Abstract
Spinal cord injury (SCI) often leads to impaired breathing. In most cases, such severe respiratory complications lead to morbidity and death. However, in the last few years there has been extensive work examining ways to restore this vital function after experimental spinal cord injury. In addition to finding strategies to rescue breathing activity, many of these experiments have also yielded a great deal of information about the innate plasticity and capacity for adaptation in the respiratory system and its associated circuitry in the spinal cord. This review article will highlight experimental SCI resulting in compromised breathing, the various methods of restoring function after such injury, and some recent findings from our own laboratory. Additionally, it will discuss findings about motor and CNS respiratory plasticity and adaptation with potential clinical and translational implications.
Spinal cord injuries often occur at the cervical level. For the injured patient, this is detrimental because of the immediate threat to the important function of breathing. The final common pathway motor neurons from the ventral horn to the two halves of the diaphragm, the phrenic nuclei (PN), are located bilaterally at the C3 to C6 level of the spinal cord. Therefore, cervical SC trauma can potentially disrupt axons from the respiratory regions of the brainstem to the PN or damage the phrenic motor neurons themselves - resulting in paralysis of the diaphragm and impaired breathing (Fuller et al, 2009, Goshgarian, 2003, Moreno, et al., 1992). In addition, compromised respiration makes the SCI patient more susceptible to respiratory related illness and disease, including pneumonia and atelectasis. Clearly, investigating interventions to restore breathing and understanding the adaptive changes, which take place after SCI are of high priority to the SCI community.
The majority of studies exploring the ramifications of SCI on breathing and potential therapies utilize the lateral C2 hemisection model (Fuller, et al., 2009, Goshgarian, 2003, Zimmer, et al., 2007). With this injury type, projections from the rostral ventral respiratory group (rVRG) in the medulla, which provide the excitatory inspiratory drive to the ipsilateral PN, are disrupted (Fig. 1) (Goshgarian and Rafols, 1981, Goshgarian and Rafols, 1984, Moreno, et al., 1992, Chitravanshi and Sapru, 1996). As a result, the hemidiaphragm ipsilateral to the lesion becomes paralyzed. The injured animal is able to breathe independent of a mechanical ventilator since the hemidiaphragm contralateral to the hemisection is still active and compensates for the decrement in respiratory capacity. From here, strategies to restore function can be examined and the plasticity and adaptation which takes place after SCI associated with hemiparalysis of a major muscle can be uncovered.
Figure 1. A schematic of the medullary-spinal cord respiratory circuitry.
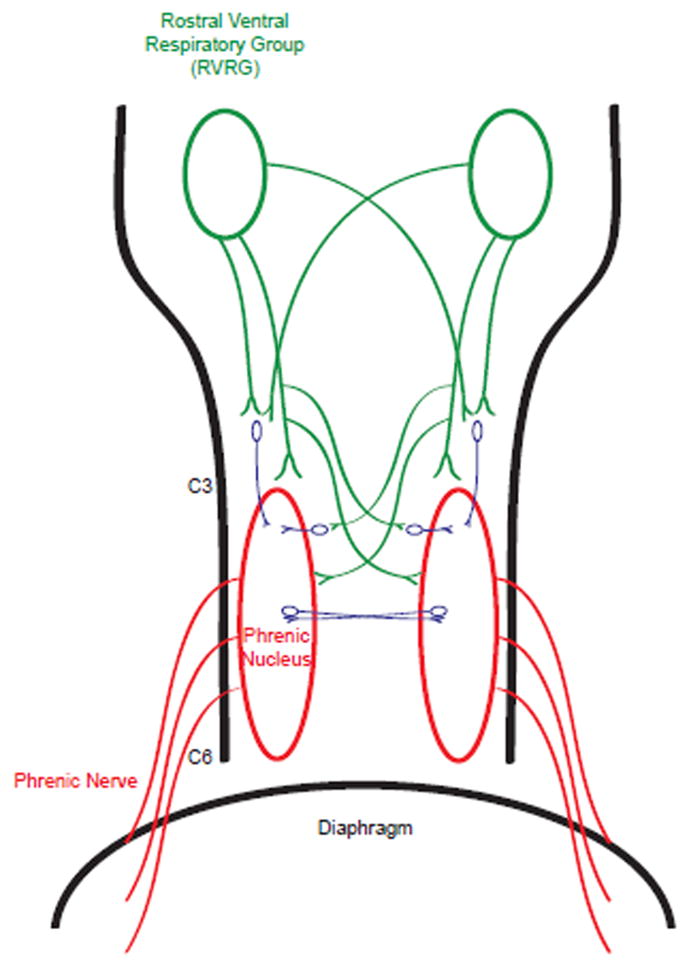
From the brainstem, bilateral projections are made from the RVRG (green) to the phrenic nucleus (red), providing the inspiratory drive. These pathways decussate at the medulla, as well as at the cervical spinal cord, which comprises the crossed phrenic pathway and is normally latent. Following injury at the cervical level, these pathways become interrupted resulting in impaired diaphragm activity. Furthermore, these bulbospinal projections are not exclusively monosynaptic, with interneurons dispersed in the circuitry (blue). These interneurons have become a target to restore function after injury.
It was demonstrated by Porter in 1895 that following C2 hemisection, activity could be restored to the paralyzed hemidiaphragm via activation of a spared, but diminutive and latent pathway to the denervated phrenic nucleus. Specifically, in these early experiments, it was found that when the contralateral phrenic nerve of a C2 hemisected animal was transected, instead of killing the animal due to complete paralysis of the diaphragm, the initially paralyzed hemidiaphragm became rapidly active again, allowing the animal to survive (Goshgarian, 2003, Goshgarian, 1981, Rosenbluth and Ortiz 1936, Porter, 1895). This recovery following hemisection and contralateral phrenicotomy was called the “crossed phrenic phenomenon” (Goshgarian, 2003). The pathway mediating the recovery (the crossed phrenic pathway) circumvents the hemisection by descending contralateral to the lesion and crossing over at the level of the PN (Goshgarian, et al., 1991, Moreno, et al., 1992). Although the pathway continues to be a potential path for descending input following C2 hemisection alone, this pathway is normally latent and ineffective, since after hemisection, there is no function in the hemidiaphragm.
It was later demonstrated that activation of this pathway is dependent upon strongly increasing respiratory drive (Lewis and Brookhart, 1951). Since this discovery, there have been numerous studies to increase respiratory drive and activate this pathway without a contralateral phrenicotomy by utilizing pharmacological treatments, intermittent hypoxia, and reducing plasticity inhibiting substrates. In addition, this model presents an opportunity to examine methods to increase the effectiveness of spared pathways potentially remaining after any type of SCI to improve function or behavior.
Strategies to restore respiratory function after cervical SCI
Since it had been shown that increasing central respiratory drive via contralateral phrenicotomy after C2 hemisection reveals and activates this latent pathway, it was logical to explore whether pharmacological treatments that increase respiratory drive would also work in the C2 hemisected animal to restore function. Indeed, it was shown that administration of the methylxanthine, theophylline; a respiratory stimulant long used as a treatment for asthma, could partially restore function to the paralyzed hemidiaphragm. Through a series of experiments it was determined that the mechanism behind the restoration of activity was through theophylline’s non-specific antagonism of adenosine (and specifically adenosine A1) receptors (Nantwi, et al., 1996, Nantwi and Goshgarian, 1998). Focusing on the adenosine system, it appears that manipulation of both central and peripheral receptors can restore as well as modulate respiratory activity (Nantwi, 2009, Nantwi and Goshgarian, 2005). Additionally, chronic treatment with theophylline results in restored activity that persists even after weaning off the drug, suggesting that plasticity of the spinal respiratory circuitry could be induced via long term drug mediated recovery (Nantwi, et al., 2003). Additionally, the inhibitory effect of theophylline on phosphodiesterase, the enzyme which degrades cAMP, has also led to further experiments showing that upregulation of cAMP can lead to plasticity of the spinal respiratory circuitry and restoration of function (Kajana and Goshgarian, 2008, Kajana and Goshgarian, 2008).
As the theophylline experiments showed, pharmacologically increasing respiratory drive can result in restored hemidiaphragmatic activity following C2 hemisection. However, drug intervention is not the only way to increase CNS respiratory drive.
Intermittent hypoxia and plasticity of the intact respiratory system
Exposure to repeated episodes of hypoxia (i.e. low oxygen levels) interspersed with a return to normal oxygen levels has been shown to elicit a long-lasting increase in respiratory motor output or drive, a phenomenon known as long term facilitation (LTF) (for a review, see Fuller et. al, 2000). LTF is a form of respiratory plasticity, generally studied in phrenic and hypoglossal nerves, that lasts at least one hour (although this threshold varies) and is primarily expressed as an enhanced nerve burst amplitude leading to increased inspiratory motor output (Bocchiaro and Feldman 2004, Fuller et al 2001a). LTF patterns in the phrenic nucleus are known as phrenic LTF (pLTF), and are the focus of this section, although LTF in the hypoglossal nerve and ventilatory LTF (vLTF) will also be discussed.
LTF was originally discovered as a phenomenon arising from periodic stimulation of the carotid sinus nerve that persisted despite normal blood arterial gas levels (Millhorn et al, 1980a). This pattern of plasticity has also been shown to be elicited by intermittent hypoxia (Hayashi et al, 1993). Mostly developed and elucidated in the seminal studies from the Gordon Mitchell laboratory, it has been shown an array of protocols that present hypoxia to animals have been shown to elicit respiratory plasticity, making this a technique of interest. Understanding and elucidating the mechanisms of this phenomena may lead to understanding and even harnessing this latent potential for plasticity in the spinal cord, especially after injury. Different protocols induce contrasting effects, but the mechanisms seem to share common pathways.
The most extensively studied paradigm to induce phrenic LTF is acute intermittent hypoxia (AIH) which is characterized by 3–5 episodes of hypoxia each lasting for 5 minutes (Vinit, 2009; Hayashi et al, 1993). Less well studied is chronic intermittent hypoxia (CIH), which differs from AIH in that it may have over 500 such episodes over a period of days or weeks (Vinit, 2009). CIH pretreatment can enhance the effects of an AIH protocol by increasing sensitivity to hypoxia as well as increasing the phrenic response during hypoxia (Ling et al, 2001). It also increases the duration of LTF in AIH exposed rats but without changing baseline ventilation or metabolism (McGuire et al, 2003). These effects indicate that CIH induces metaplasticity (i.e., previous plastic changes which alter the current plasticity of circuits). This metaplasticity renders hypoxia more effective in inducing LTF when preceded with CIH (McGuire et al, 2003). Unfortunately, CIH protocols have several pernicious side effects including high blood pressure (Fletcher et al., 1992), cognitive deficits (Row et al, 2007; Row, 2007), as well as the so-called metabolic syndrome (Tasali and Ip, 2008).
Several other experimental protocols exist including daily acute intermittent hypoxia (dAIH) (Wilkerson and Mitchell, 2009) and early postnatal CIH (Reeves and Gozal, 2006). Of course, each protocol elicits different results and dAIH may especially allow researchers to circumvent the problems of CIH side effects (some dAIH results are discussed below). A final experimental protocol of note is chronic or sustained hypoxia exposure, which does not induce synaptic plasticity in the phrenic nucleus (Castro-Moure and Goshgarian, 1997).
It has been known since the discovery of LTF that the mechanism is intimately involved with the serotonergic system (Millhorn et al 1980b). Two years after Castro-Moure and Goshgarian’s results, Kinkead and Mitchell discovered that 5-HT2A/C receptor activation is necessary for the short-term phrenic response, but post-hypoxia frequency decline (which would be absent in chronic hypoxia) is also necessary for LTF (Kinkead and Mitchell, 1999). It also was demonstrated that intermittent serotonin activation of 5-HT receptors appears to trigger protein synthesis required for LTF (Baker-Herman and Mitchell, 2002). Further, it has been shown that periodic intraspinal injections of 5-HT receptor agonists directly into the spinal cord can induce phrenic motor facilitation (MacFarlane and Mitchell, 2009). While serotonin is necessary for initiating phrenic LTF, it is not necessary to maintain it (Fuller et al, 2001a).
Blockade of NMDA receptors via pretreatment with the antagonist MK-801 in the phrenic motor nucleus has been shown to eliminate pLTF in anesthetized rats (McGuire et al, 2005). It has also been demonstrated that (2R)-2-amino-5-phosphonovaleric acid (APV)(an NMDA antagonist) can prevent AIH-induced vLTF and attenuate or abolish vLTF in conscious rats if administered at the time of AIH or even 20 minutes after AIH (McGuire et al, 2008). Unlike serotonin, NMDA receptor activation seems necessary to maintain LTF patterns. Nonetheless, both play major roles in mediating recovery after injury.
Overall, the pathway for pLTF seems to start when hypoxic episodes activate serotonergic medullary raphe neurons (Erickson and Millhorn, 1994), which in turn trigger serotonin release near phrenic motor neurons in the spinal cord. This release activates 5-HT2 receptors, which are coupled to Gq proteins (for a review, see Bockaert et al. 2006). These G proteins (through the Phospholipase C pathway leading to activation of PKC) stimulate new protein synthesis and trigger a release of BDNF. The ensuing activation of BDNF’s high affinity receptor TrkB has been shown to be necessary and sufficient for pLTF (Baker-Herman et al 2004). Changes occurring downstream of TrkB activation are less well understood, but it seems that extracellular regulated kinases 1 and 2 (ERK1/2) play important roles. BDNF increases their phosphorylation in motor neurons (Kishino and Nakayama, 2003) and AIH does the same in areas associated with the phrenic motor nucleus (Wilkerson and Mitchell, 2009). Further, when spinal MEK (the enzyme responsible for phosphorylating ERKs) is inhibited, pLTF is abolished (Dale-Nagle et. al, 2010). BDNF has been shown to modulate NMDA receptors by phosphorylating its subunit 1 (NR1) via this ERK pathway (Slack et al., 2004); possibly being the mechanism by which modulation of NMDA receptors induces pLTF patterns in phrenic motor neurons.
The reason that hypoxia must be intermittent is that sustained hypoxia induces certain serine/threonine phosphatases that prevent pLTF from developing. Inhibition of those phosphatases allows pLTF to develop during sustained hypoxia but has no effect on the patterns evoked by AIH (Wilkerson et al, 2008). This phosphatase activity can be blocked by the formation of reactive oxygen species (ROS), which is what seems to happen in AIH when 5-HT activated protein kinase C induces NADPH oxidase activity to increase ROS levels (MacFarlane et al, 2009; Mahamed and Mitchell, 2007). It appears the reason ROS levels are higher during intermittent hypoxia (vs. sustained hypoxia) is due to NADPH oxidase exhibiting a burst of ROS formation during re-oxygenation after oxidative stress (Abramov et al, 2007). Furthermore, inhibitors of the NADPH oxidase complex have been shown to attenuate pLTF indicating a crucial regulatory role of this complex in IH-induced LTF (MacFarlane et al, 2009).
In CIH, plasticity of the carotid bodies (the group of cells that play a chemosensory role and detect arterial blood gas levels) is also serotonin dependent, but appears to utilize a broader range of 5-HT receptors including the 5-HT7 receptor (McGuire et al, 2004). CIH can cause LTF of carotid body afferent nerve activity (i.e., sensory LTF (sLTF) (Peng et al, 2003). sLTF requires conditioning with CIH, is reversible after exposure to normoxia, preventable with anti-oxidants (Peng et al, 2003) and like AIH, requires NADPH oxidase (Peng et al, 2009) suggesting similarities between the sLTF and pLTF pathways.
There are several other mechanisms and pathways by which phrenic motor output can be enhanced as well involving other pathways (Dale-Nagle et al, 2010), but these mechanisms do not appear to be central to hypoxia induced LTF.
Intermittent hypoxia and SCI
In the context of cervical SCI, intermittent hypoxia improves respiratory output. This improvement has been demonstrated by a protocol of AIH applied 4 to 8 weeks after C2 hemisection resulting in a serotonin-dependent plasticity (Golder and Mitchell, 2005), implying that AIH improves functional recovery even after chronic spinal cord injury. Further downstream in the putative pathway, Golder et al discovered that mimicking the impact of BDNF on TrkB receptors by activating adenosine 2A receptors also improved respiratory function after C2H. (Golder et al, 2008). Overall, this work points to a possibility of harnessing the AIH-induced serotonin dependent plasticity pathway to improve respiratory function after spinal cord injury.
CIH has also been demonstrated to improve phrenic motor output after C2 hemisection. Specifically, CIH given for 7 days after C2 hemisection improved spontaneous ipsilateral phrenic motor output via the crossed phrenic pathway in sub-chronically injured (2 weeks) rats. However, CIH preconditioning had no effect, and CIH also had no effect on contralateral outputs or in uninjured control rats (Fuller et al, 2003). Further, short latency phrenic potentials are stronger after CIH which points to increased efficiency of synapses in a single or pauci-synaptic pathway (Fuller et al, 2003). The CIH mediated plasticity has also been demonstrated to be serotonin-dependent (McGuire et al 2004). Interestingly, cervical spinal cord injury has been shown to upregulate spinal 5-HT2A receptors in ventral segments ipsilateral to C2H associated with the phrenic motor nucleus (Fuller et al 2005). These results collectively suggest that CIH takes advantage of and strengthens crossed phrenic pathway circuitry that develops after chronic SCI through a serotonin-dependent mechanism. The upregulation of 5-HT2A receptors may allow for greater plasticity after SCI than is available normally and improve synaptic efficiency in spared pathways.
Daily treatments of AIH (dAIH), which can avoid the negative side effects of CIH (Wilkerson and Mitchell, 2009), have also shown some exciting results in inducing plasticity and restoring ventilatory output. In C2H rats, dAIH returned tidal volume to the same level as sham-operated rats, although it did not entirely restore normal breathing patterns (Barr et al 2007).
Importantly, there is some evidence that intermittent hypoxia may improve non-respiratory motor function after spinal cord injury as well. Preliminary evidence taken from studies done on two SCI incomplete patients showed an increase in voluntary motor function in the ankle after treatment with 15 episodes of hypoxia. (Rymer et al, 2007, Trumbower et al, 2011).
Gaining a further understanding of the mechanisms by which intermittent hypoxia induces plasticity in the spinal cord offers a very intriguing and exciting avenue of research to help devise strategies to maximize the intrinsic capacity for spinal cord plasticity after injury. Furthermore, it seems that there is even greater potential for IH induced plasticity to improve both respiratory and non-respiratory related synaptic circuitry if sprouting or bona fide regeneration of damaged bulbospinal respiratory pathways can be achieved.
Sprouting and regeneration of respiratory CNS circuitry
Following SCI, growth of severed axons is very limited without intervention. In the face of the injury-induced environment of the “glial scar”, which is composed of potently inhibitory reactive astrocytes and extracellular matrix molecules, transected axons terminate in dystrophic endbulbs unable to reach their proper neuronal targets (Busch and Silver, 2007, Cajal, 1928, Silver and Miller, 2004). This, however, does not mean that CNS axons do not have the intrinsic ability to grow and regenerate. When introduced to an environment favorable to regeneration, or a “growth permissive” environment, injured axons can indeed grow. In their classic work, David and Aguayo (1981) showed that a segment of the peripheral nervous system (PNS) is one such substrate that can support and re-myelinate the lengthy regrowth of a large number of central axons (David and Aguayo, 1981). In the context of SCI, grafts of PNS segments can be used to bridge around a lesion and provide a new and growth-permissive route to denervated neurons. Re-entry of axons from the PNS graft back into a CNS environment, however, remains an issue in this approach.
There have been several attempts to bridge a lesion with peripheral nerve grafts to the phrenic motor nucleus in order to restore respiratory function. In their pioneering work, Gauthier and colleagues found that respiratory related bulbospinal axons can readily regenerate into a peripheral nerve bridge placed near the rVRG or into the funiculi that contain the descending respiratory tracts (Decherchi, et al., 1996, Gauthier and Lammari-Barreault, 1992, Gauthier and Rasminsky, 1988, Lammari-Barreault, et al., 1991). However, they also showed that a paucity of fiber penetration back into the CNS produced only minimal impact on restoring spontaneous phrenic nerve activity after a C3 hemi-lesion (Gauthier, et al., 2002). The aforementioned inhibitory ECM molecules could potentially contribute to the failure of axons to regenerate back into the CNS.
Chondroitinase ABC (ChABC) is a bacterial enzyme that degrades the inhibitory glycosaminoglycan (GAG) chains of chondroitin sulfate proteoglycans (CSPGs) found in the glial scar. In recent experiments, treatment with ChABC allowed for strong penetration of regenerating axons back into the CNS from a peripheral nerve bridging a C3 lesion, resulting in significant behavioral improvements in the ipsilateral forelimb (Houle, et al., 2006, Tom and Houle, 2008, Tom, et al., 2009). Treatment with ChABC is required because of the rapid deposition of CSPGs at the site of CNS trauma and bridge insertion near the vicinity of the denervated target neurons. Additionally, CSPGs are a component of the perineuronal net, which increases around denervated neurons, including motor neurons well away from the site of damage. The appearance of CSPGs within the perineuronal net during development coincides with the cessation of developmental plasticity (Pizzorusso, et al., 2002, Pizzorusso, et al., 2006). In CNS trauma, it has been shown that CSPGs strongly inhibit regeneration and that plasticity through ChABC treatment can promote behavioral recovery (Barritt, et al., 2006, Bradbury, et al., 2002, Busch and Silver, 2007, Cafferty, et al., 2008, Massey, et al., 2006, Silver and Miller, 2004, Steinmetz, et al., 2005, Tester and Howland, 2008). In our own experiments we are currently using ChABC treatment alone and in conjunction with peripheral nerve grafting to promote strong recovery of respiratory function. Briefly, there is an increased presence of lesion scar, as well as perineuronal net associated CSPGs, at the level of the phrenic nucleus, which powerfully inhibit regeneration/plasticity of respiratory function. Degradation of the glial scar allowing for regeneration can promote recovery, which is augmented by potential modification of the inherent spinal circuitry and sprouting of spared tracts (Alilain et al., 2006, Alilain et al., 2007 and Alilain et al., 2011)
The quick and intense reaction of the CNS to trauma in particular, the increased presence of CSPGs, makes natural regeneration of severed axons near impossible. In addition to promoting sprouting and regeneration of essential tracts to respiration to promote recovery, manipulation of the spinally located respiratory neurons is another strategy to restore function.
Plasticity and modification of respiratory spinal neurons
Paralysis of the hemidiaphragm following C2 hemisection is often a transitory condition. At chronic time points following injury, there is normally a very modest return of respiratory function (Nantwi, et al., 1999, Pitts, 1940, Fuller, et al., 2008). Coinciding with this spontaneous return of activity are synaptic changes in or near the phrenic motor pool (mentioned earlier in this review) including plasticity of the serotonergic and excitatory glutamatergic systems.
For the serotonergic system, at chronic time points there is an increase of presynaptic 5-HT terminals from the caudal Raphe nucleus as well as an increase in its receptors, in particular the 5-HT 2A subtype (Fuller, et al., 2005, Golder and Mitchell, 2005, Tai, et al., 1997). In addition to its effect on LTF, as noted earlier in this review, an increase in serotonergic neurotransmission is important because it has been shown that 5-HT is a requirement for the induction of the crossed phrenic phenomenon itself. Depletion of 5-HT results in abolishment of the morphological changes associated with the phenomenon and failure to induce it (Hadley, et al., 1999, Hadley, et al., 1999). Treatment with 5-HT receptor agonists is also sufficient to induce activation of the crossed phrenic pathway and restore function (Ling, et al., 1994, Zhou, et al., 2001, Zhou and Goshgarian, 1999, Zhou and Goshgarian, 2000, Zimmer and Goshgarian, 2006). While 5-HT may have a modulatory role in respiratory motor output, glutamate from the rVRG is the excitatory neurotransmitter that directs the phrenic motor neuron to fire (Chitravanshi and Sapru, 1996). Similar to 5-HT, at chronic time points there is an increase in glutamatergic terminal length (Tai and Goshgarian, 1996). For the glutamate receptors, an increase in the 2A subunit of the NMDA receptor and a decrease of the AMPA GluR1 subunit on phrenic motor neurons correlates with the onset of spontaneous activity (Alilain and Goshgarian, 2008). This finding is also observed in other models of SCI (Grossman et al., 1999, Grossman et al., 2000). Furthermore, pharmacologically increasing the 2A subunit can result in recovery with the NMDA receptor antagonist MK-801 through “disuse hypersensitization”. This treatment has unwanted side effects on the animals, but suggests the importance of this receptor subunit (Alilain and Goshgarian, 2007). Overall, similar to the series of experiments examining LTF and plasticity of phrenic output, the same elements of 5-HT and glutamate neurotransmission play major roles in mediating recovery after injury.
The role of interneurons in respiratory plasticity
In addition to the phrenic motor neurons, there have been recent studies regarding the importance of pre-phrenic spinal interneurons in the spontaneous recovery process (Fig. 1). A recent elegant series of experiments showed that there are a number of different types of pre-phrenic interneurons located bilaterally between the brainstem and the SC (Lane, et al., 2009, Lane, et al., 2008). In the unlesioned animal, these interneurons can potentially enhance or amplify phrenic output (Hayashi, et al., 2003). In the injured animal at time points where spontaneous recovery occurs, these interneurons may play a role in mediating spontaneous recovery. Recent studies suggest either recruitment or strengthening of these cells (or possibly both) and a capacity of the CNS respiratory circuitry to adapt and reorganize in response to injury. These interneurons, when harnessed, can play an important and complex role in mediating recovery. Optogenetic control of this interneuron population is discussed below. Other experiments attempting to utilize these interneurons and the spinal phrenic circuitry include fetal/donor cell transplants and grafts directly into the area near these cells to potentially act as a cell replacement or influence on the spinal respiratory circuitry (White, et al. 2010). Indeed, the results have shown an impact on respiratory output; however, more work still needs to be done regarding this approach.
Optogenetic approaches to induce plasticity of lesioned respiratory pathways
With the emergence of the light sensitive cation channel, channelrhodopsin-2 (ChR2), new ways of precisely controlling CNS activity are now possible (Herlitze and Landmesser, 2007, Zheng et al 2007). In SCI, which can result in denervated neurons, this tool becomes immensely useful. Expression of ChR2 and subsequent photostimulation can lead to neuronal depolarization and/or induction of action potentials, independent of presynaptic input. Indeed, it was shown that when stimulated with light, ChR2 expressing spinal cells at the cervical level after C2 hemisection can lead to a near complete restoration of respiratory activity which persisted long after the cessation of light exposure (Alilain, et al., 2008, Alilain and Silver, 2009). Perhaps more interesting, the activity that was evoked in the hemidiaphragm ipsilateral to the lesion was not entrained to the pattern of light stimulation (0.5 Hz) but was synchronized to the contralateral side. These results suggest that repeated photostimulation and spinal cell depolarization can potentiate these cells to spared and weak pathways with sparse neurotransmitter release, in this case the crossed phrenic pathway.
Interestingly, the cell types that expressed the construct included both motor neurons and interneurons (as well as some astrocytes). Furthermore, recovery was achieved through a unique pattern of oscillating waves between both sides of the diaphragm, although ChR2 was expressed on only one side. Potentially serving as the anatomical substrate for this bilateral phenomenon were both interneuron and motor neuron populations with neurite extensions projecting toward the contralateral side of the SC (Fig. 1). When MK-801 was administered immediately prior to photostimulation, the induction of the oscillating phenomenon and restoration of activity was totally abolished - indicating an important role of the NMDA receptor and its ability to facilitate plasticity. These results clearly provide further data demonstrating the potential of the inherent plasticity of the spinal respiratory circuitry. Because this experiment utilized the general cytomegalovirus (CMV) promoter, it will be interesting to see whether future experiments, employing more specific promoters, will define which cell types contribute to the return of function.
With regards to future optogenetic approaches to manipulate spinal cord plasticity, there are many strategies. Combinatorial treatment with ChABC, as well as, photostimulation of supraspinal centers which directly influence phrenic motor output including the raphe nucleus and rVRG could potentially enhance the plasticity of the spinal cord and augment recovery.
Other models of SCI with respiratory complications
While the C2 hemisection model of SCI allows for investigation of spared nerve pathways and the reinnervation of the phrenic nucleus, comparatively little has been done about restoring function to the accessory muscles of respiration. These muscles act in concert with the diaphragm not only in rhythmic breathing but also in augmented functions such as sighing, yawning, sneezing, and coughing. The internal intercostals and abdominal muscles (T1–T11) are the accessory muscles of expiration. The external intercostals (T1–T11), scalenes (C4–C6), and sternocleidomastoid (CN XI) are the accessory muscles of inspiration.
It is important to study these muscles and the effect SCI has on them, because: 1) optimal diaphragm function depends on its ability to act in concert with muscles of the thoracic cage and abdominal wall, 2) augmented inspiration and forced expiration are independent of diaphragmatic function and essential for sighing and coughing, and 3) many actual incidents are thoracic SCIs which do not involve denervation of the phrenic nucleus but still impose respiratory insufficiency. The methods and applications for investigating intercostal and abdominal respiratory function are discussed below.
One must consider that the C2 hemisection model cannot be advanced to a full transection thereby affecting all respiratory related muscles, as it is essentially impossible to model a ventilator dependent human in an animal model. Primarily due to logistical issues, it cannot be applied to higher vertebrates and non-human primates (Philips 2004). Lower cervical or upper thoracic injury, in contrast, preserves function of the diaphragm while still allowing study of respiratory dysfunction and the plasticity of mammalian models similar to humans such as dogs (DiMarco et. al. 1995, Kowalski et. al. 2007, Walter et. al. 2010). One method involves contusion of the spinal cord at T8. With a T8 contusion there ensues a significantly decreased tidal volume and an increased respiratory rate in the rat model (Teng et. al. 1999, Teng et. al. 2003). Perhaps, not surprisingly, 5-HT also plays a role in reversing respiratory deficits in this particular model (Teng, et al., 2003, Teng, et al., 1999). More recently, contusion injuries at the cervical level are being investigated. For more on the contusion model at the C2 level please see the article by Lane et al., in this issue and the recent article by Golder et al. (2011). Furthermore, besides being more clinically relevant in terms of animal model, utilization of the contusion injury, at any level, may help to translate the model to actual injuries encountered by humans. Other models use a full transection in cats and dogs. In an interesting study by DiMarco et al., they demonstrated that in addition to activating expiratory muscles and large positive airway pressures, stimulation of the SC at caudal thoracic levels can lead to activation of diaphragmatic activity before and after C2 section, clearly demonstrating the plastic nature of the circuitry of the SC, at least acutely, which can be harnessed for restoration of activity (DiMarco et. al., 1999, DiMarco et. al., 2008).
Transition from the laboratory bench to the bedside
It is clear from these experiments, the rat provides an excellent model for respiratory motor dysfunction, CNS plasticity, and repair after spinal cord injury. However, there are some limitations. It has been well established by patient models that diaphragm function alone is not enough to sustain life and quality of life in patients with SCI who also have an impaired ability to augment their inspiratory capacity in response to the hypoxic state: individuals are mechanically ventilated which only allows rhythmic activation of diaphragm contractions (DiMarco 2005, Sander et. al., 2010). Although restoration of diaphragm activity following SCI is of high importance, there are other issues that need to be considered. The diaphragm’s contractile properties are not sufficient to restore full inspiratory capacity; adequate ventilation requires simultaneous contraction of the external intercostals to allow adequate lung expansion during inhalation (Walter et. al., 2010, Philips 2004). In addition, without periodic stretching of the ribcage in breathing, the joints themselves become calcified and rigid, resulting in reduced ventilation and expedited diaphragmatic fatigue. Furthermore, the intercostal muscles are essential for deep inhalation to relieve atelectasis in an able-bodied individual; normal respiration would need restoration of the periodic sigh provided by external intercostal contraction.
Forceful expiration may be an even more important role for accessory muscles of respiration (DiMarco et. al, 1995). Many studies describe forced expiration as the ability to react to stimuli irritating the respiratory tract, as in a cough (Lim et. al. 2007, Kowalski et. al. 2007, Jefferson et. al. 2010). Cough allows for expulsion of harmful stimuli and possible pathogens, of which the SCI patient is already at potential risk (Dimarco et. al. 2009). When the coughing mechanism is suppressed, probability of upper respiratory infection in the already immunocompromised patient is a certainty. These same studies that emphasize the importance of the muscles of expiration (the internal intercostals and abdominals) are attempting to develop electrical stimulation to restore impulses to these muscles based on the principle of muscle priming to restore automatic function (Jefferson et. al., 2010, Lee et. al, 2008, Martin et. al, 1992, Schilero et. al. 2009) or stimulation upon command (Lim et. al., 2007, Sander et. al., 2010). For instance, DiMarco et. al. 2002 and DiMarco 2008 successfully produced positive airway pressure in both the dog and cat model by stimulating spinal cord levels between T9 through T10 to generate a cough. Such promising studies support the importance of a holistic approach to restoration of muscle function in spinal cord injury.
In concert with the notion that phrenic innervation is not the only goal of SCI respiration recovery is the idea that many patients are either ineligible for phrenic nerve pacing or need support other than diaphragmatic innervations. As described by DiMarco et. al, patients with unilateral phrenic nerve function may not be able to adopt phrenic nerve pacing because of the remaining functioning hemidiaphragm. However, because cough is still suppressed in these individuals, new techniques such as functional electrical stimulation, surface stimulation, or magnetic stimulation may be able to isolate expiratory muscles to preserve cough (DiMarco et. al. 2005).
Conclusions
As one can clearly see, there has been much progress in investigating potential therapeutic strategies to restore respiratory function after SCI. This includes uncovering an examination of the inherent plasticity of the spinal cord and respiratory activity, which can be harnessed and exploited to restore function after injury. However, there is still much work to be done in further characterizing these processes and the basic science behind these mechanisms. Potentially in the end, a multi-faceted strategy that combines many of the described approaches in this review will fully restore respiratory function and aid in improving the outcomes of the SCI community and their quality of life.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramov AY, Scorziello A, Duchen MR. Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J Neurosci. 2007;27:1129–38. doi: 10.1523/JNEUROSCI.4468-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alilain WJ, Horn KP, Dick TE, Silver J. 2006 Neuroscience Meeting Planner. Atlanta, GA: Society for Neuroscience; 2006. Chondroitinase ABC-induced reduction of chondroitin sulfate proteoglycans and the perineuronal net following C2 hemisection. Program No. 720.7. Online. [Google Scholar]
- Alilain WJ, Goshgarian HG. MK-801 upregulates NR2A protein levels and induces functional recovery of the ipsilateral hemidiaphragm following acute C2 hemisection in adult rats. J Spinal Cord Med. 2007;30:346–354. doi: 10.1080/10790268.2007.11753950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alilain WJ, Horn KP, Dick TE, Silver J. 2007 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience; 2007. Chondroitinase ABC treatment following C2 hemisection results in dramatically enhanced ipsilateral hemi-diaphragmatic recovery. Program No. 137.4. Online. [Google Scholar]
- Alilain WJ, Goshgarian HG. Glutamate receptor plasticity and activity-regulated cytoskeletal associated protein regulation in the phrenic motor nucleus may mediate spontaneous recovery of the hemidiaphragm following chronic cervical spinal cord injury. Exp Neurol. 2008;212:348–357. doi: 10.1016/j.expneurol.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alilain WJ, Li X, Horn KP, Dhingra R, Dick TE, Herlitze S, Silver J. Light-induced rescue of breathing after spinal cord injury. J Neurosci. 2008;28:11862–11870. doi: 10.1523/JNEUROSCI.3378-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alilain WJ, Silver J. Shedding light on restoring respiratory function after spinal cord injury. Front Mol Neurosci. 2009;2:18. doi: 10.3389/neuro.02.018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alilain WJ, Horn KP, Hu H, Dick TE, Silver J. Functional regeneration of respiratory pathways after spinal cord injury. Nature. 2011;475:196–200. doi: 10.1038/nature10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci. 2002;22:6239–46. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2004;7:48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- Barritt AW, Davies M, Marchand F, Hartley R, Grist J, Yip P, McMahon SB, Bradbury EJ. Chondroitinase ABC promotes sprouting of intact and injured spinal systems after spinal cord injury. J Neurosci. 2006;26:10856–10867. doi: 10.1523/JNEUROSCI.2980-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchiaro CM, Feldman JL. Synaptic activity-independent persistent plasticity in endogenously active mammalian motorneurons. Proc Natl Acad Sci USA. 2004;101:4292–5. doi: 10.1073/pnas.0305712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockaert J, Claeysen S, Becamel C, Dumuis A, Marin P. Neuronal 5-HT metabotropic receptors: fine-tuning of their structure, signaling, and roles in synaptic modulation. Cell Tissue Res. 2006;326:553–72. doi: 10.1007/s00441-006-0286-1. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- Busch SA, Silver J. The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol. 2007;17:120–127. doi: 10.1016/j.conb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Cafferty WB, Bradbury EJ, Lidierth M, Jones M, Duffy PJ, Pezet S, McMahon SB. Chondroitinase ABC-mediated plasticity of spinal sensory function. J Neurosci. 2008;28:11998–12009. doi: 10.1523/JNEUROSCI.3877-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajal RS. Degeneration and regeneration of the nervous system. London: Oxford UP; 1928. [Google Scholar]
- Castro-Moure F, Goshgarian HG. Chronic hypoxia does not induce synaptic plasticity in the phrenic nucleus. Exp Neurol. 1997;148:293–8. doi: 10.1006/exnr.1997.6649. [DOI] [PubMed] [Google Scholar]
- Chitravanshi VC, Sapru HN. NMDA as well as non-NMDA receptors mediate the neurotransmission of inspiratory drive to phrenic motoneurons in the adult rat. Brain Res. 1996;715:104–112. doi: 10.1016/0006-8993(95)01565-5. [DOI] [PubMed] [Google Scholar]
- Dale-Nagle EA, Hoffman MS, MacFarlane PM, Mitchell GS. Multiple pathways to long-lasting phrenic motor facilitation. Adv Exp Med Biol. 2010;669:225–30. doi: 10.1007/978-1-4419-5692-7_45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science. 1981;214:931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- Decherchi P, Lammari-Barreault N, Gauthier P. Regeneration of respiratory pathways within spinal peripheral nerve grafts. Exp Neurol. 1996;137:1–14. doi: 10.1006/exnr.1996.0001. [DOI] [PubMed] [Google Scholar]
- DiMarco AF. Phrenic nerve stimulation in patients with spinal cord injury. Respiratory Physiology & Neurobiology. 2009 Nov 30;169(2):200–209. doi: 10.1016/j.resp.2009.09.008. [DOI] [PubMed] [Google Scholar]
- DiMarco AF. Restoration of respiratory muscle function following spinal cord injury: Review of electrical and magnetic stimulation techniques. Respiratory Physiology & Neurobiology. 2005 Jul 28;147(2–3):273–287. doi: 10.1016/j.resp.2005.03.007. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE. Effects of chronic electrical stimulation on paralyzed expiratory muscles. J Appl Physiol. 2008 Jun;104 (6):1634–40. doi: 10.1152/japplphysiol.01321.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE, Supinski G, Romaniuk JR. Mechanism of expiratory muscle activation during lower thoracic spinal cord stimulation. J Appl Physiol. 2002 Jun 1;92:2341–2346. doi: 10.1152/japplphysiol.01231.2001. published ahead of print February 1 2002. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE, Romaniuk JR. Effects of diaphragm activation on airway pressure generation during lower thoracic spinal cord stimulation. Respir Physiol Neurobiol. 2007;159:102–107. doi: 10.1016/j.resp.2007.06.007. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Romaniuk, Supinski GS. Electrical activation of the expiratory muscles to restore cough. Am J Respir Crit Care Med. 1995 May;151 (5 ):1466–71. doi: 10.1164/ajrccm.151.5.7735601. [DOI] [PubMed] [Google Scholar]
- Erickson JT, Millhorn DE. Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity with catecholaminergic and serotonergic neurons of the rat brainstem. J Comp Neurol. 1994;348:161–82. doi: 10.1002/cne.903480202. [DOI] [PubMed] [Google Scholar]
- Fletcher EC, Lesske J, Behm R, Miller CC, 3rd, Stauss H, Unger T. Carotid chemoreceptors, systemic blood pressure, and chronic episodic hypoxia mimicking sleep apnea. J Appl Physiol. 1992;72:1978–84. doi: 10.1152/jappl.1992.72.5.1978. [DOI] [PubMed] [Google Scholar]
- Frankel HL, Coll JR, Charlifue SW, Whiteneck GG, Gardner BP, Jamous MA, Krishnan KR, Nuseibeh I, Savic G, Sett P. Long-term survival in spinal cord injury: a fifty-year investigation. Spinal Cord. 1998;36:266–74. doi: 10.1038/sj.sc.3100638. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Bach KB, Baker TL, Kinkead R, Mitchell GS. Long term facilitation of phrenic motor output. Respir Physiol. 2000;121:135–46. doi: 10.1016/s0034-5687(00)00124-9. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Zabka AG, Baker TL, Mitchell GS. Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol. 2001;90:2001–6. doi: 10.1152/jappl.2001.90.5.2001. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Baker TL, Behan M, Mitchell GS. Expression of hypoglossal long-term facilitation differs between substrains of Sprague-Dawley rat. Physiol Genomics. 2001;4:175–81. doi: 10.1152/physiolgenomics.2001.4.3.175. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Baker-Herman TL, Golder FJ, Doperalski NJ, Watters JJ, Mitchell GS. Cervical spinal cord injury upregulates ventral spinal 5-HT2A receptors. J Neurotrauma. 2005;22:203–213. doi: 10.1089/neu.2005.22.203. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Doperalski NJ, Dougherty BJ, Sandhu MS, Bolser DC, Reier PJ. Modest spontaneous recovery of ventilation following chronic high cervical hemisection in rats. Exp Neurol. 2008;211:97–106. doi: 10.1016/j.expneurol.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Sandhu MS, Doperalski NJ, Lane MA, White TE, Bishop MD, Reier PJ. Graded unilateral cervical spinal cord injury and respiratory motor recovery. Respir Physiol Neurobiol. 2009;165:245–253. doi: 10.1016/j.resp.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier P, Lammari-Barreault N. Central respiratory neurons of the adult rat regrow axons preferentially into peripheral nerve autografts implanted within ventral rather than within dorsal parts of the medulla oblongata. Neurosci Lett. 1992;137:33–36. doi: 10.1016/0304-3940(92)90291-e. [DOI] [PubMed] [Google Scholar]
- Gauthier P, Rasminsky M. Activity of medullary respiratory neurons regenerating axons into peripheral nerve grafts in the adult rat. Brain Res. 1988;438:225–236. doi: 10.1016/0006-8993(88)91341-8. [DOI] [PubMed] [Google Scholar]
- Gauthier P, Rega P, Lammari-Barreault N, Polentes J. Functional reconnections established by central respiratory neurons regenerating axons into a nerve graft bridging the respiratory centers to the cervical spinal cord. J Neurosci Res. 2002;70:65–81. doi: 10.1002/jnr.10379. [DOI] [PubMed] [Google Scholar]
- Golder FJ, Mitchell GS. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J Neurosci. 2005;25:2925–2932. doi: 10.1523/JNEUROSCI.0148-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Ranganathan L, Satriotomo I, Hoffman M, Lovett-Barr MR, Watters JJ, Baker-Herman TL, Mitchell GS. Spinal adenosine A2a receptor activation elicits long-lasting phrenic motor facilitation. J Neurosci. 2008;28:2033–42. doi: 10.1523/JNEUROSCI.3570-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Fuller DD, Lovett-Barr MR, Vinit S, Resnick DK, Mitchell GS. Breathing patterns after mid-cervical spinal contusion in rats. Exp Neurol. 2011 doi: 10.1016/j.expneurol.2011.05.020. E Pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshgarian HG. The role of cervical afferent nerve fiber inhibition of the crossed phrenic phenomenon. Exp Neurol. 1981;72:211–225. doi: 10.1016/0014-4886(81)90139-4. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG. The crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury. J Appl Physiol. 2003;94:795–810. doi: 10.1152/japplphysiol.00847.2002. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG. The crossed phrenic phenomenon and recovery of function following spinal cord injury. Respir Physiol Neurobiol. 2009;169:85–93. doi: 10.1016/j.resp.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshgarian HG, Ellenberger HH, Feldman JL. Decussation of bulbospinal respiratory axons at the level of the phrenic nuclei in adult rats: a possible substrate for the crossed phrenic phenomenon. Exp Neurol. 1991;111:135–139. doi: 10.1016/0014-4886(91)90061-g. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Rafols JA. The phrenic nucleus of the albino rat: a correlative HRP and Golgi study. J Comp Neurol. 1981;201:441–456. doi: 10.1002/cne.902010309. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Rafols JA. The ultrastructure and synaptic architecture of phrenic motor neurons in the spinal cord of the adult rat. J Neurocytol. 1984;13:85–109. doi: 10.1007/BF01148320. [DOI] [PubMed] [Google Scholar]
- Grossman SD, Wolfe BB, Yasuda RP, Wrathall JR. Alterations in AMPA receptor subunit expression after experimental spinal cord contusion injury. J Neurosci. 1999;19:5711–5720. doi: 10.1523/JNEUROSCI.19-14-05711.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman SD, Wolfe BB, Yasuda RP, Wrathall JR. Changes in NMDA receptor subunit expression in response to contusive spinal cord injury. J Neurochem. 2000;75:174–184. doi: 10.1046/j.1471-4159.2000.0750174.x. [DOI] [PubMed] [Google Scholar]
- Hadley SD, Walker PD, Goshgarian HG. Effects of serotonin inhibition on neuronal and astrocyte plasticity in the phrenic nucleus 4 h following C2 spinal cord hemisection. Exp Neurol. 1999;160:433–445. doi: 10.1006/exnr.1999.7238. [DOI] [PubMed] [Google Scholar]
- Hadley SD, Walker PD, Goshgarian HG. Effects of the serotonin synthesis inhibitor p-CPA on the expression of the crossed phrenic phenomenon 4 h following C2 spinal cord hemisection. Exp Neurol. 1999;160:479–488. doi: 10.1006/exnr.1999.7240. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Coles SK, Bach KB, Mitchell GS, McCrimmon DR. Time-dependent phrenic nerve responses to carotid afferent activation: intact vs. decerebellate rats Am J Physiol. 1993;265:R811–9. doi: 10.1152/ajpregu.1993.265.4.R811. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Hinrichsen CF, McCrimmon DR. Short-term plasticity of descending synaptic input to phrenic motoneurons in rats. J Appl Physiol. 2003;94:1421–1430. doi: 10.1152/japplphysiol.00599.2002. [DOI] [PubMed] [Google Scholar]
- Herlitze S, Landmesser LT. New optical tools for controlling neuronal activity. Curr Opin Neurobiol. 2007;17:87–94. doi: 10.1016/j.conb.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Houle JD, Tom VJ, Mayes D, Wagoner G, Phillips N, Silver J. Combining an autologous peripheral nervous system “bridge” and matrix modification by chondroitinase allows robust, functional regeneration beyond a hemisection lesion of the adult rat spinal cord. J Neurosci. 2006;26:7405–7415. doi: 10.1523/JNEUROSCI.1166-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson SC, Tester NJ, Rose M, Blum AE, Howland BG, Bolser DC, Howland DR. Cough following low thoracic hemisection in the cat. Exp Neurol. 2010 Mar;222(1):165–70. doi: 10.1016/j.expneurol.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajana S, Goshgarian HG. Administration of phosphodiesterase inhibitors and an adenosine A1 receptor antagonist induces phrenic nerve recovery in high cervical spinal cord injured rats. Exp Neurol. 2008;210:671–680. doi: 10.1016/j.expneurol.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajana S, Goshgarian HG. Spinal activation of the cAMP-PKA pathway induces respiratory motor recovery following high cervical spinal cord injury. Brain Res. 2008;1232:206–213. doi: 10.1016/j.brainres.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkead R, Mitchell GS. Time-dependent hypoxic ventilatory responses in rats: effects of ketanserin and 5-carboxamidotryptamine. Am J Physiol. 1999;277:R658–66. doi: 10.1152/ajpregu.1999.277.3.R658. [DOI] [PubMed] [Google Scholar]
- Kishino A, Nakayama C. Enhancement of BDNF and activated-ERK immunoreactivity in spinal motor neurons after peripheral administration of BDNF. Brain Res. 2003;964:56–66. doi: 10.1016/s0006-8993(02)04066-0. [DOI] [PubMed] [Google Scholar]
- Kowalski KE, Romaniuk JR, DiMarco AF. Changes in expiratory muscle function following spinal cord section. J Appl Physiol. 2007 Apr;102(4):1422–8. doi: 10.1152/japplphysiol.00870.2006. [DOI] [PubMed] [Google Scholar]
- Lammari-Barreault N, Rega P, Gauthier P. Axonal regeneration from central respiratory neurons of the adult rat into peripheral nerve autografts: effects of graft location within the medulla. Neurosci Lett. 1991;125:121–124. doi: 10.1016/0304-3940(91)90006-f. [DOI] [PubMed] [Google Scholar]
- Lane MA, Lee KZ, Fuller DD, Reier PJ. Spinal circuitry and respiratory recovery following spinal cord injury. Respir Physiol Neurobiol. 2009;169:123–132. doi: 10.1016/j.resp.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, White TE, Coutts MA, Jones AL, Sandhu MS, Bloom DC, Bolser DC, Yates BJ, Fuller DD, Reier PJ. Cervical prephrenic interneurons in the normal and lesioned spinal cord of the adult rat. J Comp Neurol. 2008;511:692–709. doi: 10.1002/cne.21864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis LJ, Brookhart JM. Significance of the crossed phrenic phenomenon. Am J Physiol. 1951;166:241–254. doi: 10.1152/ajplegacy.1951.166.2.241. [DOI] [PubMed] [Google Scholar]
- Lee BB, Boswell-Ruys C, Butler JE, Gandevia SC. Surface functional electrical stimulation of the abdominal muscles to enhance cough and assist tracheostomy decannulation after high-level spinal cord injury. J Spinal Cord Med. 2008;31(1):78–82. doi: 10.1080/10790268.2008.11753985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Gorman RB, Saboisky JP, Gandevia SC, Butler JE. Optimal electrode placement for noninvasive electrical stimulation of human abdominal muscles. J Appl Physiol. 2007 Apr;102(4):1612–7. doi: 10.1152/japplphysiol.00865.2006. [DOI] [PubMed] [Google Scholar]
- Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB, Jr, Mitchell GS. Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the central neural control of breathing. J Neurosci. 2001;21:5381–8. doi: 10.1523/JNEUROSCI.21-14-05381.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L, Bach KB, Mitchell GS. Serotonin reveals ineffective spinal pathways to contralateral phrenic motoneurons in spinally hemisected rats. Exp Brain Res. 1994;101:35–43. doi: 10.1007/BF00243214. [DOI] [PubMed] [Google Scholar]
- Lovett-Barr MR, Windelborn JA, Sibigtroth CM, Mitchell GS. Daily acute intermittent hypoxia differentially affects BDNF and TrkB expression in uninjured and spinally injured rats. Soc Neuro. 2008:Abstract. 352.2/Y31. [Google Scholar]
- MacFarlane PM, Mitchell GS. Episodic spinal serotonin receptor activation elicits long-lasting phrenic motor facilitation by an NADPH oxidase-dependent mechanism. J Physiol. 2009;587:5469–81. doi: 10.1113/jphysiol.2009.176982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane PM, Satriotomo I, Windleborn JA, Mitchell GS. NADPH oxidase activity is necessary for acute intermittent hypoxia-induced phrenic long term facilitation. J Physiol. 2009;587:1931–42. doi: 10.1113/jphysiol.2008.165597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahamed S, Mitchell GS. Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnoea? Exp Physiol. 2007;92:27–37. doi: 10.1113/expphysiol.2006.033720. [DOI] [PubMed] [Google Scholar]
- Martin TP, Stein RB, Hoeppner PH, Reid DC. Influence of electrical stimulation on the morphological and metabolic properties of paralyzed muscle. J Appl Physiol. 1992 Apr;72(4):1401–6. doi: 10.1152/jappl.1992.72.4.1401. [DOI] [PubMed] [Google Scholar]
- Massey JM, Hubscher CH, Wagoner MR, Decker JA, Amps J, Silver J, Onifer SM. Chondroitinase ABC digestion of the perineuronal net promotes functional collateral sprouting in the cuneate nucleus after cervical spinal cord injury. J Neurosci. 2006;26:4406–4414. doi: 10.1523/JNEUROSCI.5467-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire M, Zhang Y, White DP, Ling L. Chronic intermittent hypoxia enhances ventilatory long-term facilitation in awake rats. J Appl Physiol. 2003;95:1499–508. doi: 10.1152/japplphysiol.00044.2003. [DOI] [PubMed] [Google Scholar]
- McGuire M, Zhang Y, White DP, Ling L. Serotonin receptor subtypes required for ventilator long-term facilitation and its enhancement after chronic intermittent hypoxia in awake rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R334–41. doi: 10.1152/ajpregu.00463.2003. [DOI] [PubMed] [Google Scholar]
- McGuire M, Zhang Y, White DP, Ling L. Phrenic long-term facilitation requires NMDA receptors in the phrenic motornucleus in rats. J Physiol. 2005;576:599–611. doi: 10.1113/jphysiol.2005.087650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire M, Liu C, Cao Y, Ling L. Formation and maintenance of ventilatory long-term facilitation require NMDA but not non-NMDA receptors in awake rats. J Appl Physiol. 2008;105:942–50. doi: 10.1152/japplphysiol.01274.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by a new central neural mechanism. Respir Physiol. 1980;41:87–103. doi: 10.1016/0034-5687(80)90025-0. [DOI] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by endogenous central serotonin. Respir Physiol. 1980;42:171–88. doi: 10.1016/0034-5687(80)90113-9. [DOI] [PubMed] [Google Scholar]
- Moreno DE, Yu XJ, Goshgarian HG. Identification of the axon pathways which mediate functional recovery of a paralyzed hemidiaphragm following spinal cord hemisection in the adult rat. Exp Neurol. 1992;116:219–228. doi: 10.1016/0014-4886(92)90001-7. [DOI] [PubMed] [Google Scholar]
- Nantwi KD, El Bohy A, Schrimsher GW, Reier PJ, Goshgarian HG. Spontaneous Functional Recovery in Paralyzed Hemidiaphragm Following Upper Cervical Spinal Cord Injury in Adult Rats. Neurorehabilitation and Repair. 1999;13:225–234. [Google Scholar]
- Nantwi KD. Recovery of respiratory activity after C2 hemisection (C2HS): involvement of adenosinergic mechanisms. Respir Physiol Neurobiol. 2009;169:102–114. doi: 10.1016/j.resp.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nantwi KD, Basura GJ, Goshgarian HG. Effects of long-term theophylline exposure on recovery of respiratory function and expression of adenosine A1 mRNA in cervical spinal cord hemisected adult rats. Exp Neurol. 2003;182:232–239. doi: 10.1016/s0014-4886(03)00109-2. [DOI] [PubMed] [Google Scholar]
- Nantwi KD, El-Bohy A, Goshgarian HG. Actions of systemic theophylline on hemidiaphragmatic recovery in rats following cervical spinal cord hemisection. Exp Neurol. 1996;140:53–59. doi: 10.1006/exnr.1996.0114. [DOI] [PubMed] [Google Scholar]
- Nantwi KD, Goshgarian HG. Theophylline-induced recovery in a hemidiaphragm paralyzed by hemisection in rats: contribution of adenosine receptors. Neuropharmacology. 1998;37:113–121. doi: 10.1016/s0028-3908(97)00190-1. [DOI] [PubMed] [Google Scholar]
- Nantwi KD, Goshgarian HG. Adenosinergic mechanisms underlying recovery of diaphragm motor function following upper cervical spinal cord injury: potential therapeutic implications. Neurol Res. 2005;27:195–205. doi: 10.1179/016164105X21977. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci USA. 2003;100:100073–8. doi: 10.1073/pnas.1734109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Prabhakar NR. Effect of two paradigms of chronic intermittent hypoxia on carotid body sensory activity. J Appl Physiol. 2004;96:1236–42. doi: 10.1152/japplphysiol.00820.2003. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Nanduri J, Yuan G, Wang N, Deneris E, Pendyala S, Natarajan V, Kumar GK, Prabhakar NR. NADPH oxidase is required for the sensory plasticity of the carotid body by chronic intermittent hypoxia. J Neurosci. 2009;29:4903–10. doi: 10.1523/JNEUROSCI.4768-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips LH. The epidemiology of myasthenia gravis. Semin Neurol. 2004;24:17. doi: 10.1055/s-2004-829593. [DOI] [PubMed] [Google Scholar]
- Pitts RF. The respiratory center and its descending pathways. J Comp Neurol. 1940;72:605–625. [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Landi S, Baldini S, Berardi N, Maffei L. Structural and functional recovery from early monocular deprivation in adult rats. Proc Natl Acad Sci U S A. 2006;103:8517–8522. doi: 10.1073/pnas.0602657103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter WT. The path of the respiratory impulse from the bulb to the phrenic nuclei. J Physiol. 1895;17:455–485. doi: 10.1113/jphysiol.1895.sp000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves SR, Gozal D. Respiratory and metabolic responses to early postnatal chronic intermittent hypoxia and sustained hypoxia in the developing rat. Pediatr Res. 2006;60:680–6. doi: 10.1203/01.pdr.0000246073.95911.18. [DOI] [PubMed] [Google Scholar]
- Rosenbluth A, Ortiz T. The crossed respiratory impulses to the phrenic. Am J Physiol. 1936;117:495–513. [Google Scholar]
- Row BW. Intermittent hypoxia and cognitive function: implications from chronic animal models. Adv Exp Med Biol. 2007;618:51–67. doi: 10.1007/978-0-387-75434-5_5. [DOI] [PubMed] [Google Scholar]
- Row BW, Kheirandish L, Cheng Y, Rowell PP, Gozal D. Impaired spatial working memory and altered choline acetyltransferase (CHAT) immunoreactivity and nicotinic receptor binding in rats exposed to intermittent hypoxia during sleep. Behav Brain Res. 2007;177:308–14. doi: 10.1016/j.bbr.2006.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymer WZ, Hornby T, Mitchell GS, Schmit BD, Trumbower RD. Effects of intermittent hypoxia on motor function in persons with incomplete SCI. Soc Neuro. 2007:Abstract. 82.18/LL2. [Google Scholar]
- Sander BH, Dieck T, Homrighausen F, Tschan CA, Steffens J, Raymondos K. Electromagnetic ventilation: first evaluation of a new method for artificial ventilation in humans. Muscle Nerve. 2010 Sep;42(3):305–10. doi: 10.1002/mus.21698. [DOI] [PubMed] [Google Scholar]
- Schilero GJ, Spungen AM, Bauman WA, Radulovic M, Lesser M. Pulmonary function and spinal cord injury. Respir Physiol Neurobiol. 2009 May 15;166(3):129–41. doi: 10.1016/j.resp.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Slack SE, Pezet S, McMahon SB, Thompson SW, Malcangio M. Brain-derived neurotrophic factor induces NMDA receptor subunit one phosphorylation via ERK and PKC in the rat spinal cord. Eur J Neurosci. 2004;20:1769–78. doi: 10.1111/j.1460-9568.2004.03656.x. [DOI] [PubMed] [Google Scholar]
- Steinmetz MP, Horn KP, Tom VJ, Miller JH, Busch SA, Nair D, Silver DJ, Silver J. Chronic enhancement of the intrinsic growth capacity of sensory neurons combined with the degradation of inhibitory proteoglycans allows functional regeneration of sensory axons through the dorsal root entry zone in the mammalian spinal cord. J Neurosci. 2005;25:8066–8076. doi: 10.1523/JNEUROSCI.2111-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai Q, Goshgarian HG. Ultrastructural quantitative analysis of glutamatergic and GABAergic synaptic terminals in the phrenic nucleus after spinal cord injury. J Comp Neurol. 1996;372:343–355. doi: 10.1002/(SICI)1096-9861(19960826)372:3<343::AID-CNE2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Tai Q, Palazzolo KL, Goshgarian HG. Synaptic plasticity of 5-hydroxytryptamine-immunoreactive terminals in the phrenic nucleus following spinal cord injury: a quantitative electron microscopic analysis. J Comp Neurol. 1997;386:613–624. [PubMed] [Google Scholar]
- Tasali E, Ip MS. Obstructive sleep apnea and metabolic syndrome: alterations in glucose metabolism and inflammation. Proc Am Thorac Soc. 2008;5:207–17. doi: 10.1513/pats.200708-139MG. [DOI] [PubMed] [Google Scholar]
- Teng YD, Bingaman M, Taveira-DaSilva AM, Pace PP, Gillis RA, Wrathall JR. Serotonin 1A receptor agonists reverse respiratory abnormalities in spinal cord-injured rats. J Neurosci. 2003;23:4182–4189. doi: 10.1523/JNEUROSCI.23-10-04182.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng YD, Mocchetti I, Taveira-DaSilva AM, Gillis RA, Wrathall JR. Basic fibroblast growth factor increases long-term survival of spinal motor neurons and improves respiratory function after experimental spinal cord injury. J Neurosci. 1999;19:7037–7047. doi: 10.1523/JNEUROSCI.19-16-07037.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinit S, Lovett-Barr MR, Mitchell GS. Intermittent hypoxia induces functional recovery following cervical spinal injury. Respir Physiol Neurobiol. 2009;169:210–7. doi: 10.1016/j.resp.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tester NJ, Howland DR. Chondroitinase ABC improves basic and skilled locomotion in spinal cord injured cats. Exp Neurol. 2008;209:483–496. doi: 10.1016/j.expneurol.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumbower RD, Jayaraman A, Mitchell GS, Rymer WZ. Exposure to Acute Intermittent Hypoxia Augments Somatic Motor Function in Humans With Incomplete Spinal Cord Injury. Neurorabil Neural Repair. 2011 doi: 10.1177/1545968311412055. In Press. [DOI] [PubMed] [Google Scholar]
- Tom VJ, Houle JD. Intraspinal microinjection of chondroitinase ABC following injury promotes axonal regeneration out of a peripheral nerve graft bridge. Exp Neurol. 2008;211:315–319. doi: 10.1016/j.expneurol.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom VJ, Sandrow-Feinberg HR, Miller K, Santi L, Connors T, Lemay MA, Houle JD. Combining peripheral nerve grafts and chondroitinase promotes functional axonal regeneration in the chronically injured spinal cord. J Neurosci. 2009;29:14881–14890. doi: 10.1523/JNEUROSCI.3641-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinit S, Lovett-Barr MR, Mitchell GS. Intermittent hypoxia induces functional recovery following cervical spinal injury. Respir Physiol Neurobiol. 2009;169:210–7. doi: 10.1016/j.resp.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter JS, Wurster RD, Zhu Q, Staunton C, Laghi F. Stimulating multiple respiratory muscles with intramuscular Permaloc electrodes. J Spinal Cord Med. 2010;33(2):135–43. doi: 10.1080/10790268.2010.11689688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TE, Lane MA, Sandhu MS, O’Steen BE, Fuller DD, Reier PJ. Neuronal progenitor transplantation and respiratory outcomes following upper cervical spinal cord injury in adult rats. Exp Neurol. 225:231–236. doi: 10.1016/j.expneurol.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson JE, Satriotomo I, Baker-Herman TL, Watters JJ, Mitchell GS. Okadaic acid-sensitive protein phosphatases constrain phrenic long-term facilitation after sustained hypoxia. J Neurosci. 2008;28:2949–58. doi: 10.1523/JNEUROSCI.5539-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson JE, Mitchell GS. Daily intermittent hypoxia augments spinal BDNF levels, ERK phosphorylation and respiratory long-term facilitation. Exp Neurol. 2009;217:116–23. doi: 10.1016/j.expneurol.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, Wang L, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, Deisseroth K. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- Zhou SY, Basura GJ, Goshgarian HG. Serotonin(2) receptors mediate respiratory recovery after cervical spinal cord hemisection in adult rats. J Appl Physiol. 2001;91:2665–2673. doi: 10.1152/jappl.2001.91.6.2665. [DOI] [PubMed] [Google Scholar]
- Zhou SY, Goshgarian HG. Effects of serotonin on crossed phrenic nerve activity in cervical spinal cord hemisected rats. Exp Neurol. 1999;160:446–453. doi: 10.1006/exnr.1999.7213. [DOI] [PubMed] [Google Scholar]
- Zhou SY, Goshgarian HG. 5-Hydroxytryptophan-induced respiratory recovery after cervical spinal cord hemisection in rats. J Appl Physiol. 2000;89:1528–1536. doi: 10.1152/jappl.2000.89.4.1528. [DOI] [PubMed] [Google Scholar]
- Zimmer MB, Goshgarian HG. Spinal activation of serotonin 1A receptors enhances latent respiratory activity after spinal cord injury. J Spinal Cord Med. 2006;29:147–155. doi: 10.1080/10790268.2006.11753868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer MB, Nantwi K, Goshgarian HG. Effect of spinal cord injury on the respiratory system: basic research and current clinical treatment options. J Spinal Cord Med. 2007;30:319–330. doi: 10.1080/10790268.2007.11753947. [DOI] [PMC free article] [PubMed] [Google Scholar]


