Abstract
Embryonic stem (ES) cells are pluripotent cells that can differentiate into all three main germ layers: endoderm, mesoderm, and ectoderm. Although a number of methods have been developed to differentiate ES cells into neuronal phenotypes such as sensory and motor neurons, the efficient generation of GABAergic interneurons from ES cells still presents an ongoing challenge. Because the main output of inhibitory GABAergic interneurons is the gamma-aminobutyric-acid (GABA), a neurotransmitter whose controlled homeostasis is required for normal brain function, the efficient generation in culture of functional interneurons may have future implications on the treatment of neurological disorders such as epilepsy, autism, and schizophrenia. The goal of this work was to examine the generation of GABAergic neurons from mouse ES cells by comparing an embryoid body-based methodology versus a hydrogel-based encapsulation protocol that involves the use of all-trans-retinoid acid (RA). We observed that 1) there was a 2-fold increase in neuronal differentiation in encapsulated versus non-encapsulated cells and 2) there was an increase in the specificity for interneuronal differentiation in encapsulated cells, as assessed by mRNA expression and electrophysiology approaches. Furthermore, our results indicate that most of the neurons obtained from encapsulated mouse ES cells are GABA-positive (~87%). Thus, these results suggest that combining encapsulation of ES cells and RA treatment provide a more efficient and scalable differentiation strategy for the generation in culture of functional GABAergic interneurons. This technology may have implications for future cell replacement therapies and the treatment of CNS disorders.
Keywords: stem cell, neuron, encapsulation, GABAergic, differentiation
Introduction
Embryonic stem (ES) cells have the potential to differentiate into all three cell lineages (i.e., endoderm, mesoderm, and ectoderm), providing a new perspective not only for embryonic development but also for their application in cell replacement therapies (Murry et al., 2008; Weitzer et al., 2006). ES cells are derived from the inner cell mass of blastocyte-stage (day 3.5) embryos (Williams et al., 1988) and have been experimentally differentiated into various cell types including cardiac, skeletal, and neuronal cells (Choi et al., 2005). A number of studies have shown that treatment of ES cells with RA enhances their efficiency of neuronal differentiation (Bain et al., 1995; Fraichard et al., 1995; Strübing et al., 1995; see Clagett-Dame et al., 2006 and Soprano et al., 2007 for recent reviews). However, the generation in culture of GABAergic neurons is difficult and very challenging, and usually requires the employment of expensive growth factors such as basic Fibroblast Growth Factor (bFGF) and Epidermal Growth Factor (EGF) (Chatzi et al., 2009). Similarly, other strategies involve complex cell manipulations such as the generation of neural progenitors and subsequent withdrawal of mitogens (Westmoreland et al., 2001) or sequential RA treatment followed by potassium chloride depolarization (Bosch et al., 2004).
GABAergic interneurons release gamma-aminobutyric-acid (GABA), which is the main inhibitory neurotransmitter in the central nervous system (CNS). A number of neurological conditions such as Huntington’s disease, epilepsy, chronic pain, anxiety and other mood disorders are associated with a remarkable dysfunctional GABAergic inhibition and neuronal hyperexcitability in the CNS (Benes and Berreta, 2001, Benes et al., 2007; Brambilla et al., 2003, Cicchetti and Parent, 1996, Kumar and Buckmaster, 2006). Because stem cell-based therapies that involve the transplantation of inhibitory interneurons are now being developed to treat a spectrum of neurological conditions, the efficient generation of GABAergic neurons in culture is germane to the success of these cell replacement strategies.
Most of the current ES to GABAergic differentiation strategies employ the generation of embryoid bodies (EBs), which are cell aggregates comprised of all three germ layers. However, these strategies possess inherent limitations that affect the efficiency of ES differentiation due to 1) a wide variability in EB size (Gerami-Naini et al., 2004), 2) aggregation among EBs in high concentration static cultures (Dang et al., 2004), and 3) limited diffusion of inducing morphogens into the EBs (Carpenedo et al., 2010), among others. A strategy currently employed to overcome this problem is the encapsulation of cells in biologically compatible hydrogels. Hydrogels composed of materials such as agarose (Bauwens et al., 2005), dextran (Doetschman et al., 1985), and gelatin (Akasha et al., 2008) have been used for culturing ES cells. However, alginic acid or alginate, a polysaccharide obtained from brown algae (Hwang et al., 2009; Maguire et al., 2006; Magyar et al., 2001), remains the encapsulation material of choice because of its intrinsic properties (Orive et al., 2003). Alginates are natural linear polysaccharides with 1, 4-linked β-d-mannuronate and α-l-guluronate residues arranged as blocks of similar and alternating residues (Silva et al., 2010). Alginate is an appealing material for the construction of “biohybrid organs” and “micro-bioreactors” because its hydrated 3D network allows cells to adhere, spread, migrate and interact with other cells (Zimmermann et al., 2001). The alginate technology has also been applied to the generation of well-vascularized EBs (Gerecht-Nir et al., 2004; Magyar et al., 2001). Similarly, other researchers have demonstrated that the alginate-based encapsulation of ES cells can increase the efficiency of differentiation along various cell lineages (Bauwens et al., 2005; Hwang et al., 2009; Jing et al., 2010; Li et al., 2011; Maguire et al., 2007; Wang et al., 2009). Thus, encapsulation of cells in hydrogels represents an important method for improving current stem cell differentiation strategies.
In this work, we generated GABAergic neurons from mouse ES cells using an alginate-based encapsulation protocol. We show that up to 87% GABAergic neurons are obtained using this protocol without the need of growth factors. We also show that the differentiated GABAergic neurons produce large outward K+ currents typical of mature neurons. Our results shed a new light on the effect of cell environment during the neuronal differentiation of mouse ES cells and may provide new therapeutic opportunities for the future treatment of neurological diseases.
Materials and methods
ES cell culture
The cells used in this study were the EMC-ES-Hoxa1-1 (E1) and JI Wild type mouse ES cell-lines. The generation and characterization of the E1 ES cell line from C57Bl/6 mice was previously described (Martinez-Ceballos et al., 2005). J1 is a commonly-used ES cell line obtained from the American Type Culture Collection (ATCC). The ES cells were maintained in an undifferentiated state by culturing them in ES medium (Dulbecco’s modified Eagle’s medium (Invitrogen) supplemented with 10% ES qualified fetal bovine serum (Atlanta Biologicals), 100 μM MEM nonessential Amino acids, 0.1 mM β-mercaptoethanol, Penicillin and Streptomycin (Invitrogen), 1 mM sodium pyruvate, and 1 × 103 U/ml leukemia inhibitory factor (LIF, Esgro, Millipore)). To avoid spontaneous differentiation, the ES cells were passaged every two days for up to three passages before induction of differentiation.
Generation of embryoid bodies
To determine the percentage of neurons obtained using a standard protocol that requires EB formation, 3 × 106 cells were plated onto 100 mm non-adherent dishes in the absence of LIF as previously described (Martinez-Ceballos and Gudas, 2008). The aggregates were cultured in ES medium for 8 days and the medium was changed every two days. RA (5 μM, 5RA) or vehicle-only (ethanol, 0RA) was added on days 4 and 6 of EB formation. On day 8, the EBs were harvested, disaggregated with Accutase (Millipore), and cell viability was determined by the trypan blue exclusion method using a Cellometer (Nexcellom, Inc.). In separate experiments, day 8 EBs were either fixed for sectioning as previously described (Martinez-Ceballos and Gudas, 2008) or disaggregated and fixed for immunofluorescence analyses.
ES cell encapsulation
Undifferentiated ES cells were suspended in a solution of sterile 1.1% (w/v) alginic acid and 0.1% (v/v) porcine gelatin at a final concentration of 2.5 × 104 cells/ml. Using an 18-gauge (18G) needle, the cell suspension was dropped into a sterile alginate gelation solution (100 mM CaCl2, 10 mM HEPES, 0.01% (v/v) Tween 20) at pH 7.4 with stirring as described by Hwang and coworkers (2009). The average volume of the cell suspension drops was 50 μl. The hydrogels were allowed to solidify in the CaCl2 solution for 5 minutes and were washed 3 times with ES medium. The encapsulated ES cells were then cultured in ES medium without LIF for 8 days. Medium was replenished every 2 days and RA (5 μM, 5RA) was added at days 4 and 6. Control cells in hydrogels were treated with vehicle only (0RA). On day 8, depolymerization buffer (50 mM tri-sodium citrate dihydrate, 77 mM sodium chloride and 10 mM HEPES) was added to the hydrogels to harvest the cells for the appropriate assays.
Neuronal Differentiation
To induce neuronal differentiation, both day 8 encapsulated and non-encapsulated cells were harvested and plated at a density of 1.5 × 105 cells per cm2 on tissue culture dishes pre-coated with poly-D-lysine and laminin (PDL/laminin) as described by Bibel and coworkers (2004). The cells were cultured in N2 medium (Neurobasal medium containing N2 supplement (Invitrogen)) to allow for the selection of neuronal lineages. The N2 medium was changed 2 hrs after plating and then again after 24 hrs. The total culture time in N2 medium was 2 days (2N, Bibel et al., 2004) In order to promote neuronal maturation, the N2 medium was removed and cells were cultured for four days in maturation medium (B27-supplemented Neurobasal medium) to induce neuronal maturation (4M stage). For the first two days of this maturation step, 5 μM RA was also added to the cells. For the remaining two days of this 4M stage, cells were cultured in the absence of RA. The cell fate of the differentiated cells obtained at 4M was examined by immunofluorescence.
Immunofluorescence analyses
ES cells were fixed in 4% formalin for 15 min, followed by permeabilization for 20 min in 0.1% Triton X-100. Samples were blocked with goat or horse serum and incubated with the appropriate primary antibodies for 1 hr. The primary antibodies used include rabbit anti-β-tubulin III (Covance, Berkeley, CA), mouse anti-Nestin (Rat401, Developmental Studies Hybridoma Bank, Iowa City, IA), guinea pig anti-GABA (Millipore), rabbit anti-GAD 65/67 (Millipore), goat anti-Parvalbumin (Santa Cruz Biotechnologies), and goat anti-Somatostatin (Santa Cruz Biotechnologies). All primary antibody dilutions were 1:1000. Secondary antibodies included goat anti-rabbit AlexaFluor 488, goat anti-rabbit AlexaFluor 594, chicken anti-goat AlexaFluor 488, and goat anti-guinea pig AlexaFluor 488. Immunostained cells were examined using a Nikon E400 fluorescence microscope equipped with a Diagnostic Instruments RT-Spot Slider digital camera. To determine the percentage of GABAergic neurons obtained from independent triplicate experiments, cells were harvested by trypsinization at day 4M and the expression of various GABAergic markers was examined by immunofluorescence. The total number of cells was determined by nuclear visualization using 6-diamidino-2-phenylindole (DAPI). A similar procedure was employed to determine the percentage of neurons expressing parvalbumin or somatostatin, except that in this case the percentage of cells positive for either of these markers was determined based on the total number of cells positive for β-tubulin III. Statistical significance was determined by one-way ANOVA.
RT-PCR analyses
Semi-quantitative RT-PCR was employed to investigate the mRNA expression of various neuronal and non-neuronal markers in RA-treated versus control encapsulated or non-encapsulated cells. For this purpose, total RNA from day 8 samples was extracted using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. The concentration of RNA was determined using a NanoDrop (Thermo Scientific). RT-PCR amplification mixtures (20 μl) contained 10 ng of template cDNA. PCR reactions were performed using the following primers: 36B4, forward 5′-AGAACAACCCAGCTCTGGAGAA-3′, reverse 5′-ACACCCTCCAGAAAGCGAGAGT-3′, 25 cycles, 58°C, (Martinez-Ceballos et al., 2008); Rex-1, forward 5′-TGACAAAGGGGACGAAGCAAGAG-3′, reverse 5′-GCCATCAAAAGGACACACAAAG-3′, 25 cycles, 58°C (Shi et al., 2006); Nanog, forward 5′-GCGGACTGTGTGTTCTCTCAGGC-3′, reverse 5′-TTCCAGATCCGTTCACCAGATAG-3′, 25 cycles, 58°C (Shi et al., 2006); GFAP, forward 5′-ACAGAGGAGTGGTATCGGTCTA-3′, reverse 5′-CTCCTCCAGCCGAGCAAGT-3′, 28 cycles, 58°C; GAD1, forward 5′-CAGAACCAGAATCATCGGCCAT-3′, reverse 5′-CTGTAGTTGCTTGCGAGATGGT-3′, 28 cycles, 58°C; TH, forward 5′-TGGGGAGCTGAAGGCTTACGGTGC-3′, reverse 5′-ACAGCATGAAGGGCAGGAGGAATGC-3′, 30 cycles, 58°C; GLT1, forward 5′-AGAGGCTGCCCGTTAAATACCG-3, reverse 5′-GTAATACACCATAGCTCTCGT-3′, 30 cycles, 52°C (NM_001077514.3). Amplified PCR products were analyzed on 2% agarose gels, visualized by staining with ethidium bromide, and sequenced to verify their identity. Reactions were performed at least two times from independent RNA samples.
Electrophysiology recordings
Cells were maintained in external solution of the following composition (in mM): NaCl 130, KCl 3, CaCl2 2, MgCl2 0.6, NaHCO3 1, HEPES 10, glucose 5, pH 7.4 adjusted with NaOH. The internal solution contained (in mM): KCl 140, CaCl2 0.1, EGTA 1, MgCl2 2, ATP 2, HEPES 10, pH 7.2 adjusted with Tris. The osmolarity of the solutions were ~300 mOsm/L adjusted with sucrose. Voltage-gated delayed rectifier K+ currents were recorded in the tight-seal whole-cell configuration mode at 21-25°C. High-resolution current recordings were acquired by a computer-based patch-clamp amplifier system (EPC-10, HEKA, Lambrecht, Germany). Patch pipettes had resistances between 3-5 MΩ. Immediately following establishment of the whole-cell configuration, voltage ramps of 50 ms duration spanning the voltage range of −100 to +100 mV were delivered from a holding potential of −80 mV at a rate of 0.5 Hz over a period of 60 s. Data were extracted and analyzed at +50 mV. To demonstrate the effect of depolarization on voltage-gated delayed rectifier K+ currents, the holding potential was changed to 0 mV. Data are shown as means + S.E.M. or by representative traces, and were plotted using Igor Pro 5 software program (Wavemetrics, Portland, OR, USA). Peak currents were analyzed using paired Student’s t-test. Statistical significance was established at P<0.05.
Results
Encapsulation of mouse ES cells increases the efficiency of neuronal differentiation
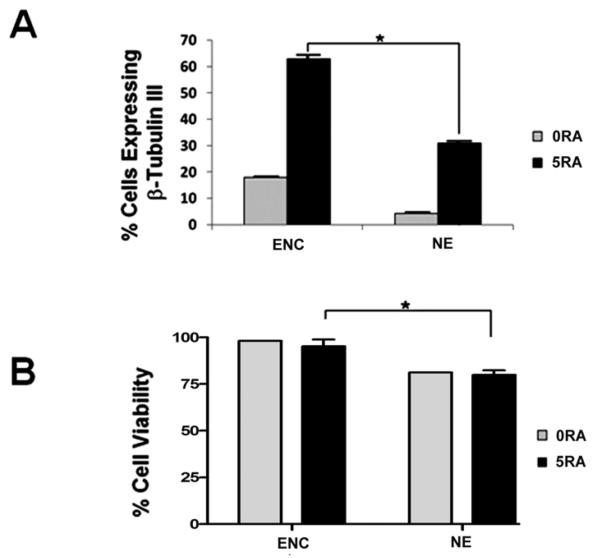
Cell encapsulation has been employed for the directed differentiation of murine embryonic stem cells into mature cells of all three lineages (Hwang at al., 2009; Jing et al., 2010; Li et al., 2011; Maguire et. al., 2006, Maguire et al., 2007). To determine if ES cell encapsulation can increase the efficiency of neuronal differentiation, we designed a differentiation protocol that involves the treatment of encapsulated mouse ES cells with retinoic acid and compared these results to those obtained using a traditional differentiation protocol that involves the formation of embryoid bodies. Encapsulated cells (Enc) and EBs (non-encapsulated or NE) were treated with vehicle only or RA on days 4 and 6. At day 8 of culture, cells were harvested, trypsinized, and fixed for immunofluorescence to examine the expression of the β-tubulin III neuronal marker. After imaging, the number of β-tubulin III-expressing cells was determined from three different fields in triplicate experiments. As shown in figure 1A for non-encapsulated cells, RA treatment increased the number of β-tubulin III-expressing cells by about 7-fold as compared to control NE cells. In encapsulated E1 ES cells, RA treatment resulted in a 3.5-fold increase in the number of β-tubulin III-expressing cells. The highest percentage of neuronal cell generation was obtained from RA-treated encapsulated cells (Enc, 5RA). As shown in figure 1A, there was a 2-fold increase in the efficiency of neuronal differentiation in the presence of 5μM RA in encapsulated ES cells (~60%) as compared to non-encapsulated cells (~30%). Interestingly, cell encapsulation increased the neuronal differentiation of ES cells by about 3.5-fold in the absence of RA treatment, which indicates that the hydrogels used in this study possess intrinsic properties that promote the differentiation of mouse ES cells along a neuroectodermal lineage. These results indicate that cell encapsulation, together with 5μM RA treatment, can be employed to increase the efficiency of neuronal differentiation in mouse ES cells.
Figure 1. Cell encapsulation increases the efficiency of ES neuronal differentiation and improves ES cell viability.
A) Quantitative analysis of immunofluorescence experiments showing the percentage of neuronal differentiation in encapsulated (Enc) versus non-encapsulated (NE) ES cells. Cells treated with vehicle only (0RA) or 5μM RA (5RA) were examined at day 8 for the expression of β-tubulin III by immunofluorescence. The percentage of cells expressing β-tubulin III was calculated based on the total number of cells as determined by nuclear staining with DAPI. Quantitative analyses from triplicate experiments demonstrated that there was a 2-fold increase in β-tubulin expression in encapsulated versus non-encapsulated cells after RA treatment. B) Cell viability was determined by the trypan blue exclusion method. These results demonstrated that encapsulation significantly increased the viability of mouse ES cells as compared to non-encapsulated cells. *p<0.05 by one-way ANOVA. Means ± SEM are shown.
Cell viability in encapsulated versus non-encapsulated ES cells
To determine if differences in cell viability account for the higher percentage of neuronal differentiation observed in encapsulated versus non-encapsulated ES cells, day 8 encapsulated and non-encapsulated cell samples were harvested and disaggregated with Accutase to determine the percentage of cell death using the trypan blue exclusion method. Live cells were counted using a Cellometer. As shown in figure 1B, there was no difference in the percentage of cell viability between control and RA-treated encapsulated cells (about 95-98%). Similarly, RA treatment did not affect the cell viability of NE cells (about 82%). However, there was a clear reduction in cell viability in NE cells as compared to encapsulated cells. Thus, encapsulation increased the viability of mouse ES cells in a significant manner (p<0.05) as compared to the non-encapsulation conditions after 8 days of culture and RA treatment. Taken together, the results shown in figure 1 suggest that an increase in cell viability may be one of the factors that contribute to the higher efficiency of neuronal differentiation observed in encapsulated versus non-encapsulated cells.
Encapsulation increases the number of neuronal precursors
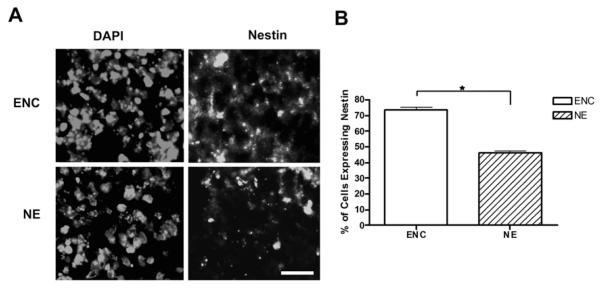
Our findings that encapsulation of ES cells increased the percentage of immature neurons, which express β-tubulin III, prompted us to investigate whether this effect was due to an increase in the number of neuronal precursors or was the result of a more efficient differentiation of neuronal precursors into immature neurons. For this purpose, we examined the expression of Nestin, an intermediate filament protein expressed in neuronal progenitors (Frederiksen and McKay, 1988; Lendahl et al., 1990), in day 8 encapsulated versus non-encapsulated cells cultured in the presence of RA. As shown in figure 2, a higher percentage of Nestin-positive cells was observed in encapsulated versus non-encapsulated cells (about 75% and 47%, respectively), which roughly corresponds to a 1.6-fold increase. Since this increase is similar to that obtained after examining β-tubulin III expression (see Figure 1, panel A), these results suggest that encapsulation enhances the efficiency of neuronal differentiation mainly by increasing the number of neuronal precursor rather than promoting the differentiation of precursors into immature neurons.
Figure 2. Encapsulation enhances the generation of neuronal precursors in RA-treated cells.
Encapsulated ES cells or EBs were treated with 5 μM RA and harvested at day 8. Encapsulated aggregates and EBs were either sectioned or disaggregated for the determination of Nestin expression by immunofluorescence. A) Representative sections of encapsulated (ENC) or non-encapsulated (NE) cells showing Nestin expression. B) Statistical analysis showing the percentage of Nestin-positive ENC or NE cells in disaggregated samples. The total number of cells was determined by nuclear staining with DAPI. These experiments demonstrated that encapsulation increased the percentage of neuronal precursors by ~1.6-fold. Scale bar, 10 μm. *p<0.05.
Expression of neuronal and glial markers in differentiated ES cells by RT-PCR
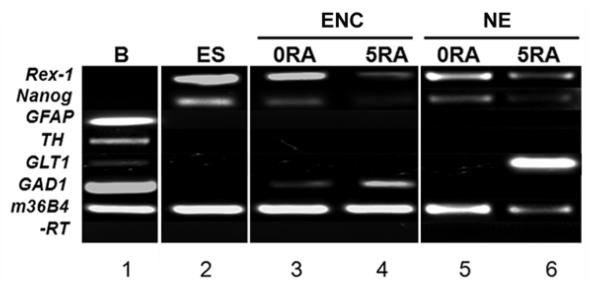
Since β-tubulin III expression does not discriminate among the different types of neurons generated from ES cells, we next employed RT-PCR to determine the type of neurons generated from encapsulated versus non-encapsulated cells at day 8 of culture. In addition, we also examined the expression of the stem cell markers Nanog and Rex-1 in order to compare the degree of ES cell differentiation between the two methodologies tested. While expression of both Nanog and Rex-1 mRNA was detected in both encapsulated and non-encapsulated control cells (no RA, Fig. 3, lanes 3 and 5), RA treatment resulted in a reduction of the mRNA levels of these two stem cell markers, which confirms the inducive effect that RA exerts on ES cell differentiation. In encapsulated cells, expression of the GABAergic marker GAD1 was detected at low levels in control cells and at higher levels in RA treated cells (compare lane 3 with 4). No expression of GFAP (astrocyte marker), TH (Tyrosine Hydroxylase, dopaminergic marker), or GLT1 (astroglial and glutamatergic marker) was detected in control or RA-treated encapsulated cells. In non-encapsulated cells, expression of GLT1 mRNA was detected only in the presence of RA treatment (lane 6), which indicates that RA supports the differentiation of non-encapsulated mouse ES cells along a glutamatergic lineage. No mRNA expression of GFAP, TH, or GAD1 was detected in non-encapsulated cells either in the presence or absence of RA treatment. Together, our results suggest that encapsulation alone is capable of promoting the differentiation of ES cells into GABAergic neurons even in the absence of RA treatment. In turn, RA may function either as an enhancer or as a co-activator of GABAergic differentiation in encapsulated cells.
Figure 3. Characterization of day 8 encapsulated versus non-encapsulated ES cells by RT-PCR.
The mRNA expression levels of various neuronal and non-neuronal markers was examined by non-quantitative RT-PCR in day 8 Encapsulated (Enc) or Non-encapsulated (NE) cells treated with vehicle-only (0RA) or 5 μM RA (5RA). Total RNA was reverse transcribed and cDNA was used as template for PCR employing specific primer pairs for the following markers: Rex1 and Nanog (stem cell markers), GFAP (differentiated astrocytes), TH (dopaminergic neurons), GLT1 (astroglial and glutamatergic neurons), GAD1 (also called GAD67; GABAergic neurons), and 36B4 (a ubiquitously-expressed gene used as a loading control). -RT: no reverse transcriptase. B= Adult mouse brain tissue. ES = Embryonic stem cells.
GABAergic neuronal differentiation of mouse ES cells
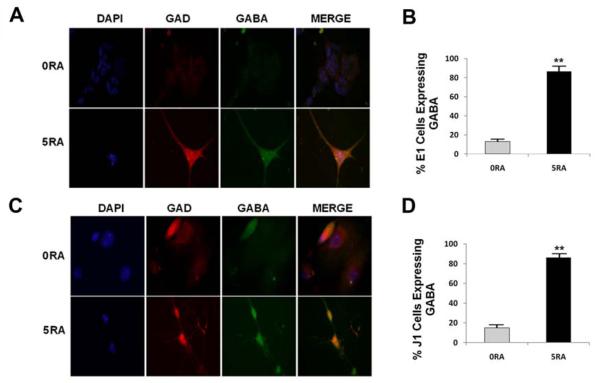
Our RT-PCR results suggest that, after eight days of differentiation, our protocol using cell encapsulation plus RA treatment results in the generation of GABAergic neurons, but not of astrocytes or dopaminergic neurons. To further confirm these results at a later time point of this protocol, encapsulated E1 cells were transferred to PDL/laminin-coated plates and were allowed to differentiate up to the 4M stage (i.e., after 4 days in maturation medium). Next, we examined the expression of the GABAergic markers GABA and glutamic acid decarboxylase (GAD 65/67) by immunofluorescence analyses (Fig. 4). As shown in representative images from these experiments (Fig. 4A), and from the corresponding statistical analyses shown in figure 4B, we observed that there was a 6.6-fold increase in the percentage of GABA-expressing cells after RA treatment (86.5%), as compared to untreated controls (13%). Similarly, RA treatment of encapsulated ES cells resulted in a 6.3-fold increase in the percentage of GAD 65/67-expressing cells (77%) as compared to the untreated controls (12.2%) (data not shown).
Figure 4. Neurons Generated from Encapsulated mouse ES Cells are GABA-positive.
Neurons obtained at the 4M stage were fixed and the expression of GABAergic markers was examined by immunofluorescence. A) Co-expression of the GABAergic markers GAD65/67 (GAD) and GABA in differentiated E1 ES cells treated with vehicle-only (0RA) or 5 μM RA (5RA). Encapsulated E1 cells were harvested at day 8 followed by plating on PDL/laminin-coated dishes. The expression of GAD and GABA was examined by immunofluorescence at 4M. B) Quantitative analysis from independent triplicate experiments showing the percentage of GABA-expressing cells based on the total number of cells as determined by nuclear DAPI staining. C) GABAergic marker expression in differentiated J1 cells at 4M. D) The percentage of GABA-expressing cells was determined from triplicate experiments. The results obtained using E1 and J1 ES cells were similar. **p<0.001 by one-way ANOVA. Means + SEM are shown.
To verify that our results can be reproduced using another mouse ES cell line, we next determined whether the widely employed J1 mouse ES cell line (ATCC) could also produce GABAergic neurons after being subjected to our encapsulation protocol. As shown in figures 4C and 4D, our differentiation methodology resulted in a 5.7-fold increase in the number of GABA-expressing cells in the RA treated group (86%) as compared to the control (15%). This amounts to a 5.7-fold increase in the number of GABA-expressing cells due to the RA treatment of encapsulated J1 ES cells (Fig. 4D). Thus, these results indicate that the differentiating capabilities of our E1 mouse ES cell line are similar to those of a standard mouse ES cell line. Our results also indicate that our protocol involving mouse ES cell encapsulation plus 5μM RA treatment results in the efficient generation of GABAergic neurons without the employment of growth factors such as bFGF and EGF.
Expression of somatostatin and parvalbumin in 4M neurons
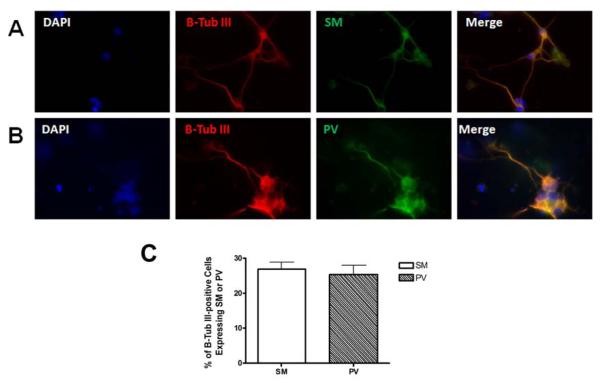
The neuropeptide somatostatin (SM) is a marker for cortical GABAergic interneurons (Gonchar and Burkhalter, 1997; Kawaguchi and Kubota, 1997), while parvalbumin (PV) is a Ca2+-binding protein that is expressed in fast-spiking cortical interneurons (Cauli et al., 1997; Freund and Buzsaki, 1996; Kawaguchi and Kubota, 1997, 1998). Since SM and PV are not co-expressed in the same subtype of GABAergic neurons, SM is useful in identifying PV-independent neurons (Cauli et al., 1997; Gonchar and Burkhalter, 1997; Kawaguchi and Kubota, 1997), and vice versa. Thus, to determine which GABAergic subpopulation is generated using our encapsulation protocol, we performed immunofluorescence on 4M cells using SM, PV, and β-tubulin III antibodies. As shown in figure 5, both neuronal subtypes expressing SM (panel A) or PV (panel B) were detected after four days of culture in maturation medium. In average, about 27% and 25% of the neurons obtained expressed SM and PV, respectively (panel C). The co-expression of β-tubulin III with SM, or with PV, demonstrated the neuronal identity of cells stained with these GABAergic markers. Taken together, these results indicate that our differentiation protocol generates GABAergic neurons of mixed subtypes.
Figure 5. Neurochemical characterization of differentiated GABAergic neurons.
Neurons obtained from mouse E1 ES cells were examined at 4M for the expression of A) somatostatin (SM) and B) parvalbumin (PV) using immunofluorescence. Both SM- and PV-expressing GABAergic subtypes were identified among the differentiated neurons. Co-staining with β-tubulin III demonstrates the neuronal identity of cells expressing SM or PV. C) The percentage of β-tubulin III-positive cells expressing SM or PV was determined from triplicate experiments.
Electrophysiological properties of differentiated GABAergic neurons
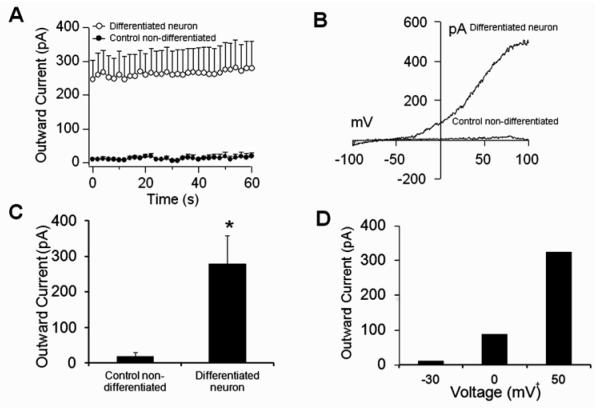
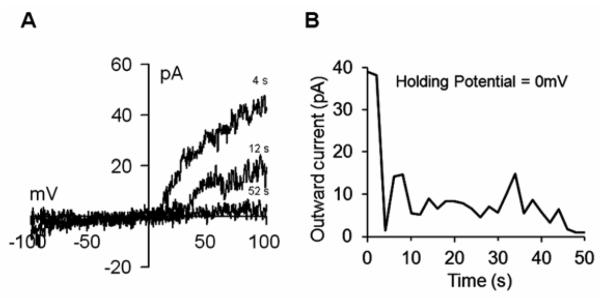
One important property of mature neurons is the expression of ion channels, in particular of the voltage-gated delayed rectifier K+ channel, which is a hallmark of excitable cells. To examine the electrophysiological properties of the differentiated GABAergic neurons, we performed patch-clamp recordings on differentiated versus control (no RA treatment) cells at the 4M stage of differentiation. We detected the presence of large outward currents in differentiated cells but not in controls (Fig. 6A and C). The observed current-voltage relationship (I/V) values were typical of voltage-gated delayed rectifier K+ channels (Fig. 6B and D). Another characteristic of these channels is their voltage sensitivity, where depolarization inhibits channel functioning. Therefore, we performed patch-clamp recordings with the holding potential set at 0 mV. Under this condition, we clearly observed suppression of K+ currents upon establishment of whole-cell configuration (Fig. 7A and B). These results demonstrated that differentiated cells produced large outward K+ currents typical of mature neurons (Chen et al., 2008; Falk et al., 2006; Nisenbaum and Wilson, 1995).
Figure 6. Whole-cell patch-clamp recordings in differentiated embryonic stem cells.
A) Average outward currents recorded from neuronal differentiated cells and undifferentiated controls for 60 s. Currents were extracted at +50 mV from a voltage ramp ranging from −100 mV to +100 mV and −80 mV holding potential (n=4 cells/group; mean+SEM). B) The current-voltage relationship (I/V) is typical for voltage-gated delayed rectifier K+ channels in the neuronal differentiated cell, but was absent in control undifferentiated cells. C) Average peak currents from panel A at 60 s after establishment of whole-cell configuration (*P<0.05). D) Data extracted at the respective voltage from the differentiated neuronal cell in panel B.
Figure 7. Effect of depolarization on Voltage-Gated Delayed Rectifier K+ currents.
A) Current-voltage relationship (I/V) from a differentiated neuronal cell extracted at +50mV after establishment of whole-cell configuration with a holding potential of 0mV. Note the inhibitory effect of voltage on channel currents. B) Outward current from panel A showing the rapid channel closure after establishment of whole-cell configuration.
Discussion
Pluripotent stem cells hold great promise for basic research experiments and future therapeutic applications due to their intrinsic ability to differentiate into all cell types. A number of research groups have described various techniques to differentiate stem cells into different lineages using growth factors or extracellular matrix proteins (Bain et al., 1995; Kitazawa et al., 2005; Okabe et al., 1996; Tian and Kaufman, 2005; Ying et al., 2003). Recent technologies involve the use of alginate-based encapsulation to generate specific cell types from ES cells. For instance, Hwang and coworkers used mouse ES cells and alginate encapsulation to generate bone tissue (Hwang et al., 2009). Similarly, other researchers have demonstrated that alginate encapsulation can increase the efficiency of ES cell differentiation into pancreatic insulin-producing cells (Wang et al., 2009). In the present study, we first demonstrated that cell encapsulation in alginate hydrogels increased the efficiency of neuronal differentiation in mouse ES cells. We observed that, even in the absence of RA treatment, neuronal differentiation occurred at higher levels than in non-encapsulated controls. Furthermore, encapsulation alone was shown to promote ES cell differentiation, albeit at low levels, along a GABAergic neuronal subtype (see Figure 3, lane 3). Since ES cell differentiation is regulated by microenvironment stimuli such as cell-cell contacts, cell-extracellular matrix interactions, and diffusion of cell cell-soluble factors (Choi et al., 2010), the observed effect of encapsulation on neuronal differentiation could be explained by 1) the presence of a hydrogel microenvironment that permits optimum cell-cell contacts (Hwang et al., 2009; Li et al., 2011) or improved cell-cell communication via paracrine signaling (Lin and Anseth, 2011), 2) a better diffusion of nutrients and/or neuronal-inducing morphogenes from the culture medium into the growing encapsulated cells (Elisseeff et al., 2002; Gerecht-Nir, 2004; Torres et al., 2000), and 3) the conjugation of the hydrogels with gelatin, which contains collagen peptides (Bailey and Paul, 1998). Collagens such as Collagen IV have been shown to promote neuronal and inhibit astroglial differentiation in cortical neuronal progenitors (Ali et al., 1998).
It could be argued that the differences in initial cell seeding between encapsulation and EB formation (2.5 × 105 cells/ml and 3 × 106 cells/ml, respectively) may be an additional factor that influences the outcome between these two technologies. Although this is an important concern, it is difficult to test since EBs failed to form when cells were seeded in non-adherent dishes at a density of 2.5 × 105 cells/ml (see Supplemental Figure S1). However, we observed that aggregates formed from encapsulated cells seeded at 2.5 × 105 cells/ml and EBs seeded at 3 × 106 cells/ml possess similar average sizes after 8 days in culture (see Supplemental Figure S1), which suggests that aggregates formed from encapsulated cells grow at a higher rate than EBs. The higher growth rate of encapsulated aggregates may result from a more permissive microenvironment that decreases cell turnover or death.
As shown here and elsewhere (Bain et al., 1995, Bibel et al., 2004; Martinez-Ceballos and Gudas, 2008), high-dose RA treatment increased the percentage of β-tubulin III-expressing cells as compared to untreated controls at day 8 of cell differentiation. Although the complete RA signaling pathway responsible for this effect on neuronal differentiation is not well understood, the recent employment of cDNA microarray technologies have identified a number of putative RA target genes that may play a role as mediators of the RA-induced neuronal differentiation of ES and embryonal carcinoma cells (Freemantle et al., 2002, Martinez-Ceballos and Gudas, 2005; for recent reviews see Clagett-Dame et al., 2006 and Soprano et al., 2007). In addition to being a direct regulator of neuronal differentiation, RA may also facilitate the neuronal differentiation of encapsulated and non-encapsulated cells by repressing genes responsible for maintaining ES cell pluripotency such as Nanog and Rex-1 (Fig. 3) or Wnt family members (Katoh, 2002; Elizalde et al., 2011), and/or by repressing ES cell differentiation along the mesodermal or endodermal lineages (Bain et al., 1996; Bibel et al., 2004, Martinez-Ceballos and Gudas, 2008).
We demonstrated by RT-PCR that the main type of neurons obtained at day 8 from encapsulated cells correspond to the GABAergic type, as shown by the mRNA expression of the GABAergic marker GAD1. The low expression levels of Rex-1 mRNA observed by RT-PCR indicate that some undifferentiated cells may remain in RA treated encapsulated cells by day 8 of differentiation. It would be of interest to determine whether these undifferentiated cells occupy a specific ES cell niche or whether they are randomly dispersed throughout the encapsulated aggregates. In contrast to what we observed in encapsulated cells, the main neuronal types obtained at day 8 from RA-treated EBs were GLT1-expressing glutamatergic cells. None of the neuronal markers assayed by RT-PCR showed detectable mRNA expression levels in control EBs (no RA), which suggests that control EBs are composed mainly of non-neuronal cells types plus an unknown number of undifferentiated ES cells. Nevertheless, the results observed in non-encapsulated cells confirm previous results obtained by Bibel and coworkers (2004) who demonstrated that, in embryoid bodies, 5μM RA induce the differentiation of mouse ES cells preferentially into glutamatergic neurons.
To further verify and extend our results obtained at day 8 of ES cell differentiation, we cultured the day 8 encapsulated cells up to the 4M stage as described in Materials and Methods. During the first two days of the 4M stage, we again treated the differentiated cells with 5 μM RA. The purpose of this additional RA treatment was to induce any remaining neuronal precursors to differentiate into GABA-expressing neurons and to aid in the terminal maturation of all GABAergic neurons present in the cultures. Support for this strategy comes from a number of reports indicating that high doses of RA promote the formation of GABAergic neurons from undifferentiated ES cells (Chatzi et al., 2009; Shan et al., 2011). Thus, we speculate that this additional RA treatment supports the enrichment and further maturation of GABAergic neurons in the absence of growth factors such as bFGF or EGF. The precise molecular mechanism of action of this second RA dose is unknown and is currently being investigated in our lab.
To determine which type of GABAergic neurons is generated using our protocol, we examined the expression by immunofluorescence of two chemical markers for specific GABAergic interneurons: PV and SM. We found that both types of GABAergic neurons are present at 4M, which indicates that our differentiation protocol generates GABAergic neurons of mixed subtypes. It is possible that an additional step involving the treatment of the differentiating cells with a factor such as BMP4 would lead to a more homogeneous population of GABAergic interneurons expressing PV (Mukhopadhyay et al., 2009).
Finally, we confirmed the maturity and in vitro functionality of the differentiated cells at 4M using the patch-clamp technique. Undifferentiated stem cells are considered non-excitable cells because they lack the expression of a particular set of ion channels (e.g. voltage-gated delayed rectifier K+ channels). These channels are critical for returning the depolarized cell to their resting membrane potential during an action potential, which is normally present in excitable cells such as GABAergic neurons. In our study, we showed that differentiated neurons developed currents typical of the voltage-gated delayed rectifier K+ channel and that were voltage sensitive. In conclusion, we have demonstrated that GABAergic neurons can be generated from encapsulated mouse ES cells after treatment with 5μM RA. Our differentiation protocol is scalable and affordable because, unlike other ES-to-GABAergic differentiation protocols, it does not require the use of expensive growth factors such as bFGF and EGF. By using this methodology, more GABAergic neurons can be obtained than by using traditional differentiation protocols. Thus, the future employment of our differentiation methodology may result in useful applications for cell replacement therapies and tissue engineering.
Supplementary Material
Figure S1. Effect of cell density on EB formation. To determine the effect of cell concentration on the formation of EBs, cells were plated in low-attachment dishes at two different concentrations: 2.5 × 105 cells/ml and 3 × 106 cells/ml. Cells were cultured for 8 days with medium changes every two days. A) EBs from cells plated at 2.5 × 105 cells/ml. B) EBs from cells plated at 3 × 106 cells/ml. C) Day 8 cell aggregates formed from cells encapsulated at a density of 2.5 × 105 cells/ml. Scale bars, 100 μm.
Highlights.
We report a protocol for the differentiation of mouse ES cells into GABAergic neurons.
The method employs hydrogel-based cell encapsulation but not expensive growth factors.
The efficiency of differentiation was increased by two-fold versus a standard protocol.
Up to 87% of the mature neurons generated are GABAergic.
The mature neurons generated with this protocol contain functional K+ channels.
Acknowledgment
The project described was supported by grants from the National Center for Research Resources (5P20RR016456-11) and the National Institute of General Medical Sciences (8 P20GM103424-11) from the National Institutes of Health. We thank Dr. Konstantin Kousoulas and Dr. Nicolas Bazan for their thoughtful advice and mentorship.
Role of the funding source
This work was supported by the NIH. No separate funding was used specifically for this study. The funding source did not have any involvement on the conduct of the research and/or the preparation of the article.
Abbreviations
- EB
embryoid body
- LIF
Leukemia Inhibitory Factor
- NE
non-encapsulated
- PDL
poly-D-lysine
- RA
all-trans-retinoic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
None of the authors have any conflicts of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Akasha AA, Sotiriadou I, Doss MX, Halbach M, Winkler J, Baunach JJ, Katsen-Globa A, Zimmermann H, Choo Y, Hescheler J, Sachinidis A. Entrapment of embryonic stem cells-derived cardiomyocytes in macroporous biodegradable microspheres: preparation and characterization. Cell. Physiol. Biochem. 2008;22:665–672. doi: 10.1159/000185550. [DOI] [PubMed] [Google Scholar]
- Ali SA, Pappas IS, Parnavelas JG. Collagen type IV promotes the differentiation of neuronal progenitors and inhibits astroglial differentiation in cortical cell cultures. Dev. Brain Res. 1998;110:31–38. doi: 10.1016/s0165-3806(98)00091-1. [DOI] [PubMed] [Google Scholar]
- Bailey AJ, Paul RG. Collagen: a not so simple protein. J Soc Leather Technol Chem. 1998;82:104–110. [Google Scholar]
- Bain G, Ray WJ, Yao M, Gottlieb DI. Retinoic acid promotes neural and represses mesodermal gene expression in mouse embryonic stem cells in culture. Biochem. Biophys. Res. Commun. 1996;223:691–694. doi: 10.1006/bbrc.1996.0957. [DOI] [PubMed] [Google Scholar]
- Bain G, Kitchens D, Yao M, Huettner JE, Gottlieb DI. Embryonic stem cells express neuronal properties in vitro. Dev. Biol. 1995;168:342–357. doi: 10.1006/dbio.1995.1085. [DOI] [PubMed] [Google Scholar]
- Bauwens C, Yin T, Dang S, Peerani R, Zandstra PW. Development of a perfusion fed bioreactor for embryonic stem cell-derived cardiomyocyte generation: oxygen-mediated enhancement of cardiomyocyte output. Biotechnol. Bioeng. 2005;90:452–461. doi: 10.1002/bit.20445. [DOI] [PubMed] [Google Scholar]
- Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Benes FM, Lim B, Matzilevich D, Walsh JP, Subburaju S, Minns M. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proc. Natl. Acad. Sci. 2007;104:10164–10169. doi: 10.1073/pnas.0703806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibel M, Richter J, Schrenk K, Tucker KL, Staiger V, Korte M, Goetz M, Barde YA. Differentiation of mouse embryonic stem cells into a defined neuronal lineage. Nat. Neurosci. 2004;7:1003–1009. doi: 10.1038/nn1301. [DOI] [PubMed] [Google Scholar]
- Bosch M, Pineda JR, Sunol C, Petriz J, Cattaneo E, Alberch J, Canals JM. Induction of GABAergic phenotype in a neural stem cell line for transplantation in an excitotoxic model of Huntington’s disease. Exp. Neurol. 2004;190:42–58. doi: 10.1016/j.expneurol.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Perez J, Barale F, Schettini G, Soares JC. GABAergic dysfunction in mood disorders. Mol. Psychiatry. 2003;8:721–37. doi: 10.1038/sj.mp.4001362. [DOI] [PubMed] [Google Scholar]
- Carpenedo RL, Seaman SA, McDevitt TC. Microsphere size effects on embryoid body incorporation and embryonic stem cell differentiation. J Biomed Mater Res A. 2010;94:466–475. doi: 10.1002/jbm.a.32710. [DOI] [PubMed] [Google Scholar]
- Cauli B, Audinat E, Lambolez B, Angulo MC, Ropert N, Tsuzuki K, Hestrin S, Rossier J. Molecular and physiological diversity of cortical nonpyramidal cells. J. Neurosci. 1997;17:3894–3906. doi: 10.1523/JNEUROSCI.17-10-03894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzi C, Scott RH, Pu J, Lang B, Nakamoto C, McCaig CD, Shen S. Derivation of homogeneous GABAergic neurons from mouse embryonic stem cells. Exp. Neurol. 2009;217:407–416. doi: 10.1016/j.expneurol.2009.03.032. [DOI] [PubMed] [Google Scholar]
- Chen LF, Yin ZQ, Chen S, Chen ZS. Differentiation and production of action potentials by embryonic rat retina stem cells in vitro. Invest. Ophthalmol. Vis. Sci. 2008;49:5144–5150. doi: 10.1167/iovs.08-1907. [DOI] [PubMed] [Google Scholar]
- Choi D, Lee HJ, Jee S, Jin S, Koo SK, Paik SS, Jung SC, Hwang SY, Lee KS, Oh B. In vitro differentiation of mouse embryonic stem cells: enrichment of endodermal cells in the embryoid body. Stem Cells. 2005;23:817–827. doi: 10.1634/stemcells.2004-0262. [DOI] [PubMed] [Google Scholar]
- Choi YY, Chung BG, Lee DH, Khademhosseini A, Kim JH, Lee SH. Controlled-size embryoid body formation in concave microwell arrays. Biomaterials. 2010;31:4296–4303. doi: 10.1016/j.biomaterials.2010.01.115. [DOI] [PubMed] [Google Scholar]
- Cicchetti F, Parent A. Striatal interneurons in Huntington’s disease: selective increase in the density of calretinin-immunoreactive medium-sized neurons. Mov. Disord. 1996;11:619–26. doi: 10.1002/mds.870110605. [DOI] [PubMed] [Google Scholar]
- Clagett-Dame M, McNeill EM, Muley PD. Role of all-trans retinoic acid in neurite outgrowth and axonal elongation. J. Neurobiol. 2006;66:739–756. doi: 10.1002/neu.20241. [DOI] [PubMed] [Google Scholar]
- Dang SM, Gerecht-Nir S, Chen J, Itskovitz-Eldor J, Zandstra PW. Controlled, scalable embryonic stem cell differentiation culture. Stem Cells. 2004;22:275–282. doi: 10.1634/stemcells.22-3-275. [DOI] [PubMed] [Google Scholar]
- Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J. Embryol. Exp. Morphol. 1985;87:27–45. [PubMed] [Google Scholar]
- Elisseeff JH, Yamada Y, Langer R. Biomaterials for tissue engineering. In: Lewandrowski KU, et al., editors. Tissue engineering and biodegradable equivalents: Scientific and clinical applications. New York; Marcel Dekker, Inc.: 2002. pp. 1–23. [Google Scholar]
- Elizalde C, Campa VM, Caro M, Schlangen K, Aransay AM, Vivanco M, Kypta RM. Distinct roles for Wnt-4 and Wnt-11 during retinoic acid-induced neuronal differentiation. Stem Cells. 2011;29:141–153. doi: 10.1002/stem.562. [DOI] [PubMed] [Google Scholar]
- Falk T, Zhang S, Erbe EL, Sherman SJ. Neurochemical and electrophysiological characteristics of rat striatal neurons in primary culture. J. Comp. Neurol. 2006;494:275–289. doi: 10.1002/cne.20819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraichard A, Chassande O, Bilbaut G, Dehay C, Savatier P, Samarut J. In vitro differentiation of embryonic stem cells into glial cells and functional neurons. J. Cell Sci. 1995;108:3181–3188. doi: 10.1242/jcs.108.10.3181. [DOI] [PubMed] [Google Scholar]
- Frederiksen K, McKay RD. Proliferation and differentiation of rat neuroepithelial precursor cells in vivo. J. Neurosci. 1988;8:1144–1151. doi: 10.1523/JNEUROSCI.08-04-01144.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freemantle SJ, Kerley JS, Olsen SL, Gross RH, Spinella MJ. Developmentally-related candidate retinoic acid target genes regulated early during neuronal differentiation of human embryonal carcinoma. Oncogene. 2002;21:2880–2889. doi: 10.1038/sj.onc.1205408. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Gerami-Naini B, Dovzhenko OV, Durning M, Wegner FH, Thomson JA, Golos TG. Trophoblast differentiation in embryoid bodies derived from human embryonic stem cells. Endocrinology. 2004;145:1517–1524. doi: 10.1210/en.2003-1241. [DOI] [PubMed] [Google Scholar]
- Gerecht-Nir S, Cohen S, Ziskind A, Itskovitz-Eldor J. Three-dimensional porous alginate scaffolds provide a conducive environment for generation of well-vascularized embryoid bodies from human embryonic stem cells. Biotechnol. Bioeng. 2004;88:313–320. doi: 10.1002/bit.20248. [DOI] [PubMed] [Google Scholar]
- Gonchar Y, Burkhalter A. Three distinct families of GABAergic neurons in rat visual cortex. Cereb. Cortex. 1997;7:347–358. doi: 10.1093/cercor/7.4.347. [DOI] [PubMed] [Google Scholar]
- Hwang YS, Cho J, Tay F, Heng JY, Ho R, Kazarian SG, Williams DR, Boccaccini AR, Polak JM, Mantalaris A. The use of murine embryonic stem cells, alginate encapsulation, and rotary microgravity bioreactor in bone tissue engineering. Biomaterials. 2009;30:499–507. doi: 10.1016/j.biomaterials.2008.07.028. [DOI] [PubMed] [Google Scholar]
- Jing D, Parikh A, Tzanakakis ES. Cardiac cell generation from encapsulated embryonic stem cells in static and scalable culture systems. Cell Transplant. 2010;19:1397–1412. doi: 10.3727/096368910X513955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh M. Regulation of WNT signaling molecules by retinoic acid during neuronal differentiation in NT2 cells: threshold model of WNT action. Int. J. Mol. Med. 2002;10:683–687. review. [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb. Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. Neurochemical features and synaptic connections of large physiologically-identified GABAergic cells in the rat frontal cortex. Neuroscience. 1998;85:677–701. doi: 10.1016/s0306-4522(97)00685-4. [DOI] [PubMed] [Google Scholar]
- Kitazawa A, Shimizu N. Differentiation of mouse embryonic stem cells into neurons using conditioned medium of dorsal root ganglia. J. Biosci. Bioeng. 2005;100:94–99. doi: 10.1263/jbb.100.94. [DOI] [PubMed] [Google Scholar]
- Kumar SS, Buckmaster PS. Hyperexcitability, interneurons, and loss of GABAergic synapses in entorhinal cortex in a model of temporal lobe epilepsy. J. Neurosci. 2006;26:4613–23. doi: 10.1523/JNEUROSCI.0064-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- Li L, Davidovich AE, Schloss JM, Chippada U, Schloss RR, Langrana NA, Yarmush ML. Neural lineage differentiation of embryonic stem cells within alginate microbeads. Biomaterials. 2011;32:4489–4497. doi: 10.1016/j.biomaterials.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Lin CC, Anseth KS. Cell-cell communication mimicry with poly(ethylene glycol) hydrogels for enhancing beta-cell function. Proceedings of the National Academy of Sciences. 2011;108:6380–6385. doi: 10.1073/pnas.1014026108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire T, Novik E, Schloss R, Yarmush M. Alginate-PLL microencapsulation: effect on the differentiation of embryonic stem cells into hepatocytes. Biotechnol. Bioeng. 2006;93:581–591. doi: 10.1002/bit.20748. [DOI] [PubMed] [Google Scholar]
- Maguire T, Davidovich AE, Wallenstein EJ, Novik E, Sharma N, Pedersen H, Androulakis IP, Schloss R, Yarmush M. Control of hepatic differentiation via cellular aggregation in an alginate microenvironment. Biotechnol. Bioeng. 2007;98:631–644. doi: 10.1002/bit.21435. [DOI] [PubMed] [Google Scholar]
- Magyar JP, Nemir M, Ehler E, Suter N, Perriard JC, Eppenberger HM. Mass production of embryoid bodies in microbeads. Ann. N. Y. Acad. Sci. 2001;944:135–143. doi: 10.1111/j.1749-6632.2001.tb03828.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Ceballos E, Gudas LJ. Hoxa1 is required for the retinoic acid-induced differentiation of embryonic stem cells into neurons. J. Neurosci. Res. 2008;86:2809–2819. doi: 10.1002/jnr.21729. [DOI] [PubMed] [Google Scholar]
- Martinez-Ceballos E, Chambon P, Gudas LJ. Differences in gene expression between wild type and Hoxa1 knockout embryonic stem cells after retinoic acid treatment or leukemia inhibitory factor (LIF) removal. J. Biol. Chem. 2005;280:16484–16498. doi: 10.1074/jbc.M414397200. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay A, McGuire T, Peng CY, Kessler JA. Differential effects of BMP signaling on parvalbumin and somatostatin interneuron differentiation. Development. 2009;136:2633–2642. doi: 10.1242/dev.034439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Nisenbaum ES, Wilson CJ. Potassium currents responsible for inward and outward rectification in rat neostriatal spiny projection neurons. J. Neurosci. 1995;15:4449–4463. doi: 10.1523/JNEUROSCI.15-06-04449.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe S, Forsberg-Nilsson K, Spiro AC, Segal M, McKay RD. Development of neuronal precursor cells and functional postmitotic neurons from embryonic stem cells in vitro. Mech. Dev. 1996;59:89–102. doi: 10.1016/0925-4773(96)00572-2. [DOI] [PubMed] [Google Scholar]
- Orive G, Hernandez RM, Gascon AR, Calafiore R, Chang TM, De Vos P, Hortelano G, Hunkeler D, Lacik I, Shapiro AM, Pedraz JL. Cell encapsulation: promise and progress. Nat. Med. 2003;9:104–107. doi: 10.1038/nm0103-104. [DOI] [PubMed] [Google Scholar]
- Shan ZY, Liu F, Lei L, Li QM, Jin LH, Wu YS, Li X, Shen JL. Generation of dorsal spinal cord GABAergic neurons from mouse embryonic stem cells. Cell Reprogram. 2011;13:85–91. doi: 10.1089/cell.2010.0055. [DOI] [PubMed] [Google Scholar]
- Shi W, Wang H, Pan G, Geng Y, Guo Y, Pei D. Regulation of the pluripotency marker Rex-1 by Nanog and Sox2. J. Biol. Chem. 2006;281:23319–23325. doi: 10.1074/jbc.M601811200. [DOI] [PubMed] [Google Scholar]
- Silva SS, Mano JF, Reis RL. Potential applications of natural origin polymer-based systems in soft tissue regeneration. Crit. Rev. Biotechnol. 2010;30:200–221. doi: 10.3109/07388551.2010.505561. [DOI] [PubMed] [Google Scholar]
- Soprano DR, Teets BW, Soprano KJ. Role of retinoic acid in the differentiation of embryonal carcinoma and embryonic stem cells. Vitam. Horm. 2007;75:69–95. doi: 10.1016/S0083-6729(06)75003-8. [DOI] [PubMed] [Google Scholar]
- Strubing C, Ahnert-Hilger G, Shan J, Wiedenmann B, Hescheler J, Wobus AM. Differentiation of pluripotent embryonic stem cells into the neuronal lineage in vitro gives rise to mature inhibitory and excitatory neurons. Mech. Dev. 1995;53:275–287. doi: 10.1016/0925-4773(95)00446-8. [DOI] [PubMed] [Google Scholar]
- Tian X, Kaufman DS. Hematopoietic development of human embryonic stem cells in culture. Methods Mol .Biol. 2005;290:149–162. doi: 10.1385/1-59259-838-2:149. [DOI] [PubMed] [Google Scholar]
- Torres DS, Freyman TM, Yannas IV, Spector M. Tendon cell contraction of collagen-GAG matrices in vitro: effect of cross-linking. Biomaterials. 2000;21:1607–1619. doi: 10.1016/s0142-9612(00)00051-x. [DOI] [PubMed] [Google Scholar]
- Wang N, Adams G, Buttery L, Falcone FH, Stolnik S. Alginate encapsulation technology supports embryonic stem cells differentiation into insulin-producing cells. J. Biotechnol. 2009;144:304–312. doi: 10.1016/j.jbiotec.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Weitzer G. Embryonic stem cell-derived embryoid bodies: an in vitro model of eutherian pregastrulation development and early gastrulation. Handb. Exp. Pharmacol. 2006;174:21–51. [PubMed] [Google Scholar]
- Westmoreland JJ, Hancock CR, Condie BG. Neuronal development of embryonic stem cells: a model of GABAergic neuron differentiation. Biochem. Biophys. Res .Commun. 2001;284:674–680. doi: 10.1006/bbrc.2001.5031. [DOI] [PubMed] [Google Scholar]
- Williams RL, Hilton DJ, Pease S, Willson TA, Stewart CL, Gearing DP, Wagner EF, Metcalf D, Nicola NA, Gough NM. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336:684–687. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- Ying QL, Stavridis M, Griffiths D, Li M, Smith A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat. Biotechnol. 2003;21:183–186. doi: 10.1038/nbt780. [DOI] [PubMed] [Google Scholar]
- Zimmermann U, Thurmer F, Jork A, Weber M, Mimietz S, Hillgartner M, Brunnenmeier F, Zimmermann H, Westphal I, Fuhr G, Noth U, Haase A, Steinert A, Hendrich C. A novel class of amitogenic alginate microcapsules for long-term immunoisolated transplantation. Ann. N. Y. Acad. Sci. 2001;944:199–215. doi: 10.1111/j.1749-6632.2001.tb03833.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Effect of cell density on EB formation. To determine the effect of cell concentration on the formation of EBs, cells were plated in low-attachment dishes at two different concentrations: 2.5 × 105 cells/ml and 3 × 106 cells/ml. Cells were cultured for 8 days with medium changes every two days. A) EBs from cells plated at 2.5 × 105 cells/ml. B) EBs from cells plated at 3 × 106 cells/ml. C) Day 8 cell aggregates formed from cells encapsulated at a density of 2.5 × 105 cells/ml. Scale bars, 100 μm.