Abstract
Relations among antecedant biomarkers of AD were evaluated using causal modeling; although correlation cannot be equated to causation, causation does require correlation. Individuals aged 43 to 89 years (N = 220) enrolled as cognitively normal controls in longitudinal studies had clinical and psychometric assessment, structural magnetic resonance imaging (MRI), cerebrospinal fluid (CSF) biomarkers, and brain amyloid imaging via positron emission tomography with Pittsburgh Compound B (PIB) obtained within 1 year. CSF levels of Aβ42 and tau were minimally correlated, indicating they represent independent processes. Aβ42, tau, and their interaction explained 60% of the variance in PIB. Effects of APOE genotype and age on PIB were indirect, operating through CSF markers. Only spurious relations via their common relation with age were found between the biomarkers and regional brain volumes or cognition. Hence, at least two independent hypothesized processes, one reflected by CSF Aβ42 and one by CSF tau, contribute to the development of fibrillar amyloid plaques preclinically. The lack of correlation between these two processes and brain volume in the regions most often affected in AD suggests the operation of a third process related to brain atrophy.
Keywords: preclinical Alzheimer disease, amyloid-β, tau, PIB, amyloid plaque, APOE, brain volumetry, memory, biomarkers, cerebrospinal fluid
1. Introduction
It is increasingly accepted that the pathologic changes that lead to the eventual diagnosis of symptomatic Alzheimer disease (AD) begin long before there is sufficient cognitive impairment to warrant a clinical diagnosis of the disease (Jack et al. 2009; Price et al., 2009). Recent advances (Klunk et al., 2004) make it possible to image fibrillar amlyoid plaques, a pathologic hallmark of AD, providing one avenue to detection of pathology prior to clinical diagnosis.
There is a strong inverse relation between fibrillar amyloid plaque burden as assessed by positron emission tomography (PET) imaging using the amyloid tracer, Pittsburgh Compound-B (PIB), with levels of cerebrospinal fluid (CSF) Aβ42 in cognitively healthy individuals (Fagan et al., 2006,Fagan et al., 2009; Tolbloom, 2009). This has been interpreted as suggesting that an early step in the process leading to AD is sequestering of Aβ42 in plaques (Hong et al., 2011), thereby reducing the level in the CSF. The amount of plaque burden also is associated with increased levels of CSF total tau and phospho-tau181 (ptau; Fagan et al., 2009b). This relation has often been interpreted in terms of the amyloid cascade hypothesis (Selkoe, 1991). In its simplest form the hypothesis states that Aβ42 peptides aggregate to form amyloid plaques which, in turn, lead to synaptic loss and cell death, reflected in elevated CSF tau, thereby causing dementia. Recent reviews, however, suggest that the process may not be that simple (Holtzman et al., 2011; Hyman, 2011; Pimplikar, 2009; Small and Duff, 2008).
Other variables associated with one or more of the CSF biomarkers and PIB include age and apolipoprotein (APOE) genotype, the major genetic susceptibility factor associated with late-onset AD (Morris et al., 2010; Rowe et al., 2010; Sunderland et al., 2004; Vemuri et al., 2010). Mixed results have been reported for forebrain structure (Apostolova et al., 2010; Becker et al., 2010; Chetelat, et al., 2010; Fagan et al., 2009a; Mormino et al., 2009; Oh et al., 2011; Tosun et al., 2010). Concurrent measures of cognition, however, are uncorrelated with the CSF measures (Fagan et al., 2009) or PIB (Mormino et al., 2009; Oh et al., 2011; Storandt et al., 2009) in cognitively normal individuals.
We examined all of these variables in cognitively normal individuals using causal modeling in an effort to explore theoretical models of their interrelations. To the best of our knowledge there has been no prior attempt to do so. Causal modeling is a statistical procedure using regression analysis that is designed to determine if empirical data are consistent with a theoretical model. It requires that three conditions exist if X is a potential cause of Y (Cohen et al. 2003). One, there must be a correlation between X and Y; that is, although correlation cannot be equated to causation, causation does require correlation. Two, X must precede Y in time. Three, the relation between X and Y must not be spurious; a spurious relation is one in which X and Y are related because both are influenced by a third variable, Z. For example, wrinkled skin and slowed reaction times are correlated because both are associated with age, not because either causes the other. Of course, although correlation cannot be equated to causation, causation does require correlation.
Longitudinal study ultimately is required to verify causality, but those results for preclinical AD may not be available for many years. Similarly, longitudinal study is necessary to determine the temporal order of appearance of the various processes, even if they are independent. In the meantime models built on cross-sectional data can provide useful suggestions about avenues of investigation of various underlying pathophysiolocal processes.
2. Methods
2.1. Participants
The sample included 220 participants (64% women) aged 45 to 89 years (M = 65.8, SD = 9.7) enrolled in longitudinal studies at the Knight Alzheimer’s Disease Research Center, Washington University in St. Louis. Their mean years of education was 15.7 years (SD = 2.6). Only participants with PIB imaging and lumbar puncture (LP) to obtain CSF within 1 year of each other (M = 1.7 months, SD = 4.4) between December, 2003 and April, 2010 were included. Participants were cognitively normal (Clinical Dementia Rating [CDR] = 0; Morris, 1993) at the time of assessment; 14 subsequently progressed to a CDR > 0 indicating cognitive impairment. A subset (n = 164) comparable to the total sample in terms of age, gender, education, and APOE allele distribution had structural brain assessment with magnetic resonance imaging (MRI) within 1 year of LP (M = 1.2 months, SD = 3.6) and PIB imaging (M = 0.7 months, SD =2.9). All procedures were approved by the university’s Human Research Protection Office; written informed consent was obtained from participants and their collateral sources. Data from many of these participants have appeared in previous reports from the center.
2.2. Clinical evaluation
Experienced clinicians determined if the person was demented (CDR > 0) or not (CDR = 0) based solely on semistructured interviews with participants and their knowledgeable collateral sources (usually spouse or adult child) followed by a neurological examination of the participant. Clinicians determined if any cognitive problems represented decline from former level of function for that individual and interfered to some degree with the person’s ability to carry out accustomed activities. Assessment included a health history, medication inventory, and assessment of depression and aphasia. Clinicians were unaware of the results of previous clinical evaluations and of previous and current psychometric test results. The CDR staging and diagnostic protocol is sensitive to clinical progression and highly predictive (93%) of autopsy-confirmed AD (Berg et al., 1998).
2.3. CSF collection, processing, and biomarker measurement
CSF (20 – 30 ml) free from blood contamination was collected by LP in polypropylene tubes at 8:00 AM after overnight fasting as described previously (Fagan et al., 2006). Samples were gently inverted to avoid gradient effects, briefly centrifuged at low speed to pellet any cellular elements, and aliquoted (500μl) into polypropylene tubes before freezing at −84°C. Analyses for Aβ42, total tau, and ptau were performed using commercial enzyme-linked immunosorbant assay (INNOTEST; Innogenetics, Ghent, Belgium). Samples were continuously kept on ice with only a single thaw after initial freezing before assays.
2.4. PET PIB imaging
In vivo fibrillar amyloid imaging via PET with PIB ([N-methyl-[11C]]2-(4′-methylaminophenyl)-6-hydroxybenzothiazole) was performed as described previously (Mintun et al., 2006). Approximately 12 mCi of [11C]PIB was administered intravenously simultaneous with initiation of a 60-minute dynamic PET scan in three-dimensional mode. Measured attenuation factors and a ramp filter were used to reconstruct dynamic PET images. Three-dimensional regions of interest (ROIs) were created for each participant based on their individual MRI scans (T1-weighted 1×1×1.25mm MPRAGE). A binding potential for each region-of-interest was calculated (Logan et al., 1996) to express regional binding values in a manner proportional to number of binding sites. Values from prefrontal cortex, gyrus rectus, lateral temporal, and precuneus regions-of-interest were averaged to calculate a mean cortical binding potential value based on brain regions known to have high PIB uptake among participants with AD.
2.5. APOE genotyping
TaqMan assays (Applied Biosystems, Foster City, CA) for both rs429358 (ABI#C_3084793_20) and rs7412 (ABI#C_904973_10) were used for APOE genotyping. Allele calling was performed using the allelic discrimination analysis module of ABI Sequence Detection Software. Positive controls for each of six possible APOE genotypes were included on the genotyping plate.
2.6. MRI acquisition
One to four T1-weighted images were acquired in one scanning session in 168 participants on either a Sonata 1.5T (n = 17), Vision 1.5T (n = 23), or Trio 3.0T scanner (n = 128). Cushions reduced head movement during scanning; a scout image was acquired first in order to center the field of view on the brain.
2.7. Regional volumetry
Regional volumes were obtained using Freesurfer software (Desikan et al., 2006; Fischl et al., 2002). During processing each voxel is assigned a neuroanatomical label based on probabilistic information derived from a manually labeled training set, which included healthy young and older adults. ROIs included lateral parietal (combined inferior parietal and supramarginal regions), temporal neocortical (combined superior, middle, and inferior temporal gyri), anterior cingulate, posterior cingulate, precuneus, hippocampal, entorhinal cortex and parahippocampal cortex. Previous work indicates that this technique generates volumes with a high correspondence to manually generated volumes (Desikan et al., 2006; Fischl et al., 2002). As there were no hypotheses regarding laterality effects, volumes were summed across the left and right hemispheres. Total intracranial volume (ICV) was used to adjust volumes used in the analyses for body size differences via a formula based on the analyses of covariance approach: Adjusted volume = raw volume − (b× [ICV − mean ICV]), where b is the slope of the regression of the ROI volume on ICV. There is evidence of reliability of Freesurfer-derived estimates of cortical thickness and volumes across scanner upgrades, different manufacturers, and number of MP-RAGE acquisitions (Fennema-Notestine et al., 2007; Jovicichet al., 2009); cross-scanner aggregation has been successfully used previously (McEvoy et al., 2009; Storandt et al., 2009).
2.8. Psychometric assessment
Participants received one of two batteries of cognitive measures a few weeks after the clinical assessment. The four measures common to the two batteries were examined here. Selective Reminding Test free recall (Grober et al., 1988) measures episodic memory; Animal Naming for 1 minute (Goodglass and Kaplan, 1983) assesses semantic memory. Trailmaking A and B (Armitage, 1948) are speeded visuospatial tests; the score for each was the number of connections per second.
2.9. Statistical analyses
Analyses were conducted using SPSS 18.0 with alpha set at .05. The distributions of the quantitative variables were assessed for normality using the Kolmogorov-Smirnov one-sample tests. Pearson product-moment correlations among the variables as well as partial correlations controlling for age were computed. Scatter plots were examined and tests for curvilinear relations were conducted using hierarchical regression, after converting quantitative variables to z scores; APOEε4 and ε2 were scored 1 if present, otherwise 0. Additional hierarchical regression analyses were conducted first regressing PIB on Aβ42 and tau including a curvilinear component for Aβ42 as well as interactions between the two CSF measures. The analysis was repeated substituting ptau for tau. Analysis was then conducted entering the organismic variables (APOE and age) prior to the CSF measures. Partial correlations among all the variables including four cognitive measures and brain volume in eight ROIs controlling for age were then examined. Finally, regression analyses examining the linear and quadratic relation between age and each of the eight brain ROIs were conducted.
3. Results
3.1 Distributions of variables
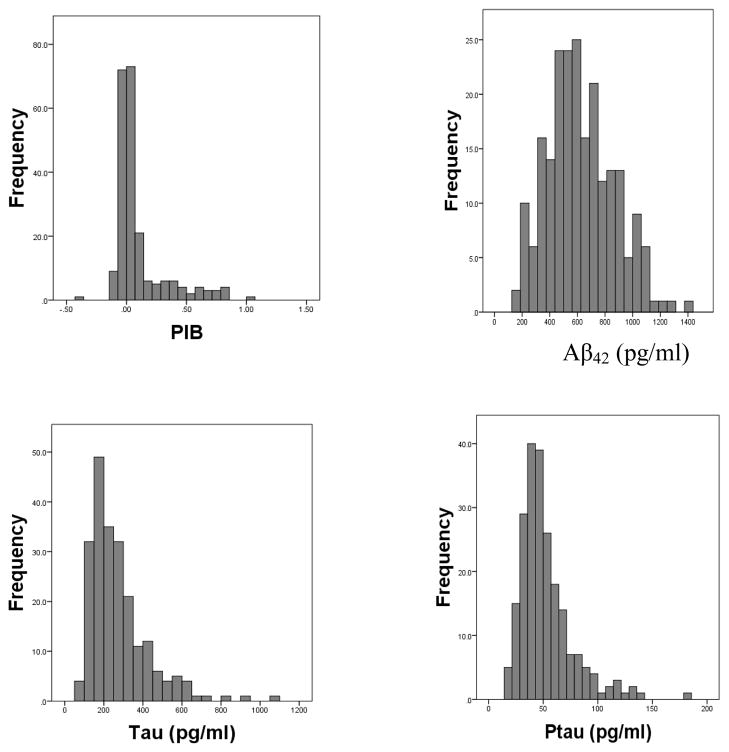
Kolmogrov-Smirnov one-sample tests revealed that the quantitative variables were normally distributed with the exception of PIB, tau, and ptau. Histograms for PIB, Aβ42, tau, and ptau are shown in Figure 1. The APOE genotypes were as follows: 22, 1%; 23, 9%, 24, 1%, 33, 55%, 34, 28%, 44, 6%.
Figure 1.
Histograms showing distributions of PIB and the CSF biomarkers (Aβ42, tau, ptau)
3.2 Correlations among variables
Table 1 shows the zero-order correlations among the measures above the diagonal. All the variables except APOE were significantly correlated with age. Therefore the partial correlations among the variables controlling for age are shown below the diagonal. Only one regional brain volume (hippocampus) and one memory measure (SRT free recall) are included here; the others are reported in Supplemental Tables 1 and 2.
Table 1.
Zero-order (above diagonal) and partial correlations controlling for age (below diagonal)
| PIB | Aβ42 | Tau | Ptau | APOEε4 | APOEε2 | Hippocampus | Memory | |
|---|---|---|---|---|---|---|---|---|
| Age | .26 | −.17 | .37 | .37 | −.13 | .12 | −.54 | −.38 |
| PIB | −.49 | .54 | .44 | .22 | −.17 | −.20 | −.18 | |
| Aβ42 | −.46 | −.06 | .01 | −.22 | .22 | .07 | .11 | |
| Tau | .51 | −.01 | .85 | .03 | −.01 | .13 | −.24 | |
| Ptau | .41 | .06 | .83 | .03 | −.04 | −.14 | −.24 | |
| APOEε4 | .26 | −.24 | .09 | .08 | −.18 | −.01 | .03 | |
| APOEε2 | −.20 | .24 | −.04 | −.07 | −.16 | .02 | −.07 | |
| Hippocampus | −.09 | .01 | .05 | .02 | −.10 | .07 | −.21 | |
| Memory | −.09 | .05 | −.10 | −.14 | −.02 | −.03 | −.02 | |
| Mean | .10 | 626 | 274 | 52.4 | 7654 | 31.3 | ||
| Median | .02 | 595 | 235 | 46.1 | 7675 | 32.0 | ||
| SD | .22 | 241 | 150 | 24.4 | 875 | 6.0 |
Note: Correlations with p < .05 are shown in bold. The memory measure is the Selective Reminding Test. Means, medians, and SDs for quantitative variables are shown in the last three rows.
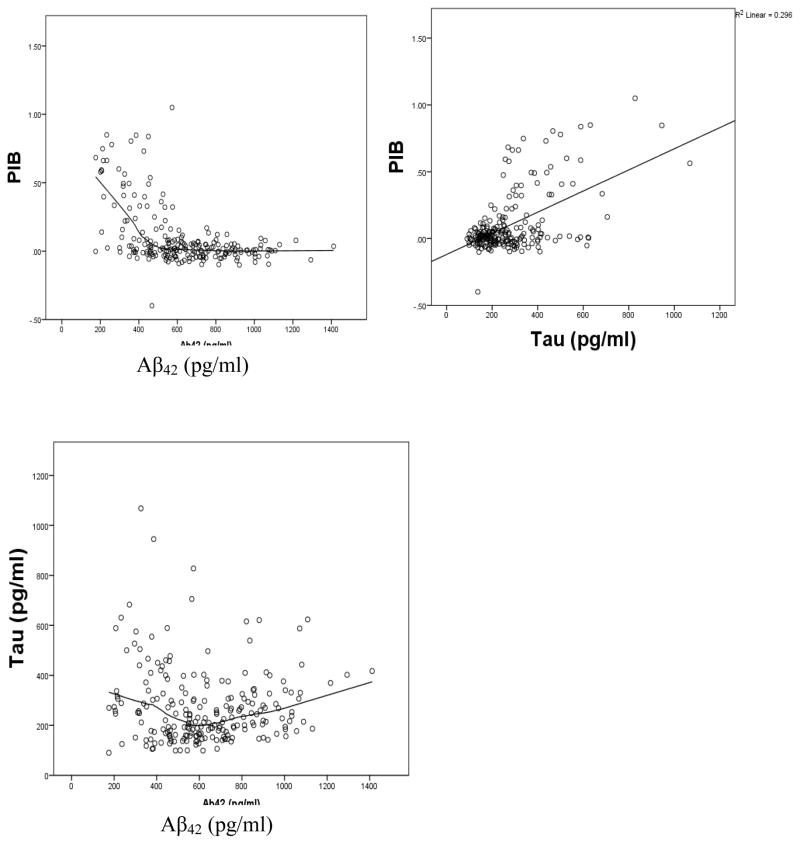
As previously reported (Fagan, 2009; Tolboom, 2009), PIB was strongly correlated with CSF Aβ42, tau, and ptau; the correlations changed very little when controlled for age (Table 1, Figure 2). In addition to a linear relation between PIB and Aβ42, there was also a significant (p < .0001) quadratic component; this was not the case for the relation between PIB and tau (quadratic p = .53).
Figure 2.
Scatter plots of relations among PIB, Aβ42, and tau
As shown in Table 1, CSF Aβ42 and tau were uncorrelated linearly (r = −.06), although there was a small quadratic relation(p = .001, Figure 2) indicating 4% shared variance between the two variables after controlling for age. Using a break point of 500 pg/ml on Aβ42 (Fagan et al., 2009), piecewise regression revealed a significant difference in slopes before and after that point. The partial r controlling for age between Aβ42 and tau for those with lower values of Aβ42 was −.20 (p = .10) compared with +.25 (p = .003) for those with higher values. Analogous results were obtained substituting ptau for tau (r = −.15 for Aβ42 values < 500 pg/ml compared with r = +.27 for Aβ42 values > 500 pg/ml).
Given the pattern of observed relations (minimal relation between Aβ42 and tau but strong relations of each with PIB) the next analysis determined how much of the total variance in fibrillar amyloid plaque formation was explained by the two biomarkers in combination. A hierarchical regression analysis was conducted with PIB as the dependent variable and the independent variables entered in the following order: Aβ42 linear, Aβ42 quadratic, tau, Aβ42 linear × tau interaction, and Aβ42 quadratic × tau interaction. Each of the first four predictors produced a significant increment in the R2, producing a total R2 of .60 (Table 2). Unique contributions of significant predictors (i.e., beta weights from the model at the fourth step) were as follows: Aβ42 linear = − .47, Aβ42 quadratic = .20, tau = .36, Aβ42 linear × tau interaction = .24. When the analysis was repeated using ptau instead of tau the R2 was .55.
Table 2.
Hierarchical regression model of PIB on Aβ42 (linear and quadratic), tau, and their interactions (N = 220)
| Step | Predictor added | R2 increment | F | df error | p |
|---|---|---|---|---|---|
| 1 | Aβ42 (linear) | .24 | 67.48 | 218 | < .0001 |
| 2 | Aβ42 (quadratic) | .11 | 36.17 | 217 | < .0001 |
| 3 | Tau | .20 | 93.29 | 216 | < .0001 |
| 4 | Aβ42 (linear)× Tau | .05 | 27.15 | 215 | < .0001 |
| 5 | Aβ42 (quadratic) × Tau | .00 | 2.29 | 314 | .13 |
Note. R2 = .60
The significant interaction between Aβ42 and tau indicates that the magnitude of the relation between tau and PIB depends on the level of Aβ42, even though tau and Aβ42 are largely independent; this is illustrated in Figure 3. The simple regression lines relating tau and PIB are plotted at low, medium, and high values of Aβ42 as defined by −1 SD (373 pg/ml), mean (610 pg/ml), +1 SD (983 pg/ml). The relation between tau and PIB was stronger as Aβ42 decreased. Because of the curvilinear nature of the relationship between Aβ42 and PIB, the rate of increase in PIB as tau increased was especially dramatic at low values of Aβ42.
Figure 3.
Interaction between CSF Aβ42 and tau as related to amyloid plaque burden (PIB)
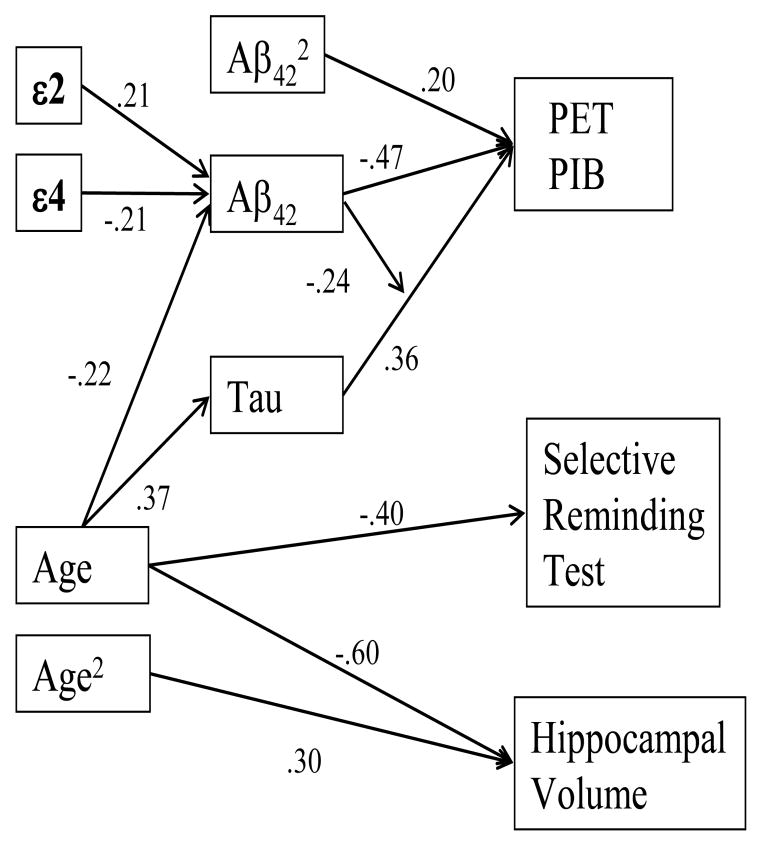
Both APOE variables and age were modestly correlated with PIB and Aβ42; only age was correlated with tau (Table 1). Because both APOE variables exist from conception and therefore precede the biomarkers, the hierarchical regression analysis was repeated entering them at Steps 1 and 2. Similar logic applies to age in a cross-sectional analysis; it precedes measurement of the biomarkers; therefore, it was entered at Step 3. The Age × ε4 interaction (Morris et al., 2010) was added at Step 4. Order of entry thereafter was as in the previous regression analysis: Aβ42 linear, Aβ42 quadratic, tau, Aβ42 linear × Tau interaction. The Aβ42 quadratic × Tau interaction was not included because it was not significant in the previous analysis.
The increment in the R2 was significant at each step. The R2 was .18 after the first four steps, but the beta weights for these four variables were no longer significant after inclusion of the remaining terms representing the two CSF markers: APOE ε4 β = .07, APOE ε2 β = −.05, age β = .02, Age × ε4 β = .10. These results indicate that the effects of APOE and age on plaque formation are indirect, operating through their influence on Aβ42 and tau. They may have a causal effect on the two CSF biomarkers, but they have no additional causal effect on fibillar amyloid plaque burden as detected by PIB. The demographic variables of gender and education were not correlated with any of the biomarkers and, therefore, were not included in the model.
The sample was randomly split in half and the model was tested on each half with similar results in terms of the pattern of increments in the R2 and significant beta weights in the final step. The R2 was .62 for Sample 1 and .59 for Sample 2.
As shown in Table 1, the significant zero-order correlations (above the diagonal) of episodic memory, hippocampus volume, tau, and PIB were spurious; they disappeared when controlled for age (below the diagonal). The partial rs between memory and the other brain regions ranged from −.06 to .06. None of the partial rs controlling for age between the other three cognitive measures and Aβ42, tau, ptau, PIB, or any of the brain regions were significant (all ps> .05).
Because brain volume was available for only a subset of the sample, its role was examined in separate analyses. Hippocampus volume was associated with PIB (r = −.20), but not with either CSF measure (Table 1) This single significant correlation was spurious, however, in that it resulted from the correlation of each variable with age. As shown below the diagonal in Table 1 their partial r controlling for age was −.09 (p = .27). A similar pattern was observed in the other brain regions examined. Although some modest zero-order correlations were significant, partial rs controlling for age were not (range = −.16 to .16). There was a significant curvilinear component to the relation between age and brain region volume (Table 3), which has been reported previously (Raz and Rodrigue, 2006) for the hippocampus and some, but not all, of the other brain regions that were examined.
Table 3.
Results of hierarchical regression analyses of regional brain volumes on age linear and quadratic (n = 164).
| Age (linear) | Age (quadratic) | |||||
|---|---|---|---|---|---|---|
| Region | R2 | F (1, 162) | p | R2 increment | F(1,161) | p |
| Hippocampus | .28 | 62.90 | < .0001 | .09 | 22.04 | <.0001 |
| Temporal | .25 | 52.88 | < .0001 | .07 | 16.00 | < .0001 |
| Lateral parietal | .21 | 44.11 | < .0001 | .06 | 13.10 | < .0001 |
| Precuneus | .21 | 43.42 | < .0001 | .05 | 10.79 | .001 |
| Posterior cingulate | .09 | 16.03 | < .0001 | .03 | 5.34 | .02 |
| Anterior cingulate | .00 | 0.08 | .77 | .03 | 5.07 | .03 |
| Entorhinal | .00 | 0.37 | .54 | .05 | 8.29 | .005 |
| Parahipppocampus | .01 | 1.22 | .27 | .02 | 3.91 | .05 |
Figure 4 provides a summary of the relations among the domains examined in this report using the results for the hippocampus and SRT to represent brain structure and cognition.
Figure 4.
Model showing relations among all of the variables. The beta weights relating APOE and age to Aβ42 are from the simultaneous regression of those three variables on Aβ42. The beta weights relating Aβ42 and tau to PIB are from the final step of the hierarchical regression analysis reported in Table 2. The beta weights relating age to hippocampus volume are from the second step of the analysis reported on the first line of Table 3.
4. Discussion
The distributions of both fibrillar amyloid plaques and tau were skewed, as would be expected if these two biomarkers reflect underlying pathology in some of these cognitively healthy participants. Only a small number of people are in the beginning stage of the disease and therefore have higher values of these two indices. In contrast, the distribution of CSF Aβ42 was not skewed. Instead, it was not significantly different from a normal distribution. Normal distributions are more often seen in individual difference variables such as height. Perhaps there are two influences on CSF Aβ42 level. One may reflect normal human biological variability, whereas the other indicates onset of pathology. The median value of CSF Aβ42 was slightly less than the mean (594 vs. 626; index of skewness = 0.40, see Figure 1). Whether this is due to sampling variability or indicates that CSF Aβ42 levels have dropped from prior levels for a small portion of the participants can only be determined by future studies, particularly longitudinal ones.
CSF Aβ42 and tau were not correlated linearly. There was a very small nonlinear relation between these two biomarkers indicating only 4% shared variation. Although we cannot find any report in the literature referring to this correlation in cognitively normal people, we expected it to be negative (low CSF Aβ42 associated with high CSF tau) if both CSF markers occurred as described by the amyloid cascade hypothesis. As indicated in the introduction, that hypothesis suggests that Aβ42 peptides aggregate to form amyloid plaques which, in turn, lead to synaptic loss and cell death, reflected in elevated CSF tau, thereby causing cognitive impairment.
The model observed in the analyses reported here indicates, as suggested previously (Holtzman et al., 2011; Hyman, 2011; Pimplikar, 2009; Small and Duff, 2008), that there are at least two independent processes, one represented by CSFAβ42 and the other by CSF tau, related to fibrillar amyloid plaque burden. These two biomarkers, and the processes they represent, were associated with an impressive amount of the variance in PIB binding (R2 = .60) in this cognitively normal sample, 17% of whom had MCBP values ≥ .18. This value was used previously to describe individuals as PIB positive (Fagan et al., 2009; Mintun et al, 2006) or having fibrillar amyloid plaque burden similar to those with AD. Similarly, Shaw et al. (2009) reported that these two biomarkers made independent contributions to the differentiation of autopsy-verified AD cases from normal controls. There may be, however, still more processes contributing to plaque formation or, perhaps more important, to brain atrophy and dementia (Rowe et al., 2010). In this cross-sectional sample fibrillar amyloid plaque formation had only a spurious relation with brain atrophy via their mutual association with age.
In addition to their main effects on PIB, there was also an interaction between CSF tau and Aβ42. The correlation between CSF tau and PIB was much stronger when Aβ42 was low than when Aβ42 was moderate or high. This is analogous to the text book example of an interaction provided by Cohen et al. (2002) showing a strong negative correlation between age and endurance on a treadmill that is much weaker in people with a history of aerobic exercise.
Multiple previous studies of these two CSF biomarkers in cognitively normal samples with a wide age range have alluded to the possibility of their interaction when they examined how the ratio of tau to Aβ42 was related to other variables. Long ignored by researchers in many fields, Pearson (1897) pointed out over a century ago that the ratio of two variables can produce spurious results. The ratio actually represents a combination of the main effects of each of the variables in the ratio as well as their interaction. The analysis reported here used the appropriate procedure by first entering the main effects of Aβ42 and tau followed by their product, representing the interaction, in a subsequent step of a hierarchical regression analysis (Kronmal, 1993). Each CSF biomarker was associated with unique, unrelated portions of the variance in PIB, and their interaction was associated with an additional, unrelated portion. This result, along with the minimal association between the two CSF biomarkers, again suggests the operation of at least two independent processes, one associated with lower levels of CSF Aβ42 and one associated with increased levels of CSF tau. One does not cause the other, but when both are present the effect is enhanced. A recent study (Desikan et al., 2011) identified an interaction between Aβ42 and ptau in the prediction of longitudinal changes in the volume of the entorhinal cortext in healthy controls and individuals with amnestic mild cognitive impairment.
The effects of APOE and age on plaque burden were indirect through CSF Aβ42 and tau. It should be noted, however, that APOE and age together accounted for only 13% of the variance in Aβ42 and 14% of tau. There clearly are other important variables to be identified in explaining the processes associated with CSF levels of Aβ42 and tau, and their inclusion may change the model.
Brain volumes in the eight ROIs were uncorrelated with CSF measures or with PIB after controlling for age. As noted in the introduction, previous studies have reported mixed results in efforts to relate PIB to brain structure. There are likely numerous reasons for the differences. One involves the adjustment for age, which has not always been done. Others probably relate to brain regions examined, the measure of structural integrity (e.g., cortical thickness vs. volume), age range of the sample, and inclusion criteria. One study (Chetelat et al., 2010), for example, found no correlation when people with subjective memory complaints were excluded. Similarly, in a study based on neuropathological data (Price et al., 2001) little atrophy was observed in those without a diagnosis of at least very mild symptomatic AD.
The strengths of the current study include the wide age range and large sample size of carefully characterized cognitively normal individuals for whom a number of the biomarkers shown previously to be present prior to clinical diagnosis of AD were obtained in relatively close temporal proximity. A primary limitation is its cross-sectional nature, which allows only modeling of potential causative relations. These relations may be affected if individual biomarkers become abnormal at different times in the AD process. Further, reliability of some of the measures used in the analyses has not been well studied; failure to detect relations may reflect measurement error. A similar limitation is that PIB measures only fibrillar Aβ deposits; the model does not address earlier potential processes such as Aβ oligomerization and aggregation into diffuse plaques. Because the biology of AD undoubtedly is complex and may not be well-represented by the assumptions of our model, any potential causative relations must be confirmed by longitudinal studies. Our findings do, however, highlight the importance of considering multiple contributors to AD pathogenesis as that longitudinal research is pursued.
Supplementary Material
Acknowledgments
Supported by National Institute on Aging grants P50 AG05861, P01 AG03991, and P01 AG026276, and by the Charles F. and Joanne Knight Alzheimer Disease Research Center of Washington University (St. Louis, MO). The authors thank the center’s Clinical Core for the clinical and psychometric assessments, Biomarker Core for the CSF measures, Imaging Core for the PET and structural MRI data, and Genetics Core for the APOE data.
Footnotes
Disclosure Statement: The authors have no actual or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apostolova LG, Hwang KS, Andrawis JP, Green AE, Babakchanian S, Morra JH, Cummings JL, Toga AW, Trojanowski JQ, Shaw LM, Jack CR, Petersen RC, Aisen PS, Jagust WJ, Koeppe RA, Mathis CA, Weiner MW, Thompson PM Alzheimer’s Disease Neuroimaging Initiative. 3D PIB and CSF biomarker associations with hippocampal atrophy in ADNI subjects. Neurobiol Aging. 2010;31:1452–1462. doi: 10.1016/j.neurobiolaging.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage SG. An analysis of certain psychological tests used for the evaluation of brain injury. Psych Monogr. 1945;60:1–48. [Google Scholar]
- Becker JA, Hedden T, Carmasin J, Maye J, Rentz DM, Putcha D, Fischl B, Greve DN, Marshall GA, Salloway S, Marks D, Buckner RL, Sperling RA, Johnson KA. Ann. Neurol. 2011;69:1032–1042. doi: 10.1002/ana.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg L, McKeel DW, Jr, Miller JP, Storandt M, Rubin EH, Morris JC, Baty J, Coats M, Norton J, Goate AM, Price JL, Gearing M, Mirra SS, Saunders AM. Clinicopathologic studies in cognitively healthy aging and Alzheimer disease: Relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch Neurol. 1998;55:326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- Chetelat G, Villemagne VL, Pike K, Baron JC, Bourgeat P, Jones G, Faux NG, Ellis KA, Salvado O, Szoeke C, Martins RN, Ames D, Masters CL, Rowe CC Australian Imaging Biomarkers, Lifestyle, Study of Ageing (AIBL) Research Group. Larger temporal volume in elderly with high versus low beta-amyloid deposition. Brain. 2010;133:3349–3358. doi: 10.1093/brain/awq187. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. 3. Lawrence Erlbaum Associates; Mahway, NJ: 2002. [Google Scholar]
- Desikan RS, McEvoy LK, Thompson WK, Holland D, Roddey JC, Blennow K, Aisen PS, Brewer JB, Hyman BT, Dale AM Alzheimer’s Disease Neuroimaging Initiative. Amyloid-β associated volume loss occurs only in the present of phospho-tau. Ann Neuro. 2011;70:657–661. doi: 10.1002/ana.22509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Mintun M, Mach RH, Lee SY, Dence CS, Shah AR, LaRossa GN, Spinner ML, Klunk WE, Mathis CA, DeKosky ST, Morris JC, Holtzman DM. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Aβ42 in humans. Ann Neuro. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Head D, Shah AR, Marcus D, Mintun M, Morris JC, Holtzman DM. Decreased cerebrospinal fluid Aβ42 correlates with brain atrophy in cognitively normal elderly. Ann Neurol. 2009a;65:176–183. doi: 10.1002/ana.21559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Mintun MA, Shah AR, Aldea P, Roe CM, Mach RH, Marcus D, Morris JC, Holtzman DM. Cerebrospinal fluid tau and ptau181 increase with cortical amyloid deposition in cognitively normal individuals: Implications for future clinical trials of Alzheimer’s disease. EMBO Mol Med. 2009b;1:371–380. doi: 10.1002/emmm.200900048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennema-Notestine C, Garnst C, Quinn BT, Pacheco J, Jernigan TL, Thal L, Buckner R, Killiany R, Blacker D, Dale AM, Fischl B, Dickerson B, Gollub RL. Feasibility of multi-site clinical structural neuroimaging studies of aging using legacy data. Neuroinformatics. 2007;5:235–245. doi: 10.1007/s12021-007-9003-9. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. The Assessment of Aphasia and Related Disorders. 2. Lee & Febiger; Philadelphia, PA: 1983. [Google Scholar]
- Grober E, Buschke H, Crystal H, Bang S, Dresner R. Screening for dementia by memory testing. Neurology. 1988;3:900–903. doi: 10.1212/wnl.38.6.900. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Morris JC, Goate AM. Alzheimer’s disease: The challenge of the second century. Sci Transl Med. 2011;3:77sr1. doi: 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Quintero-Monzon O, Ostaszewski BL, Podlisny DR, Cavanaugh WT, Yang T, Holtzman DM, Cirrito JR, Selkoe DJ. Dynamic analysis of amyloid β-protein in behaving mice reveals opposing changes in ISF versus parenchymal Aβ during age-related plaque formation. J Neurosci. 2011;31:15861–15869. doi: 10.1523/JNEUROSCI.3272-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman BT. Amyloid-dependent and amyloid-independent stages of Alzheimer disease. Arch Neurol. 2011;68:1062–1064. doi: 10.1001/archneurol.2011.70. [DOI] [PubMed] [Google Scholar]
- Jack CR, Lowe VJ, Wegland SD, Wiste HJ, Senjem ML, Knopman DS, Shiung MM, Gunter JL, Boeve BF, Kemp BJ, Weiner M, Petersen RC the Alzheimer’s Disease Neuroimaging Initiative. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: Implications for sequence of pathological events in Alzheimer’s disease. Brain. 2009;132:1355–1365. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Han X, Salat D, van der Kouwe A, Quinn B, Pacheco J, Albert M, Killiany R, Blacker D, Maguire P, Rosas D, Makris N, Gollub R, Dale A, Dickerson BC, Fischl B. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: Reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuroimage. 2009;46:177–192. doi: 10.1016/j.neuroimage.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergström M, Savitcheva I, Huang GF, Estrada S, Ausén B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Långström B. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Kronmal RA. Spurious correlations and the fallacy of the ratio standard revisited. J Royal Statistical Society (Series A) 1993;156:379–392. [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb, Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- McEvoy LK, Fennema-Notestine C, Roddey JC, Hagler DJ, Jr, Holland D, Karow DS, Pung CJ, Brewer JB, Dale AM Alzheimer’s Disease Neuroimaging Initiative. Alzheimer’s disease: Quantitative structural neuroimaging for detection and prediction of clinical and structural changes in mild cognitive impairment. Radiology. 2009;251:195–205. doi: 10.1148/radiol.2511080924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, Klunk WE, Mathis CA, DeKosky ST, Morris JC. [11C]PIB in a nondemented population: Potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, Koeppe RA, Mathis CA, Weiner MW, Jagust WJ Alzheimer’s Disease Neuroimaging Initiative. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain. 2009;132:1310–1323. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR) - current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, Mintun MA. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67:122–131. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Mormino EC, Madison C, Hayenga A, Smiljic A, Jagust WJ. β-amyloid affects frontal and posterior brain networks in normal aging. NeuroImage. 2011;54:1887–1895. doi: 10.1016/j.neuroimage.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson K. Mathematical contributions to the theory of evolution--on a form of spurious correlation which may arise when indices are used in the measurements of organs. Proc R Soc London. 1897;60:489–497. [Google Scholar]
- Pimplikar SW. Reassessing the amyloid cascade hypothesis of Alzheimer’s disease. Int J Biochem Cell Biol. 2009;41:1261–1268. doi: 10.1016/j.biocel.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Ko AI, Wade MJ, Tsou SK, McKeel DW, Jr, Morris JC. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Arch Neurol. 2001;58:1395–1402. doi: 10.1001/archneur.58.9.1395. [DOI] [PubMed] [Google Scholar]
- Price JL, McKeel DW, Jr, Buckles VD, Roe CM, Ziong C, Grundman M, Hansen LA, Petersen RC, Parisi JE, Dickson DW, Smith CD, Davis DG, Schmitt FA, Markesbeery WR, Kaye J, Kurlan R, Hulette C, Kurland BF, Higdon R, Kukull W, Morris JC. Neuropathology of nondemented aging: Presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging. 2009;30:1026–1036. doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neurosci Biohav Rev. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, Fripp J, Tochon-Danguy H, Morandeau L, O’Keefe G, Price R, Raniga P, Robins P, Acosta O, Lenzo N, Szoeke C, Salvado O, Head R, Martins R, Masters CL, Ames D, Villemagne VL. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31:1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter W, Lee VM, Trojanowski JQ Alzheimer’s Disease Neuroimaging Initiative. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neuro. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small S, Duff K. Linking Aβ and tau in late-onset Alzheimer’s disease: A dual pathway hypothesis. Neuron. 2008;600:534–542. doi: 10.1016/j.neuron.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storandt M, Minton MA, Head D, Morris JC. Cognitive decline and brain volume loss are signatures of preclinical Alzheimer’s disease. Arch Neurol. 2009;66:1476–1481. doi: 10.1001/archneurol.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderland T, Mirza N, Putnam KT, Linker G, Bhupali D, Durham R, Soares H, Kimmel L, Friedman D, Bergeson J, Csako G, Levy JA, Bartko JJ, Cohen RM. Cerebrospinal fluid beta-amyloid1–42 and tau in control subjects at risk for Alzheimer’s disease: The effect of APOE epsilon4 allele. Biol Psychiatry. 2004;56:670–676. doi: 10.1016/j.biopsych.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Tolboom N, van der Flier WM, Yaqub M, Boellaard R, Verwey NA, Blankenstein MA, Windhorst AD, Scheltens P, Lammertsma AA, van Berckel BN. Relationship of cerebrospinal fluid markers to 11C-PiB and 18F-FDDNP binding. J Nucl Med. 2009;50:1464–1470. doi: 10.2967/jnumed.109.064360. [DOI] [PubMed] [Google Scholar]
- Tosun D, Schuff N, Truran-Sacrey D, Shaw LM, Trojanowski JQ, Aisen P, Peterson R, Weiner MW Alzheimer’s Disease Neuroimaging Initiative. Relations between brain tissue loss, CSF biomarkers, and the APOE genetic profile: A longitudinal MRI study. Neurobiol Aging. 2010;31:1340–1354. doi: 10.1016/j.neurobiolaging.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuri P, Wiste HJ, Weigand SD, Knopman DS, Shaw LM, Trojanowski JQ, Aisen PS, Weiner M, Petersen RC, Jack CR Alzheimer’s Disease Neuroimaging Initiative. Effect of apolipoprotein E on biomarkers of amyloid load and neuronal pathology in Alzheimer disease. Ann Neurol. 2010;67:308–316. doi: 10.1002/ana.21953. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.