developmentally-treated mice more fully, later cohorts were also tested in a DRL schedule, which does not depend on auditory stimulus processing. To familiarize mice with the liquid reward, each mouse was placed in an empty cage and given 30 min access to a capful of 50% Vanilla Ensure solution. Once mice reliably consumed the Ensure, they were given a single session of magazine training in the new operant boxes (5 min habituation and 15 min with reward delivered every 30 sec). For the DRL task, mice were randomly assigned either the left or right nosepoke as their `active' nosepoke (i.e., responses there were rewarded), while the remaining nosepoke port was `inactive' (i.e., no consequence). Mice were then placed on a fixed ratio (FR1) schedule with the inactive nosepoke port blocked for one session. They were then run on an FR1 schedule with both nosepoke ports available until they learned to make responses in the active, but not inactive, nosepoke port for rewards in the magazine (typically one or two sessions). Finally, mice were run for 12 days in a DRL12 schedule. That is, the ITI was 12 sec and any responses in the active nosepoke port during this time would reset the ITI. Responses after the 12 sec ITI were rewarded with delivery of liquid reward (24 μl) in the magazine. The end of the ITI was not signaled.
Figure 4. Differential reinforcement of low rate task.
(A) Mice were trained to make head entries into the Active nosepoke port, while ignoring the Inactive nosepoke port (top). Nosepoke entries after the end of the 12 sec ITI resulted in presentation of liquid reward in the magazine at the opposite side of the operant arena (bottom). Nosepoke responses in the Active nosepoke port before 12 sec had passed reset the ITI. (B) Typical training and testing schedule. Behavioral training was carried out during single daily sessions. Mice were first trained to approach the magazine for food (`Mag'). Next, mice were rewarded only after making a single nosepoke entry in the Active port. During the first `FR1' session, the Inactive nosepoke port was blocked. It was revealed during the second `FR1' session. Finally, the ability of mice to withhold responding during the 12 sec ITI was tested (`DRL Testing') over twelve sessions.
2.4.3. Behavioral measures of DRL performance
Measures used to quantify performance in the DRL task included number of rewards earned, efficiency (number of rewarded active / number of total active), total response rate (total number of responses per min in a 20 min session), burst response rate (per min), and pause response rate (per min). Burst responding was defined as responses occurring with ITIs of less than 1 sec. Pause responses were responses with ITIs 1 sec or longer.
2.4.4. Statistical analyses for DRL performance
Data were organized and tested for normality and homogeneity of variance as described for the auditory discrimination procedure. For DRL performance, rmANOVAs were run over sessions to compare response rates (untransformed), efficiency (arcsine-transformed), and rewards earned (square-root transformed) across experimental groups. As the DRL task is dependent on learning of a fixed interval schedule, previous experience in the fixed vs. random interval schedule in the auditory discrimination task was included as a between-subjects factor in the analysis. To assess the timing (i.e., session effects) and direction of differences between groups, we examined pair-wise comparisons of the estimated marginal means using Fisher's LSD [51]. For all analyses, significance was set at p<0.05.
3. Results
3.1. Auditory discrimination learning is altered in mice exposed to perinatal nicotine
One saccharin-treated and two nicotine-treated mice failed to acquire the simple nosepoke response. Two saccharin-treated mice were removed from the study due to completing insufficient trials. Two mice from each treatment group were excluded due to a power outage that wiped out the data from one of their discrimination sessions. Exploratory analysis (see Methods) on the remaining mice revealed five animals in the saccharin group and six in the nicotine group with a Discrimination Ratio that was outside of 1.5 × the interquartile range on at least one day, and the data from these animals were excluded from further analysis. The final analysis therefore included 24 saccharin- and 21 nicotine-treated mice.
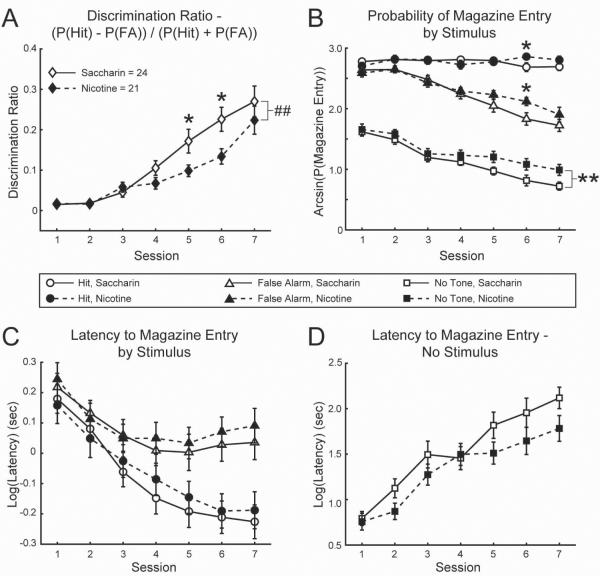
Mice from both groups learned to discriminate between 12 kHz and 15 kHz auditory tones, as evidenced by an overall increase in the Discrimination Ratio over days (see Table 1 for rmANOVA results; Session 7>Session 1: 0.25±0.026>0.015±0.004, LSD p<0.001; Figure 2A). This effect was driven by a reduction in False Alarm responding over days (Table 1; Session 7<Session 1: 1.81±0.079<2.62±0.039, LSD p<0.001; Figure 2B), as the rate of Hit responding remained stable throughout training (Table 1; Figure 2B). Over sessions, mice also learned to withhold nosepoke port (Table 1; Session 7<Session 1: 0.41±0.052<0.75±0.049, LSD p<0.001) and magazine (Table 1; Session 7<Session 1: 0.87±0.054<1.65±0.052, LSD p<0.001; Figure 3B) entries during the ITI, responded more rapidly following tone delivery (Table 1; Hit latency, Session 7<Session 1: −0.22±0.042<0.17±0.040, LSD p<0.001; False Alarm latency, Session 7<Session 1: 0.063±0.042<0.23±0.038, LSD p=0.001; Figure 2C), and slowed responding in the magazine when no tone was delivered (Table 1; Session 7>Session 1: 1.92±0.090>0.77±0.061, LSD p<0.001; Figure 2D). Thus, mice with either perinatal nicotine exposure or their saccharin-treated counterparts were able to improve all aspects of auditory discrimination performance over training.
Table 1.
Auditory Discrimination Performance in Mice Exposed Perinatally to Nicotine
| Statistical Tests | Disc Ratio | Hit | False Alm | No Stim | Hit Lat | False Lat | No Lat | NPs / min | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Comparisons | F(1,6 | P | F(1,6 | P | F(1,6 | P | F(1,6 | P | F(1,6 | P | F(1,6 | P | F(1,6 | P | F(1,6 | P |
| Within-subjects | ||||||||||||||||
| Session | 49.9 | * | 0.7 | 0.6 | 46. | * | 51. | † | 84. | * | 10. | * | 46. | * | 16. | * |
| Sess × Ttmt | 2.92 | * | 2.3 | † | 2.3 | * | 1.9 | 0.0 | 1.0 | 0.3 | 0.3 | 0.8 | 1.3 | 0.2 | 2.1 | * |
| Sess × Stim | 0.66 | 0.6 | 0.9 | 0.4 | 0.3 | 0.8 | 1.0 | 0.3 | 1.1 | 0.3 | 0.7 | 0.5 | 1.1 | 0.3 | 0.6 | 0.6 |
| Sess × Sched | 0.53 | 0.7 | 1.2 | 0.2 | 0.8 | 0.5 | 1.0 | 0.4 | 0.9 | 0.4 | 0.7 | 0.5 | 0.5 | 0.7 | 1.6 | 0.1 |
| Sess × Ttmt x Stim | 0.28 | 0.8 | 0.6 | 0.6 | 0.3 | 0.8 | 1.2 | 0.2 | 0.7 | 0.5 | 0.8 | 0.4 | 1.3 | 0.2 | 1.0 | 0.3 |
| Sess × Ttmt × Sched | 0.25 | 0.8 | 0.9 | 0.4 | 0.3 | 0.9 | 2.1 | 0.0 | 1.1 | 0.3 | 0.6 | 0.6 | 2.1 | 0.0 | 2.0 | 0.0 |
| Sess × Stim × Sched | 1.90 | 0.1 | 2.9 | † | 1.6 | 0.1 | 0.6 | 0.7 | 1.1 | 0.3 | 0.3 | 0.8 | 1.2 | 0.3 | 0.8 | 0.5 |
| Sess × Ttmt × Stim × Sched | 3.29 | * | 1.1 | 0.3 | 1.2 | 0.3 | 1.2 | 0.3 | 1.2 | 0.2 | 0.4 | 0.8 | 1.9 | 0.0 | 0.5 | 0.7 |
| Between-subjects | ||||||||||||||||
| Ttmt | 3.92 | 0.0 | 0.6 | 0.4 | 2.2 | 0.1 | 6.1 | * | 0.0 | 0.9 | 0.0 | 0.9 | 3.4 | 0.0 | 0.0 | 0.8 |
| Stim | 0.02 | 0.8 | 0.6 | 0.4 | 0.1 | 0.7 | 1.1 | 0.3 | 0.8 | 0.3 | 0.9 | 0.3 | 0.7 | 0.4 | 0.7 | 0.4 |
| Sched | 0.33 | 0.5 | 0.6 | 0.4 | 0.0 | 0.7 | 7.0 | * | 0.0 | 0.9 | 0.1 | 0.6 | 0.3 | 0.5 | 0.0 | 0.7 |
| Ttmt × Stim | 0.06 | 0.8 | 1.0 | 0.3 | 0.0 | 0.9 | 0.1 | 0.6 | 0.0 | 0.8 | 0.0 | 0.8 | 0.1 | 0.7 | 0.1 | 0.7 |
| Ttmt × Sched | 0.68 | 0.4 | 0.3 | 0.5 | 1.7 | 0.2 | 3.0 | 0.0 | 0.0 | 0.9 | 0.0 | 0.9 | 1.4 | 0.2 | 0.6 | 0.4 |
| Stim × Sched | 3.04 | 0.0 | 3.6 | 0.0 | 4.8 | * | 9.2 | * | 1.2 | 1.2 | 0.2 | 1.4 | 5.2 | * | 3.0 | 0.0 |
| Ttmt × Stim × Sched | 2.52 | 0.1 | 0.3 | 0.5 | 0.8 | 0.3 | 0.3 | 0.5 | 2.6 | 0.1 | 1.4 | 0.2 | 0.0 | 0.7 | 2.4 | 0.1 |
Results of rmANOVA. “Disc Ratio” = untransformed Discrimination Ratio, ‘Hit’ = arcsine-transformed P(Hit), ‘False Alm’ = arcsine-transformed P(False Alarm), ‘No Stim’ = arcsine-transformed P(magazine response in absence of tone), ‘Hit Lat’ = log(latency of Hit response), ‘False Lat’ = log(latency of False Alarm), ‘No Lat’ = log(latency of response in absence of tone), ‘NPs / min’ = log(nosepokes per minute).
= p<0.05, Huynh-Feldt.
= data passes Mauchly's Test of Sphericity and p-values are reported with sphericity assumed.
Figure 2. Auditory discrimination performance in mice exposed perinatally to nicotine.
A) Discrimination Ratio indicating auditory discrimination performance in saccharin- and nicotine-treated mice. B) Arcsine-transformed probabilities of magazine entry (attempted reward retrieval) following the Rewarded Tone (`Hit'), Unrewarded Tone (`False Alarm'), and after a nosepoke response when no stimulus was presented in saccharin- and nicotine-treated mice. C–D) Log-transformed response latencies between nosepoke / stimulus presentation and magazine entry for tone presentation trials (C) and trials in which no tone was presented (D). # = p<0.06, trend by pairwise LSD. * = p<0.05, significant by pairwise LSD. ## = p<0.06, trend toward main effect of treatment by rmANOVA. ** = p<0.05, significant main effect of treatment by rmANOVA.
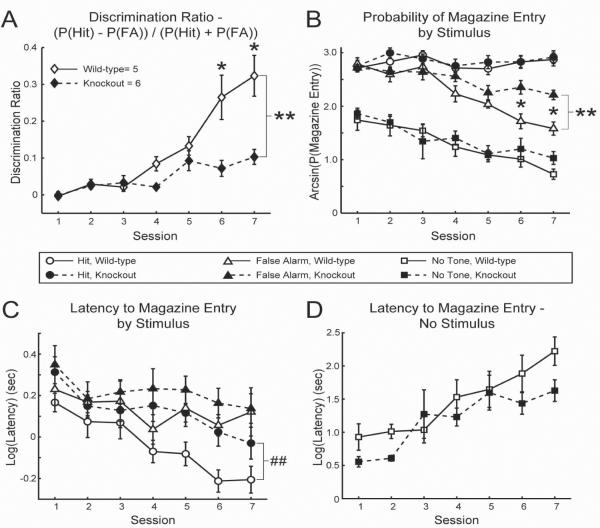
Figure 3. Auditory discrimination performance in mice lacking the β2 nAChR subunit.
A) Discrimination Ratio indicating auditory discrimination performance in β2 wild-type and knockout mice. B) Arcsine-transformed probabilities of magazine entry (attempted reward retrieval) following the Rewarded Tone (`Hit'), Unrewarded Tone (`False Alarm'), and after a nosepoke response when no stimulus was presented in β2 wild-type and knockout mice. C–D) Log-transformed response latencies between nosepoke / stimulus presentation and magazine entry for tone presentation trials (C) and trials in which no tone was presented (D). * = p<0.05, significant by pairwise LSD. ## = p<0.06, trend toward main effect of genotype by rmANOVA. ** = p<0.05, significant main effect of genotype by rmANOVA.
There was a strong trend toward a main effect of treatment (Table 1; nicotine<saccharin: 0.087±0.014<0.13±0.013, LSD p=0.055; Figure 2A) and a significant interaction between treatment and session on the Discrimination Ratio (Table 1). Nicotine-treated mice had lower Discrimination Ratios in later sessions (Session 5, nicotine<saccharin: 0.097±0.024<0.18±0.023, LSD p=0.013; Session 6, nicotine<saccharin: 0.13±0.028<0.23±0.026, LSD p=0.015; Figure 2A), but performed comparably to saccharin control mice by the seventh day of training (Session 7, nicotine=saccharin: 0.23±0.038=0.28±0.036, LSD p=0.32; Figure 2A). In summary, perinatal nicotine exposure disrupted learning of the auditory discrimination procedure, as measured by Discrimination Ratio.
The treatment-related difference in Discrimination Ratio over sessions could be driven either by reduced probability of a Hit response or increased probability of a False Alarm response. There were significant session x treatment interactions in both of these measures (Table 1). However, LSD pair-wise comparisons revealed significant post hoc elevation on both of these measures only on the sixth day of training (Session 6: Hit, nicotine>saccharin: 2.87±0.069<2.67±0.065, LSD p=0.041; False Alarm, nicotine>saccharin: 2.13±0.096>1.81±0.089, LSD p=0.020; Figure 2B). Therefore, it would appear that non-significant differences between these measures contributed to the treatment-related differences in the Discrimination Ratio, with elevated False Alarm responding contributing significantly on session 6. There were no treatment-related differences in the speed with which mice responded to the tones (Table 1; Figure 2C).
To determine whether mice of either treatment group tended to check more frequently for the end of the ITI, the overall nosepoke frequency was assessed. There was a significant session x treatment effect on ITI nosepoking (Table 1), but no significant post hoc effects. However, there was a significant main effect of treatment on the proportion of responses in the magazine during the ITI when no stimulus was delivered (Table 1). Mice exposed to nicotine during perinatal development made more such responses than control mice (nicotine>saccharin: 1.31±0.056>1.12±0.052, LSD p=0.018; Figure 2B). In addition, there was a main effect of schedule assignment, and an interaction between assigned stimulus and schedule (Table 1). Mice assigned to the fixed interval schedule tended to respond more during the ITI than mice assigned to the random interval schedule (fixed (n=22) > random (n=23): 1.32±0.055>1.12±0.053, LSD p=0.012). This difference was highly significant in the group assigned to the 15 kHz Rewarded Tone (fixed (n=11) > random (n=13), 15 kHz group: 1.48±0.078>1.04±0.069, LSD p<0.001). This schedule-related difference is not unexpected, as the exponential distribution used for the random interval schedule has a greater probability of trials shorter than the 20 sec of the fixed interval schedule, decreasing the likelihood for ITI nosepoke and magazine entry observed after a longer wait. There was no interaction between treatment and schedule, nor was there an effect of treatment on the latency to respond in the magazine after an ITI nosepoke entry (Table 1; Figure 2D). Mice treated with nicotine during perinatal development were therefore equally able to withhold responding in the nosepoke port during the ITI, but once such a response was made, they were more likely to approach the reward magazine.
3.2. Auditory discrimination learning is altered in mice lacking the β2 nAChR subunit
All β2 nAChR subunit knockout mice were included in the initial exploratory analysis. Two β2 knockout mice and one wild-type control mouse had a Discrimination Ratio that was outside of 1.5 × the interquartile range on at least one day, and the data from these animals were excluded from further analysis (see Methods). The final analysis included 6 β2 knockout mice and 5 wild-type controls. Because of the small number of subjects in this study, only main effects and second order interactions are considered.
Both β2 knockout mice and their wild-type controls learned to discriminate between the Rewarded and Unrewarded Tones as measured by an increase in the Discrimination Ratio over days (see Table 2 for rmANOVA results; Session 7>Session 1: 0.21±0.032>−0.005±0.007, LSD p=0.003; Figure 3A). The increase in Discrimination Ratio was primarily due to reduction of the False Alarm rate (Table 2; Session 7<Session 1: 1.93±0.072<2.77±0.064, LSD p=0.003; Figure 3B), as the probability of a Hit response remained stable over days (Table 2; Figure 3B). Mice of both genotypes decreased ITI responding in the nosepoke port (Table 2; Session 7<Session 1: 0.59±0.089<0.87±0.11, LSD p=0.022) and magazine (Table 2; Session 7<Session 1: 0.88±0.12<1.77±0.15, LSD p=0.003; Figure 3B) across training. Response latencies for the Rewarded Tone decreased over days (Table 2; Session 7<Session 1: −0.112±0.077<0.21±0.020, LSD p=0.013; Figure 3C), while there was no change in False Alarm latencies (Table 2; Figure 3C) and slowing of magazine responses in the absence of tone delivery (Table 2; Session 7>Session 1: 1.96±0.18>0.76±0.11, LSD p=0.001; Figure 3D). By these measures, mice of either genotype were able to improve auditory discrimination performance over sessions.
Table 2.
Auditory Discrimination Performance in Mice Lacking the β2 nAChR Subunit
| Statistical Tests | Disc Ratio | Hit | False Alm | No Stim | Hit Lat | False Lat | No Lat | NPs / min | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Comparisons | F(1,6 | P | F(1,6 | P | F(1,6 | P | F(1,6 | P | F(1,6 | P | F(1,6 | P | F(1,6 | P | F(1,6 | P |
| Within-subjects | ||||||||||||||||
| Session | 24.0 | * | 1.8 | 0.1 | 18. | * | 13. | * | 9.7 | * | 2.4 | 0.0 | 9.1 | * | 4.2 | * |
| Sess × Geno | 9.22 | * | 1.3 | 0.2 | 5.8 | * | 3.1 | * | 1.0 | 0.4 | 0.9 | 0.5 | 1.8 | 0.1 | 2.9 | * |
| Sess × Stim | 1.37 | 0.2 | 0.7 | 0.6 | 1.1 | 0.3 | 1.4 | 0.2 | 1.2 | 0.3 | 1.3 | 0.2 | 0.8 | 0.5 | 2.2 | 0.0 |
| Sess × Sched | 1.09 | 0.4 | 0.7 | 0.5 | 1.1 | 0.3 | 2.9 | * | 0.4 | 0.8 | 2.2 | 0.0 | 1.4 | 0.2 | 3.8 | * |
| Between-subjects | ||||||||||||||||
| Geno | 25.9 | * | 0.3 | 0.5 | 16. | * | 0.5 | 0.5 | 7.5 | 0.0 | 1.8 | 0.2 | 3.2 | 0.1 | 0.1 | 0.7 |
| Stim | 4.43 | 0.1 | 1.9 | 0.2 | 5.4 | 0.0 | 1.2 | 0.3 | 6.9 | 0.0 | 8.2 | * | 0.6 | 0.4 | 0.6 | 0.4 |
| Sched | 0.40 | 0.5 | 0.8 | 0.4 | 0.0 | 0.8 | 1.7 | 0.2 | 0.3 | 0.6 | 2.3 | 0.2 | 9.3 | * | 0.7 | 0.4 |
| Geno × Stim | 0.86 | 0.4 | 1.2 | 0.3 | 1.1 | 0.3 | 0.4 | 0.5 | 0.1 | 0.7 | 0.0 | 0.7 | 0.0 | 0.9 | 0.4 | 0.5 |
| Geno × Sched | 0.06 | 0.8 | 0.0 | 0.8 | 0.3 | 0.5 | 1.2 | 0.3 | 0.1 | 0.7 | 0.3 | 0.5 | 0.9 | 0.4 | 0.3 | 0.5 |
| Stim × Sched | 0.07 | 0.8 | 0.0 | 0.7 | 0.3 | 0.5 | 0.0 | 0.9 | 0.5 | 0.4 | 0.1 | 0.6 | 0.7 | 0.4 | 0.0 | 0.8 |
Results of rmANOVA. 'Disc Ratio' = untransformed Discrimination Ratio, 'Hit' = arcsine-transformed P(Hit), 'False Alm' = arcsine-transformed P(False Alarm), 'No Stim' = arcsine-transformed P(magazine response in absence of tone), 'Hit Lat' = log(latency of Hit response), 'False Lat' = log(latency of False Alarm), 'No Lat' = log(latency of response in absence of tone), 'NPs / min' = log(nosepokes per minute).
=p<0.05, Huynh-Feldt.
There was a main effect of genotype on the Discrimination Ratio, with β2 knockout mice showing a discrimination impairment compared to wild-type controls (Table 2; knockout<wild-type: 0.049±0.010<0.13±0.012, LSD p=0.005; Figure 3A). There was also a significant session × genotype interaction (Table 2), with β2 knockout mice discriminating significantly less well than wild-type controls in later sessions (Session 6, knockout<wild-type: 0.063±0.034<0.30±0.040, LSD p=0.011; Session 7, knockout<wild-type: 0.099±0.042<0.35±0.049, LSD p=0.018; Figure 3A). Whereas wild-type mice discriminated similarly to both perinatally-treated groups at session 7 (two-tailed t-test of independent samples, wild-type control mice=developmentally treated mice: 0.25±0.026=0.32±0.055, t(48)=−0.93, p=0.36), demonstrating that performance at this level can reasonably be expected by this point in training, mice lacking the β2 nAChR subunit continued to be impaired at discriminating between Rewarded and Unrewarded Tones.
The impaired performance of the β2 knockout mice was driven primarily by elevated False Alarm rate overall in this group (Table 2; knockout>wild-type: 2.49±0.040>2.24±0.047, LSD p=0.015; Figure 3B). There was also a significant session × genotype effect (Table 2), revealing a greater probability of False Alarm responding in β2 nAChR subunit knockout mice in later sessions (Session 6, knockout>wild-type: 2.41±0.13>1.66±0.15, LSD p=0.021; Session 7, knockout>wild-type: 2.23±0.094>1.53±0.11, LSD p=0.009; Figure 3B). In addition, there was a significant main effect of stimulus assignment on False Alarm latency (Table 2). However, the comparatively quicker responding to the 12 kHz tone was only significant at a trend level by LSD pair-wise comparison (12 kHz (n=4) <15 kHz (n=7): 0.089±0.050<0.26±0.040, LSD p=0.057). There were no genotype-related differences in the probability of a Hit response (Table 2; Figure 3B) or on latencies to respond to the Unrewarded Tone (Table 2; Figure 3C). There was, however, a strong trend toward slower responding in the knockout mice for the Rewarded Tone (Table 2; knockout>wild-type: 0.11±0.038>−0.050±0.045, LSD p=0.050; Figure 3C). Thus, β2 knockout mice failed to suppress responding to the Unrewarded Tone and responded with equal speed to both tones.
There were session × genotype effects on ITI nosepoke rates and on the probability of responding in the magazine during the ITI (Table 2). However, there were no significant differences by pair-wise LSD (Figure 3B). There was also a significant main effect of schedule on the latency to respond in the magazine after an ITI nosepoke (Table 2). Mice assigned to the fixed interval schedule were faster to respond (fixed (n=6) < random (n=5): 1.09±0.078<1.53±0.081, LSD p=0.018). There were no genotype-related differences in the response latencies in the absence of a tone (Table 2; Figure 3D). It therefore appears that the absence of the β2 nAChR subunit does not affect the ability to suppress responding significantly during periods of waiting, nor does it appear to impact the ability of mice to detect the presence of a tone or to withhold magazine approach when no tone is delivered.
3.3. Neither perinatal nicotine exposure nor absence of the β2 nAChR subunit affects DRL performance
Cohorts of mice trained initially in the auditory discrimination task (saccharin = 12, nicotine = 12, wild-type = 5, knockout = 6) were subsequently trained on a DRL schedule. This task was used to assess learning in a task that does not depend on discrimination between sensory stimuli and to examine potential differences in impulsive responding, both measured as differences in rates of responding during the 12 sec delay.
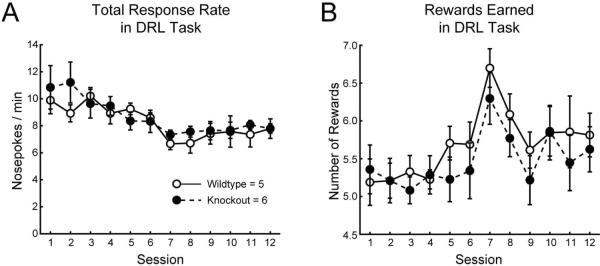
There were no differences in performance between the treatment groups on the DRL schedule (Table 3; Figure 5A–B). Mice exposed to either nicotine or saccharin during perinatal development were able to reduce responding (Table 3; total response rate, Day 12<Day 1: 7.71±0.37<10.1±0.67, LSD p=0.006; Figure 5A) and increase the number of rewards earned (Table 3; rewards, Day 12>Day 1: 5.74±0.12>5.09±0.14, LSD p=0.027; Figure 5B) over twelve days of DRL training (see Table 3 for rmANOVA results).
Table 3.
DRL Performance in Mice Exposed Perinatally to Nicotine
| Response rates |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Statistical Tests | Rewards | Efficiency | Total | Pause | Burst | |||||
| Comparisons | F(1,6) | P | F(1,6) | P | F(1,6) | P | F(1,6) | P | F(1,6) | P |
| Within-subjects | ||||||||||
| Session | 3.23 | * | 3.00 | * | 8.88 | * | 5.41 | * | 10.8 | * |
| Sess × Ttmt | 1.10 | 0.36 | 0.83 | 0.52 | 0.72 | 0.58 | 0.62 | 0.63 | 1.05 | 0.40 |
| Sess × Sched | 0.76 | 0.60 | 0.97 | 0.44 | 0.81 | 0.52 | 0.90 | 0.46 | 0.38 | 0.89 |
| Sess × Ttmt × Sched | 1.25 | 0.29 | 0.89 | 0.48 | 0.44 | 0.78 | 0.28 | 0.87 | 0.66 | 0.68 |
| Between-subjects | ||||||||||
| Ttmt | 1.81 | 0.19 | 1.45 | 0.24 | 0.35 | 0.56 | 0.36 | 0.55 | 0.04 | 0.84 |
| Sched | 1.20 | 0.29 | 0.53 | 0.48 | 0.55 | 0.47 | 0.66 | 0.43 | 0.04 | 0.85 |
| Ttmt × Sched | 0.05 | 0.82 | 0.13 | 0.73 | 1.07 | 0.31 | 0.31 | 0.59 | 1.07 | 0.31 |
Results of rmANOVA.
=p<0.05, Huynh-Feldt.
Figure 5. DRL performance in mice exposed perinatally to nicotine.
A) Total response rate over sessions in the DRL task in saccharin- and nicotine-treated mice. B) Square-root transformed number of rewards earned over sessions in the DRL task in saccharin- and nicotine-treated mice.
Deletion of the β2 nAChR subunit did not impair performance in the DRL task in any measure (see Table 4 for rmANOVA results; Figure 6A–B). All mice learned to withhold responding during the 12 sec delay (Table 4; total response rate, Day 12<Day 1: 7.66±0.32<10.3±1.10, LSD p=0.038; Figure 6A). Interestingly, there was a main effect of previous assignment to either fixed or random interval schedules on total response rate in the DRL task (Table 4; total response rate, fixed (n=6) > random (n=5): 9.32±0.44<7.42±0.50, LSD p=0.025). This was driven by an increase in the burst (Table 4; burst response rate, fixed (n=6) > random (n=5): 1.76±0.24<0.70±0.26, LSD p=0.020), but not the pause (Table 4), response rate in the fixed interval group over the random interval group. The number of rewards earned increased over sessions (Table 4), but the difference between rewards earned in the first vs. last session did not reach significance (rewards, Day 12>Day 1: 5.75±0.25<5.24±0.27, LSD p=0.187; Figure 6B).
Table 4.
DRL Performance in Mice Lacking the β2 nAChR Subunit
| Response Rates |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Statistical Tests | Rewards | Efficiency | Total | Pause | Burst | |||||
| Comparisons | F(1,6) | P | F(1,6) | P | F(1,6) | P | F(1,6) | P | F(1,6) | P |
| Within-subjects | ||||||||||
| Session | 4.28 | * | 3.81 | * | 6.46 | * | 4.42 | * | 8.84 | * |
| Sess × Geno | 0.35 | 0.97 | 0.58 | 0.83 | 0.66 | 0.70 | 0.43 | 090 | 1.56 | 0.19 |
| Sess × Sched | 0.65 | 0.78 | 1.10 | 0.37 | 0.72 | 0.65 | 0.64 | 0.74 | 1.97 | 0.10 |
| Sess × Geno × Sched | 0.67 | 0.77 | 1.48 | 0.16 | 2.24 | 0.05 | 2.25 | * | 2.02 | 0.09 |
| Between-subjects | ||||||||||
| Geno | 0.37 | 0.57 | 0.06 | 0.82 | 0.76 | 0.41 | 0.10 | 0.76 | 0.80 | 0.40 |
| Sched | 0.31 | 0.60 | 0.66 | 0.44 | 8.12 | * | 1.02 | 0.35 | 9.07 | * |
| Geno × Sched | 0.15 | 0.71 | 0.17 | 0.69 | 0.01 | 0.94 | 0.04 | 0.84 | 0.42 | 0.54 |
Results of rmANOVA.
=p<0.05, Huynh-Feldt.
Figure 6. DRL performance in mice lacking the β2 nAChR subunit.
A) Total response rate over sessions in the DRL task in β2 knockout and wild-type mice. B) Square-root transformed number of rewards earned over sessions in the DRL task in β2 knockout and wild-type mice.
4. Discussion
The results presented here provide evidence that perinatal nicotine exposure alters auditory processing in an appetitive context in mice. Animals were trained in an auditory discrimination procedure in which they were rewarded for responding after delivery of one of two possible tones. Mice exposed to nicotine in early development showed impaired discrimination in this task. Interestingly, mice lacking the β2 nAChR subunit exhibited similar impairments in auditory discrimination. These results suggest either that β2-containing nAChRs are critical for normal auditory discrimination learning or that exposure to nicotine during a critical period of development can disrupt the normal expression or function of β2-containing nAChRs at a time when this receptor is necessary for the maturation of neural circuits involved in auditory processing. By contrast, neither developmentally-treated or β2 knockout mice displayed treatment- or genotype-dependent differences in performance in a DRL schedule, suggesting that the impaired performance we observed in auditory discrimination learning was not due to learning impairments or to increases in overall response rates.
4.1. Perinatal nicotine exposure leads to persistent alterations in auditory processing
The perinatal exposure-induced auditory discrimination impairments reported in this study were observed months after the termination of nicotine administration. Therefore, the differences in behavior observed in these experiments are likely to be a consequence of alterations during neurodevelopment. One of the persistent consequences of gestational tobacco exposure in human children is an elevated risk for developing ADHD [7, 52–54]. Several rodent studies have used open field locomotor activity as a proxy for the hyperactivity observed in this disorder [55], and have reported increased locomotion in adult mice following perinatal nicotine treatment [41, 56, 57], although another group found no differences in locomotor activity [58]. In the auditory discrimination paradigm used here, poor discrimination as measured by a lack of difference in responding to the Rewarded and Unrewarded Tones could therefore be due to an overall increase in response rate. However, we found little evidence of increased magazine entries in the absence of tone delivery in the auditory discrimination task and no evidence of elevated nosepoke responding in the DRL procedure in our developmentally exposed mice, suggesting that the behavioral impairment observed was primarily in the auditory discrimination.
It is possible that developmentally exposed animals are less able to learn the contingencies of the auditory discrimination task. Contrary to this hypothesis, enhanced learning has been reported in a fear conditioning task [57] and in performance of passive avoidance [27], while no differences in learning were observed in a 5-choice serial reaction time paradigm [32]. To identify any potential differences in learning versus sensory discrimination in our mice, a subset of mice were trained in a DRL paradigm. Perinatal nicotine exposure did not affect learning in the DRL task. Impairments in acquisition of auditory discrimination may therefore depend upon specific task requirements, such as the need for subjects to process auditory stimuli, rather than on learning per se. For instance, if early tone processing is compromised, then brain regions that are involved in learning about tone contingencies will receive degraded representations, which could impact the speed of learning in exposed subjects.
It is also possible that the differential impact of perinatal nicotine exposure or β2 nAChR knockout on auditory discrimination vs. DRL performance was due to non-auditory dependent differences in the brain circuitry underlying the acquisition of these tasks. Successful performance of both tasks depends upon behavioral inhibition [59]; the auditory discrimination task requires mice to withhold responding to the Unrewarded Tone, while the DRL task requires mice to withhold responding over the 12 sec interval. Consistent with the partial overlap in the behavioral requirements of the two tasks, there are both similarities and differences in the neural substrates involved in their performance. Previous studies have implicated the anterior cingulate region of prefrontal cortex [60] and the dorsomedial striatum [44, 45] in sensory discrimination when multiple stimuli differentially predict reward and when responding to an unrewarded stimulus must be inhibited [59]. Prefrontal cortex, including anterior cingulate cortex, is also implicated in successful DRL performance [59, 61], but function of the hippocampus [59, 61] and ventral (rather than dorsal) striatum [59, 62] are essential for performance of this task. Our results suggest that learning is impaired specifically when task performance depends on sensory discrimination for successful behavioral inhibition, and since the brain regions involved differ from those underlying `waiting' inhibition, we believe that it is likely that functional changes occur in the networks that do not rely on the anterior cingulate, hippocampus or ventral striatum.
It seems likely, therefore, that the diminished auditory discrimination performance observed here may be a consequence of impaired processing of auditory stimuli. Jacobsen and colleagues [18] observed elevated hemodynamic activity in brain regions that process auditory stimuli in human adolescents after gestational tobacco exposure. It is uncertain at what stage in the auditory system aberrant stimulus processing begins to occur, with some studies reporting alterations in auditory brainstem responses in neonates and 6 month old offspring exposed to tobacco in utero [63, 64] and others finding no effect at this level [65]. It is important to note that aberrant sensory processing does not imply that animals are unable to detect sensory stimuli. For instance, mice lacking the β2 subunit were unimpaired in an auditory-cued fear conditioning paradigm [66] and showed hypersensitivity to a somatosensory stimulus in passive avoidance [27, 28]. Mice in our auditory discrimination task appear to have intact hearing, as they approach the reward magazine nearly 100% of the time when a Rewarded Tone is played (Figures 2B, 3B: circles), but much less than this when no tone is played (Figures 2B, 3B: squares). At least one human study of auditory brainstem responses provided evidence for the absence of altered signal detection based on a lack of difference in auditory brainstem responses [65], suggesting the involvement of central mechanisms in observed auditory impairments.
Evidence from rodent studies supports the hypothesis that perinatal nicotine exposure via nAChRs alters the maturation of the thalamocortical neurons that relay sensory information from the thalamus to primary sensory cortex [11, 29]. Corticothalamic neurons that provide cortical feedback to modulate thalamic sensory input have also been implicated in the altered sensory processing observed following developmental exposure to nicotine [27]. This is supported by evidence of nicotine-exposure induced alterations in the electrophysiological characteristics of corticothalamic projection neurons [67]. Since both the ascending and descending connections between sensory thalamus and primary sensory cortex are vulnerable to nicotine exposure, it is likely that the initial cortical relay of sensory stimuli in nicotine-exposed individuals is compromised which in turn impairs overall sensory processing.
Given the wide expression of nAChRs in the developing central nervous system, the initial cortical relay of sensory stimuli is unlikely to be the only component of the sensory processing circuitry affected by developmental nicotine exposure. For example, it has been reported recently that a cortico-thalamo-cortical circuit exists between primary and secondary somatosensory cortices that allows information transfer in the absence of direct cortico-cortical connections [68]. This circuit includes a corticothalamic relay from layer VB of primary somatosensory cortex to the posterior medial thalamic nucleus and a thalamocortical relay from this nucleus to layer IV of secondary somatosensory cortex [68]. It has been suggested that similar circuitry is present in the auditory and visual systems and that therefore information transfer through the cortex may occur via both cortico-cortical and cortico-thalamo-cortical routes [68–70]. Due to the dependence of this circuitry on thalamocortical and corticothalamic projections, it is likely that it is also altered in individuals exposed to nicotine during development. Therefore, the sensory representations initially relayed to primary sensory cortex may be disrupted further by subsequent relay to higher cortical areas.
Clearly, altered sensory processing can lead to differential stimulus representations in the higher cortical areas responsible for executive function and cognitive control and therefore result in altered behavioral responses; however, direct adverse effects of developmental nicotine exposure on these higher cortical areas cannot be excluded. For instance, the corticothalamic projections between rodent prefrontal cortex and the medial dorsal thalamic nucleus can be altered by nicotine administration in a slice preparation [67], suggesting that activity in this higher cortical area can be affected directly by nicotine exposure. While some evidence indicates that nicotine exposure during early development can affect performance in tasks that depend upon higher order cortex [32], others have failed to find differences in performance of decision-making tasks [38, 71]. It should be noted that the nicotine exposure window in these studies did not extend beyond the first postnatal week, which could imply that the behavioral differences observed in this study are due to nicotine exposure-induced effects on developmental processes that occur exclusively in postnatal weeks 2 and 3. This later period coincides with the peak nAChR-mediated currents exhibited by corticothalamic neurons projecting from medial prefrontal cortex [67].
It is significant that the β2 nAChR subunit knockout mice exhibited a behavioral profile similar to the nicotine-exposed mice in the auditory discrimination task, since it suggests that expression of β2* nAChRs is required for normal sensory processing in adulthood. While the current study did not determine directly if expression of the β2 subunit is required during development or adulthood for normal performance in the auditory discrimination task, the hypersensitive passive avoidance performance behavior exhibited by β2 knockout mice [33] can be normalized by transgenic expression of this subunit exclusively in corticothalamic neurons during development [27, 28]. Subsequent suppression of β2 subunit expression in these neurons in adult animals yields normal passive avoidance performance [28]. These data suggest that β2* nAChRs have an essential role in the maturation of the sensory processing circuitry and that it is dispensable once this process is completed [27, 28]. Therefore, we would hypothesize that the altered auditory discrimination performance of the β2 nAChR subunit knockout mice in this study is a consequence of altered maturation of the sensory processing circuitry induced by absence of this subunit. The lack of β2 nAChR expression in these animals may therefore be functionally equivalent to desensitization of β2* nAChRs by chronic nicotine exposure during early development. Further studies using transgenic mice with regulated expression of nAChRs will be required to confirm this hypothesis.
In summary, this study identifies a novel and persistent behavioral consequence of developmental nicotine exposure in mice. The impaired performance in an operant auditory discrimination task observed in this study extends the consequences of developmental nicotine exposure beyond paradigms involving aversive and/or predominantly visuo-spatial stimuli. The absence of an effect of developmental nicotine exposure on performance in the DRL task in this study also adds further support to the hypothesis that the primary mechanism through which developmental nicotine exposure persistently affects behavioral performance is through altered sensory processing, as opposed to compromised learning and memory processes. The consistency of the behavioral profile of β2 nAChR subunit knockout mice in the auditory discrimination task also implies a critical role for this subunit in the neural circuitry required for task performance. Taken together, these data emphasize the critical role of nAChRs in the development of neural circuits involved in sensory processing and that nicotine-mediated disruption of the refinement of this circuitry can have long-lasting consequences for exposed individuals.
Highlights
Perinatal nicotine exposure impairs auditory discrimination learning in mice.
β2 nAChR subunit knockout similarly impairs auditory discrimination learning.
Neither perinatal nicotine or β2 nAChR subunit knockout impair DRL performance.
Acknowledgements
This work was supported by grants DA10455, DA14241, and DA00436 from the National Institutes of Health, and funds from the State of Connecticut Department of Mental Health and Addiction Services, and the Yale University Kavli Center to MRP. NKH was supported by a post-doctoral fellowship from the Yale University Interdisciplinary Research Consortium on Stress, Self-Control and Addiction (Research Education (RL5, 12 of 14) NIH IRL5 DA024858).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Substance Abuse and Mental Health Services Administration . Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2011. HHS Publication No. (SMA) 11-4658. [Google Scholar]
- [2].Salihu HM, Wilson RE. Epidemiology of prenatal smoking and perinatal outcomes. Early Hum Dev. 2007;83:713–20. doi: 10.1016/j.earlhumdev.2007.08.002. [DOI] [PubMed] [Google Scholar]
- [3].Heath CJ, Picciotto MR. Nicotine-induced plasticity during development: modulation of the cholinergic system and long-term consequences for circuits involved in attention and sensory processing. Neuropharmacology. 2009;56(Suppl 1):254–62. doi: 10.1016/j.neuropharm.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Biederman J, Monuteaux MC, Faraone SV, Mick E. Parsing the associations between prenatal exposure to nicotine and offspring psychopathology in a nonreferred sample. J Adolesc Health. 2009;45:142–8. doi: 10.1016/j.jadohealth.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rogers JM. Tobacco and pregnancy. Reprod Toxicol. 2009;28:152–60. doi: 10.1016/j.reprotox.2009.03.012. [DOI] [PubMed] [Google Scholar]
- [6].Zammit S, Thomas K, Thompson A, Horwood J, Menezes P, Gunnell D, et al. Maternal tobacco, cannabis and alcohol use during pregnancy and risk of adolescent psychotic symptoms in offspring. Br J Psychiatry. 2009;195:294–300. doi: 10.1192/bjp.bp.108.062471. [DOI] [PubMed] [Google Scholar]
- [7].Nomura Y, Marks DJ, Halperin JM. Prenatal exposure to maternal and paternal smoking on attention deficit hyperactivity disorders symptoms and diagnosis in offspring. J Nerv Ment Dis. 2010;198:672–8. doi: 10.1097/NMD.0b013e3181ef3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Key AP, Ferguson M, Molfese DL, Peach K, Lehman C, Molfese VJ. Smoking during pregnancy affects speech-processing ability in newborn infants. Environ Health Perspect. 2007;115:623–9. doi: 10.1289/ehp.9521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cornelius MD, Day NL. Developmental consequences of prenatal tobacco exposure. Curr Opin Neurol. 2009;22:121–5. doi: 10.1097/WCO.0b013e328326f6dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hunter SK, Kisley MA, McCarthy L, Freedman R, Ross RG. Diminished cerebral inhibition in neonates associated with risk factors for schizophrenia: parental psychosis, maternal depression, and nicotine use. Schizophr Bull. 2011;37:1200–8. doi: 10.1093/schbul/sbq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Metherate R. Nicotinic acetylcholine receptors in sensory cortex. Learn Mem. 2004;11:50–9. doi: 10.1101/lm.69904. [DOI] [PubMed] [Google Scholar]
- [12].Fried PA, Makin JE. Neonatal behavioural correlates of prenatal exposure to marihuana, cigarettes and alcohol in a low risk population. Neurotoxicol Teratol. 1987;9:1–7. doi: 10.1016/0892-0362(87)90062-6. [DOI] [PubMed] [Google Scholar]
- [13].Fried PA, Watkinson B, Gray R. A follow-up study of attentional behavior in 6-year-old children exposed prenatally to marihuana, cigarettes, and alcohol. Neurotoxicol Teratol. 1992;14:299–311. doi: 10.1016/0892-0362(92)90036-a. [DOI] [PubMed] [Google Scholar]
- [14].McCartney JS, Fried PA, Watkinson B. Central auditory processing in school-age children prenatally exposed to cigarette smoke. Neurotoxicol Teratol. 1994;16:269–76. doi: 10.1016/0892-0362(94)90048-5. [DOI] [PubMed] [Google Scholar]
- [15].Fried PA, Watkinson B, Siegel LS. Reading and language in 9- to 12-year olds prenatally exposed to cigarettes and marijuana. Neurotoxicol Teratol. 1997;19:171–83. doi: 10.1016/s0892-0362(97)00015-9. [DOI] [PubMed] [Google Scholar]
- [16].Fried PA, Watkinson B, Gray R. Differential effects on cognitive functioning in 9- to 12-year olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol. 1998;20:293–306. doi: 10.1016/s0892-0362(97)00091-3. [DOI] [PubMed] [Google Scholar]
- [17].Fried PA, Watkinson B, Gray R. Differential effects on cognitive functioning in 13- to 16-year-olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol. 2003;25:427–36. doi: 10.1016/s0892-0362(03)00029-1. [DOI] [PubMed] [Google Scholar]
- [18].Jacobsen LK, Slotkin TA, Mencl WE, Frost SJ, Pugh KR. Gender-specific effects of prenatal and adolescent exposure to tobacco smoke on auditory and visual attention. Neuropsychopharmacology. 2007;32:2453–64. doi: 10.1038/sj.npp.1301398. [DOI] [PubMed] [Google Scholar]
- [19].Zoli M, Le Novere N, Hill JA, Jr., Changeux JP. Developmental regulation of nicotinic ACh receptor subunit mRNAs in the rat central and peripheral nervous systems. J Neurosci. 1995;15:1912–39. doi: 10.1523/JNEUROSCI.15-03-01912.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hellstrom-Lindahl E, Gorbounova O, Seiger A, Mousavi M, Nordberg A. Regional distribution of nicotinic receptors during prenatal development of human brain and spinal cord. Brain Res Dev Brain Res. 1998;108:147–60. doi: 10.1016/s0165-3806(98)00046-7. [DOI] [PubMed] [Google Scholar]
- [21].Dwyer JB, Broide RS, Leslie FM. Nicotine and brain development. Birth Defects Res C Embryo Today. 2008;84:30–44. doi: 10.1002/bdrc.20118. [DOI] [PubMed] [Google Scholar]
- [22].Dwyer JB, McQuown SC, Leslie FM. The dynamic effects of nicotine on the developing brain. Pharmacol Ther. 2009;122:125–39. doi: 10.1016/j.pharmthera.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Winzer-Serhan UH. Long-term consequences of maternal smoking and developmental chronic nicotine exposure. Front Biosci. 2008;13:636–49. doi: 10.2741/2708. [DOI] [PubMed] [Google Scholar]
- [24].Aramakis VB, Metherate R. Nicotine selectively enhances NMDA receptor-mediated synaptic transmission during postnatal development in sensory neocortex. J Neurosci. 1998;18:8485–95. doi: 10.1523/JNEUROSCI.18-20-08485.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Aramakis VB, Hsieh CY, Leslie FM, Metherate R. A critical period for nicotine-induced disruption of synaptic development in rat auditory cortex. J Neurosci. 2000;20:6106–16. doi: 10.1523/JNEUROSCI.20-16-06106.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hsieh CY, Leslie FM, Metherate R. Nicotine exposure during a postnatal critical period alters NR2A and NR2B mRNA expression in rat auditory forebrain. Brain Res Dev Brain Res. 2002;133:19–25. doi: 10.1016/s0165-3806(01)00314-5. [DOI] [PubMed] [Google Scholar]
- [27].Heath CJ, King SL, Gotti C, Marks MJ, Picciotto MR. Cortico-thalamic connectivity is vulnerable to nicotine exposure during early postnatal development through alpha4/beta2/alpha5 nicotinic acetylcholine receptors. Neuropsychopharmacology. 2010;35:2324–38. doi: 10.1038/npp.2010.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].King SL, Marks MJ, Grady SR, Caldarone BJ, Koren AO, Mukhin AG, et al. Conditional expression in corticothalamic efferents reveals a developmental role for nicotinic acetylcholine receptors in modulation of passive avoidance behavior. J Neurosci. 2003;23:3837–43. doi: 10.1523/JNEUROSCI.23-09-03837.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Liang K, Poytress BS, Chen Y, Leslie FM, Weinberger NM, Metherate R. Neonatal nicotine exposure impairs nicotinic enhancement of central auditory processing and auditory learning in adult rats. Eur J Neurosci. 2006;24:857–66. doi: 10.1111/j.1460-9568.2006.04945.x. [DOI] [PubMed] [Google Scholar]
- [30].Genedani S, Bernardi M, Bertolini A. Sex-linked differences in avoidance learning in the offspring of rats treated with nicotine during pregnancy. Psychopharmacology (Berl) 1983;80:93–5. doi: 10.1007/BF00427504. [DOI] [PubMed] [Google Scholar]
- [31].Vaglenova J, Parameshwaran K, Suppiramaniam V, Breese CR, Pandiella N, Birru S. Long-lasting teratogenic effects of nicotine on cognition: gender specificity and role of AMPA receptor function. Neurobiol Learn Mem. 2008;90:527–36. doi: 10.1016/j.nlm.2008.06.009. [DOI] [PubMed] [Google Scholar]
- [32].Schneider T, Ilott N, Brolese G, Bizarro L, Asherson PJ, Stolerman IP. Prenatal exposure to nicotine impairs performance of the 5-choice serial reaction time task in adult rats. Neuropsychopharmacology. 2011;36:1114–25. doi: 10.1038/npp.2010.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Picciotto MR, Zoli M, Lena C, Bessis A, Lallemand Y, Le Novere N, et al. Abnormal avoidance learning in mice lacking functional high-affinity nicotine receptor in the brain. Nature. 1995;374:65–7. doi: 10.1038/374065a0. [DOI] [PubMed] [Google Scholar]
- [34].McClure FD, Gordon M. Performance of disturbed hyperactive and nonhyperactive children on an objective measure of hyperactivity. J Abnorm Child Psychol. 1984;12:561–71. doi: 10.1007/BF00916850. [DOI] [PubMed] [Google Scholar]
- [35].Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, et al. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacology. 2010;35:388–400. doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lovic V, Keen D, Fletcher PJ, Fleming AS. Early-life maternal separation and social isolation produce an increase in impulsive action but not impulsive choice. Behav Neurosci. 2011;125:481–91. doi: 10.1037/a0024367. [DOI] [PubMed] [Google Scholar]
- [37].Brunzell D, Russell D, Picciotto M. In vivo nicotine treatment regulates mesocorticolimbic CREB and ERK signaling in C57Bl/6J mice. J Neurochem. 2003;84:1431–41. doi: 10.1046/j.1471-4159.2003.01640.x. [DOI] [PubMed] [Google Scholar]
- [38].Brunzell D, Chang J, Schneider B, Olausson P, Taylor J, Picciotto M. beta2-Subunit-containing nicotinic acetylcholine receptors are involved in nicotine-induced increases in conditioned reinforcement but not progressive ratio responding for food in C57BL/6 mice. Psychopharmacology (Berl) 2006;184:328–38. doi: 10.1007/s00213-005-0099-z. [DOI] [PubMed] [Google Scholar]
- [39].Caldarone B, King S, Picciotto M. Sex differences in anxiety-like behavior and locomotor activity following chronic nicotine exposure in mice. Neurosci Lett. 2008;439:187–91. doi: 10.1016/j.neulet.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].King S, Caldarone B, Picciotto M. Beta2-subunit-containing nicotinic acetylcholine receptors are critical for dopamine-dependent locomotor activation following repeated nicotine administration. Neuropharmacology. 2004;47(Suppl 1):132–9. doi: 10.1016/j.neuropharm.2004.06.024. [DOI] [PubMed] [Google Scholar]
- [41].Pauly JR, Sparks JA, Hauser KF, Pauly TH. In utero nicotine exposure causes persistent, gender-dependant changes in locomotor activity and sensitivity to nicotine in C57Bl/6 mice. Int J Dev Neurosci. 2004;22:329–37. doi: 10.1016/j.ijdevneu.2004.05.009. [DOI] [PubMed] [Google Scholar]
- [42].Heath CJ, Horst NK, Picciotto MR. Oral nicotine consumption does not affect maternal care or early development in mice but results in modest hyperactivity in adolescence. Physiol Behav. 2010;101:764–9. doi: 10.1016/j.physbeh.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ehret G. Development of absolute auditory thresholds in the house mouse (Mus musculus) J Am Audiol Soc. 1976;1:179–84. [PubMed] [Google Scholar]
- [44].Kimchi EY, Laubach M. The dorsomedial striatum reflects response bias during learning. J Neurosci. 2009;29:14891–902. doi: 10.1523/JNEUROSCI.4060-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kimchi EY, Laubach M. Dynamic encoding of action selection by the medial striatum. J Neurosci. 2009;29:3148–59. doi: 10.1523/JNEUROSCI.5206-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Li HS, Borg E. Age-related loss of auditory sensitivity in two mouse genotypes. Acta Otolaryngol. 1991;111:827–34. doi: 10.3109/00016489109138418. [DOI] [PubMed] [Google Scholar]
- [47].Willott JF. Changes in frequency representation in the auditory system of mice with age-related hearing impairment. Brain Res. 1984;309:159–62. doi: 10.1016/0006-8993(84)91022-9. [DOI] [PubMed] [Google Scholar]
- [48].Willott JF. Effects of aging, hearing loss, and anatomical location on thresholds of inferior colliculus neurons in C57BL/6 and CBA mice. J Neurophysiol. 1986;56:391–408. doi: 10.1152/jn.1986.56.2.391. [DOI] [PubMed] [Google Scholar]
- [49].Willott JF, Aitkin LM, McFadden SL. Plasticity of auditory cortex associated with sensorineural hearing loss in adult C57BL/6J mice. J Comp Neurol. 1993;329:402–11. doi: 10.1002/cne.903290310. [DOI] [PubMed] [Google Scholar]
- [50].Willott JF, Carlson S. Modification of the acoustic startle response in hearing-impaired C57BL/6J mice: prepulse augmentation and prolongation of prepulse inhibition. Behav Neurosci. 1995;109:396–403. doi: 10.1037//0735-7044.109.3.396. [DOI] [PubMed] [Google Scholar]
- [51].Howell DC. Statistical methods for psychology. 5th ed Pacific Grove; Duxbury: 2002. [Google Scholar]
- [52].Button TM, Maughan B, McGuffin P. The relationship of maternal smoking to psychological problems in the offspring. Early Hum Dev. 2007;83:727–32. doi: 10.1016/j.earlhumdev.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ernst M, Moolchan ET, Robinson ML. Behavioral and neural consequences of prenatal exposure to nicotine. J Am Acad Child Adolesc Psychiatry. 2001;40:630–41. doi: 10.1097/00004583-200106000-00007. [DOI] [PubMed] [Google Scholar]
- [54].Linnet KM, Dalsgaard S, Obel C, Wisborg K, Henriksen TB, Rodriguez A, et al. Maternal lifestyle factors in pregnancy risk of attention deficit hyperactivity disorder and associated behaviors: review of the current evidence. Am J Psychiatry. 2003;160:1028–40. doi: 10.1176/appi.ajp.160.6.1028. [DOI] [PubMed] [Google Scholar]
- [55].Wickstrom R. Effects of nicotine during pregnancy: human and experimental evidence. Curr Neuropharmacol. 2007;5:213–22. doi: 10.2174/157015907781695955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ajarem JS, Ahmad M. Prenatal nicotine exposure modifies behavior of mice through early development. Pharmacol Biochem Behav. 1998;59:313–8. doi: 10.1016/s0091-3057(97)00408-5. [DOI] [PubMed] [Google Scholar]
- [57].Paz R, Barsness B, Martenson T, Tanner D, Allan AM. Behavioral teratogenicity induced by nonforced maternal nicotine consumption. Neuropsychopharmacology. 2007;32:693–9. doi: 10.1038/sj.npp.1301066. [DOI] [PubMed] [Google Scholar]
- [58].Chistyakov V, Patkina N, Tammimaki A, Talka R, Salminen O, Belozertseva I, et al. Nicotine exposure throughout early development promotes nicotine self-administration in adolescent mice and induces long-lasting behavioural changes. Eur J Pharmacol. 2010;640:87–93. doi: 10.1016/j.ejphar.2010.04.044. [DOI] [PubMed] [Google Scholar]
- [59].Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–94. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- [60].Cardinal RN, Parkinson JA, Marbini HD, Toner AJ, Bussey TJ, Robbins TW, et al. Role of the anterior cingulate cortex in the control over behavior by Pavlovian conditioned stimuli in rats. Behav Neurosci. 2003;117:566–87. doi: 10.1037/0735-7044.117.3.566. [DOI] [PubMed] [Google Scholar]
- [61].Cho YH, Jeantet Y. Differential involvement of prefrontal cortex, striatum, and hippocampus in DRL performance in mice. Neurobiol Learn Mem. 2010;93:85–91. doi: 10.1016/j.nlm.2009.08.007. [DOI] [PubMed] [Google Scholar]
- [62].Pothuizen HH, Jongen-Relo AL, Feldon J, Yee BK. Double dissociation of the effects of selective nucleus accumbens core and shell lesions on impulsive-choice behaviour and salience learning in rats. Eur J Neurosci. 2005;22:2605–16. doi: 10.1111/j.1460-9568.2005.04388.x. [DOI] [PubMed] [Google Scholar]
- [63].Kable JA, Coles CD, Lynch ME, Carroll J. The impact of maternal smoking on fast auditory brainstem responses. Neurotoxicol Teratol. 2009;31:216–24. doi: 10.1016/j.ntt.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Peck JD, Neas B, Robledo C, Saffer E, Beebe L, Wild RA. Intrauterine tobacco exposure may alter auditory brainstem responses in newborns. Acta Obstet Gynecol Scand. 2010;89:592–6. doi: 10.3109/00016340903511068. [DOI] [PubMed] [Google Scholar]
- [65].Trammer RM, Aust G, Koster K, Obladen M. Narcotic and nicotine effects on the neonatal auditory system. Acta Paediatr. 1992;81:962–5. doi: 10.1111/j.1651-2227.1992.tb12154.x. [DOI] [PubMed] [Google Scholar]
- [66].Wehner JM, Keller JJ, Keller AB, Picciotto MR, Paylor R, Booker TK, et al. Role of neuronal nicotinic receptors in the effects of nicotine and ethanol on contextual fear conditioning. Neuroscience. 2004;129:11–24. doi: 10.1016/j.neuroscience.2004.07.016. [DOI] [PubMed] [Google Scholar]
- [67].Kassam SM, Herman PM, Goodfellow NM, Alves NC, Lambe EK. Developmental excitation of corticothalamic neurons by nicotinic acetylcholine receptors. J Neurosci. 2008;28:8756–64. doi: 10.1523/JNEUROSCI.2645-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Theyel BB, Llano DA, Sherman SM. The corticothalamocortical circuit drives higher-order cortex in the mouse. Nat Neurosci. 2010;13:84–8. doi: 10.1038/nn.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Reichova I, Sherman SM. Somatosensory corticothalamic projections: distinguishing drivers from modulators. J Neurophysiol. 2004;92:2185–97. doi: 10.1152/jn.00322.2004. [DOI] [PubMed] [Google Scholar]
- [70].Sherman SM. The thalamus is more than just a relay. Curr Opin Neurobiol. 2007;17:417–22. doi: 10.1016/j.conb.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Mitchell MR, Mendez IA, Vokes CM, Damborsky JC, Winzer-Serhan UH, Setlow B. Effects of developmental nicotine exposure in rats on decision-making in adulthood. Behav Pharmacol. 2012;23:34–42. doi: 10.1097/FBP.0b013e32834eb04a. [DOI] [PMC free article] [PubMed] [Google Scholar]